Kyoh® Rocket Leaf Extract Regulates Proliferation and VEGF and FGF7 Expression in Human Dermal Follicle Papilla Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of Kyoh® Extract by HPLC-DAD-MS
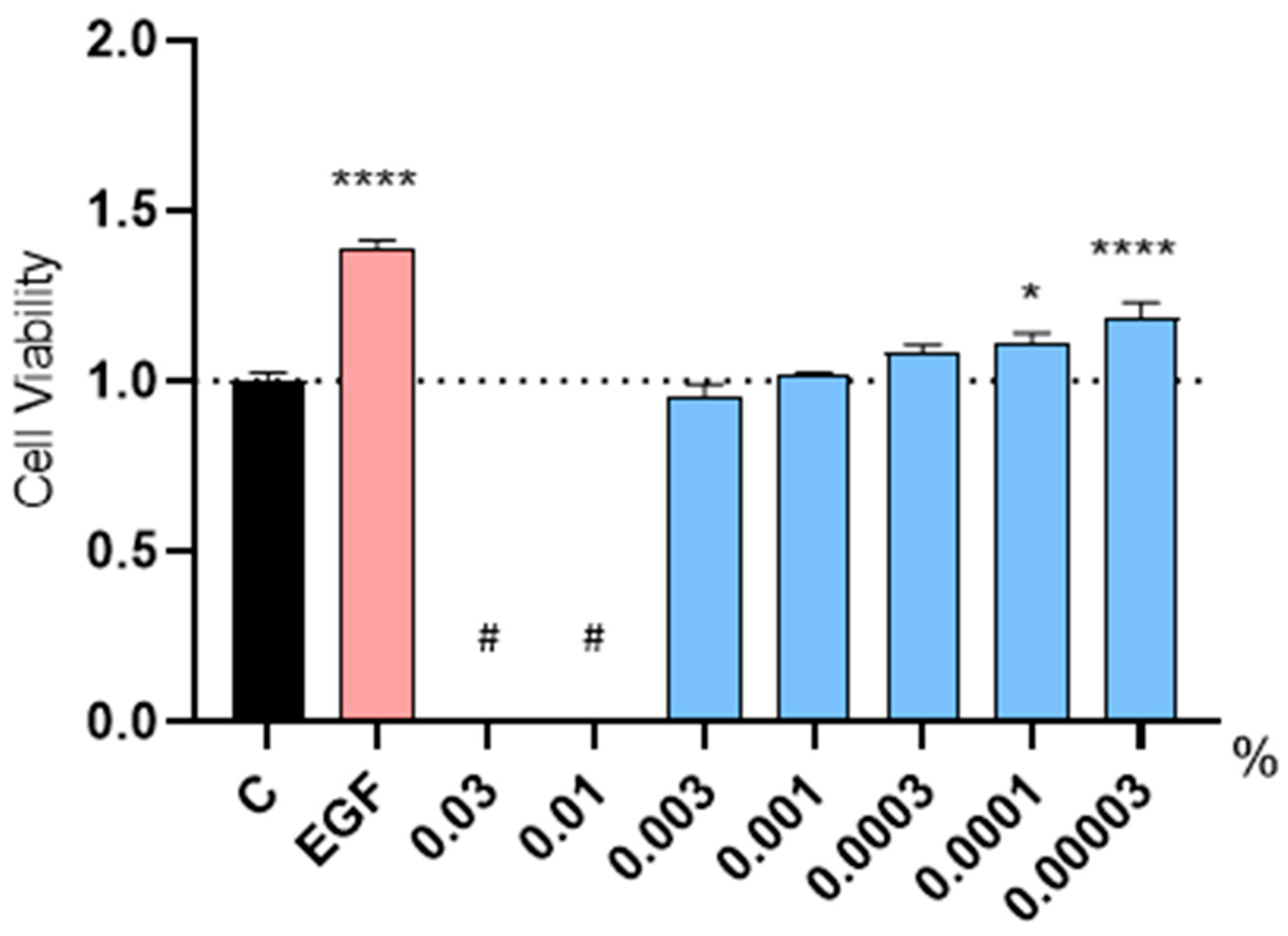
2.2. Evaluation of the Proliferative Capacity in Human Follicle Dermal Papilla Cells (HFDPC)
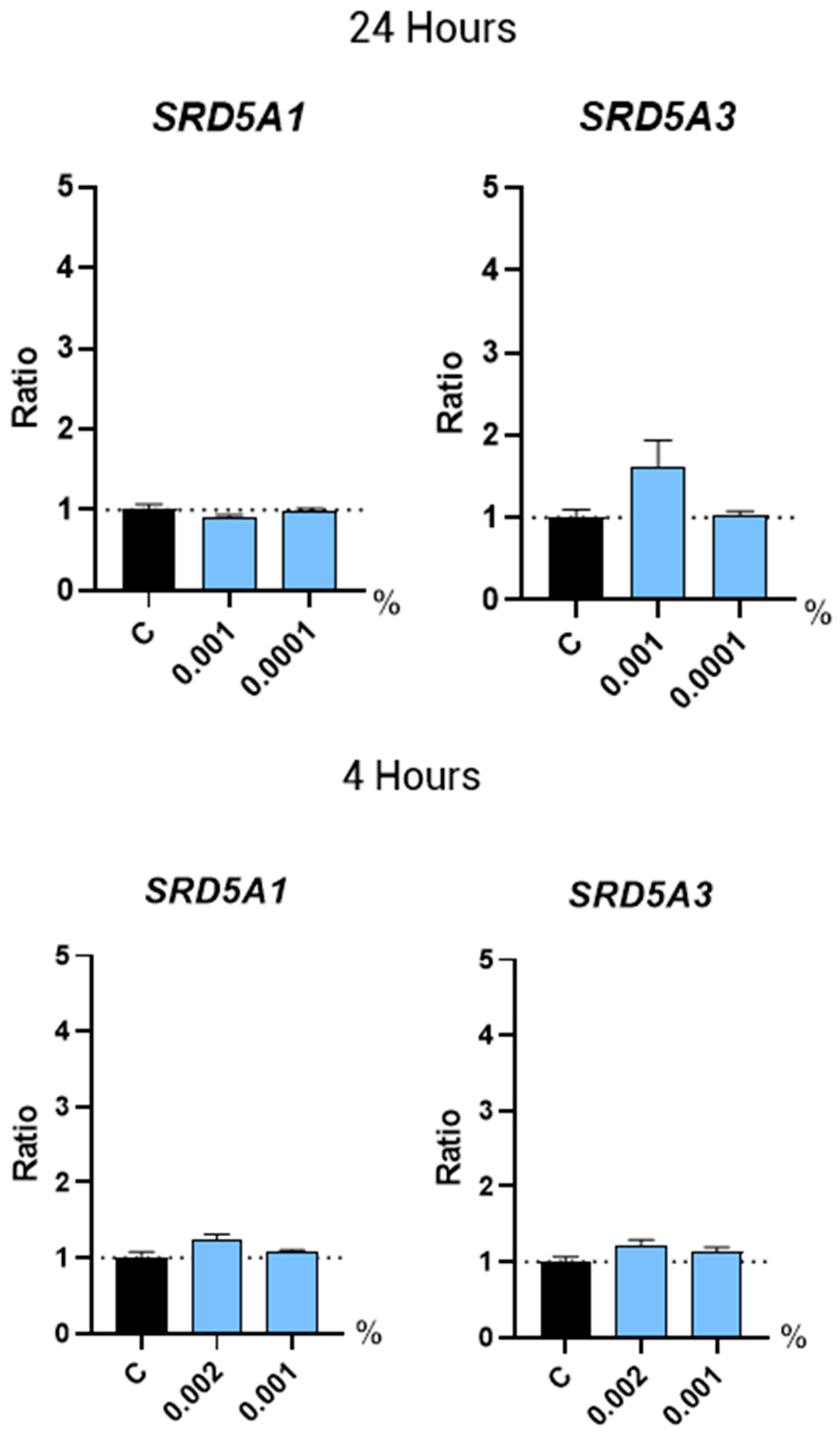
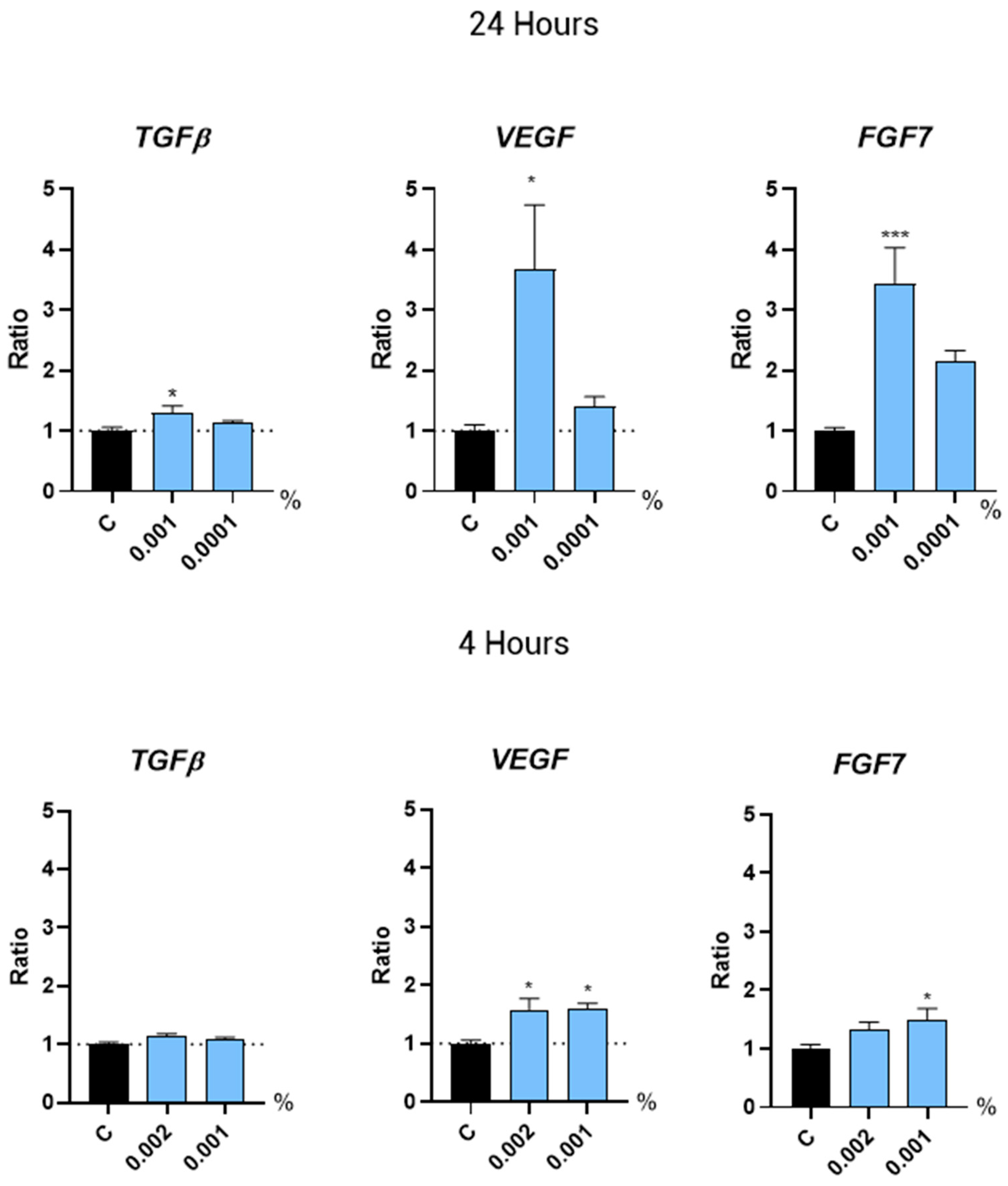
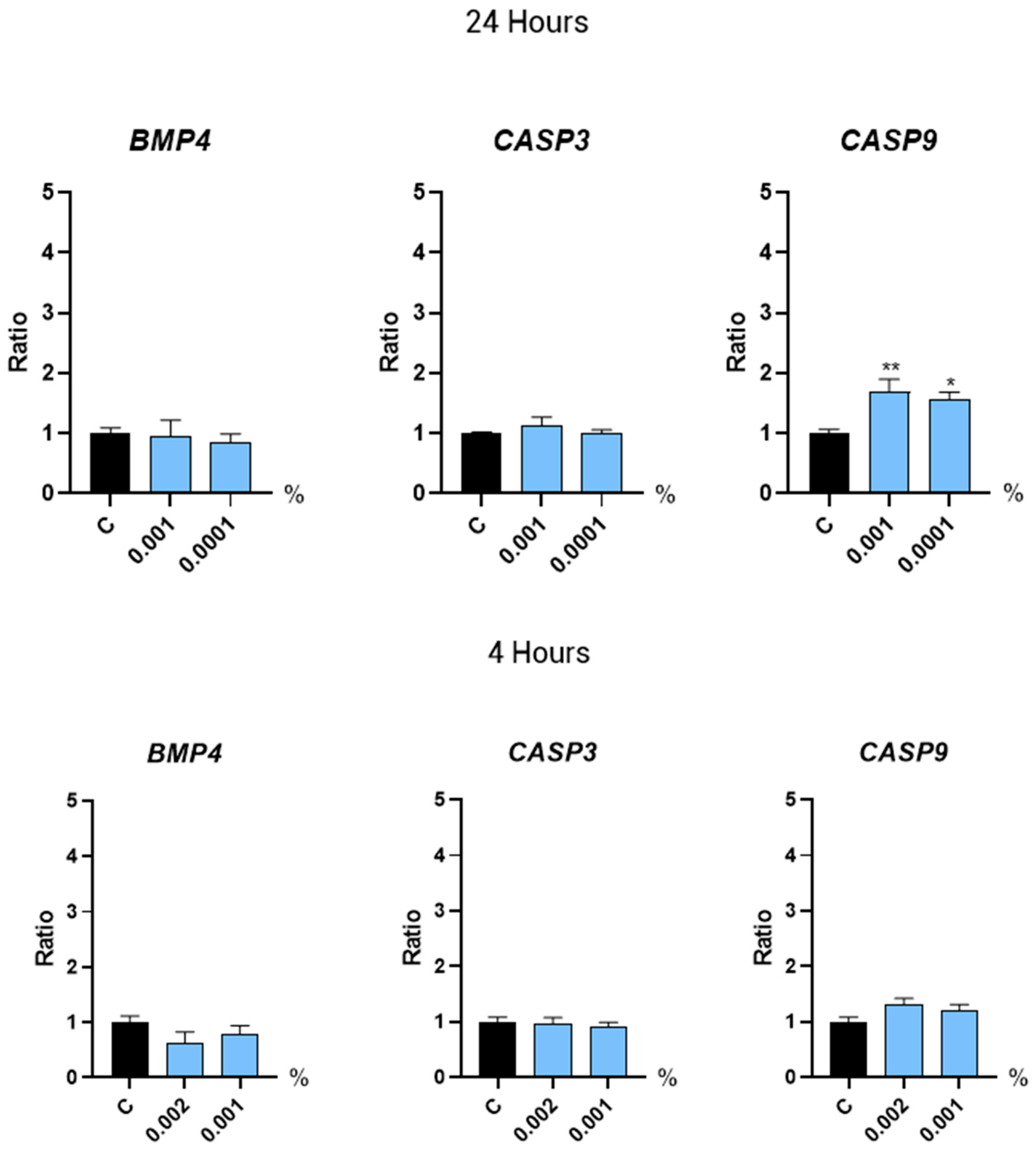
2.3. Expression Analysis of Genes Involved in Hair Growth and Health in Human Follicle Dermal Papilla Cells
2.3.1. 5α-Reductases
2.3.2. Hair Growth Regulation by Growth Factors
2.3.3. Regulators of the Hair Growth Cycle
2.3.4. Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents and Standards
4.3. Sample and Sample Preparation
4.4. HPLC-DAD-HRMS Characterization
4.5. Cell Proliferation Assay
4.6. Analysis of Gene Expression by qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Actin |
| ANOVA | Analysis of Variance |
| BMP | Bone Morphogenic Proteins |
| CASP3 | Caspase 3 |
| CASP9 | Caspase 9 |
| DAD | Diode Array Detector |
| DHT | Hormone Dihydrotestosterone |
| DMB | Dimeric 4-mercaptobutyl-glucosinolate |
| DMSO | Dimethylsulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| EGF | Epidermal Growth factor |
| FBS | Fetal Bovine Serum |
| FDA | Food and Drug Administration |
| FGF7 | Fibroblast Growth Factor 7 |
| HFDPC | Human Follicle Dermal Papilla Cells |
| HPLC-DAD-MS | High-Performance Liquid Chromatography–Diode Array Detector–Mass Spectrometry |
| HPLC-DAD-HRMS | High-Performance Liquid Chromatography–Diode Array Detector–High-Resolution Mass Spectrometry |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| MW | Molecular Weight |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| ROUT | Robust Regression and Outliner Detection |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SEM | Standard Error of Mean |
| SRD5A1 | Steroid 5 alpha-reductase 1 |
| SRD5A2 | Steroid 5 alpha-reductase 1 |
| SRD5A3 | Steroid 5 alpha-reductase 3 |
| TGBb | Transforming Growth Factor beta |
| TGF | Transforming Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
References
- McElwee, K.J.; Shapiro, J.S. Promising therapies for treating and/or preventing androgenic alopecia. Skin Ther. Lett. 2012, 17, 1–4. [Google Scholar]
- Martinez-Jacobo, L.; Villarreal-Villarreal, C.D.; Ortiz-López, R.; Ocampo-Candiani, J.; Rojas-Martínez, A. Genetic and molecular aspects of androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263–268. [Google Scholar]
