Synthesis, Characterization, and Comparative Study on Norbornene Polymerization of CNN and PCN Pincer Palladium Complexes
Abstract
1. Introduction
2. Results and Discussion
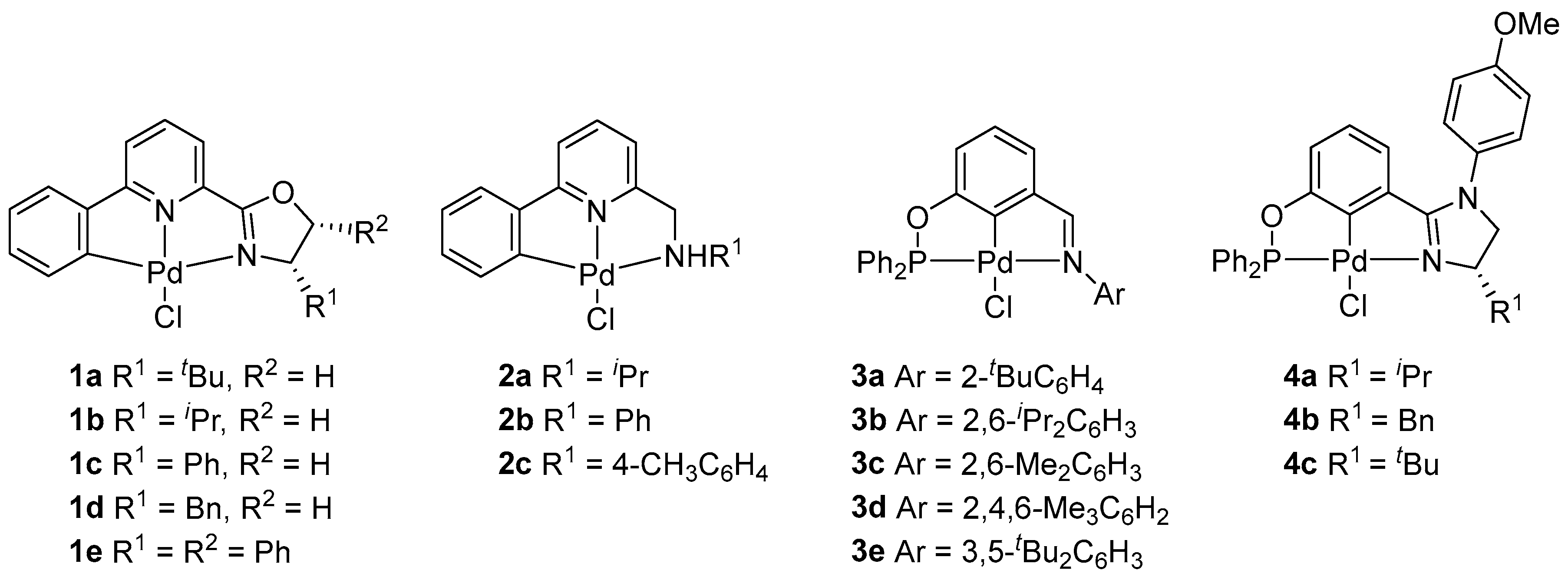
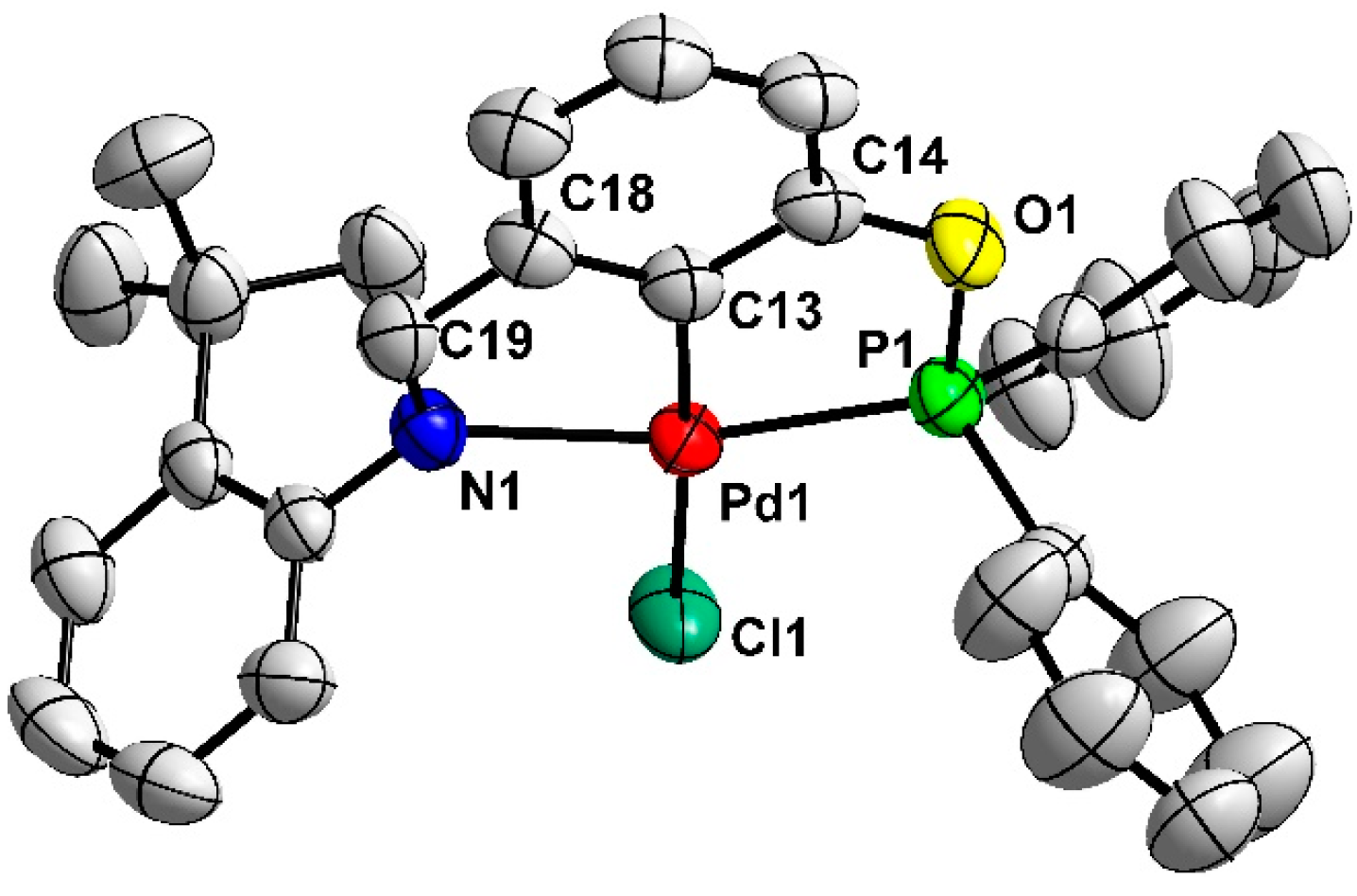
2.1. Synthesis and Characterization of New Achiral PCN Pincer Pd (II) Complexes 3a–e
2.2. Norbornene Polymerization
2.2.1. NB Polymerization Optimization with CNN Pd(II)/EtAlCl2
2.2.2. NB Polymerization Optimization with CNN Pd(II)/Et2AlCl
2.2.3. NB Polymerization Optimization with PCN Pd(II)/EtAlCl2
2.2.4. NB Polymerization Optimization with PCN Pd(II)/MAO
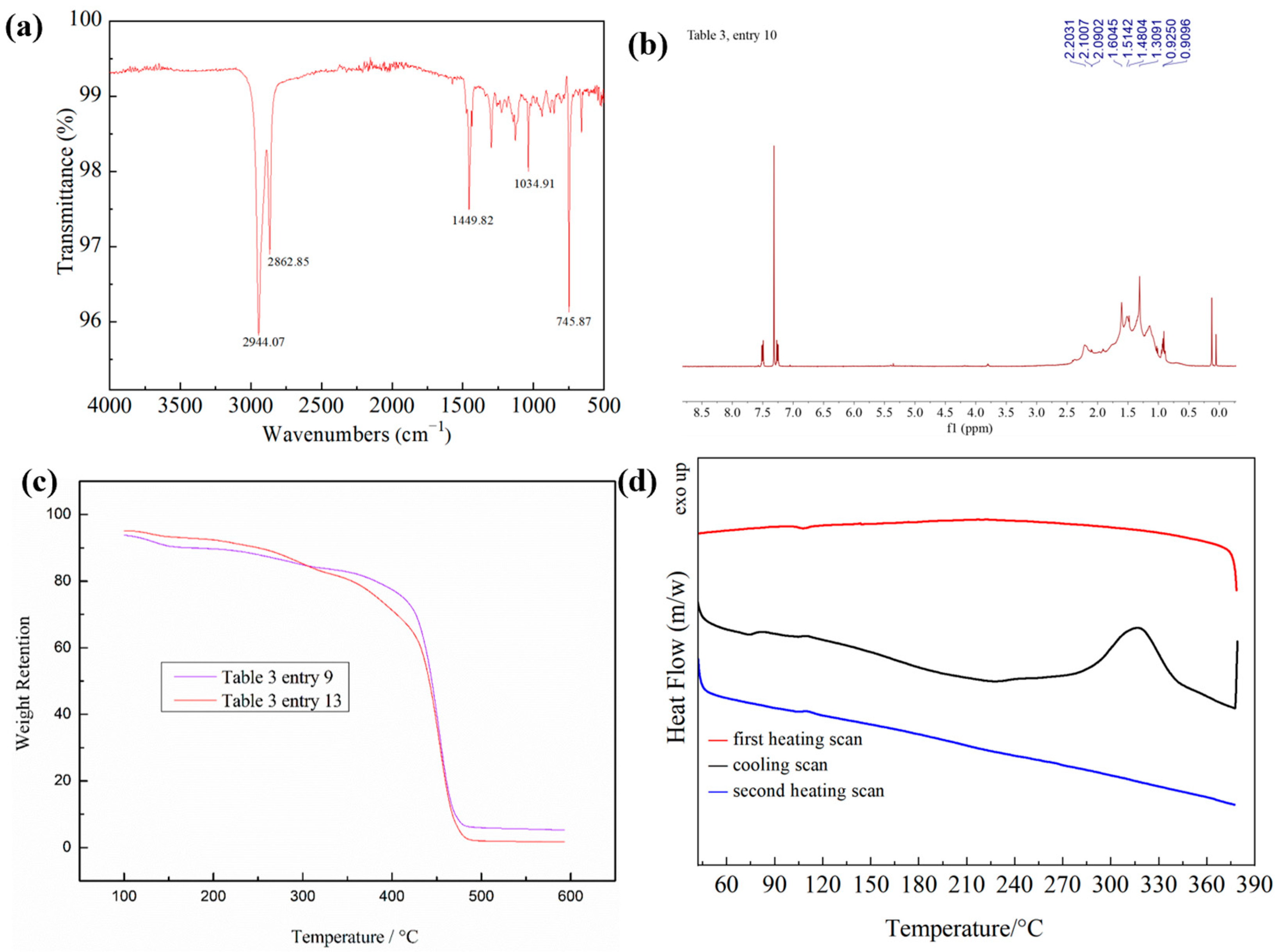
2.3. Polymer Characterization
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of m-Hydroxybenzaldimines 3a′–e′
- 3-(((2-(tert-butyl)phenyl)imino)methyl)phenol (3a′): Brown liquid (2.3 g, 10 mmol, and a 91% yield based on 2-(tert-butyl)aniline). 1H NMR (400 MHz, CDCl3): δ 8.18 (s, 1H, CH=N); 7.40–7.36 (m, 3H, and Ar-H); 7.26 (t, J = 7.8 Hz, 1H, and Ar-H); 7.22–7.13 (m, 2H, and Ar-H); 6.92–6.89 (m, 1H, and Ar-H); 6.80 (dd, J = 1.7, 7.4 Hz, 1H, and Ar-H); and 1.42 (s, 9H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 158.9, 156.2, 151.5, 143.0, 138.1, 130.3, 127.3, 126.3, 125.9, 122.2, 119.8, 118.9, 114.8, 35.8, and 30.7 ppm. HRMS (positive ESI): [M + H]+ calculated for C17H20NO+: 254.1539 was found to be 254.1543.
