Metabolomic Profiles and Differential Constituents of Andrographis paniculata (Burm. f.) in Different Growth Stages and Parts
Abstract
1. Introduction
2. Experiment
2.1. Reagents and Materials
2.2. Preparation of Reference and Sample Solutions
2.3. Chromatographic Conditions and Mass Spectrometry Parameters
2.4. Chemical Characterization
2.5. Data Analysis
3. Results and Discussion
3.1. Optimization of Chromatographic Conditions and Mass Spectrometry Parameters
3.2. Identification of Chemical Constituents in Different Growth Stages and Plant Parts of A. paniculata
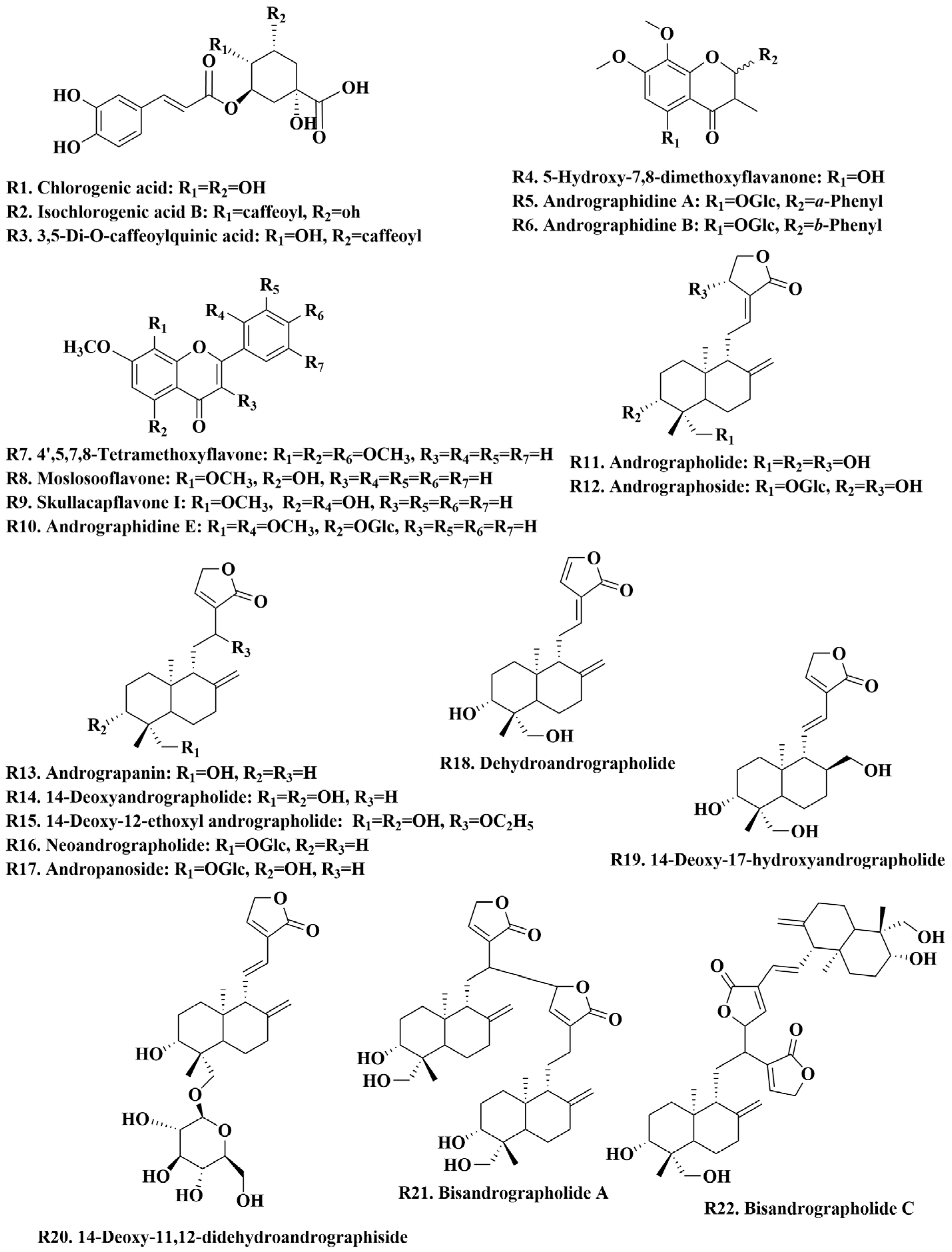
3.2.1. Analysis of Diterpene Lactones in A. paniculata
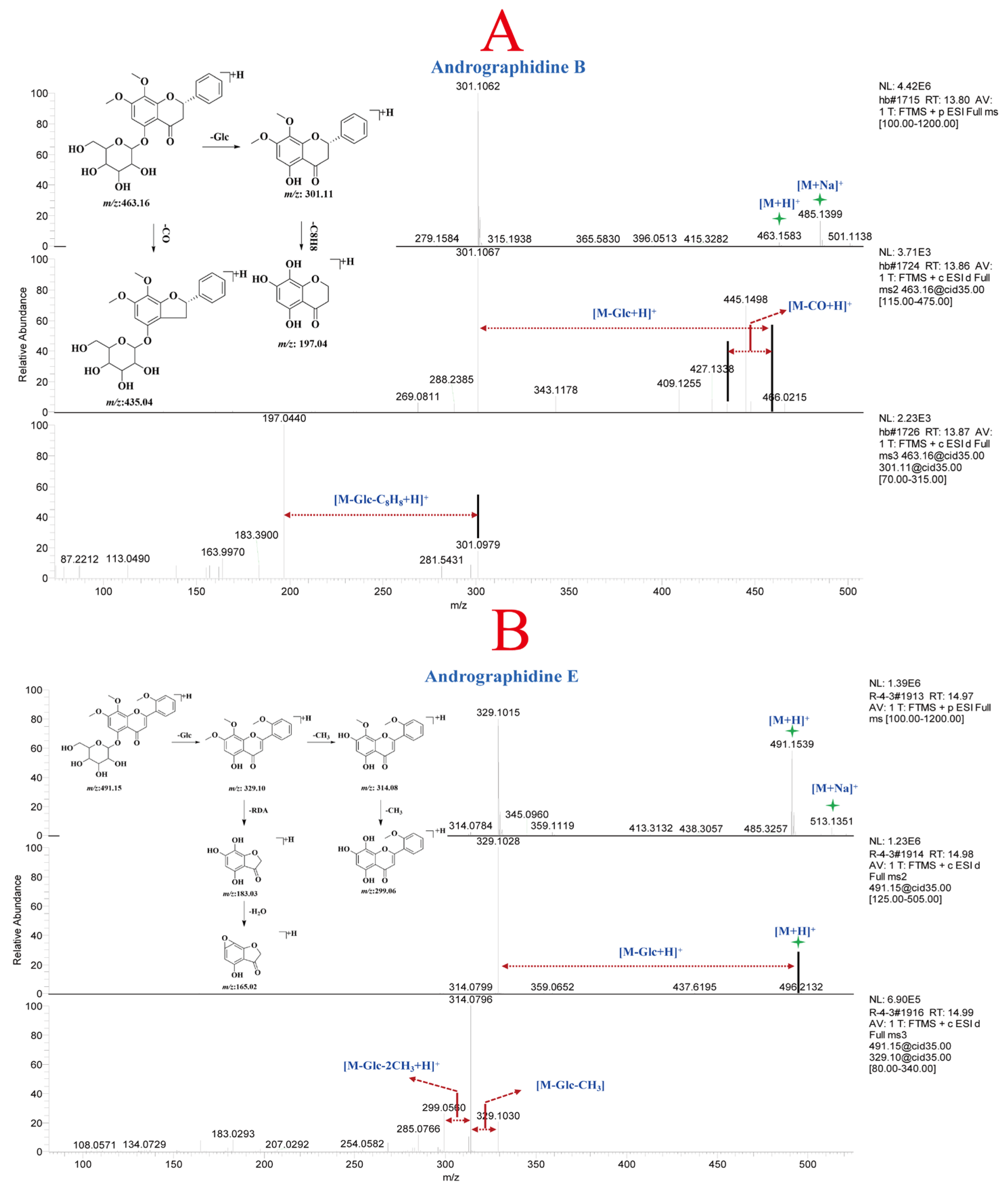
3.2.2. Analysis of Flavonoids in A. paniculata
3.2.3. Analysis of Organic Acids in A. paniculata
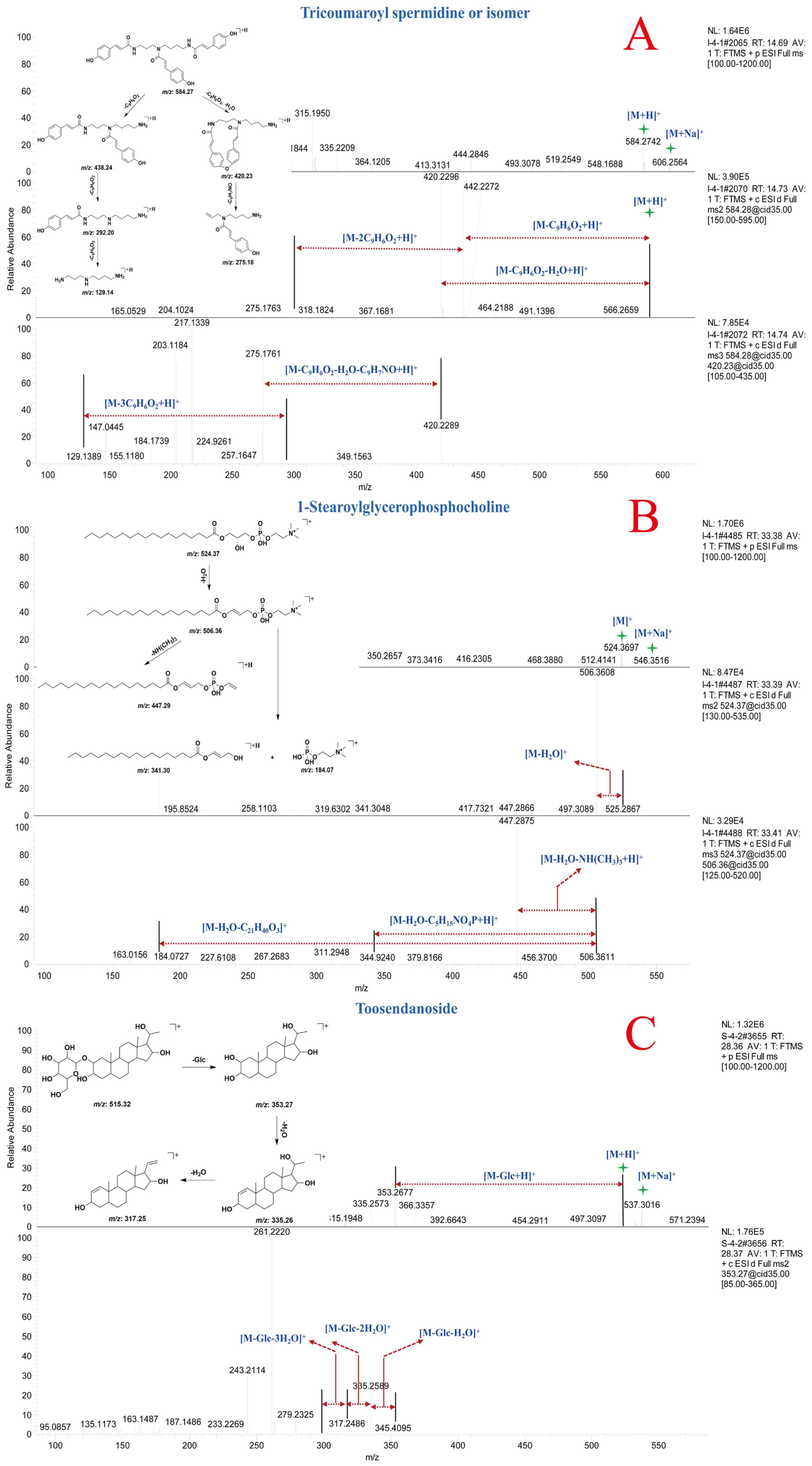
3.2.4. Analysis of Other Components in A. paniculata
3.3. Metabolite Analysis of A. paniculata at Different Growth Stages and Plant Parts
3.3.1. Targeted Analysis of Major Compounds in A. paniculata
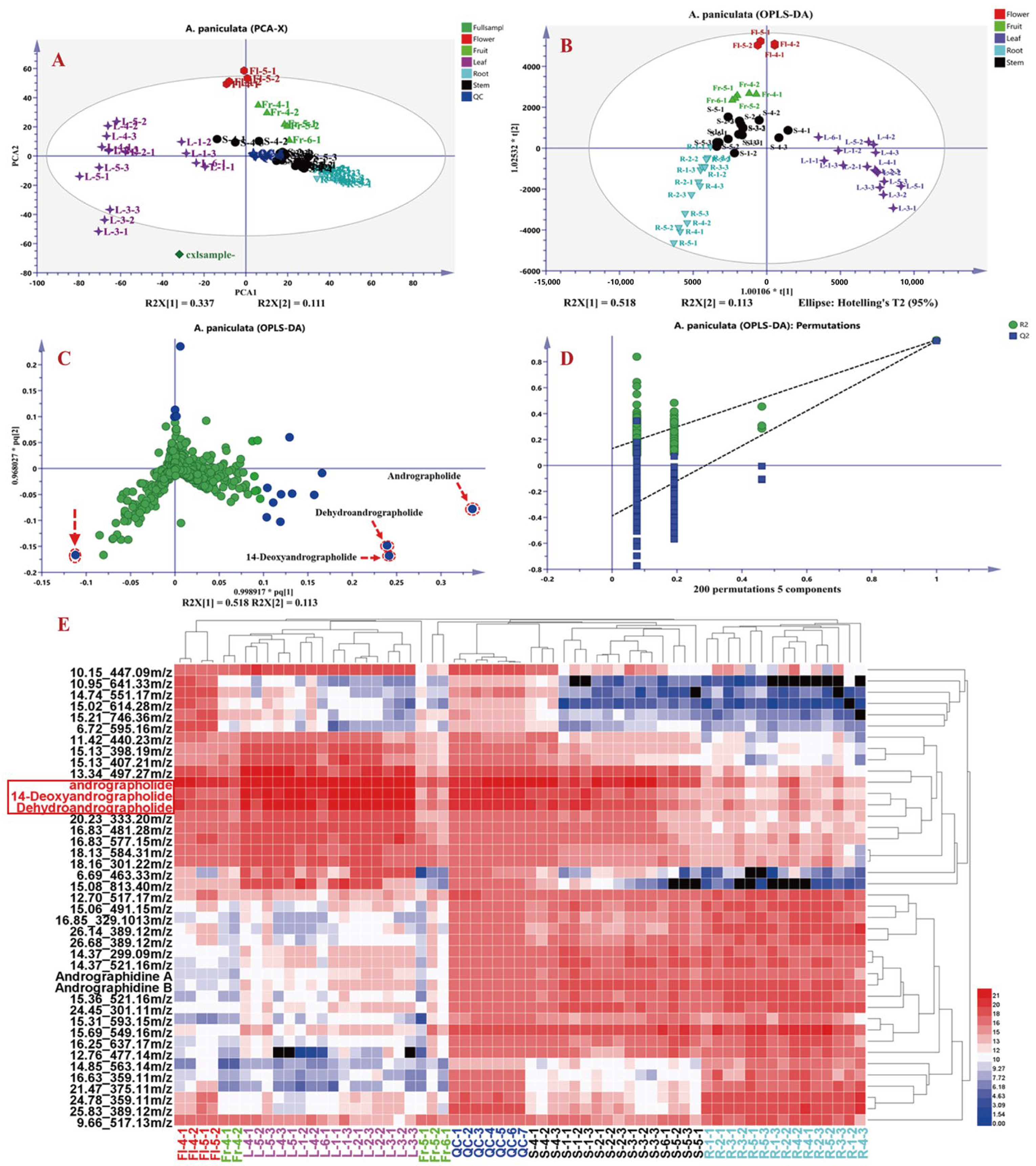
3.3.2. Non-Targeted Metabolomics Analysis of Chemical Constituents in A. paniculata
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-ard, P. A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules 2022, 27, 4479. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis paniculata (Burm.f.) Nees: Traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [PubMed]
- Jiang, M.; Sheng, F.; Zhang, Z.; Ma, X.; Gao, T.; Fu, C.; Li, P. Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents. J. Ethnopharmacol. 2021, 272, 113954. [Google Scholar] [PubMed]