- Ntshingila, S.; Oputu, O.; Arowolo, A.T.; Khumalo, N.P. Androgenetic alopecia: An update. JAAD Int. 2023, 13, 150–158. [Google Scholar]
- Owecka, B.; Tomaszewska, A.; Dobrzeniecki, K.; Owecki, M. The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines 2024, 12, 513. [Google Scholar] [CrossRef]
- Liang, B.; Yang, C.; Zuo, X.; Li, Y.; Ding, Y.; Sheng, Y.; Zhou, F.; Cheng, H.; Zheng, X.; Chen, G.; et al. Genetic variants at 20p11 confer risk to androgenetic alopecia in the Chinese Han population. PLoS ONE 2013, 8, e71771. [Google Scholar] [CrossRef]
- Rinaldi, S.; Bussa, M.; Mascaro, A. Update on the treatment of androgenetic alopecia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 54–58. [Google Scholar]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Mazzarella, G.; Loconsole, G.; Cammisa, G.; Mastrolonardo, G.; Vena, G. Topical finasteride in the treatment of androgenic alopecia. Preliminary evaluations after a 16-month therapy course. J. Dermatol. Treat. 1997, 8, 189–192. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Blume-Peytavi, U.; Scarci, F.; Jansat, J.M.; Falqués, M.; Otero, R.; Tamarit, M.L.; Galván, J.; Tebbs, V.; Massana, E.; et al. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: A phase III, randomized, controlled clinical trial. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 286–294. [Google Scholar]
- Arif, T.; Dorjay, K.; Adil, M.; Sami, M. Dutasteride in Androgenetic Alopecia: An Update. Curr. Clin. Pharmacol. 2017, 12, 31–35. [Google Scholar]
- Blume-Peytavi, U.; Lönnfors, S.; Hillmann, K.; Garcia Bartels, N. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatol. 2012, 66, 794–800. [Google Scholar] [CrossRef]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar]
- Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules 2024, 29, 2288. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, S.Y.; Yoo, M.; Park, W.S.; Lee, S.J.; Boo, Y.C.; Koh, J.S. Effects of a new mild shampoo for preventing hair loss in Asian by a simple hand-held phototrichogram technique. Int. J. Cosmet. Sci. 2011, 33, 491–496. [Google Scholar]
