Manganese Phthalocyanine-Based Magnetic Core–Shell Composites with Peroxidase Mimetic Activity for Colorimetric Detection of Ascorbic Acid and Glutathione
Abstract
1. Introduction
2. Experimental Sections
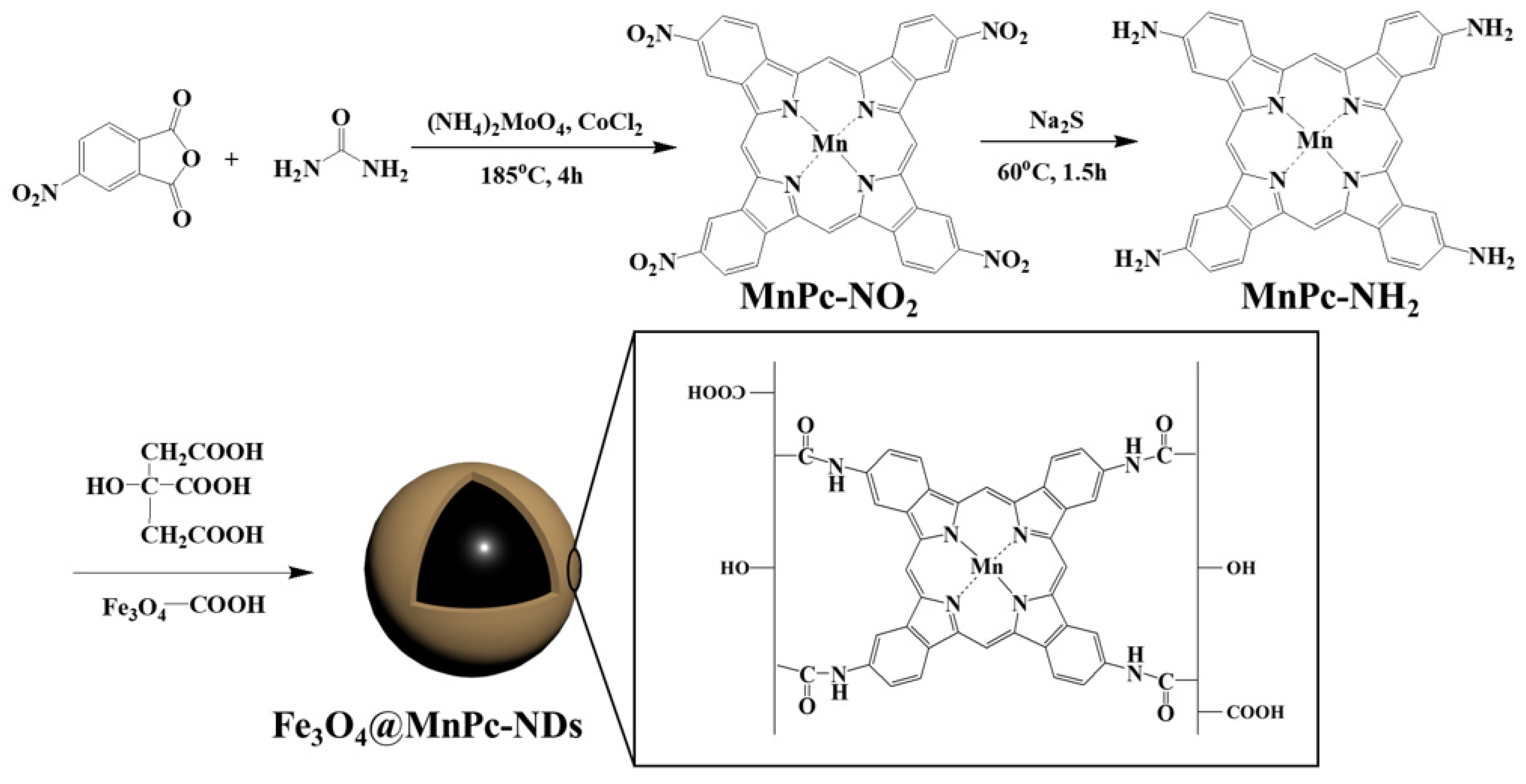
2.1. Synthesis of Manganese Tetranitro Phthalocyanine
2.2. Synthesis of Manganese Tetraamino Phthalocyanine
2.3. Synthesis of Magnetic Fe3O4 Nanoparticles
2.4. Synthesis of Fe3O4@MnPc-NDs
2.5. Peroxidase Like Catalytic Activity of Fe3O4@MnPc-NDs
2.6. Kinetic Measurements
2.7. Detection of H2O2 by the Colorimetric Method
2.8. Determination of AA and GSH by the Colorimetric Method
2.9. Identification of AA and GSH Concentrations in Real Samples
3. Results and Discussion
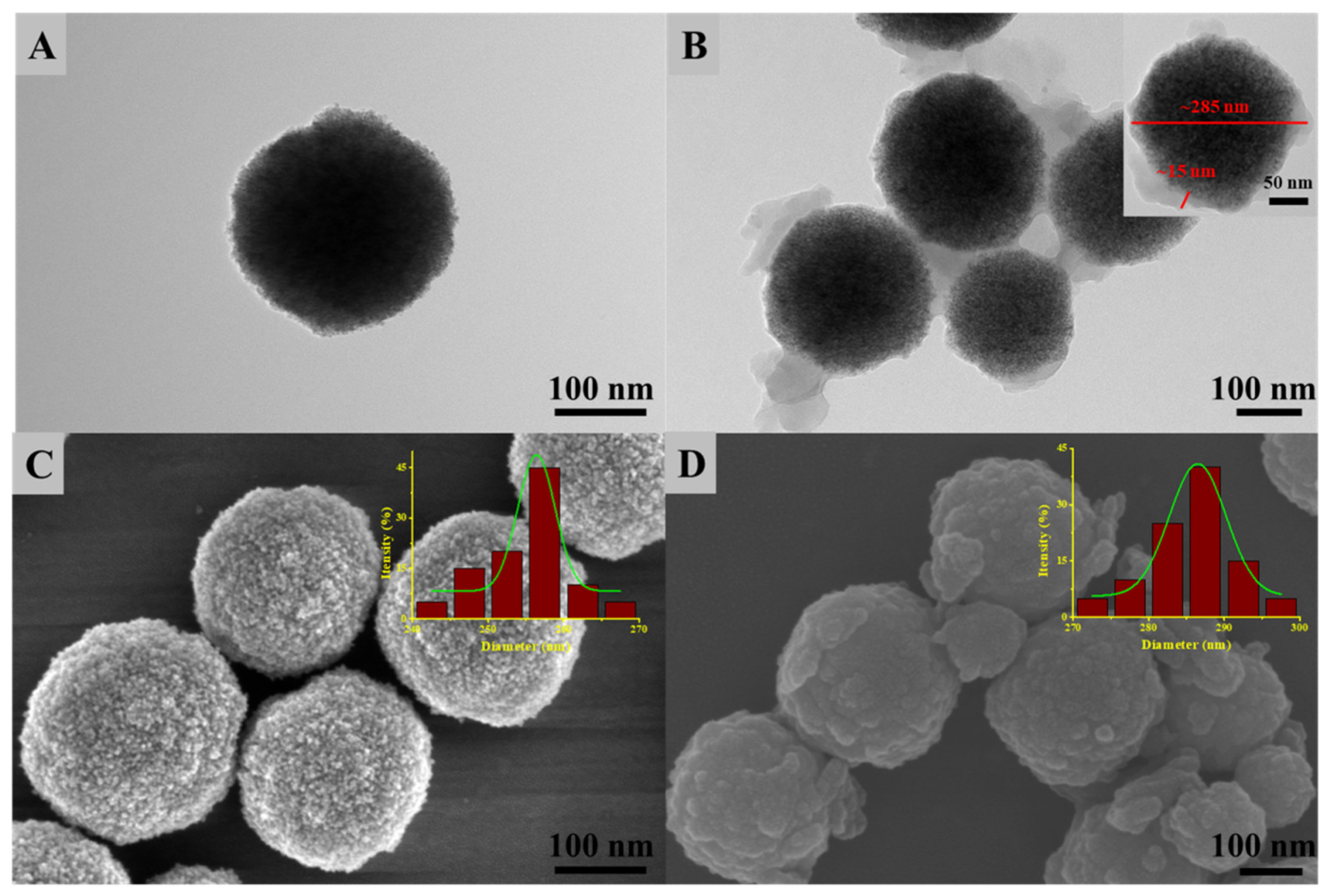
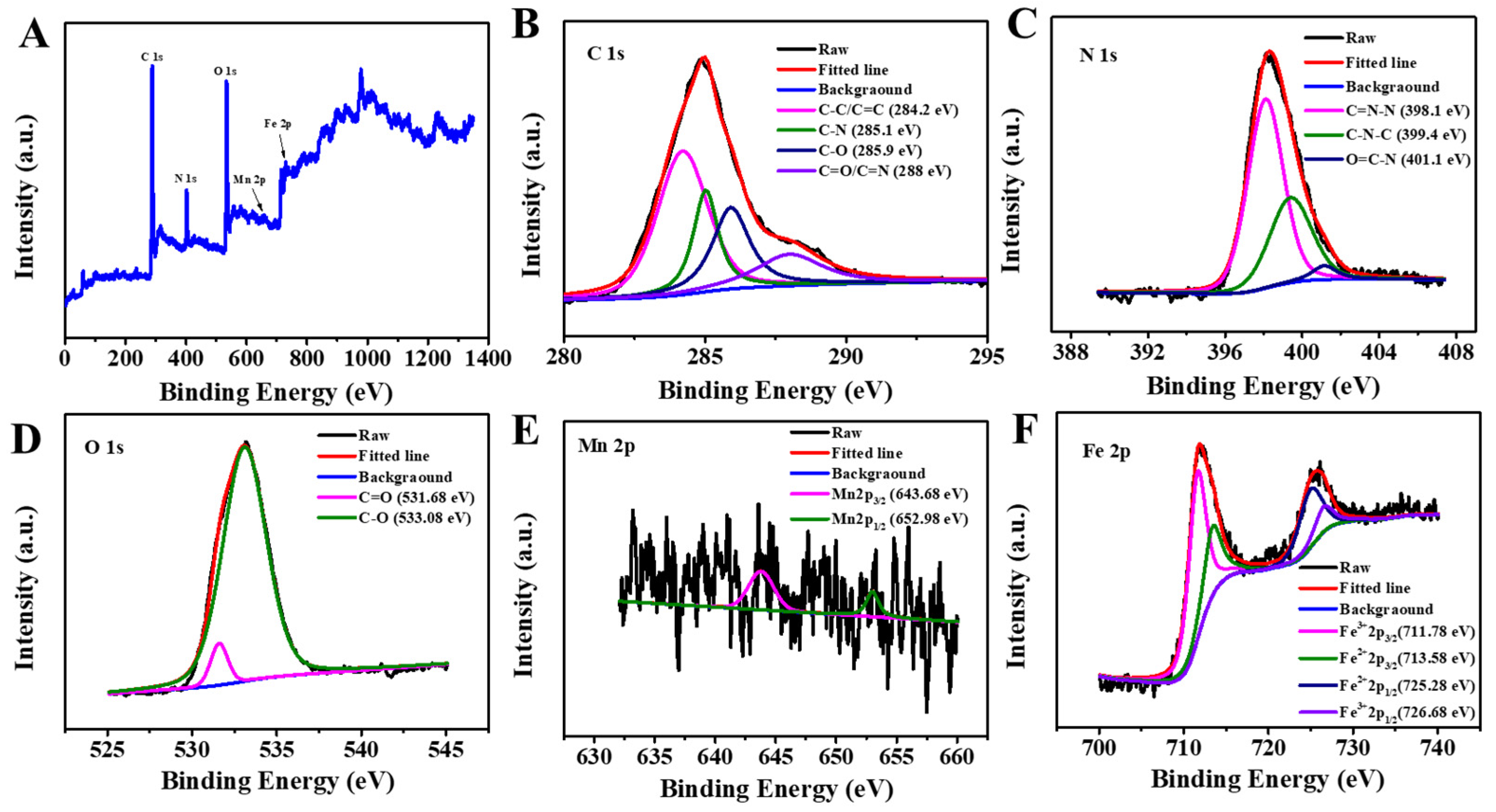
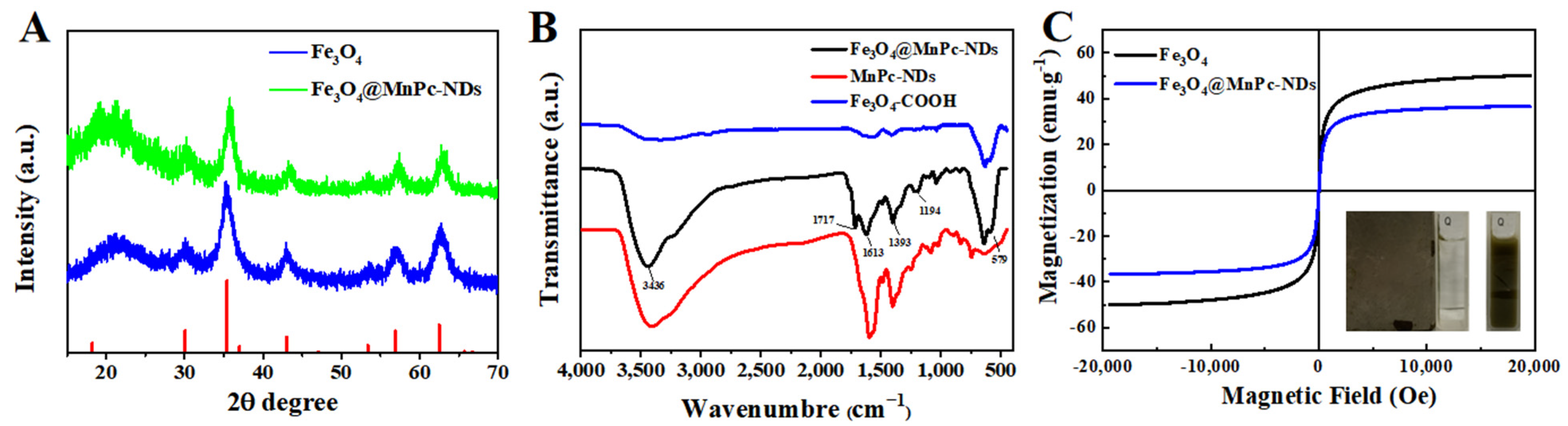
3.1. Characterization of Fe3O4@MnPc-NDs
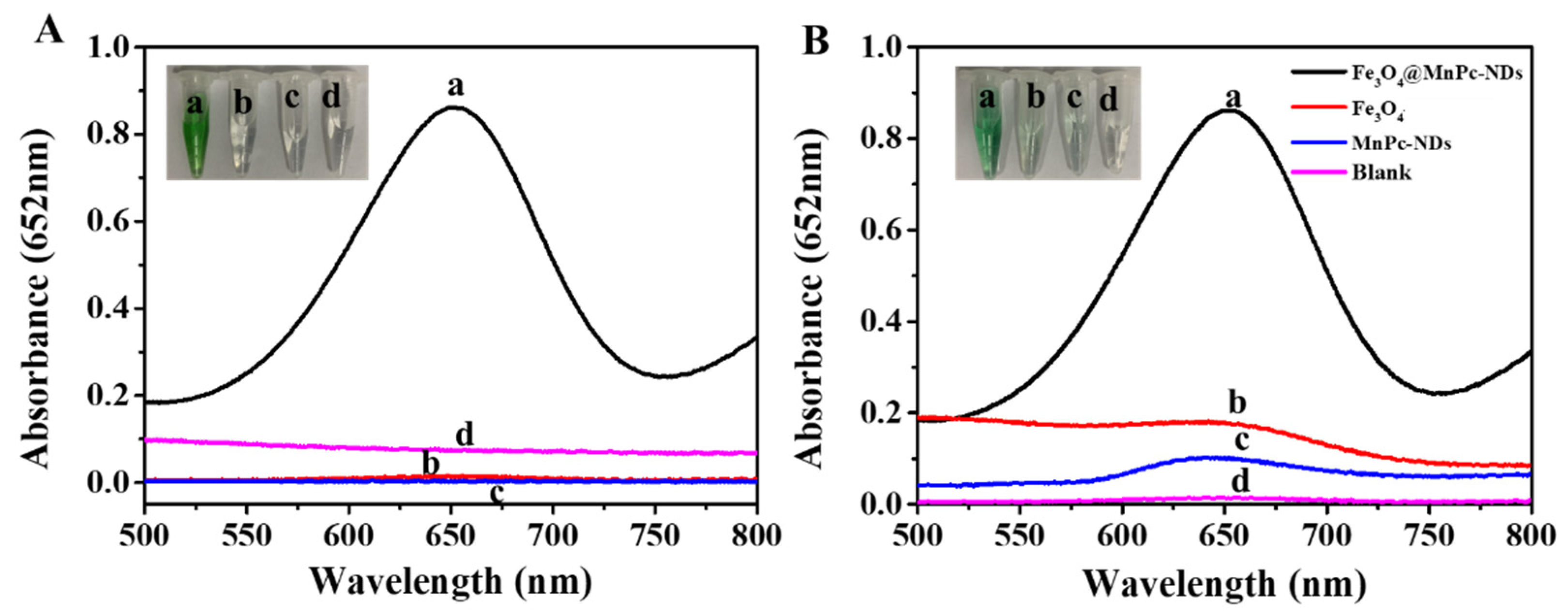
3.2. Peroxidase-Mimicking Activity of Fe3O4@MnPc-NDs
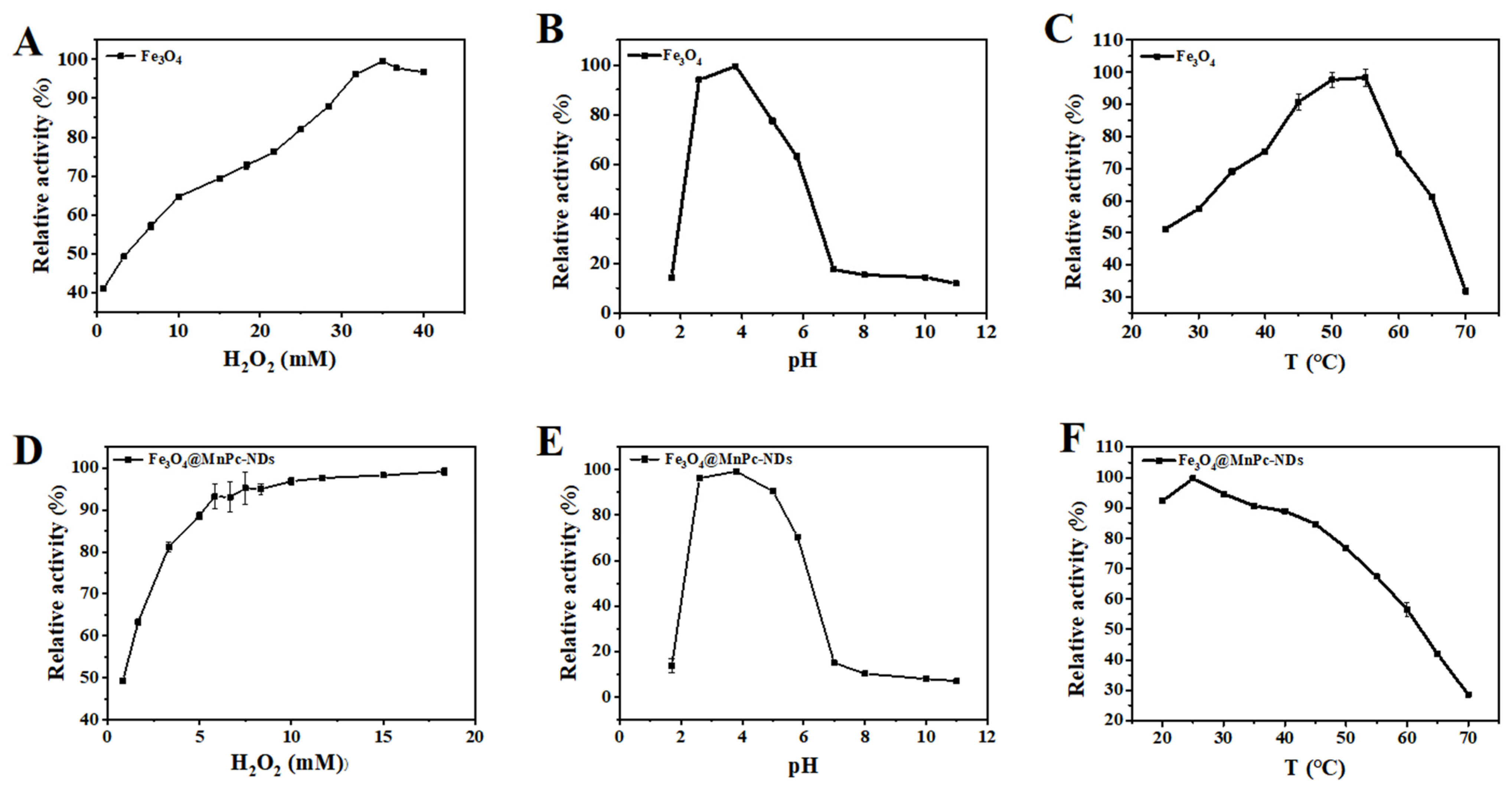
3.3. Optimization of the Catalyzed Conditions
3.4. Steady-State Kinetic Determination of Fe3O4@MnPc-NDs
3.5. Detection of H2O2
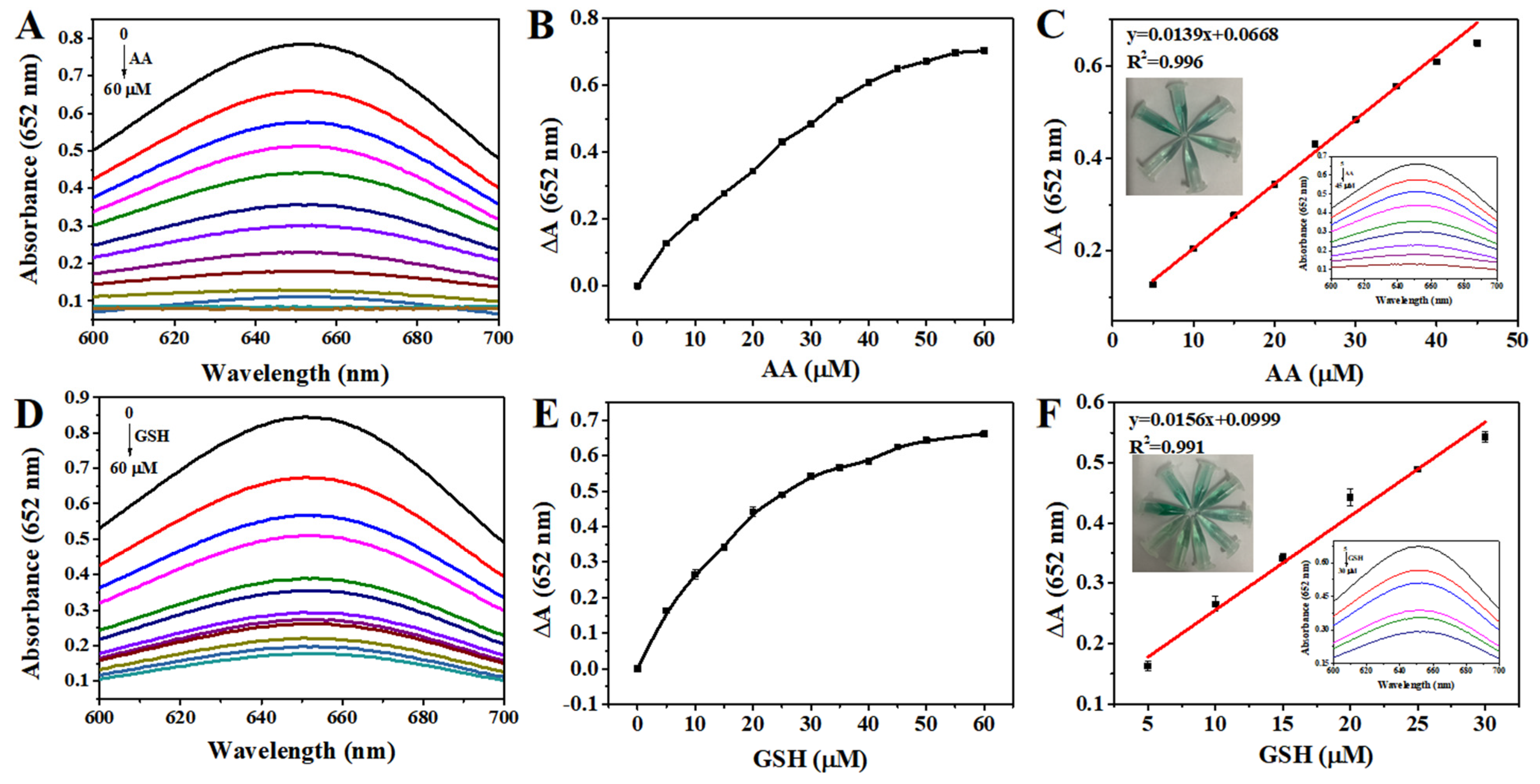
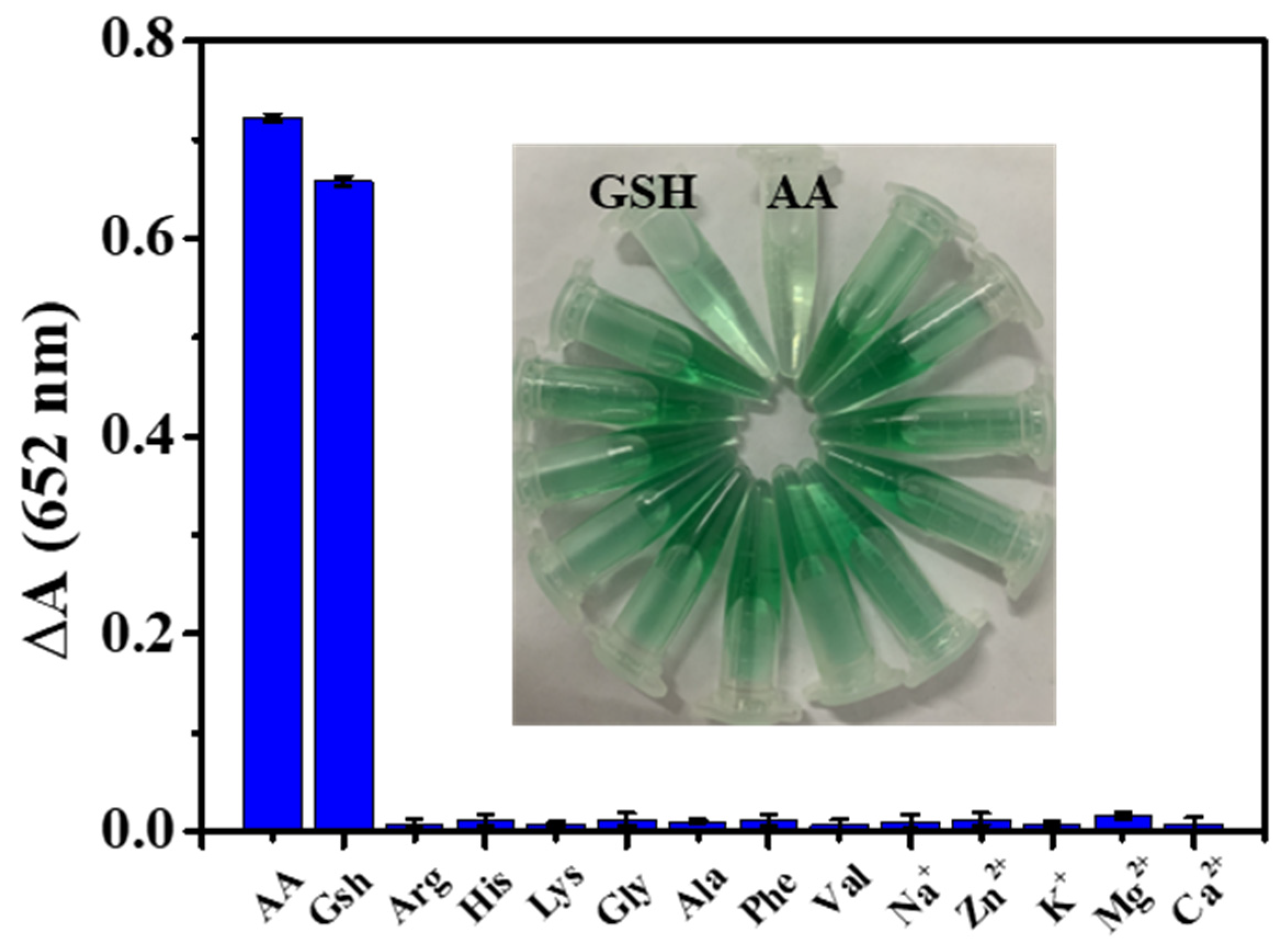
3.6. Detection of AA and GSH
3.7. Detection of Ascorbic Acid and Glutathione in Actual Samples
3.8. Recyclability Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, B.; Zhao, L.; Yu, J.; Hou, Y.; Ai, N.; Lu, J.-J.; Ge, W.; Chen, X. Exogenous iron impairs the anti-cancer effect of ascorbic acid both in vitro and in vivo. J. Adv. Res. 2023, 46, 149–158. [Google Scholar] [CrossRef]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.Y.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.N.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 2018, 34, 700–706. [Google Scholar] [CrossRef]