- 3-(((3,5-di-tert-butylphenyl)imino)methyl)phenol (3e′): Pale-brown solid (687.0 mg, 2.5 mmol, and a 89% yield based on 3,5-di-tert-butylaniline). mp 144–145 °C. 1H NMR (400 MHz, CDCl3): δ 8.42 (s, 1H, and CH=N); 7.46–7.45 (m, 1H, and Ar-H); 7.43–7.31 (m, 3H, and Ar-H); 7.05 (d, 2H, J = 1.7 Hz, and Ar-H); 6.99–6.96 (m, 1H, and Ar-H); and 1.35 (s, 18H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 160.0, 156.2, 151.9, 137.7, 130.1, 122.2, 120.4, 118.8, 115.2, 114.1, 110.1, 35.0, and 31.5 ppm.
3.1.2. Synthesis of PCN Pincer Palladium(II) Complexes 3a–e
- 2-{N-(2-tert-butylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3a): Yellow solid (66.9 mg, 58% yield). mp 248–249 °C. 1H NMR (400 MHz, CDCl3): δ 8.16 (d, J = 4.9 Hz, 1H, and CH=N); 8.05–8.00 (m, 4H, and Ph-H); 7.50–7.47 (m, 7H, Ph-H, and Ar-H); 7.24–7.13 (m, 4H, and Ar-H); 7.04–6.97 (m, 2H, and Ar-H); 1.51 (s, 9H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 175.2 (d, J = 4.4 Hz); 162.2 (d, J = 9.4 Hz); 155.5, 148.4, 145.6, 141.3, and 133.0 (d, J = 55.2 Hz); 132.4, 132.2, 132.1, and 131.6 (d, J = 14.5 Hz); 128.9 (d, J = 11.8 Hz); 127.6, 126.9, 126.8, 126.5, 124.2, 123.2, and 115.5 (d, J = 17.6 Hz); 36.1; and 32.6 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 155.4 ppm. Anal. Calcd for C29H27ClNOPPd.0.5H2O: C, 59.30; H, 4.80; N, 2.38; Found: C, 59.05; H, 4.88; N, 2.15.
- 2-{N-(2,6-diisopropylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3b): Yellow solid (36.4 mg, 30% yield). mp 319–320 °C. 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 5.4 Hz, 1H, and CH=N); 8.07–8.02 (m, 4H, and Ph-H); 7.50–7.44 (m, 6H, and Ph-H); 7.21–7.14 (m, 5H, and Ar-H); 7.04 (d, J = 7.6 Hz, 1H, and Ar-H); 3.30–3.23 (m, 2H, and CH(CH3)2); 1.36 (d, J = 6.8 Hz, 6H, and CH(CH3)2); and 1.17 (d, J = 6.9 Hz, 6H, and CH(CH3)2) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 176.1 (d, J = 4.4 Hz); 162.1 (d, J = 9.5 Hz); 155.6 (d, J = 2.3 Hz); 145.4, 144.3, 140.5, and 133.1 (d, J = 54.7 Hz); 132.2 (d, J = 2.3 Hz); 131.9 (d, J = 14.6 Hz); 128.9 (d, J = 12.4 Hz); 127.2, 127.0, 123.2, 123.1, and 115.6 (d, J = 17.5 Hz); 28.6; 24.2; and 23.1 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 154.8 ppm. Anal. Calcd for C31H31ClNOPPd.0.5H2O: C, 60.50; H, 5.24; N, 2.28. Found: C, 60.73; H, 5.23; N, 2.06.
- 2-{N-(2,6-dimethylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3c): Yellow solid (76.9 mg, 70% yield). mp 245–246 °C. 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 5.4 Hz, 1H, and CH=N); 8.06–8.01 (m, 4H, and Ph-H); 7.49–7.44 (m, 6H, and Ph-H); 7.18–7.13 (m, 2H, and Ar-H); 7.09–7.03 (m, 4H, and Ar-H); and 2.33 (s, 6H, and CH3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 176.8 (d, J = 4.6 Hz); 162.2 (d, J = 9.8 Hz); 155.3 (d, J = 2.2 Hz); 145.6, 144.5, 135.9, and 133.0 (d, J = 54.2 Hz); 132.2 (d, J = 2.5 Hz); 131.8 (d, J = 15.1 Hz); 129.6 and 128.9 (d, J = 11.8 Hz); 128.8, 127.0, 123.0, and 115.5 (d, J = 16.8 Hz); 21.0; and 19.0 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 154.5 ppm. Anal. Calcd for C27H23ClNOPPd: C, 58.93; H, 4.21; N, 2.55. Found: C, 58.66; H, 4.27; N, 2.33.