- Choi, H.C.; Nam, G.W.; Jeong, N.H.; Choi, B.Y. Hair Growth Promotion by Extracts of Inula Helenium and Caesalpinia Sappan Bark in Patients with Androgenetic Alopecia: A Pre-clinical Study Using Phototrichogram Analysis. Cosmetics 2019, 6, 66. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, W.Y.; Trinh, T.A.; Pyo, J.S.; Lee, S.; Kim, C.E.; Lee, D.H.; Park, E.S.; Kang, K.S. Hair Growth Effect of Emulsion Extracted Brevilin A, a JAK3 Inhibitor, from Centipeda minima. Processes 2020, 8, 767. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kawai, Y.; Masutani, T.; Tanaka, K.; Ito, K.; Iddamalgoda, A. Effects of watercress extract fraction on R-spondin 1-mediated growth of human hair. Int. J. Cosmet. Sci. 2022, 44, 154–165. [Google Scholar]
- Liang, C.H.; Lin, Y.H.; Lin, Y.K.; Chiang, C.F. Hair growth-promotion effects and antioxidant activity of the banana flower extract HappyAngel®: Double-blind, placebo-controlled trial. Food Sci. Hum. Wellness 2023, 12, 1917–1923. [Google Scholar]
- Ham, S.; Lee, Y.I.; Kim, I.A.; Suk, J.; Jung, I.; Jeong, J.; Lee, J.H. Efficacy and safety of persimmon leaf formulated with green tea and sophora fruit extracts (BLH308) on hair growth: A randomized, double-blind, placebo-controlled clinical trial. Skin Res. Technol. 2023, 29, e13448. [Google Scholar]
- You, J.; Woo, J.; Roh, K.; Jeon, K.; Jang, Y.; Choi, S.; Ryu, D.; Cho, E.; Park, D.; Lee, J.; et al. Evaluation of efficacy of Silybum marianum flower extract on the mitigating hair loss in vitro and in vivo. J. Cosmet. Dermatol. 2024, 23, 529–542. [Google Scholar]
- Vicente, R.; Leite ESilva, V.; Baby, A.; Velasco, M.; Bedin, V. Double-blind, randomized, placebo-controlled trial of a cream containing the Stryphnodendron adstringens (Martius) Coville bark extract for suppressing terminal hair growth. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 410–414. [Google Scholar]
- Choi, J.S.; Park, J.B.; Moon, W.S.; Moon, J.N.; Son, S.W.; Kim, M.R. Safety and Efficacy of Rice Bran Supercritical CO2 Extract for Hair Growth in Androgenic Alopecia: A 16-Week Double-Blind Randomized Controlled Trial. Biol. Pharm. Bull. 2015, 38, 1856–1863. [Google Scholar]
- Yu, J.Y.; Gupta, B.; Park, H.G.; Son, M.; Jun, J.-H.; Yong, C.S.; Kim, J.A.; Kim, J.O. Preclinical and Clinical Studies Demonstrate That the Proprietary Herbal Extract DA-5512 Effectively Stimulates Hair Growth and Promotes Hair Health. Evid. Based Complement. Altern. Med. 2017, 2017, 4395638. [Google Scholar] [CrossRef]
- Pekmezci, E.; Dündar, C.; Türkoğlu, M. A proprietary herbal extract against hair loss in androgenetic alopecia and telogen effluvium: A placebo-controlled, single-blind, clinical-instrumental study. Acta Dermatovenerol. Alp. Panon. Adriat. 2018, 27, 51–57. [Google Scholar] [CrossRef]
