Product Development Study of Freeze-Dried Apples Enriched with Sea Buckthorn Juice and Calcium Lactate
Abstract
1. Introduction
2. Results and Discussion
2.1. First Part of Research
2.1.1. Optimization of Conditions to Obtain Optimum Total Phenolic Content and Antioxidant Activities
2.1.2. Color of Freeze-Dried Apples Prepared in Optimized Conditions
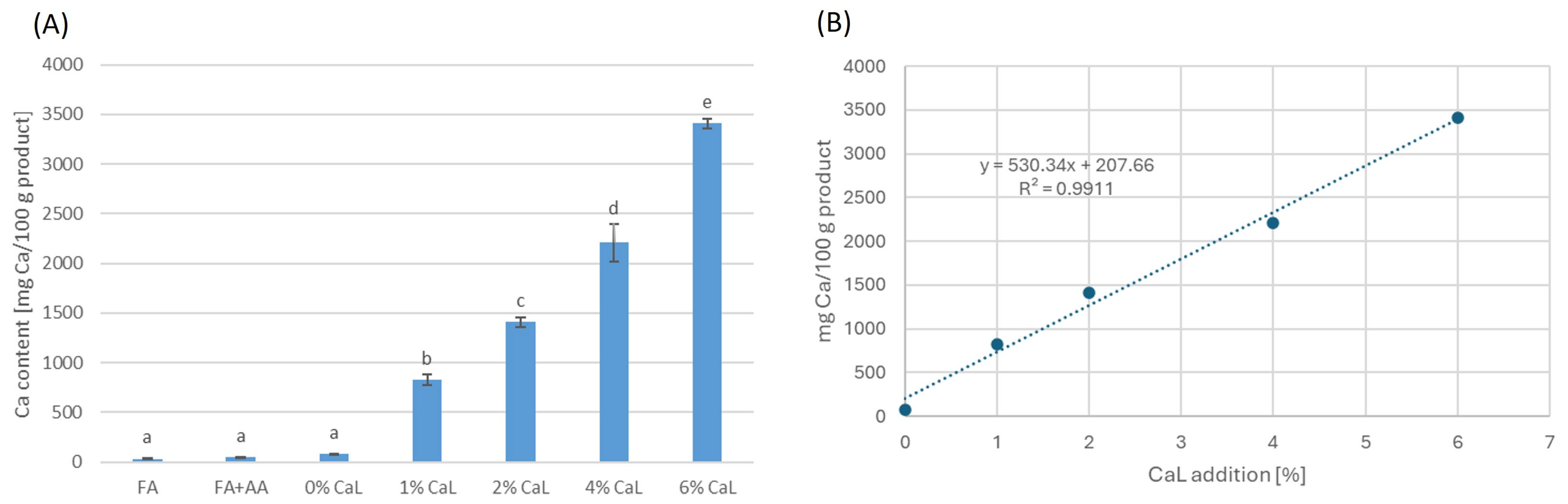
2.2. Second Part of Research
2.3. Analyses of Selected Samples
2.3.1. Quantification of Phenolic and Carotenoid Composition of Freeze-Dried Apples by UPLC-PDA
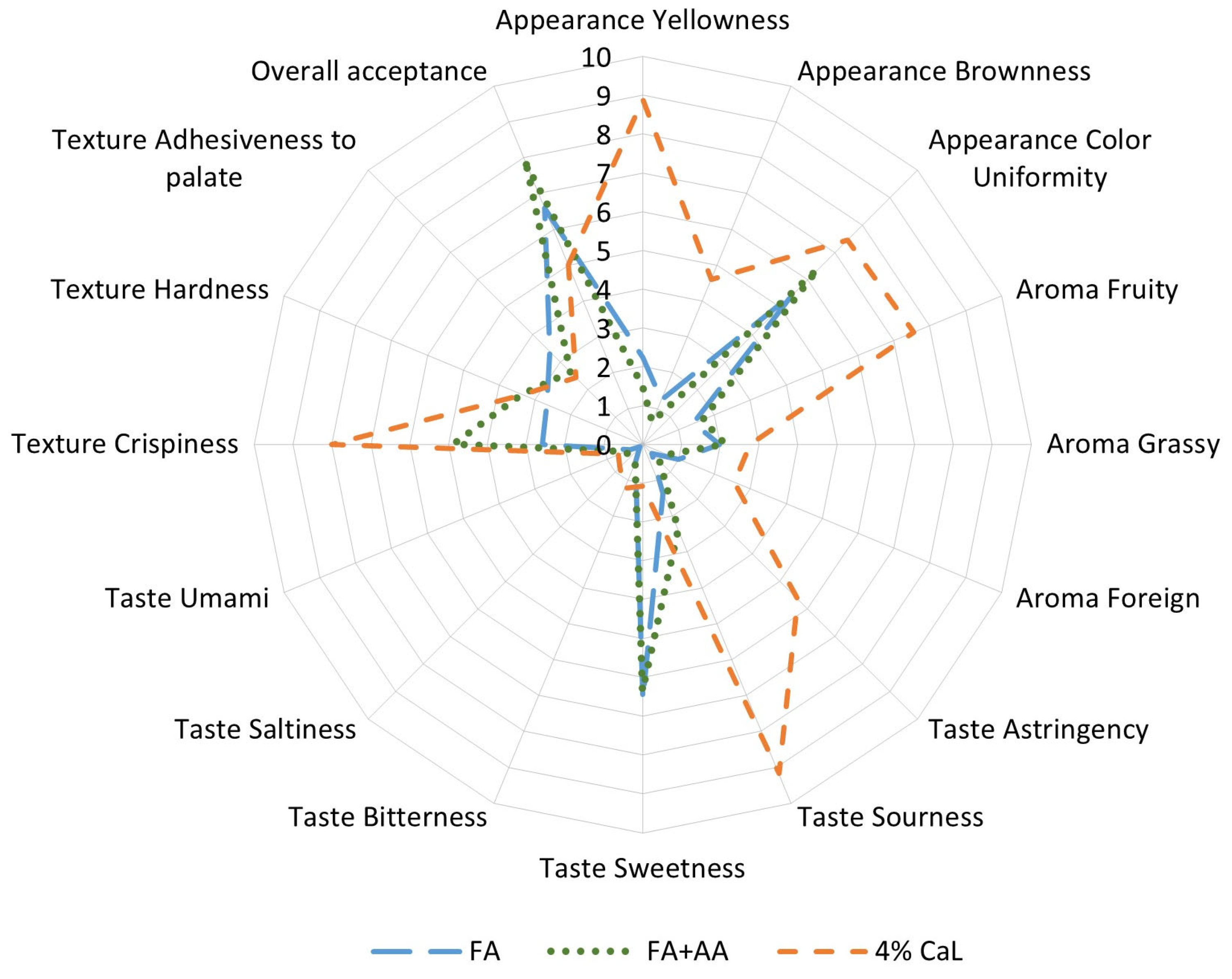
2.3.2. Sensory Profiling of Freeze-Dried Apples
2.3.3. Activity of Enzymes
2.3.4. Proximate Analysis of Freeze-Dried Apples
3. Materials and Methods
3.1. Raw Materials and Chemicals
3.2. Osmotic Dehydration or Impregnation
3.3. Analytical Methods
3.3.1. Extraction of Antioxidants
3.3.2. Determination of Total Phenolic Content and Antioxidant Activity
3.3.3. Color Measurement
3.3.4. Determination of Calcium Content
3.3.5. Analyses of Selected Samples
Quantification of Phenolic and Carotenoid Composition of Freeze-Dried Apples by UPLC-PDA
Sensory Profiling of Freeze-Dried Apples
Activity of Enzymes
Proximate Analysis of Freeze-Dried Apples
3.4. Response Surface Methodology and Statistical Analysis
- Mixture components:
- ○
- SB juice: 0–250 g;
- ○
- Water: 0–250 g;
- ○
- Inulin: 0–75 g;
- ○
- Total mixture mass fixed at 250 g.
- Process factors:
- ○
- Time: 30–120 min;
- ○
- Temperature: 30–50 °C.
- Total phenolic content (TPC, mg GAE/100 g product).
- Antioxidant activity assays:
- ○
- ABTS (mg TE/100 g product);
- ○
- DPPH (mg TE/100 g product);
- ○
- ORAC (mg TE/100 g product);
- ○
- PCL-ACL, PCL-ACW (mg TE/100 g product).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Sustainable Development Goals. Available online: https://sdgs.un.org/ (accessed on 25 May 2023).