- Chang, J.; Qin, X.; Li, S.; He, F.; Gai, S.; Ding, H.; Yang, P. Combining Cobalt Ferrite Nanozymes with a Natural Enzyme to Reshape the Tumor Microenvironment for Boosted Cascade Enzyme-Like Activities. ACS Appl. Mater. Interfaces 2022, 14, 45217–45228. [Google Scholar] [CrossRef]
- Li, H.Q.; Ma, J.; Qin, Y.M.; Sun, X.; Pei, Z.G.; Yang, R.Q.; Li, Y.M.; Zhang, Q.H. Assessment of interactions between elemental carbon and metals in black carbon: Hydroxyl radical generation and glutathione depletion. J. Hazard. Mater. 2024, 470, 134223. [Google Scholar] [CrossRef]
- Pei, P.; Shen, W.H.; Zhou, H.L.; Sun, Y.C.; Zhong, J.; Liu, T.; Yang, K. Radionuclide labeled gold nanoclusters boost effective anti-tumor immunity for augmented radio-immunotherapy of cancer. Nano Today 2021, 38, 101144. [Google Scholar] [CrossRef]
- Liu, W.L.; Zhang, Y.H.; Wei, G.; Zhang, M.X.; Li, T.; Liu, Q.Y.; Zhou, Z.J.; Du, Y.; Wei, H. Integrated Cascade Nanozymes with Antisenescence Activities for Atherosclerosis Therapy. Angew. Chem. Int. Ed. 2023, 62, e202304465. [Google Scholar] [CrossRef]
- Charisis, S.; Ntanasi, E.; Stamelou, M.; Xiromerisiou, G.; Maraki, M.; Veskoukis, A.S.; Yannakoulia, M.; Kosmidis, M.H.; Anastasiou, C.A.; Giagkou, N.; et al. Plasma Glutathione and Prodromal Parkinson’s Disease Probability. Mov. Disord. 2022, 37, 200–205. [Google Scholar] [CrossRef]
- Guo, Y.X.; Liu, Y.R.; Zhao, S.H.; Xu, W.T.; Li, Y.Q.; Zhao, P.W.; Wang, D.; Cheng, H.Q.; Ke, Y.H.; Zhang, X. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat. Commun. 2021, 12, 7094. [Google Scholar] [CrossRef]
- Zhu, X.H.; Feng, T.T.; Chen, Y.D.; Xiao, Y.; Wen, W.; Zhang, X.H.; Wang, D.; Wang, S.F.; Liang, J.C.; Xiong, H.Y. GSH-depleted Cu-covalent organic frameworks for multimodal synergistic therapy against diabetic infections. Chem. Eng. J. 2024, 488, 150770. [Google Scholar] [CrossRef]