- Abd-Elsalam, R.M.; El Badawy, S.A.; Ogaly, H.A.; Ibrahim, F.M.; Farag, O.M.; Ahmed, K.A. Eruca sativa seed extract modulates oxidative stress and apoptosis and up-regulates the expression of Bcl-2 and Bax genes in acrylamide-induced testicular dysfunction in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 53249–53266. [Google Scholar]
- Bell, L.; Wagstaff, C. Rocket science: A review of phytochemical & health-related research in Eruca & Diplotaxis species. Food Chem. X 2019, 1, 100002. [Google Scholar]
- Garg, G.; Sharma, V. Eruca sativa (L.): Botanical Description, Crop Improvement, and Medicinal Properties. J. Herbs Spices Med. Plants 2014, 20, 171–182. [Google Scholar]
- Shatalebi, M.A.; Safaeian, L.; Baradaran, A.; Alamdarian, M. Preparation and evaluation of a hair wax containing propolis and Eruca sativa seed oil for hair growth. Adv. Biomed. Res. 2016, 5, 182. [Google Scholar]
- Luo, Z.; Zhang, X. Brassica oleracea extract, glucosinlates, and sulforaphane promote hair growth in vitro and ex vivo. J. Cosmet. Dermatol. 2022, 21, 1178–1184. [Google Scholar]
- Zhang, N.N.; Park, D.K.; Park, H.J. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement. Altern. Med. 2013, 13, 9. [Google Scholar]
- Ma, L.; Shen, H.; Fang, C.; Chen, T.; Wang, J. Camellia Seed Cake Extract Supports Hair Growth by Abrogating the Effect of Dihydrotestosterone in Cultured Human Dermal Papilla Cells. Molecules 2022, 27, 6443. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Abdin, R.; Gaumond, S.I.; Issa, N.T.; Jimenez, J.J. Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1387–1406. [Google Scholar]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC–MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Verardo, V.; Caboni, M.F.; D’Antuono, L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD–MS: Evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012, 133, 1025–1033. [Google Scholar] [CrossRef]
- Martinez-Sánchez, A.; Llorach, R.; Gil, M.I.; Ferreres, F. Identification of New Flavonoid Glycosides and Flavonoid Profiles to Characterize Rocket Leafy Salads (Eruca vesicaria and Diplotaxis tenuifolia). J. Agric. Food Chem. 2007, 55, 1356–1363. [Google Scholar] [CrossRef]