- Songvut, P.; Suriyo, T.; Panomvana, D.; Rangkadilok, N.; Satayavivad, J. A comprehensive review on disposition kinetics and dosage of oral administration of Andrographis paniculata, an alternative herbal medicine, in co-treatment of coronavirus disease. Front. Pharmacol. 2022, 13, 952660. [Google Scholar]
- Rafi, M.; Karomah, A.H.; Heryanto, R.; Septaningsih, D.A.; Kusuma, W.A.; Amran, M.B.; Rohman, A.; Prajogo, B. Metabolite profiling of Andrographis paniculata leaves and stem extract using UHPLC-Orbitrap-MS/MS. Nat. Prod. Res. 2020, 36, 625–629. [Google Scholar]
- Casamonti, M.; Risaliti, L.; Vanti, G.; Piazzini, V.; Bergonzi, M.C.; Bilia, A.R. Andrographolide Loaded in Micro- and Nano-Formulations: Improved Bioavailability, Target-Tissue Distribution, and Efficacy of the “King of Bitters”. Engineering 2019, 5, 69–75. [Google Scholar]
- Pharmacopoeia of People’s Republic of China; Chinese Pharmacopoeia Commission, Ed.; China Medical Science Press: Beijing, China, 2020; Volume Part I, p. 280. [Google Scholar]
- Dalawai, D.; Aware, C.; Jadhav, J.P.; Murthy, H.N. RP-HPLC analysis of diterpene lactones in leaves and stem of different species of Andrographis. Nat. Prod. Res. 2019, 35, 2239–2242. [Google Scholar]
- Liang, X.; Ye, Y.; Zhu, Y.; Xiao, J.; Qiao, Y. Multivariate comparative analysis of chemical constituent changes and antioxidant properties of polysaccharides in ribes stenocarpum maxim. at different maturity stages on the Qinghai-Tibet Plateau. Sci. Hortic. 2023, 308, 111556. [Google Scholar]