- Cheng, X.T.; Xu, H.D.; Ran, H.H.; Liang, G.L.; Wu, F.G. Glutathione-Depleting Nanomedicines for Synergistic Cancer Therapy. Acs Nano 2021, 15, 8039–8068. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.-H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Xi, X.X.; Wang, J.H.; Wang, Y.Z.; Xiong, H.Y.; Chen, M.M.; Wu, Z.; Zhang, X.H.; Wang, S.F.; Wen, W. Preparation of Au/Pt/Ti3C2Cl2 nanoflakes with self-reducing method for colorimetric detection of glutathione and intracellular sensing of hydrogen peroxide. Carbon 2022, 197, 476–484. [Google Scholar] [CrossRef]
- Lai, X.; Shen, Y.; Gao, S.B.; Chen, Y.J.; Cui, Y.S.; Ning, D.X.; Ji, X.B.; Liu, Z.W.; Wang, L.G. The Mn-modified porphyrin metal-organic framework with enhanced oxidase-like activity for sensitively colorimetric detection of glutathione. Biosens. Bioelectron. 2022, 213, 114446. [Google Scholar] [CrossRef]
- Liao, L.H.; Lin, X.; Zhang, J.; Hu, Z.Y.; Wu, F.S. Facile preparation of carbon dots with multicolor emission for fluorescence detection of ascorbic acid, glutathione and moisture content. J. Lumin. 2023, 264, 120169. [Google Scholar] [CrossRef]
- Zhe, Y.; Wang, J.L.; Zhao, Z.Q.; Ren, G.Y.; Du, J.J.; Li, K.; Lin, Y.Q. Ascorbate oxidase-like nanozyme with high specificity for inhibition of cancer cell proliferation and online electrochemical DOPAC monitoring. Biosens. Bioelectron. 2023, 220, 114893. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhu, J.L.; Yang, B.C.; Yao, X.X.; Zhu, X.X.; Liu, Q.Y.; Lyu, X.T. Novel synthesis of NiS/MMT/GO nanocomposites with enhanced peroxidase-like activity for sensitive colorimetric detection of glutathione in solution. Adv. Compos. Hybrid Mater. 2018, 1, 612–623. [Google Scholar] [CrossRef]