- Sut, S.; Boschiero, I.; Solana, M.; Malagoli, M.; Bertucco, A.; Dall’Acqua, S. Supercritical CO2 Extraction of Eruca sativa Using Cosolvents: Phytochemical Composition by LC-MS Analysis. Molecules 2018, 23, 3240. [Google Scholar] [CrossRef]
- Junlatat, J.; Sripanidkulchai, B. Hair Growth-Promoting Effect of Carthamus tinctorius Floret Extract. Phytother. Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef]
- Lee, H.; Kim, N.H.; Yang, H.; Bae, S.K.; Heo, Y.; Choudhary, I.; Kwon, Y.C.; Byun, J.K.; Yim, H.J.; Noh, B.S.; et al. The Hair Growth-Promoting Effect of Rumex japonicus Houtt. Extract. Evid.-Based Complement. Altern. Med. ECAM 2016, 2016, 1873746. [Google Scholar]
- Wen, T.C.; Li, Y.S.; Rajamani, K.; Harn, H.J.; Lin, S.Z.; Chiou, T.W. Effect of Cinnamomum osmophloeum Kanehira Leaf Aqueous Extract on Dermal Papilla Cell Proliferation and Hair Growth. Cell Transplant. 2018, 27, 256–263. [Google Scholar]
- Serruya, R.; Maor, Y. Hair growth-promotion effects at the cellular level and antioxidant activity of the plant-based extract PhyllotexTM. Heliyon 2021, 7, e07888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, H.; Chen, T.; Ma, L. Hair growth-promoting effects of Camellia seed cake extract in human dermal papilla cells and C57BL/6 mice. J. Cosmet. Dermatol. 2022, 21, 5018–5025. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Immenschuh, S.; Baumgart-Vogt, E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid. Redox Signal. 2005, 7, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.D.; Gucek, M.; Cole, R.N.; Watkins, P.A.; Comi, A.M. Cell proliferation and oxidative stress pathways are modified in fibroblasts from Sturge-Weber syndrome patients. Arch. Dermatol. Res. 2012, 304, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione–linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle: Resting stage of the hair follicle cycle. Biol. Rev. 2015, 90, 1179–1196. [Google Scholar] [CrossRef]
- Rastegar, H.; Ashtiani, H.A.; Aghaei, M.; Barikbin, B.; Ehsani, A. Herbal Extracts Induce Dermal Papilla Cell Proliferation of Human Hair Follicles. Ann. Dermatol. 2015, 27, 667–675. [Google Scholar] [CrossRef]
- Yamana, K.; Labrie, F.; Luu-The, V. Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Horm. Mol. Biol. Clin. Investig. 2010, 2, 293–299. [Google Scholar] [CrossRef]
- Russell, D.W.; Wilson, J.D. Steroid 5 alpha-reductase: Two genes/two enzymes. Annu. Rev. Biochem. 1994, 63, 25–61. [Google Scholar] [CrossRef]
- Agis-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [PubMed]