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Sidor, A.; Brzozowska, A.; Gramza-Michałowska, A. The Role of Carotenoids in Bone Health—A Narrative Review. Nutrition 2024, 119, 112306. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.S.; Fernandes, M.A.; Mauro, M.A. Effect of Calcium on the Osmotic Dehydration Kinetics and Quality of Pineapple. J. Food Eng. 2014, 134, 37–44. [Google Scholar] [CrossRef]
- Paraskevopoulou, E.; Andreou, V.; Dermesonlouoglou, E.K.; Taoukis, P.S. Combined Effect of Pulsed Electric Field and Osmotic Dehydration Pretreatments on Mass Transfer and Quality of Air-Dried Pumpkin. J. Food Sci. 2022, 87, 4839–4853. [Google Scholar] [CrossRef]
- Wang, X.; Kahraman, O.; Feng, H. Impact of Osmotic Dehydration with/without Vacuum Pretreatment on Apple Slices Fortified with Hypertonic Fruit Juices. Food Bioprocess Technol. 2022, 15, 1588–1602. [Google Scholar] [CrossRef]
- González-Pérez, J.E.; Ramírez-Corona, N.; López-Malo, A. Mass Transfer during Osmotic Dehydration of Fruits and Vegetables: Process Factors and Non-Thermal Methods. Food Eng. Rev. 2021, 13, 344–374. [Google Scholar] [CrossRef]
- Tylewicz, U.; Oliveira, G.; Alminger, M.; Nohynek, L.; Dalla Rosa, M.; Romani, S. Antioxidant and Antimicrobial Properties of Organic Fruits Subjected to PEF-Assisted Osmotic Dehydration. Innov. Food Sci. Emerg. Technol. 2020, 62. [Google Scholar] [CrossRef]
- Jiménez-Hernández, J.; Estrada-Bahena, E.B.; Maldonado-Astudillo, Y.I.; Talavera-Mendoza, Ó.; Arámbula-Villa, G.; Azuara, E.; Álvarez-Fitz, P.; Ramírez, M.; Salazar, R. Osmotic Dehydration of Mango with Impregnation of Inulin and Piquin-Pepper Oleoresin. LWT–Food Sci. Technol. 2017, 79, 609–615. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Ciurzyńska, A.; Czajkowska, K.; Cichowska, J.; Rybak, K.; Lenart, A. Osmotic Dehydration of Honeoye Strawberries in Solutions Enriched with Natural Bioactive Molecules. LWT–Food Sci. Technol. 2017, 85, 500–505. [Google Scholar] [CrossRef]
- Haneef, N.; Hanif, N.; Hanif, T.; Raghavan, V.; Garièpy, Y.; Wang, J. Food Fortification Potential of Osmotic Dehydration and the Impact of Osmo-Combined Techniques on Bioactive Component Saturation in Fruits and Vegetables. Brazilian J. Food Technol. 2024, 27, e2023028. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Z.; Liao, X. Bioactive Compounds, Health Benefits and Functional Food Products of Sea Buckthorn: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6761–6782. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why Is Sea Buckthorn (Hippophae rhamnoides L.) so Exceptional? A Review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Binosha Fernando, W.M.A.D.; Durham, R.; Stockmann, R.; Jayasena, V. Nutritional Value, Health-Promoting Benefits and Food Application of Sea Buckthorn. Food Rev. Int. 2021, 39, 2122–2137. [Google Scholar] [CrossRef]
- Park, K.H.; Hong, J.H.; Kim, S.H.; Kim, J.C.; Kim, K.H.; Park, K.M. Anti-Osteoporosis Effects of the Fruit of Sea Buckthorn (Hippophae rhamnoides) through Promotion of Osteogenic Differentiation in Ovariectomized Mice. Nutrients 2022, 14, 3604. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus domestica)—A Review. Food Bioprocess Technol. 2024, 17, 2519–2560. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hu, L. High Efficient Freeze-Drying Technology in Food Industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 3370–3388. [Google Scholar] [CrossRef]
- Tomatis, F.; Carità, A.; de Broissia, C. Local Sourcing a Guide for the Hotel, Restaurant, Catering and Tourism Industries; FAO: Rome, Italy, 2023. [Google Scholar]
- Cichowska, J.; Żubernik, J.; Czyżewski, J.; Kowalska, H.; Witrowa-Rajchert, D. Efficiency of Osmotic Dehydration of Apples in Polyols Solutions. Molecules 2018, 23, 446. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H. Pea Protein Isolate and Inulin as Plant-Based Biomacromolecules for Reduction of Sugar Uptake in Osmotic Dehydration. J. Food Process Eng. 2023, 46, 1–10. [Google Scholar] [CrossRef]
- Kowalska, J.; Lenart, A.; Roszkowska, S.; Kowalska, H. The Influence of Chokeberry Juice and Inulin as Osmotic-Enriching Agents in Pre-Treatment on Polyphenols Content and Sensory Quality of Dried Strawberries. Agric. Food Sci. 2019, 28, 190–199. [Google Scholar] [CrossRef]
- Ramya, V.; Jain, N.K. A Review on Osmotic Dehydration of Fruits and Vegetables: An Integrated Approach. J. Food Process Eng. 2017, 40, 1–22. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Samborska, K.; Tywonek, M.; Lenart, A. Development of Apple Chips Technology. Heat Mass Transf. 2018, 54, 3573–3586. [Google Scholar] [CrossRef]
- Masztalerz, K.; Lech, K.; Wojdyło, A.; Nowicka, P.; Michalska-Ciechanowska, A.; Figiel, A. The Impact of the Osmotic Dehydration Process and Its Parameters on the Mass Transfer and Quality of Dried Apples. Dry. Technol. 2021, 39, 1074–1086. [Google Scholar] [CrossRef]
- Landim, A.P.M.; Barbosa, M.I.M.J.; Barbosa, J.L. Influence of Osmotic Dehydration on Bioactive Compounds, Antioxidant Capacity, Color and Texture of Fruits and Vegetables: A Review. Cienc. Rural 2016, 46, 1714–1722. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the Assessment of Antioxidant Activity in Foods. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 287–333. ISBN 9781782420972. [Google Scholar]
- Chen, L.Y.; Cheng, C.W.; Liang, J.Y. Effect of Esterification Condensation on the Folin-Ciocalteu Method for the Quantitative Measurement of Total Phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Gramza-Michałowska, A. Enzymatic Browning in Apple Products and Its Inhibition Treatments: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5038–5076. [Google Scholar] [CrossRef]
- Zhao, Z.; Vavrusova, M.; Skibsted, L.H. Antioxidant Activity and Calcium Binding of Isomeric Hydroxybenzoates. J. Food Drug Anal. 2018, 26, 591–598. [Google Scholar] [CrossRef]
- Tappi, S.; Mauro, M.A.; Tylewicz, U.; Dellarosa, N.; Dalla Rosa, M.; Rocculi, P. Effects of Calcium Lactate and Ascorbic Acid on Osmotic Dehydration Kinetics and Metabolic Profile of Apples. Food Bioprod. Process. 2017, 103, 1–9. [Google Scholar] [CrossRef]
- National Institutes of Health Calcium: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/#en1 (accessed on 3 November 2023).