- 2-{N-(2,4,6-trimethylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3d): Yellow solid (69.8 mg, 62% yield). mp 319–320 °C. 1H NMR (400 MHz, CDCl3): δ 8.07 (d, J = 5.5 Hz, 1H, and CH=N); 8.06–8.01 (m, 4H, and Ph-H); 7.52–7.44 (m, 6H, and Ph-H); 7.17–7.12 (m, 2H, and Ar-H); 7.04–7.02 (m, 1H, and Ar-H); 6.91 (s, 2H, and Ar-H); and 2.30–2.29 (m, 9H, and CH3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 176.9 (d, J = 4.4 Hz); 162.2 (d, J = 9.6 Hz); 155.3 (d, J = 1.7 Hz); 145.6, 144.5, 136.0, and 133.0 (d, J = 54.5 Hz); 132.2 (d, J = 2.4 Hz); 131.8 (d, J = 14.8 Hz); 129.7 and 128.9 (d, J = 11.9 Hz); 128.8, 127.0, 123.1, and 115.5 (d, J = 17.2 Hz); 21.0; and 19.0 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 154.4 ppm. Anal. Calcd for C28H25ClNOPPd.0.5H2O: C, 58.65; H, 4.57; N, 2.44. Found: C, 58.91; H, 4.52; N, 2.20.
- 2-{N-(3,5-di-tert-butylphenyl)imino}-6-(diphenylphosphinoxy)phenylchloropalladium(II) (3e): Yellow solid (57.0 mg, 45% yield). mp 247–248 °C. 1H NMR (400 MHz, CDCl3): δ 8.36 (d, J = 4.6 Hz, 1H, and CH=N); 8.08–8.03 (m, 4H, and Ph-H); 7.50–7.44 (m, 6H, and Ph-H); 7.40–7.39 (m, 3H, and Ar-H); 7.22 (d, J = 7.4 Hz, 1H, and Ar-H); 7.16–7.12 (m, 1H, and Ar-H); 7.00 (d, J = 8.0 Hz, 1H, and Ar-H); and 1.37 (s, 18H, and C(CH3)3) ppm. 13C{1H} NMR (100 MHz, CDCl3): δ 172.1 (d, J = 4.0 Hz); 162.2 (d, J = 9.4 Hz); 154.4, 151.5, 147.5, 146.2, and 132.8 (d, J = 55.6 Hz); 132.2 (d, J = 2.5 Hz); 131.8 (d, J = 14.4 Hz); 128.9 (d, J = 11.9 Hz); 127.0, 123.2, 122.1, 118.0, and 115.2 (d, J = 17.7 Hz); 35.2; and 31.5 ppm. 31P{1H} NMR (162 MHz, CDCl3): δ 153.7 ppm. Anal. Calcd for C33H35ClNOPPd.0.5H2O: C, 61.59; H, 5.64; N, 2.18. Found: C, 62.05; H, 5.65; N, 1.97.
3.2. General Procedure for Norbornene Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.-Z.; Liu, J.-Y.; Li, Y.-S.; Tong, Y.-J. Synthesis, structure and norbornene polymerization behavior of nickel complexes bearing two β-ketoiminato chelate ligands. J. Organomet. Chem. 2004, 689, 1295–1303. [Google Scholar] [CrossRef]
- Blank, F.; Scherer, H.; Ruiz, J.; Rodríguez, V.; Janiak, C. Palladium(II) complexes with pentafluorophenyl ligands: Structures, C6F5 fluxionality by 2D-NMR studies and pre-catalysts for the vinyl addition polymerization of norbornene. Dalton Trans. 2010, 39, 3609–3619. [Google Scholar] [PubMed]
- Li, M.; Song, H.; Wang, B. Synthesis, structures, and norbornene polymerization behavior of palladium methyl complexes bearing N-heterocyclic carbene-sulfonate ligands. J. Organomet. Chem. 2016, 804, 118–122. [Google Scholar]
- Bermesheva, E.V.; Medentseva, E.I.; Khrychikova, A.P.; Wozniak, A.I.; Guseva, M.A.; Nazarov, I.V.; Morontsev, A.A.; Karpov, G.O.; Topchiy, M.A.; Asachenko, A.F.; et al. Air-Stable Single-Component Pd-Catalysts for Vinyl-Addition Polymerization of Functionalized Norbornenes. ACS Catal. 2022, 12, 15076–15090. [Google Scholar] [CrossRef]
- Wang, W.; Qu, S.; Li, X.; Chen, J.; Guo, Z.; Sun, W.-H. Transition Metal Complex Catalysts Promoting Copolymers of Cycloolefin with Propylene/higher Olefins. Coord. Chem. Rev. 2023, 494, 215351. [Google Scholar]
- Li, M.; Fang, Y.; Cai, Z.; Eisen, M.S. Nickel- and Palladium-Catalyzed Copolymerizations of Norbornene with Polar α-Olefifins. ChemCatChem 2024, 16, e202301731. [Google Scholar] [CrossRef]
- Bermesheva, E.V.; Bermeshev, M.V. Single-Component Catalysts for the Vinyl-Addition Polymerization of Norbornene and its Derivatives. ChemCatChem 2023, 15, e202300818. [Google Scholar] [CrossRef]
- Lunin, A.O.; Andreyanov, F.A.; Makarov, I.S.; Bermeshev, M.V. Vinyl-Addition Homopolymeization of Norbornenes with Bromoalkyl Groups. Polymers 2023, 15, 4444. [Google Scholar] [CrossRef]
- Margaret Powell, E.; Mimna, R.A.; Day, C.S.; Day, V.W.; Long, B.K.; Rhodes, L.F. Polymerization of Ester-Functionalized Norbornenes Using Neutral Nickel Catalysts. Organometallics 2024, 43, 2565–2573. [Google Scholar] [CrossRef]