- Guo, D.; Li, C.; Liu, G.; Luo, X.; Wu, F. Oxidase Mimetic Activity of a Metalloporphyrin-Containing Porous Organic Polymer and Its Applications for Colorimetric Detection of Both Ascorbic Acid and Glutathione. Acs Sustain. Chem. Eng. 2021, 9, 5412–5421. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Wang, P.; Li, M.; Liu, D. A novel ultrasensitive electrochemiluminescence biosensor for glutathione detection based on poly-L-lysine as co-reactant and graphene-based poly(luminol/aniline) as nanoprobes. Biosens. Bioelectron. 2019, 133, 154–159. [Google Scholar] [CrossRef]
- Zeng, J.J.; Liao, L.H.; Lin, X.; Liu, G.Y.; Luo, X.G.; Luo, M.; Wu, F.S. Red-Emissive Sulfur-Doped Carbon Dots for Selective and Sensitive Detection of Mercury (II) Ion and Glutathione. Int. J. Mol. Sci. 2022, 23, 9213. [Google Scholar] [CrossRef]
- Fan, S.S.; Zhao, M.G.; Ding, L.J.; Li, H.; Chen, S.G. Preparation of Co3O4 crumpled graphene microsphere as peroxidase mimetic for colorimetric assay of ascorbic acid. Biosens. Bioelectron. 2017, 89, 846–852. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zheng, W.S.; Jiang, X.Y. Ag+-Gated Surface Chemistry of Gold Nanoparticles and Colorimetric Detection of Acetylcholinesterase. Small 2018, 14, 1801680. [Google Scholar] [CrossRef]
- Li, W.; Khan, M.; Lin, L.; Zhang, Q.; Feng, S.; Wu, Z.; Lin, J.M. Monitoring H2O2 on the Surface of Single Cells with Liquid Crystal Elastomer Microspheres. Angew. Chem. Int. Ed. 2020, 59, 9282–9287. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Guo, Z.; Hu, Z.; Xue, Z.; Lu, X. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively. Biosens. Bioelectron. 2017, 87, 101–107. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, J.; Zhao, C.L.; Zhou, F.J.; Yao, C.; Song, C. Ultra-fast colorimetric detection of glutathione by magnetic Fe NPs with peroxidase-like activity. Sens. Actuators B-Chem. 2022, 361, 131750. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, Y.Q.; Liu, X.Y.; Deng, T.T.; Dai, S.; Qu, J.F.; Yang, G.H.; Qu, L.L. Reusable ring-like Fe3O4Au nanozymes with enhanced peroxidase-like activities for colorimetric-SERS dual-mode sensing of biomolecules in human blood. Biosens. Bioelectron. 2022, 209, 114253. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.P.; Wang, Z.C.; Zhao, Y.Y.; Li, R.F.; Chen, X.Y.; Chen, H.; Wan, M.N.; Wang, X.Q. Metal-organic framework-modulated Fe3O4 composite au nanoparticles for antibacterial wound healing via synergistic peroxidase-like nanozymatic catalysis. J. Nanobiotechnology 2023, 21, 427. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Liu, B.; Liu, J. Molecular Imprinting on Inorganic Nanozymes for Hundred-fold Enzyme Specificity. J. Am. Chem. Soc. 2017, 139, 5412–5419. [Google Scholar] [CrossRef]
- Liu, S.X.; Yu, B.; Wang, S.; Shen, Y.Q.; Cong, H.L. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Matheu, R.; Gutierrez-Puebla, E.; Monge, M.Á.; Diercks, C.S.; Kang, J.; Prévot, M.S.; Pei, X.; Hanikel, N.; Zhang, B.; Yang, P.; et al. Three-Dimensional Phthalocyanine Metal-Catecholates for High Electrochemical Carbon Dioxide Reduction. J. Am. Chem. Soc. 2019, 141, 17081–17085. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Liu, Q.; Gao, W.; Xing, C.W.; Shen, J.S.; Liu, X.; Kong, X.; Li, X.Y.; Zhang, Y.X.; Chen, Y.L. A high-performance photoelectrochemical sensor for the specific detection of H2O2 and glucose based on an organic conjugated microporous polymer. J. Mater. Chem. A 2021, 9, 26216–26225. [Google Scholar] [CrossRef]