- Mahgoub, Y.A.; Shawky, E.; Ghareeb, D.A.; Darwish, F.A.; El Sebakhy, N.A.; El-Hawiet, A.M. UPLC-MS/MS multivariate data analysis reveals phenological growth stages affect silymarin bioactive components of the different organs of two Silybum marianum genotypes. Microchem. J. 2023, 187, 108436. [Google Scholar]
- Yan, J.-K.; Chen, T.-T.; Wang, Z.-W.; Wang, C.; Liu, C.; Li, L. Comparison of physicochemical characteristics and biological activities of polysaccharides from barley (Hordeum vulgare L.) grass at different growth stages. Food Chem. 2022, 389, 133083. [Google Scholar]
- Pholphana, N.; Rangkadilok, N.; Saehun, J.; Ritruechai, S.; Satayavivad, J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm.f.) Nees (Chuanxinlian). Chin. Med. 2013, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Waris, M.; Koçak, E.; Gonulalan, E.M.; Demirezer, L.O.; Kır, S.; Nemutlu, E. Metabolomics analysis insight into medicinal plant science. Trends Anal. Chem. 2022, 157, 116795. [Google Scholar] [CrossRef]
- Wu, X.; Long, H.; Li, F.; Wu, W.; Zhou, J.; Liu, C.; Hou, J.; Wu, W.; Guo, D. Comprehensive feature-based molecular networking and metabolomics approaches to reveal the differences components in Cinnamomum cassia and Cinnamomum verum. J. Sep. Sci. 2021, 44, 3810–3821. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Chen, B.; Zhao, D.; Wang, H.; Sun, M.; Li, X.; Xu, X.; Liu, J.; Wang, S.; et al. Ultra-high performance liquid chromatography/ion mobility time-of-flight mass spectrometry-based untargeted metabolomics combined with quantitative assay unveiled the metabolic difference among the root, leaf, and flower bud of Panax notoginseng. Arab. J. Chem. 2021, 14, 103409. [Google Scholar] [CrossRef]
- Wu, X.; Hou, J.; Zhang, Z.; Chen, L.; Ni, H.; Qian, Y.; Wu, W.; Long, H.; Zhang, L.; Li, F.; et al. In-depth exploration and comparison of chemical constituents from two Lilium species through offline two-dimensional liquid chromatography combined with multimode acquisition of high-resolution mass spectrometry. J. Chromatogr. A 2022, 1670, 462980. [Google Scholar] [CrossRef]
- Qian, Y.; Li, W.; Wang, H.; Hu, W.; Wang, H.; Zhao, D.; Hu, Y.; Li, X.; Gao, X.; Yang, W. A four-dimensional separation approach by offline 2D-LC/IM-TOF-MS in combination with database-driven computational peak annotation facilitating the in-depth characterization of the multicomponents from Atractylodis Macrocephalae Rhizoma (Atractylodes macrocephala). Arab. J. Chem. 2021, 14, 102957. [Google Scholar]
- Wu, X.; Ding, H.; Zhang, Z.; Zheng, M.; Ni, H.; Huang, Z.; Wu, W.; Long, H.; Zhou, Y.; Li, F.; et al. An improved strategy for identification and annotation of easily in-sourced dissociation diterpene lactones from plant natural products: Taking Andrographis paniculata (Burm. f.) as an example. Rapid Commun. Mass Spectrom. 2023, 37, e9483. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. Trends Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, C.; Li, W.; Wang, H.; Hu, Y.; Yang, W.; Jia, L.; Wang, X.; Gao, X.; Guo, D. Offline two-dimensional liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry enabling four-dimensional separation and characterization of the multicomponents from white ginseng and red ginseng. J. Pharm. Anal. 2019, 10, 597–609. [Google Scholar] [CrossRef]
- Ramabulana, A.-T.; Petras, D.; Madala, N.E.; Tugizimana, F. Mass spectrometry DDA parameters and global coverage of the metabolome: Spectral molecular networks of momordica cardiospermoides plants. Metabolomics Off. J. Metabolomic Soc. 2023, 19, 18. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, Z.; Li, Q.; Yang, K.; Huang, Z.; Wang, W.; Zhao, S.; Hu, H. What dominates the changeable pharmacokinetics of natural sesquiterpene lactones and diterpene lactones: A review focusing on absorption and metabolism. Drug Metab. Rev. 2020, 53, 41–44. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [PubMed]
- Yang, J.; Wen, K.; Cheng, K.; Nandakumar, K.; Salem, M.L.; Fang, X.; Yao, Y. Recent Research on Flavonoids and their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar]
- Qiu, X.; Zhang, Y.; Zhou, Y.; Li, G.-H.; Feng, X.-S. Progress in pretreatment and analysis of organic Acids: An update since 2010. Food Chem. 2021, 360, 129977. [Google Scholar]
- Liu, P.; Wang, L.; Li, H.; Tan, L.; Ying, X.; Ju, B. Two new organic acids from Portulaca oleracea L. and their anti-inflammatory and anticholinesterase activities. Nat. Prod. Res. 2021, 36, 4395–4403. [Google Scholar]
- Quiroga, P.R.; Nepote, V.; Baumgartner, M.T. Contribution of organic acids to α-terpinene antioxidant activity. Food Chem. 2018, 277, 267–272. [Google Scholar] [CrossRef]