- Li, M.; Cai, Z.; Eisen, M.S. Rational Design of Aldimine Imidazolidin-2-imine/guanidine Nickel Catalysts for Norbornene (Co)polymerizations with Enhanced Catalytic Performance. J. Catal. 2023, 420, 58–67. [Google Scholar] [CrossRef]
- Li, D.; Zheng, H.; Gao, H.; Wang, X.; Gao, H. Ion Pair Effects of Zwitterionic Ni/Pd Allyl Complexes Bearing an α-Sulfonate-β-diimine Ligand by Binding of B(C6F5)3 on Norbornene Polymerization. Organometallics 2024, 43, 2527–2536. [Google Scholar] [CrossRef]
- Birajdar, R.S.; Gupta, P.; Gonnade, R.G.; Chikkali, S.H. Synthesis of Imine-Phenoxy Ligated Palladium Complexes for Norbornene Homopolymerization. Inorg. Chem. 2025, 64, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pei, L.; Du, W.; Xiao, X.; Gao, H.; Zheng, H.; Gao, H. Synthesis of Branched Cyclo-Olefin Copolymers Using Neutral α-Sulfonate-β-Diimine Nickel Catalyst. Molecules 2025, 30, 157. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef]
- Dίez-González, S.; Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Late Transition Metal Catalysis. Chem. Rev. 2009, 109, 3612–3676. [Google Scholar] [CrossRef]
- Bianchini, C.; Giambastiani, G.; Luconi, L.; Meli, A. Olefin oligomerization, homopolymerization and copolymerization by late transition metals supported by (imino)pyridine ligands. Coord. Chem. Rev. 2010, 254, 431–455. [Google Scholar] [CrossRef]
- Yao, Z.-J.; Jin, G.-X. Transition metal complexes based on carboranyl ligands containing N, P, and S donors: Synthesis, reactivity and applications. Coord. Chem. Rev. 2013, 257, 2522–2535. [Google Scholar] [CrossRef]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef]
- Zhuang, R.; Liu, H.; Guo, J.; Dong, B.; Zhao, W.; Hu, Y.; Zhang, X. Highly active nickel(II) and palladium(II) complexes bearing N,N,P tridentate ligand for vinyl addition polymerization of norbornene. Eur. Polym. J. 2017, 93, 358–367. [Google Scholar] [CrossRef]
- Yang, D.; Dong, J.; Wang, B. Homo- and copolymerization of norbornene with tridentate nickel complexes bearing o-aryloxide-N-heterocyclic carbene ligands. Dalton Trans. 2018, 47, 180–189. [Google Scholar] [CrossRef]
- Santi, R.; Romano, A.M.; Sommazzi, A.; Grande, M.; Bianchini, C.; Mantovani, G. Catalytic polymerisation of ethylene with tris(pyrazolyl)borate complexes of late transition metals. J. Mol. Catal. A Chem. 2005, 229, 191–197. [Google Scholar] [CrossRef]
- Ortega-Jiménez, F.; López-Cortés, J.G.; Ortega-Alfaro, M.C.; Penieres-Carrillo, J.C.; Quijada, R.; Alvarez-Toledano, C. Evaluation of catalytic activity in ethylene polymerization and ethylene/10-undecen-1-ol copolymerization of new orthopalladated complexes derived from tridentade ligands [C,N,S]. Appl. Catal. A Gen. 2012, 417–418, 1–5. [Google Scholar] [CrossRef]
- Long, J.; Gao, H.; Song, K.; Liu, F.; Hu, H.; Zhang, L.; Zhu, F.; Wu, Q. Synthesis and Characterization of NiII and PdII Complexes Bearing N,N,S Tridentate Ligands and Their Catalytic Properties for Norbornene Polymerization. Eur. J. Inorg. Chem. 2008, 2008, 4296–4305. [Google Scholar] [CrossRef]
- Han, F.-B.; Zhang, Y.-L.; Sun, X.-L.; Li, B.-G.; Guo, Y.-H.; Tang, Y. Synthesis and Characterization of Pyrrole-imine [N-NP] Nickel(II) and Palladium(II) Complexes and Their Applications to Norbornene Polymerization. Organometallics 2008, 27, 1924–1928. [Google Scholar]
- Qiao, Y.-L.; Jin, G.-X. Nickel(II) and Palladium(II) Complexes with Tridentate [C,N,S] and [C,N,P] Ligands: Syntheses, Characterization, and Catalytic Norbornene Polymerization. Organometallics 2013, 32, 1932–1937. [Google Scholar] [CrossRef]
- Das, S.; Subramaniyan, V.; Mani, G. Nickel(II) and Palladium(II) Complexes Bearing an Unsymmetrical Pyrrole-Based PNN Pincer and Their Norbornene Polymerization Behaviors versus the Symmetrical NNN and PNP Pincers. Inorg. Chem. 2019, 58, 3444–3456. [Google Scholar] [CrossRef]