- Su, Z.; Yao, B.; Liu, G.; Fang, J. Polyphenols as Potential Preventers of Osteoporosis: A Comprehensive Review on Antioxidant and Anti-Inflammatory Effects, Molecular Mechanisms, and Signal Pathways in Bone Metabolism. J. Nutr. Biochem. 2024, 123, 1–11. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Ferreres, F.; Moreno, D.A.; Nowicka, P. UPLC-PDA-Q/TOF-MS Profiling of Phenolic and Carotenoid Compounds and Their Influence on Anticholinergic Potential for AChE and BuChE Inhibition and on-Line Antioxidant Activity of Selected Hippophaë rhamnoides L. Cultivars. Food Chem. 2020, 309. [Google Scholar] [CrossRef]
- Ma, X.; Laaksonen, O.; Zheng, J.; Yang, W.; Trépanier, M.; Kallio, H.; Yang, B. Flavonol Glycosides in Berries of Two Major Subspecies of Sea Buckthorn (Hippophaë rhamnoides L.) and Influence of Growth Sites. Food Chem. 2016, 200, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gâtlan, A.M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Samara, M.; Nasser, A.; Mingelgrin, U. Critical Examination of the Suitability of the Folin-Ciocalteu Reagent Assay for Quantitative Analysis of Polyphenols—The Case of Olive-Mill Wastewater. Am. J. Anal. Chem. 2022, 13, 476–493. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the in Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.d.A.; Oliveira, J.d.S.; Barreto, M.S.d.S.; Pinheiro, L.P.; Cantuária, P.d.C.; Borges, W.L.; da Silva, G.A.; de Souza, T.M. An HPLC Method to Determine Phenolic Compounds of Plant Extracts: Application to Byrsonima Crassifolia and Senna Alata Leaves. Pharmacognosy Res. 2022, 14, 395–404. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Tkacz, K.; Gil-Izquierdo, Á.; Medina, S.; Turkiewicz, I.P.; Domínguez-Perles, R.; Nowicka, P.; Wojdyło, A. Phytoprostanes, Phytofurans, Tocopherols, Tocotrienols, Carotenoids and Free Amino Acids and Biological Potential of Sea Buckthorn Juices. J. Sci. Food Agric. 2022, 102, 185–197. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and Carotenoid Pigments in the Peel and Flesh of Commercial Apple Fruit Varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Ampomah-Dwamena, C.; Tomes, S.; Thrimawithana, A.H.; Elborough, C.; Bhargava, N.; Rebstock, R.; Sutherland, P.; Ireland, H.; Allan, A.C.; Espley, R.V. Overexpression of PSY1 Increases Fruit Skin and Flesh Carotenoid Content and Reveals Associated Transcription Factors in Apple (Malus × Domestica). Front. Plant Sci. 2022, 13, 1–20. [Google Scholar] [CrossRef]
- Chandra, R.D.; Prihastyanti, M.N.U.; Lukitasari, D.M. Effects of PH, High Pressure Processing, and Ultraviolet Light on Carotenoids, Chlorophylls, and Anthocyanins of Fresh Fruit and Vegetable Juices. eFood 2021, 2, 113–124. [Google Scholar] [CrossRef]