- Zulkifli, N.A.; Hassan, Z.; Mustafa, M.Z.; Azman, W.N.W.; Hadie, S.N.H.; Ghani, N.; Zin, A.A.M. The potential neuroprotective effects of stingless bee honey. Front. Aging Neurosci. 2023, 14, 1048028. [Google Scholar] [CrossRef]
- Liu, T.; Qiao, N.; Ning, F.; Huang, X.; Luo, L. Identification and characterization of plant-derived biomarkers and physicochemical variations in the maturation process of Triadica cochinchinensis honey based on UPLC-QTOF-MS metabolomics analysis. Food Chem. 2023, 408, 135197. [Google Scholar]
- Loftus, N.; Miseki, K.; Iida, J.; Gika, H.G.; Theodoridis, G.; Wilson, I.D. Profiling and biomarker identification in plasma from different Zucker rat strains via high mass accuracy multistage mass spectrometric analysis using liquid chromatography/mass spectrometry with a quadrupole ion trap-time of flight mass spectrometer. Rapid Commun. Mass Spectrom. RCM 2008, 22, 2547–2554. [Google Scholar]
- Inada, A.; Somekawa, M.; Murata, H.; Nakanishi, T.; Tokuda, H.; Nishino, H.; Iwashima, A.; Darnaedi, D.; Murata, J. Phytochemical studies on meliaceous plants. VIII: Structures and inhibitory effects on Epstein-Barr virus activation of triterpenoids from leaves of Chisocheton macrophyllus KING. Chem. Pharm. Bull. 1993, 41, 617–619. [Google Scholar]
- Xu, T.; Pan, J.; Zhao, L. Simultaneous determination of four andrographolides in Andrographis paniculata Nees by silver ion reversed-phase high-performance liquid chromatography. J. Chromatogr. Sci. 2008, 46, 747–750. [Google Scholar] [PubMed]