- Bernard, B.A. Molecular approach of hair biology. C. R. Seances Soc. Biol. Fil. 1994, 188, 223–233. [Google Scholar] [PubMed]
- Wang, W.; Wang, H.; Long, Y.; Li, Z.; Li, J. Controlling Hair Loss by Regulating Apoptosis in Hair Follicles: A Comprehensive Overview. Biomolecules 2023, 14, 20. [Google Scholar] [CrossRef]
- Kulessa, H.; Turk, G.; Hogan, B.L. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000, 19, 6664–6674. [Google Scholar] [PubMed]
- Ozeki, M.; Tabata, Y. Promoted growth of murine hair follicles through controlled release of vascular endothelial growth factor. Biomaterials 2002, 23, 2367–2373. [Google Scholar]
- Alexandrescu, D.T.; Kauffman, C.L.; Dasanu, C.A. The cutaneous epidermal growth factor network: Can it be translated clinically to stimulate hair growth? Dermatol. Online J. 2009, 15, 1. [Google Scholar]
- Zhao, J.; Harada, N.; Okajima, K. Dihydrotestosterone inhibits hair growth in mice by inhibiting insulin-like growth factor-I production in dermal papillae. Growth Horm. IGF Res. 2011, 21, 260–267. [Google Scholar]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar]
- Lee, C.Y.; Su, C.H.; Chiang, C.Y.; Wu, C.N.; Kuan, Y.H. Observation of the Expression of Vascular Endothelial Growth Factor and the Potential Effect of Promoting Hair Growth Treated with Chinese Herbal BeauTop. Evid.-Based Complement. Altern. Med. ECAM 2021, 2021, 6667011. [Google Scholar]
- Rosenquist, T.A.; Martin, G.R. Fibroblast growth factor signalling in the hair growth cycle: Expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn. 1996, 205, 379–386. [Google Scholar]
- Bae, W.Y.; Jung, W.H.; Shin, S.L.; Kim, T.R.; Sohn, M.; Suk, J.; Jung, I.; Lee, Y.I.; Lee, J.H. Heat-treated Limosilactobacillus fermentum LM1020 with menthol, salicylic acid, and panthenol promotes hair growth and regulates hair scalp microbiome balance in androgenetic alopecia: A double-blind, randomized and placebo-controlled clinical trial. J. Cosmet. Dermatol. 2024, 23, 2943–2955. [Google Scholar] [CrossRef]
- Alessandrini, A.M.; Bruni, F.; Piraccini, B.M.; Starace, M. The Effectiveness and Tolerability of Preformed Growth Factors Vehiculated Through Iontophoresis on Patients with Androgenetic Alopecia and Telogen Effluvium: A Clinical Study. Dermatol. Pract. Concept. 2021, 11, e2021082. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- Shin, H.; Yoo, H.G.; Inui, S.; Itami, S.; Kim, I.G.; Cho, A.-R.; Lee, D.H.; Park, W.S.; Kwon, O.; Cho, K.H.; et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013, 46, 460–464. [Google Scholar] [CrossRef]