- Kumar, S.; Mani, G.; Mondal, S.; Chattaraj, P.K. Pyrrole-Based New Diphosphines: Pd and Ni Complexes Bearing the PNP Pincer Ligand. Inorg. Chem. 2012, 51, 12527–12539. [Google Scholar]
- Ghorai, D.; Kumar, S.; Mani, G. Mononuclear, helical binuclear palladium and lithium complexes bearing a new pyrrole-based NNN-pincer ligand: Fluxional property. Dalton Trans. 2012, 41, 9503–9512. [Google Scholar]
- Huang, Y.; He, J.; Liu, Z.; Cai, G.; Zhang, S.; Li, X. A highly active chiral (S,S)-bis(oxazoline) Pd(II) alkyl complex/activator catalytic system for vinyl polymerization of norbornene in air and water. Polym. Chem. 2017, 8, 1217–1222. [Google Scholar] [CrossRef]
- He, J.; Liu, Z.; Du, G.; Fu, Y.; Zhang, S.; Li, X. Chiral Palladium(II) and Nickel(II) Complexes with C2-Symmetrical Tridentate Bis(oxazoline) Ligands: Synthesis, Characterization, and Catalytic Norbornene Polymerization. Organometallics 2014, 33, 6103–6112. [Google Scholar]
- You, F.; Liu, H.; Luo, G.; Shi, X. Tridentate diarylamido-based pincer complexes of nickel and palladium: Sidearm effects in the polymerization of norbornene. Dalton Trans. 2019, 48, 12219–12227. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, H.; Shi, X. Synthesis of nickel and palladium complexes with diarylamido-based unsymmetrical pincer ligands and application for norbornene polymerization. Dalton Trans. 2019, 48, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, H.; You, F.; Shi, X. Synthesis of palladium complexes with quinolino-based tridentate ligands and their applications for norbornene polymerization. Inorg. Chem. Commun. 2020, 119, 108139. [Google Scholar] [CrossRef]
- Yang, D.; Tang, Y.; Song, H.; Wang, B. Synthesis, Structures, and Norbornene Polymerization Behavior of Palladium Complexes Bearing Tridentate o-Aryloxide-N-heterocyclic Carbene Ligands. Organometallics 2016, 35, 1392–1398. [Google Scholar] [CrossRef]
- Dong, J.; Wang, B. Homo- and copolymerization of norbornene using tridentate IzQO palladium catalysts with dimethylaminoethyl as a side arm. Polym. Chem. 2021, 12, 4736–4747. [Google Scholar] [CrossRef]
- Wang, T.; Hao, X.-Q.; Zhang, X.-X.; Gong, J.-F.; Song, M.-P. Synthesis, structure and catalytic properties of CNN pincer palladium(II) and ruthenium(II) complexes with N-substituted-2-aminomethyl-6-phenylpyridines. Dalton Trans. 2011, 40, 8964–8976. [Google Scholar] [CrossRef]
- Wang, T.; Hao, X.-Q.; Huang, J.-J.; Wang, K.; Gong, J.-F.; Song, M.-P. Chiral CNN Pincer Palladium(II) Complexes with 2-Aryl-6-(oxazolinyl)pyridine Ligands: Synthesis, Characterization, and Application to Enantioselective Allylation of Isatins and Suzuki-Miyaura Coupling Reaction. Organometallics 2014, 33, 194–205. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Wang, W.; Shao, D.-D.; Hao, X.-Q.; Gong, J.-F.; Song, M.-P. Unsymmetrical Chiral PCN Pincer Palladium(II) and Nickel(II) Complexes of (Imidazolinyl)aryl Phosphinite Ligands: Synthesis via Ligand C-H Activation, Crystal Structures, and Catalytic Studies. Organometallics 2010, 29, 2579–2587. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Wang, C.; Gong, J.-F.; Song, M.-P. Facile synthesis of achiral and chiral PCN pincer palladium(II) complexes and their application in the Suzuki and copper-free Sonogashira cross-coupling reactions. J. Organomet. Chem. 2009, 694, 2555–2561. [Google Scholar] [CrossRef]
- Liu, J.-K.; Gong, J.-F.; Song, M.-P. Chiral palladium pincer complexes for asymmetric catalytic reactions. Org. Biomol. Chem. 2019, 17, 6069–6098. [Google Scholar] [CrossRef]
- Huang, J.-J.; Zhang, X.-Q.; Yang, J.-J.; Gong, J.-F.; Song, M.-P. Chiral (phosphine)-(imidazoline) PCN pincer palladium(II) complexes: Synthesis and application in asymmetric hydrophosphination of 2-alkenoylpyridines with diphenylphosphine. Dalton Trans. 2022, 51, 8350–8367. [Google Scholar] [PubMed]