- Tajidin, N.E.; Shaari, K.; Maulidiani, M.; Salleh, N.S.; Ketaren, B.R.; Mohamad, M. Metabolite profiling of Andrographis paniculata (Burm. f.) Nees. young and mature leaves at different harvest ages using 1H NMR-based metabolomics approach. Sci. Rep. 2019, 9, 16766. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, E.; Cheng, R.; Liu, A.; Wang, Y.; Long, H.; Hou, J.; Wang, D.; Wu, W.; Wu, X. Metabolomic Profiles and Differential Constituents of Andrographis paniculata (Burm. f.) in Different Growth Stages and Parts. Molecules 2025, 30, 1490. https://doi.org/10.3390/molecules30071490
Hu E, Cheng R, Liu A, Wang Y, Long H, Hou J, Wang D, Wu W, Wu X. Metabolomic Profiles and Differential Constituents of Andrographis paniculata (Burm. f.) in Different Growth Stages and Parts. Molecules. 2025; 30(7):1490. https://doi.org/10.3390/molecules30071490
Chicago/Turabian StyleHu, Enming, Rui Cheng, Annian Liu, Ya Wang, Huali Long, Jinjun Hou, Daoping Wang, Wanying Wu, and Xingdong Wu. 2025. "Metabolomic Profiles and Differential Constituents of Andrographis paniculata (Burm. f.) in Different Growth Stages and Parts" Molecules 30, no. 7: 1490. https://doi.org/10.3390/molecules30071490
APA StyleHu, E., Cheng, R., Liu, A., Wang, Y., Long, H., Hou, J., Wang, D., Wu, W., & Wu, X. (2025). Metabolomic Profiles and Differential Constituents of Andrographis paniculata (Burm. f.) in Different Growth Stages and Parts. Molecules, 30(7), 1490. https://doi.org/10.3390/molecules30071490