- Inui, S.; Fukuzato, Y.; Nakajima, T.; Yoshikawa, K.; Itami, S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: A clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002, 16, 1967–1969. [Google Scholar]
- Liang, Y.; Tang, X.; Zhang, X.; Cao, C.; Yu, M.; Wan, M. Adipose Mesenchymal Stromal Cell-Derived Exosomes Carrying MiR-122-5p Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicles by Targeting the TGF-β1/SMAD3 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 5703. [Google Scholar] [CrossRef]
- Inui, S.; Fukuzato, Y.; Nakajima, T.; Yoshikawa, K.; Itami, S. Identification of androgen-inducible TGF-beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J. Investig. Dermatol. Symp. Proc. 2003, 8, 69–71. [Google Scholar] [CrossRef]
- Naruse, T.; Aoki, M.; Fujimoto, N.; Arase, S.; Oura, H.; Ueda, Y.; Ikeda, A. Novel ALK5 inhibitor TP0427736 reduces TGF-β induced growth inhibition in human outer root sheath cells and elongates anagen phase in mouse hair follicles. Pharmacol. Rep. PR 2017, 69, 485–491. [Google Scholar] [CrossRef]
- Yum, S.; Jeong, S.; Kim, D.; Lee, S.; Kim, W.; Yoo, J.W.; Kim, J.-A.; Kwon, O.S.; Kim, D.-D.; Min, D.S.; et al. Minoxidil Induction of VEGF Is Mediated by Inhibition of HIF-Prolyl Hydroxylase. Int. J. Mol. Sci. 2017, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Compound | Rt (min) | Molecular Formula | MW | Detected m/z Ions (Fragments) | Mass Error (ppm) | Standard |
|---|---|---|---|---|---|---|
| Flavonols | ||||||
| Quercetin-3,3′,4′-O-triglucoside | 6.12 | C33H40O22 | 788.2024 | 787.1946 [M-H]− (625.1521 [M-H-Glu]−) | 1.6 | No |
| Quercetin-trihexoside | 11.1 | C33H40O22 | 788.2024 | 787.1946 [M-H]− (625.1521 [M-H-Glu]− 463.1939 [M-H-2Glu]−) | 1.6 | No |
| Quercetin-3,4′-O-diglucoside | 11.4 | C27H30O17 | 626.1483 | 625.1428 [M-H]− 671.1604 [M+COO]− (463.1064 [M-H-Glu]−) | 2.6 | No |
| Kaempferol-3,4′-O-diglucoside | 11.5 | C27H30O16 | 610.1534 | 609.1474 [M-H]− | 2.4 | No |
| Isorhamnetin-3,4′-O-diglucoside | 12.0 | C28H32O17 | 640.1639 | 639.1582 [M-H]− | 2.6 | No |
| Kaempferol-3-O-sophoroside | 12.1 | C27H30O16 | 610.1534 | 609.1481 [M-H]− (447.1616 [M-H-Glu]−) | 4.1 | Yes |
| Rutin | 12.7 | C27H30O16 | 610.1534 | 609.1481 [M-H]− (447.1784 [M-H-Glu]−) | 4.4 | Yes |