- Ma, X.; Yang, W.; Laaksonen, O.; Nylander, M.; Kallio, H.; Yang, B. Role of Flavonols and Proanthocyanidins in the Sensory Quality of Sea Buckthorn (Hippophaë rhamnoides L.) Berries. J. Agric. Food Chem. 2017, 65, 9871–9879. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, S.; Yoo, B.; Nam, K. Effects of Texture Properties of Semi-Solid Food on the Sensory Test for Pharyngeal Swallowing Effort in the Older Adults. BMC Geriatr. 2020, 20, 493. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S. Physical and Sensory Properties of Ready to Eat Apple Chips Produced by Osmo-Convective Drying. J. Food Sci. Technol. 2014, 51, 3691–3701. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of Natural Antibrowning Agents on Color and Related Enzymes in Fresh-Cut Fuji Apples as an Alternative to the Use of Ascorbic Acid. J. Food Sci. 2008, 73, 267–272. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-Oxidant and Anti-Enzymatic Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Fruits Modulated by Chemical Components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, Y.; Meng, W.; Pei, L.; Zhang, X. Browning Inhibition of Seabuckthorn Leaf Extract on Fresh-Cut Potato Sticks during Cold Storage. Food Chem. 2022, 389, 133076. [Google Scholar] [CrossRef] [PubMed]
- Rather, S.A.; Mir, N.A. Effect of Carboxymethyl Cellulose Enriched with Seabuckthorn (Hippophae rhamnoides L.) Leaf Extract Edible Coatings on the Quality of Fresh Cut “Maharaji” Apple. Food Humanit. 2023, 1, 571–580. [Google Scholar] [CrossRef]
- Schulze, B.; Hubbermann, E.M.; Schwarz, K. Stability of Quercetin Derivatives in Vacuum Impregnated Apple Slices after Drying (Microwave Vacuum Drying, Air Drying, Freeze Drying) and Storage. LWT–Food Sci. Technol. 2014, 57, 426–433. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC): New Horizons in Relating Dietary Antioxidants/Bioactives and Health Benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Korczak, J. Oxygen Radical Absorbance Capacity of Selected Food Products. Acta Sci. Pol. Technol. Aliment. 2013, 12, 175–180. [Google Scholar]
- Balogh, E.; Hegedus, A.; Stefanovits-Bányai, É. Application of and Correlation among Antioxidant and Antiradical Assays for Characterizing Antioxidant Capacity of Berries. Sci. Hortic. 2010, 125, 332–336. [Google Scholar] [CrossRef]
- Suliburska, J.; Krejpcio, Z. Evaluation of the Content and Bioaccessibility of Iron, Zinc, Calcium and Magnesium from Groats, Rice, Leguminous Grains and Nuts. J. Food Sci. Technol. 2014, 51, 589–594. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food; Food Science Text Series; Springer: New York, NY, USA, 2010. [Google Scholar]
- Sikora, M.; Złotek, U.; Świeca, M. Effect of Basil Leaves and Wheat Bran Water Extracts on Enzymatic Browning of Lettuce. Int. J. Food Sci. Technol. 2020, 55, 1318–1325. [Google Scholar] [CrossRef]
- PN.75/A-04018:1975; Agricultural Food Products-Determination of Nitrogen by Kjeldahl Method and Expressing as Protein. Polish Committee for Standardization: Warsaw, Poland, 1975. (In Polish)
- PN-EN ISO 3947:2001; Starches, Native or Modified—Determination of Total Fat Content. International Organization for Standardization (ISO): Geneva, Switzerland, 2001.
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Asp, N.G.; Johansson, C.G.; Hallmer, H.; Siljestróm, M. Rapid Enzymatic Assay of Insoluble and Soluble Dietary Fiber. J. Agric. Food Chem. 1983, 31, 476–482. [Google Scholar] [CrossRef]
| Code of Sample | Name of Sample | SB Juice Concentration (w/w in Water) | Inulin–SB Juice Ratio in Solution (w/w) | Osmotic Solution Components in Grams During OD (Total Mass = 250 g) | Total Soluble Solid of Osmotic Solution [°Brix] | Time of OD [min] | Temperature of OD [°C] | ||