- Qu, J.-J.; Shi, L.-L.; Wang, Y.-B.; Yan, J.; Shao, T.; Hao, X.-Q.; Wang, J.-X.; Zhang, H.-Y.; Gong, J.-F.; Song, B. The Novel Function of Unsymmetrical Chiral CCN Pincer Nickel Complexes as Chemotherapeutic Agents Targeting Prostate Cancer Cells. Molecules 2022, 27, 3106. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, X.; Zhu, Y.; Wang, F.; Gong, J.; Song, M. Pincer iridium(III)-catalyzed enantioselective C(sp3)-H functionalization via carbenoid C–H insertion of 3-diazooxindoles with 1,4-cyclohexadiene. Chin. Chem. Lett. 2022, 33, 2437–2441. [Google Scholar]
- Jiang, H.; Zhang, C.-Y.; Liu, J.-K.; Song, M.-P.; Gong, J.-F. Rhodium-Catalyzed Direct Enantioselective Alkynylation of Trifluoropyruvates with Terminal 1,3-Diynes. Adv. Synth. Catal. 2023, 365, 3967–3972. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Wang, H.-J.; Jiang, H.; Song, M.-P.; Gong, J.-F. Chiral bis(imidazoline) NCN pincer iridium(III)-catalyzed enantioselective alkynylation of trifluoropyruvates with terminal alkynes. Green Synth. Catal. 2024; in press. [Google Scholar] [CrossRef]
- Wang, X.; Dong, B.; Yang, Q.; Liu, H.; Hu, Y.; Zhang, X. Boosting the Thermal Stability of α-Diimine Palladium Complexes in Norbornene Polymerization from Construction of Intraligand Hydrogen Bonding and Simultaneous Increasing Axial/Equatorial Bulkiness. Inorg. Chem. 2021, 60, 2347–2361. [Google Scholar]
- Kaminsky, W. The Discovery of Metallocene Catalysts and Their Present State of the Art. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3911–3921. [Google Scholar]
- Dong, J.; Yang, D.; Wang, B. Homo- and Copolymerization of Norbornene with Allyl Palladium and Nickel Complexes Bearing Imidazo[1,5-a]pyridine Sulfonate Ligands. Eur. J. Inorg. Chem. 2021, 2021, 4661–4668. [Google Scholar]


| Entry | Cat. | Temp. (°C) | t (min) | [Cat.] (μmol) | Al/Pd | PNB (g) | Conv. (%) | Activity b |
|---|---|---|---|---|---|---|---|---|
| 1 c | ― | 40 | 30 | ― | ― | 0 | 0 | 0 |
| 2 | 1a | 40 | 30 | 1.0 | 2000 | 0.7513 | 72.94 | 1.5026 |
| 3 | 1a | 40 | 30 | 1.0 | 2500 | 0.6842 | 66.43 | 1.3684 |
| 4 | 1a | 40 | 30 | 1.0 | 1500 | 0.7328 | 71.15 | 1.4656 |
| 5 | 1a | 0 | 30 | 1.0 | 2000 | 0.2606 | 25.30 | 0.5212 |
| 6 | 1a | 20 | 30 | 1.0 | 2000 | 0.8273 | 80.32 | 1.6546 |
| 7 | 1a | 60 | 30 | 1.0 | 2000 | 0.7436 | 72.19 | 1.4872 |
| 8 | 1a | 80 | 30 | 1.0 | 2000 | 0.7558 | 73.38 | 1.5116 |
| 9 | 1a | 20 | 20 | 1.0 | 2000 | 0.8197 | 79.58 | 2.4591 |
| 10 | 1a | 20 | 10 | 1.0 | 2000 | 0.8153 | 79.16 | 4.8918 |
| 11 | 1a | 20 | 5 | 1.0 | 2000 | 0.7683 | 74.59 | 9.2196 |
| 12 | 1a | 20 | 10 | 1.2 | 2000 | 0.8072 | 78.37 | 4.0360 |
| 13 | 1a | 20 | 10 | 0.8 | 2000 | 0.7264 | 70.52 | 5.4480 |
| 14 | 1b | 20 | 10 | 1.0 | 2000 | 0.7032 | 68.27 | 4.2192 |
| 15 | 1c | 20 | 10 | 1.0 | 2000 | 0.6767 | 65.70 | 4.0602 |
| 16 | 1d | 20 | 10 | 1.0 | 2000 | 0.6907 | 67.06 | 4.1442 |
| 17 | 1e | 20 | 10 | 1.0 | 2000 | 0.7018 | 68.14 | 4.2108 |
| 18 | 2a | 20 | 10 | 1.0 | 2000 | 0.7172 | 69.63 | 4.3032 |
| 19 | 2b | 20 | 10 | 1.0 | 2000 | 0.7558 | 73.38 | 4.5348 |
| 20 | 2c | 20 | 10 | 1.0 | 2000 | 0.7973 | 77.41 | 4.7838 |
| Entry | Cat. | Temp. (°C) | t (min) | [Cat.] (μmol) | Al/Pd | PNB (g) | Conv. (%) | Activity b |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | 40 | 8 | 1.0 | 2000 | 0.9654 | 93.73 | 7.2405 |
| 2 | 1a | 40 | 8 | 1.0 | 1500 | 0.9876 | 95.88 | 7.4070 |
| 3 | 1a | 40 | 8 | 1.0 | 1000 | 0.5732 | 55.65 | 4.2990 |
| 4 | 1a | 40 | 8 | 1.0 | 500 | 0.4230 | 41.07 | 3.1725 |
| 5 | 1a | 0 | 8 | 1.0 | 1500 | 0.9281 | 90.11 | 6.9608 |
| 6 | 1a | 20 | 8 | 1.0 | 1500 | 1.0253 | 99.54 | 7.6898 |
| 7 | 1a | 60 | 8 | 1.0 | 1500 | 0.1279 | 12.42 | 0.9593 |
| 8 | 1a | 80 | 8 | 1.0 | 1500 | 0.0539 | 5.23 | 0.4043 |
| 9 | 1a | 20 | 8 | 0.8 | 1500 | 0.8976 | 87.15 | 8.4150 |
| 10 | 1a | 20 | 6 | 1.0 | 1500 | 0.9597 | 93.17 | 9.5970 |
| 11 | 1a | 20 | 10 | 1.0 | 1500 | 0.9362 | 90.89 | 5.6172 |
| 12 | 1b | 20 | 8 | 1.0 | 1500 | 1.0233 | 99.35 | 7.6748 |