- Chan, J.Y.M.; Kawata, T.; Kobayashi, N.; Ng, D.K.P. Boron(III) Carbazosubphthalocyanines: Core-Expanded Antiaromatic Boron(III) Subphthalocyanine Analogues. Angew. Chem. Int. Ed. 2019, 58, 2272–2277. [Google Scholar] [CrossRef]
- Zheng, B.D.; He, Q.X.; Li, X.S.; Yoon, J.; Huang, J.D. Phthalocyanines as contrast agents for photothermal therapy. Coord. Chem. Rev. 2021, 426, 213548. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhang, X.P.; Huang, L.; Zhang, Z.Q.; Dong, S.J. One-Pot Synthesis of Fe3O4 Nanoparticle Loaded 3D Porous Graphene Nanocomposites with Enhanced Nanozyme Activity for Glucose Detection. Acs Appl. Mater. Interfaces 2017, 9, 7465–7471. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Darabdhara, G.; Sharma, B.; Das, M.R.; Boukherroub, R.; Szunerits, S. Cu-Ag bimetallic nanoparticles on reduced graphene oxide nanosheets as peroxidase mimic for glucose and ascorbic acid detection. Sens. Actuators B Chem. 2017, 238, 842–851. [Google Scholar] [CrossRef]
- Shi, Y.; Su, P.; Wang, Y.; Yang, Y. Fe3O4 peroxidase mimetics as a general strategy for the fluorescent detection of H2O2-involved systems. Talanta 2014, 130, 259–264. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Xiao, F.; Li, Y.; Zan, X.; Liao, K.; Xu, R.; Duan, H. Growth of Metal-Metal Oxide Nanostructures on Freestanding Graphene Paper for Flexible Biosensors. Adv. Funct. Mater. 2012, 22, 2487–2494. [Google Scholar] [CrossRef]
- Han, L.; Li, C.; Zhang, T.; Lang, Q.; Liu, A. Au@Ag Heterogeneous Nanorods as Nanozyme Interfaces with Peroxidase-Like Activity and Their Application for One-Pot Analysis of Glucose at Nearly Neutral pH. ACS Appl. Mater. Interfaces 2015, 7, 14463–14470. [Google Scholar] [CrossRef]