|---|---|---|---|---|---|---|---|---|---|
| SB Juice | Water | Inulin | |||||||
| FA | Freeze-dried fresh apple | - | - | - | - | - | - | ||
| FA + AA | Freeze-dried fresh apple + 1% ascorbic acid | - | - | - | - | - | - | ||
| SB0_I0 | OD of fresh apple + 1% ascorbic acid (50 g) with freeze drying | 0% | 0:100 | 0.00 | 250.00 | 0.00 | 0.00 ± 0.00 | 30, 60, 120 | 30, 50 |
| SB0_I15 | 15:85 | 0.00 | 212.50 | 37.50 | 13.90 ± 0.00 | ||||
| SB0_I30 | 30:70 | 0.00 | 175.00 | 75.00 | 28.43 ± 0.06 | ||||
| SB50_I0 | 50% | 0:100 | 125.00 | 125.00 | 0.00 | 3.83 ± 0.06 | |||
| SB50_I15 | 15:85 | 106.25 | 106.25 | 37.50 | 17.37 ± 0.06 | ||||
| SB50_I30 | 30:70 | 87.50 | 87.50 | 75.00 | 31.53 ± 0.06 | ||||
| SB100_I0 | 100% | 0:100 | 250.00 | 0.00 | 0.00 | 7.37 ± 0.06 | |||
| SB100_I15 | 15:85 | 212.50 | 0.00 | 37.50 | 20.40 ± 0.10 | ||||
| SB100_I30 | 30:70 | 175.00 | 0.00 | 75.00 | 33.43 ± 0.15 | ||||
| Optimized Conditions * | TPC | ABTS | DPPH | ORAC | PCL-ACL | PCL-ACW | |
|---|---|---|---|---|---|---|---|
| SB juice [g] | 170.35 | 225.31 | 207.35 | 234.51 | 246.92 | 250.00 | |
| Water [g] | 79.65 | 24.69 | 42.65 | 15.49 | 3.08 | 0.00 | |
| Inulin [g] | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Time [min] | 73.54 | 119.37 | 117.72 | 120.00 | 120.00 | 117.82 | |
| Temperature [°C] | 38.82 | 30.11 | 31.33 | 30.00 | 30.00 | 30.40 | |
| Freeze-dried samples | TPC [mg GAE/ 100 g product] | ABTS [mg TE/ 100 g product] | DPPH [mg TE/ 100 g product] | ORAC [mg TE/ 100 g product] | PCL-ACL [mg TE/ 100 g product] | PCL-ACW [mg TE/ 100 g product] | |
| FA | 301.08 ± 13.79 a | 449.33 ± 17.37 a | 425.99 ± 18.35 b | 3423.52 ± 52.93 a | 950.62 ± 28.04 b | 613.59 ± 5.76 b | |
| FA + AA | 623.81 ± 21.06 c | 784.18 ± 16.11 b | 877.35 ± 30.93 c | 3579.68 ± 49.61 b | 1138.25 ± 26.18 c | 762.84 ± 24.61 c | |
| Optimized conditions ** | Validation mean value | 427.35 ± 1.59 b | 464.54 ± 21.35 a | 325.03 ± 0.80 a | 3532.58 ± 17.30 b | 677.05 ± 6.25 a | 471.29 ± 5.11 a |
| Predicted mean value | 498.39 | 447.08 | 324.43 | 3627.31 | 668.41 | 465.28 | |
| 95% confidence interval | 480.75–516.66 | 427.23–467.36 | 311.65–337.45 | 3356.32–3908.84 | 644.44–692.38 | 425.72–506.62 | |
| 95% prediction interval | 449.35–551.47 | 413.71–480.35 | 289.08–361.33 | 2920.17–4392.88 | 611.31–725.51 | 377.01–560.78 | |
| Code of samples | Opt_TPC | Opt_ABTS | Opt_DPPH | Opt_ORAC | Opt_PCL-ACL | Opt_PCL-ACW | |
| Treatment | Appearance | L Value | A Value | b Value | ΔE | BI | WI | YI |
|---|---|---|---|---|---|---|---|---|
| FA |  | 70.96 ± 0.52 d | 5.99 ± 0.45 b | 29.26 ± 0.63 a | 11.17 ± 0.54 a | 58.13 ± 1.90 b | 58.33 ± 0.57 e | 58.92 ± 1.25 b |
| FA + AA |  | 81.06 ± 0.37 e | 1.58 ± 0.10 a | 27.57 ± 0.27 a | - | 41.97 ± 0.53 a | 66.51 ± 0.23 f | 48.58 ± 0.42 a |
| Opt_TPC |  | 62.19 ± 0.49 c | 20.18 ± 0.27 c | 50.74 ± 0.13 d | 35.20 ± 0.44 b | 165.13 ± 2.70 c | 33.58 ± 0.41 d | 116.57 ± 1.08 c |
| Opt_ABTS |  | 52.27 ± 1.90 b | 22.31 ± 0.47 de | 48.56 ± 1.44 cd | 41.28 ± 0.53 d | 208.94 ± 4.42 e | 28.31 ± 0.41 b | 132.76 ± 1.55 e |
| Opt_DPPH |  | 53.74 ± 2.36 b | 20.21 ± 0.37 c | 47.43 ± 0.57 bc | 38.61 ± 1.57 c | 190.16 ± 11.25 d | 30.71 ± 1.32 c | 126.25 ± 4.19 d |