| 13 | 1c | 20 | 8 | 1.0 | 1500 | 1.0025 | 97.33 | 7.6688 |
| 14 | 1d | 20 | 8 | 1.0 | 1500 | 0.9663 | 93.82 | 7.2473 |
| 15 | 1e | 20 | 8 | 1.0 | 1500 | 0.9935 | 96.46 | 7.4513 |
| 16 | 2a | 20 | 8 | 1.0 | 1500 | 0.9001 | 87.39 | 6.7508 |
| 17 | 2b | 20 | 8 | 1.0 | 1500 | 0.9268 | 89.98 | 6.9510 |
| 18 | 2c | 20 | 8 | 1.0 | 1500 | 1.0051 | 97.58 | 7.5383 |
| Entry | Cat. | Temp. (°C) | t (min) | [Cat.] (μmol) | Al/Pd | PNB (g) | Conv. (%) | Activity b |
|---|---|---|---|---|---|---|---|---|
| 1 | 3a | 40 | 10 | 1.0 | 1000 | 0.2642 | 25.65 | 1.5852 |
| 2 | 3a | 40 | 10 | 1.0 | 1500 | 0.2065 | 20.05 | 1.2390 |
| 3 | 3a | 40 | 10 | 1.0 | 2000 | 0.7095 | 68.89 | 4.2570 |
| 4 | 3a | 40 | 10 | 1.0 | 2500 | 0.5676 | 55.11 | 3.4056 |
| 5 | 3a | 0 | 10 | 1.0 | 2000 | 0.6235 | 60.53 | 1.2470 |
| 6 | 3a | 20 | 10 | 1.0 | 2000 | 0.6587 | 63.95 | 1.3174 |
| 7 | 3a | 60 | 10 | 1.0 | 2000 | 0.4025 | 39.08 | 2.4150 |
| 8 | 3a | 80 | 10 | 1.0 | 2000 | 0.5705 | 55.39 | 3.4230 |
| 9 | 3a | 40 | 30 | 1.0 | 2000 | 0.8788 | 85.32 | 1.7576 |
| 10 | 3a | 40 | 60 | 1.0 | 2000 | 0.8827 | 85.70 | 0.8827 |
| 11 | 3a | 40 | 30 | 1.2 | 2000 | 0.8735 | 84.81 | 1.4558 |
| 12 | 3a | 40 | 30 | 0.8 | 2000 | 0.7243 | 70.32 | 1.4486 |
| 13 | 3b | 40 | 30 | 1.0 | 2000 | 0.6421 | 62.34 | 1.2842 |
| 14 | 3c | 40 | 30 | 1.0 | 2000 | 0.5068 | 49.20 | 1.0136 |
| 15 | 3d | 40 | 30 | 1.0 | 2000 | 0.3625 | 35.19 | 0.7250 |
| 16 | 3e | 40 | 30 | 1.0 | 2000 | 0.2663 | 25.85 | 0.5326 |
| 17 | 4a | 40 | 30 | 1.0 | 2000 | 0.6097 | 59.19 | 1.2194 |
| 18 | 4b | 40 | 30 | 1.0 | 2000 | 0.6259 | 66.47 | 1.2518 |
| 19 | 4c | 40 | 30 | 1.0 | 2000 | 0.4715 | 45.78 | 0.9430 |
| Entry | Cat. | Temp. (°C) | t (min) | [Cat.] (μmol) | Al/Pd | PNB (g) | Conv. (%) | Activity b |
|---|---|---|---|---|---|---|---|---|
| 1 | 3a | 40 | 30 | 1.0 | 5000 | 1.0163 | 98.67 | 2.0326 |
| 2 | 3a | 40 | 30 | 1.0 | 4000 | 0.6068 | 58.91 | 1.2136 |
| 3 | 3a | 40 | 30 | 1.0 | 3000 | 0.4348 | 42.21 | 0.8696 |
| 4 | 3a | 20 | 30 | 1.0 | 5000 | 0.8762 | 85.07 | 1.7524 |
| 5 | 3a | 60 | 30 | 1.0 | 5000 | 0.7411 | 71.95 | 1.4822 |
| 6 | 3a | 80 | 30 | 1.0 | 5000 | 1.0083 | 97.89 | 2.016 |
| 7 | 3a | 40 | 15 | 1.0 | 5000 | 0.7091 | 68.84 | 2.8364 |
| 8 | 3a | 40 | 6 | 1.0 | 5000 | 0.5889 | 57.17 | 5.8890 |
| 9 | 3a | 40 | 30 | 0.8 | 5000 | 0.6063 | 58.86 | 1.5158 |
| 10 | 3b | 40 | 30 | 1.0 | 5000 | 0.2369 | 23.00 | 0.4738 |
| 11 | 3c | 40 | 30 | 1.0 | 5000 | 1.0172 | 98.76 | 2.0344 |
| 12 | 3d | 40 | 30 | 1.0 | 5000 | trace | / | / |
| 13 | 3e | 40 | 30 | 1.0 | 5000 | 0.5699 | 55.33 | 1.1398 |
| 14 | 4a | 40 | 30 | 1.0 | 5000 | 0.1045 | 10.15 | 0.2090 |
| 15 | 4b | 40 | 30 | 1.0 | 5000 | trace | / | / |
| 16 | 4c | 40 | 30 | 1.0 | 5000 | 0.2375 | 23.06 | 0.4750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, J.-K.; Wang, Y.-D.; Hao, X.-Q.; Song, M.-P.; Gong, J.-F.; Jiang, H. Synthesis, Characterization, and Comparative Study on Norbornene Polymerization of CNN and PCN Pincer Palladium Complexes. Molecules 2025, 30, 1530. https://doi.org/10.3390/molecules30071530
Wang H, Liu J-K, Wang Y-D, Hao X-Q, Song M-P, Gong J-F, Jiang H. Synthesis, Characterization, and Comparative Study on Norbornene Polymerization of CNN and PCN Pincer Palladium Complexes. Molecules. 2025; 30(7):1530. https://doi.org/10.3390/molecules30071530
Chicago/Turabian StyleWang, Huizhu, Jin-Kui Liu, Yi-Dong Wang, Xin-Qi Hao, Mao-Ping Song, Jun-Fang Gong, and Hui Jiang. 2025. "Synthesis, Characterization, and Comparative Study on Norbornene Polymerization of CNN and PCN Pincer Palladium Complexes" Molecules 30, no. 7: 1530. https://doi.org/10.3390/molecules30071530
APA StyleWang, H., Liu, J.-K., Wang, Y.-D., Hao, X.-Q., Song, M.-P., Gong, J.-F., & Jiang, H. (2025). Synthesis, Characterization, and Comparative Study on Norbornene Polymerization of CNN and PCN Pincer Palladium Complexes. Molecules, 30(7), 1530. https://doi.org/10.3390/molecules30071530