- Yadav, P.K.; Singh, V.K.; Chandra, S.; Bano, D.; Kumar, V.; Talat, M.; Hasan, S.H. Green Synthesis of Fluorescent Carbon Quantum Dots from Azadirachta indica Leaves and Their Peroxidase-Mimetic Activity for the Detection of H2O2 and Ascorbic Acid in Common Fresh Fruits. ACS Biomater. Sci. Eng. 2018, 5, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, W.; Han, Y.; Chen, X.; Zhu, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. N,S co-doped carbon dots based fluorescent “on-off-on” sensor for determination of ascorbic acid in common fruits. Food Chem. 2018, 258, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhu, H.; Chen, J.; Qiu, H. Facile synthesis of enzyme functional metal-organic framework for colorimetric detecting H2O2 and ascorbic acid. Chin. Chem. Lett. 2017, 28, 1006–1012. [Google Scholar] [CrossRef]
- Liu, J.; Bao, C.; Zhong, X.; Zhao, C.; Zhu, L. Highly selective detection of glutathione using a quantum-dot-based OFF–ON fluorescent probe. Chem. Commun. 2010, 46, 2971–2973. [Google Scholar] [CrossRef]
- Dennany, L.; Gerlach, M.; O’Carroll, S.; Keyes, T.E.; Forster, R.J.; Bertoncello, P. Electrochemiluminescence (ECL) sensing properties of water soluble core-shell CdSe/ZnS quantum dots/Nafion composite films. J. Mater. Chem. 2011, 21, 13984–13990. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zhang, X.; Chai, H.; Huang, Y. A Co,N co-doped hierarchically porous carbon hybrid as a highly efficient oxidase mimetic for glutathione detection. Sens. Actuators B Chem. 2018, 264, 312–319. [Google Scholar] [CrossRef]



| Material | Catalytic Substrate | Km (mM) | Vmax (10−8 M S−1) | Reference |
|---|---|---|---|---|
| Fe3O4@MnPc-NDs | TMB | 0.037 | 3.78 | This work |
| H2O2 | 0.47 | 4.82 | ||
| Fe3O4 | TMB | 0.098 | 3.44 | [35] |
| H2O2 | 154 | 9.78 | ||
| HRP | TMB | 0.434 | 10 | [35] |
| H2O2 | 3.7 | 8.71 | ||
| Cu-Ag/rGO | TMB | 8.61 | 7.02 | [36] |
| H2O2 | 0.634 | 4.26 |