| Opt_ORAC |  | 47.74 ± 2.25 a | 23.79 ± 1.42 f | 48.72 ± 3.09 cd | 45.43 ± 0.78 e | 248.64 ± 12.57 f | 24.61 ± 1.16 a | 145.74 ± 4.36 f |
| Opt_PCL-ACL |  | 47.64 ± 1.36 a | 23.11 ± 0.73 ef | 47.38 ± 2.16 bc | 44.48 ± 0.43 e | 237.00 ± 7.23 f | 25.67 ± 0.70 a | 142.02 ± 2.50 f |
| Opt_PCL-ACW |  | 45.14 ± 0.13 a | 21.9 ± 0.11 d | 45.71 ± 0.11 b | 45.08 ± 0.18 e | 244.59 ± 2.17 f | 25.31 ± 0.17 a | 144.69 ± 0.68 f |
| Treatment | TPC [mg GAE/100 g Product] | ABTS [mg TE/100 g Product] | DPPH [mg TE/100 g Product] | ORAC [mg TE/100 g Product] | PCL-ACL [mg TE/100 g Product] | PCL-ACW [mg TE/100 g Product] | PCL-IAC [mg TE/100 g Product] |
|---|---|---|---|---|---|---|---|
| 0% CaL | 453.33 ± 8.45 d | 525.43 ± 24.91 d | 346.51 ± 7.94 d | 3532.58 ± 17.30 d | 710.32 ± 11.84 c | 601.40 ± 15.42 d | 1311.72 ± 18.98 d |
| 1% CaL | 369.68 ± 8.98 c | 425.26 ± 3.97 c | 307.24 ± 1.90 c | 2745.31 ± 177.85 c | 583.02 ± 29.29 b | 447.85 ± 3.37 c | 1030.87 ± 26.24 c |
| 2% CaL | 347.41 ± 11.25 bc | 394.12 ± 14.29 bc | 285.48 ± 1.09 b | 2474.84 ± 131.01 bc | 503.19 ± 20.62 a | 314.31 ± 6.94 b | 817.50 ± 21.11 b |
| 4% CaL | 331.02 ± 13.99 b | 385.69 ± 3.27 b | 281.76 ± 7.25 b | 2275.52 ± 37.04 b | 475.81 ± 8.63 a | 282.12 ± 7.46 a | 757.93 ± 15.92 ab |
| 6% CaL | 280.46 ± 3.79 a | 333.18 ± 6.67 a | 262.82 ± 2.19 a | 1942.63 ± 86.43 a | 461.80 ± 25.31 a | 265.86 ± 3.01 a | 727.66 ± 28.25 a |
| Compounds | No. Compound | Total Content [mg/100 g Product] | ||
|---|---|---|---|---|
| FA | FA + AA | 4% CaL | ||
| PHENOLICS | ||||
| Phenolic acids | ||||
| p-Coumaric acid-O-hexoside | 1 | 30.71 ± 0.09 c | 35.3 ± 0.03 b | 13.04 ± 0.17 a |
| Ferulic acid-O-hexoside | 2 | 3.88 ± 0.08 b | 3.91 ± 0.05 b | 0.92 ± 0.10 a |
| Sum of phenolic acids | 34.59 ± 0.17 b | 39.21 ± 0.02 c | 13.96 ± 0.08 a | |
| Flavan-3-ols | ||||
| (+)-Catechin | 3 | 3.60 ± 0.18 a | 6.25 ± 0.13 b | 17.97 ± 0.19 c |
| Procyanidin B1 | 4 | nd | nd | 17.88 ± 0.04 |
| (-)-Epicatechin | 5 | 10.59 ± 0.63 b | 13.06 ± 0.01 c | 3.03 ± 0.10 a |
| Procyanidin C1 | 6 | 32.04 ± 1.32 b | 37.21 ± 0.57 c | 5.48 ± 0.33 a |
| Sum of flavan-3-ols | 46.24 ± 0.51 a | 56.53 ± 0.43 b | 44.36 ± 0.58 a | |
| Dihydrochalcones | ||||
| Phloretin-2-O-xyloglucoside | 7 | 2.15 ± 0.00 c | 1.61 ± 0.02 a | 1.81 ± 0.04 b |
| Derivative of dihydrochalcones | 8 | 3.83 ± 0.08 b | 3.48 ± 0.11 a | nd |
| Phloretin-2-O-glucoside | 9 | 1.47 ± 0.31 a | 1.36 ± 0.07 a | nd |
| Sum of dihydrochalcones | 7.46 ± 0.4 b | 6.44 ± 0.2 b | 1.81 ± 0.04 a | |
| Flavonols | ||||
| Isorhamnetin-3,7-O-dihexoside | 10 | nd | nd | 4.28 ± 0.08 |
| Isorhamnetin-3-O-sophoroside-7-O-rhamnoside | 11 | nd | nd | 1.93 ± 0.05 |
| Quercetin-3-O-(dirhamnosyl)hexoside | 12 | nd | nd | 3.68 ± 0.20 |
| Quercetin-3-galactoside-7-O-rhamnoside | 13 | nd | nd | 1.02 ± 0.20 |
| Quercetin-3-O-rutinoside | 14 | nd | nd | 1.24 ± 0.06 |
| Isorhamnetin-3-O-glucoside-7-O-rhamnoside | 15 | nd | nd | 2.65 ± 0.17 |
| Quercetin-3-O-glucoside | 16 | nd | nd | 4.56 ± 0.09 |
| Isorhamnetin-3-O-(2-rhamnosyl)hexoside | 17 | nd | nd | 2.50 ± 0.00 |
| Kaempferol-3-O-hexoside-7-O-rhamnoside | 18 | nd | nd | 4.06 ± 0.23 |