| Quercetin-3-O-glucoside | 13.5 | C21H20O12 | 464.0955 | 463.0896 [M-H]− | 3.7 | No |
| Kaempferol-3-O-glucoside | 14.8 | C21H20O11 | 448.1006 | 447.0947 [M-H]− | 3.2 | Yes |
| Isorhamnetin-3-O-glucoside | 15.2 | C22H22O12 | 478.1127 | 477.1050 [M-H]− | 3.2 | No |
| Kaempferol | 17.2 | C15H10O6 | 286.0477 | 285.0419 [M-H]− 345.0654 [M+COO]− | 5.0 | Yes |
| Glucosinolates | ||||||
| Glucoraphanin | 1.7 | C12H23O10S3 | 437.0484 | 436.0368 [M-H]− | −5.2 | Yes |
| Glucoerucin | 5.4 | C12H23NO9S3 | 421.0535 | 420.0488 [M-H]− | 7.3 | Yes |
| DMB | 6.5 | C22H40N2O18S6 | 812.0600 | 811.0553 [M-H]− | 4.5 | No |
| Compound | mg·g−1 Dry Weight |
|---|---|
| Quercetin-3,3′,4′-O-triglucoside | 0.88 (0.06) * |
| Quercetin-trihexoside | 0.47 (0.01) |
| Quercetin-3,4′-O-diglucoside | 5.391 (0.002) |
| Kaempferol-3,4′-O-diglucoside | 11.997 (0.005) |
| Isorhamnetin-3,4′-O-diglucoside | 1.66 (0.01) |
| Kaempferol-3-O-sophoroside | 0.51 (0.01) |
| Rutin | 0.373 (0.001) |
| Quercetin-3-O-glucoside | 0.704 (0.008) |
| Kaempferol-3-O-glucoside | 1.522 (0.002) |
| Isorhamnetin-3-O-glucoside | 0.563 (0.002) |
| Kaempferol | <LOQ |
| Total flavonols | 21.2 (0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-García, A.; Meissner, J.M.; Pajuelo, D.; Morán-Valero, M.I.; Cristos, A.; Díez-Municio, M.; Mullor, J.L. Kyoh® Rocket Leaf Extract Regulates Proliferation and VEGF and FGF7 Expression in Human Dermal Follicle Papilla Cells. Molecules 2025, 30, 1489. https://doi.org/10.3390/molecules30071489
Mena-García A, Meissner JM, Pajuelo D, Morán-Valero MI, Cristos A, Díez-Municio M, Mullor JL. Kyoh® Rocket Leaf Extract Regulates Proliferation and VEGF and FGF7 Expression in Human Dermal Follicle Papilla Cells. Molecules. 2025; 30(7):1489. https://doi.org/10.3390/molecules30071489
Chicago/Turabian StyleMena-García, Adal, Justyna M. Meissner, David Pajuelo, María Inés Morán-Valero, Ana Cristos, Marina Díez-Municio, and Jose Luis Mullor. 2025. "Kyoh® Rocket Leaf Extract Regulates Proliferation and VEGF and FGF7 Expression in Human Dermal Follicle Papilla Cells" Molecules 30, no. 7: 1489. https://doi.org/10.3390/molecules30071489
APA StyleMena-García, A., Meissner, J. M., Pajuelo, D., Morán-Valero, M. I., Cristos, A., Díez-Municio, M., & Mullor, J. L. (2025). Kyoh® Rocket Leaf Extract Regulates Proliferation and VEGF and FGF7 Expression in Human Dermal Follicle Papilla Cells. Molecules, 30(7), 1489. https://doi.org/10.3390/molecules30071489