| Material | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|
| Fe3O4@MnPc-NDs | 20–250 | 4.7 | This work |
| Fe3O4 | 5–100 | 3 | [37] |
| Copper | 10–1000 | 10 | [38] |
| Pt-MnO2/GOP | 2–13,330 | 5 | [39] |
| Au@Ag | 10–10,000 | 6 | [40] |
| Catalyst | Detection | Linear Range (µM) | Detection Limit (μM) | Reference |
|---|---|---|---|---|
| N-CQDs | AA | 5–40 | 1.77 | [41] |
| N, S-CDs | AA | 10–200 | 4.69 | [42] |
| MIL-88 | AA | 2.57–10.10 | 1.03 | [43] |
| Fe3O4@MnPc-NDs | AA | 5–45 | 0.161 | This work |
| QDs | GSH | 5–250 | 0.6 | [44] |
| Cd-Se/ZnS QDs | GSH | 10–180 | 1.5 | [45] |
| Co, N-HPC | GSH | 0.05–30 | 0.036 | [46] |
| Fe3O4@MnPc-NDs | GSH | 5–30 | 0.188 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Tian, L.; Pang, Y.; Wu, F. Manganese Phthalocyanine-Based Magnetic Core–Shell Composites with Peroxidase Mimetic Activity for Colorimetric Detection of Ascorbic Acid and Glutathione. Molecules 2025, 30, 1484. https://doi.org/10.3390/molecules30071484
Qi J, Tian L, Pang Y, Wu F. Manganese Phthalocyanine-Based Magnetic Core–Shell Composites with Peroxidase Mimetic Activity for Colorimetric Detection of Ascorbic Acid and Glutathione. Molecules. 2025; 30(7):1484. https://doi.org/10.3390/molecules30071484
Chicago/Turabian StyleQi, Junchao, Long Tian, Yudong Pang, and Fengshou Wu. 2025. "Manganese Phthalocyanine-Based Magnetic Core–Shell Composites with Peroxidase Mimetic Activity for Colorimetric Detection of Ascorbic Acid and Glutathione" Molecules 30, no. 7: 1484. https://doi.org/10.3390/molecules30071484
APA StyleQi, J., Tian, L., Pang, Y., & Wu, F. (2025). Manganese Phthalocyanine-Based Magnetic Core–Shell Composites with Peroxidase Mimetic Activity for Colorimetric Detection of Ascorbic Acid and Glutathione. Molecules, 30(7), 1484. https://doi.org/10.3390/molecules30071484