| Isorhamnetin-3-O-(rhamnosyl)hexoside | 19 | nd | nd | 0.72 ± 0.00 |
| Isorhamnetin-3-O-rutinoside | 20 | nd | nd | 8.23 ± 0.00 |
| Quercetin-3-O-rhamnoside | 21 | 1.07 ± 0.08 a | 0.96 ± 0.04 a | 1.81 ± 0.03 b |
| Isorhamnetin-3-O-glucoside | 22 | nd | nd | 8.72 ± 0.02 |
| Kaempferol-3-O-rutinoside | 23 | nd | nd | 0.63 ± 0.02 |
| Isorhamnetin-3-O-rhamnoside | 24 | nd | nd | 0.32 ± 0.05 |
| Sum of flavonols | 1.07 ± 0.08a | 0.96 ± 0.04a | 46.36 ± 0.39 b | |
| Total phenolics | 89.36 ± 0.36 a | 103.14 ± 0.26 b | 106.49 ± 0.86 c | |
| CAROTENOIDS | ||||
| Lutein isomer | 25 | 0.28 ± 0.01 a | 0.25 ± 0.04 a | nd |
| All-trans-lutein | 26 | 0.19 ± 0.12 a | 0.17 ± 0.02 a | nd |
| Zeaxanthin isomer | 27 | nd | nd | 9.75 ± 1.71 |
| Sum of carotene | 28 | 1.75 ± 0.10 a | 1.76 ± 0.05 a | 28.57 ± 0.73 b |
| Phytofluene | 29 | 0.07 ± 0.00 a | 0.05 ± 0.01 a | 0.31 ± 0.00 b |
| Total carotenoids | 2.29 ± 0.24 a | 2.23 ± 0.02 a | 38.63 ± 2.45 b | |
| Analyses | Unit | FA | FA + AA | 4% CaL |
|---|---|---|---|---|
| Activity of enzymes | ||||
| PPO activity | U/g product | 3894.81 ± 247.07 c | 3358.52 ± 175.90 b | 432.59 ± 28.57 a |
| POD activity | U/g product | 717.78 ± 26.94 b | 1642.22 ± 36.72 c | 407.41 ± 12.83 a |
| Proximate analysis | ||||
| Protein | g/100 g product | 4.38 ± 0.08 b | 3.52 ± 0.19 a | 5.14 ± 0.38 c |
| Lipid | g/100 g product | 0.53 ± 0.03 a | 0.68 ± 0.03 a | 3.64 ± 0.18 b |
| Carbohydrate | g/100 g product | 75.20 ± 0.27 b | 73.29 ± 0.36 b | 48.96 ± 1.47 a |
| Ash | g/100 g product | 1.27 ± 0.11 a | 1.12 ± 0.05 a | 8.81 ± 0.29 b |
| Moisture | g/100 g product | 6.39 ± 0.29 a | 8.95 ± 0.34 b | 15.36 ± 0.77 c |
| TDF | g/100 g product | 12.22 ± 0.29 a | 12.44 ± 0.62 a | 18.09 ± 0.31 b |
| SDF | g/100 g product | 2.57 ± 0.62 a | 3.05 ± 0.47 a | 4.18 ± 0.06 b |
| IDF | g/100 g product | 9.65 ± 0.48 a | 9.39 ± 0.36 a | 13.90 ± 0.37 b |
| Energy value | kcal/100 g product | 347.53 | 338.24 | 285.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, M.; Białas, W.; Kulczyński, B.; Multisona, R.R.; Suliburska, J.; Świeca, M.; Wojdyło, A.; Gramza-Michałowska, A. Product Development Study of Freeze-Dried Apples Enriched with Sea Buckthorn Juice and Calcium Lactate. Molecules 2025, 30, 1504. https://doi.org/10.3390/molecules30071504
Arnold M, Białas W, Kulczyński B, Multisona RR, Suliburska J, Świeca M, Wojdyło A, Gramza-Michałowska A. Product Development Study of Freeze-Dried Apples Enriched with Sea Buckthorn Juice and Calcium Lactate. Molecules. 2025; 30(7):1504. https://doi.org/10.3390/molecules30071504
Chicago/Turabian StyleArnold, Marcellus, Wojciech Białas, Bartosz Kulczyński, Ribi Ramadanti Multisona, Joanna Suliburska, Michał Świeca, Aneta Wojdyło, and Anna Gramza-Michałowska. 2025. "Product Development Study of Freeze-Dried Apples Enriched with Sea Buckthorn Juice and Calcium Lactate" Molecules 30, no. 7: 1504. https://doi.org/10.3390/molecules30071504
APA StyleArnold, M., Białas, W., Kulczyński, B., Multisona, R. R., Suliburska, J., Świeca, M., Wojdyło, A., & Gramza-Michałowska, A. (2025). Product Development Study of Freeze-Dried Apples Enriched with Sea Buckthorn Juice and Calcium Lactate. Molecules, 30(7), 1504. https://doi.org/10.3390/molecules30071504












