Influence of Copigmentation and Encapsulation on Stability and Antioxidant Activity of Anthocyanins from Blue and Pink Cornflower (Centaurea cyanus L.) Flowers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Anthocyanin Extracts from Blue and Pink Cornflower Flowers
2.2. Copigmentation and Encapsulation of Anthocyanins from Blue and Pink Cornflowers
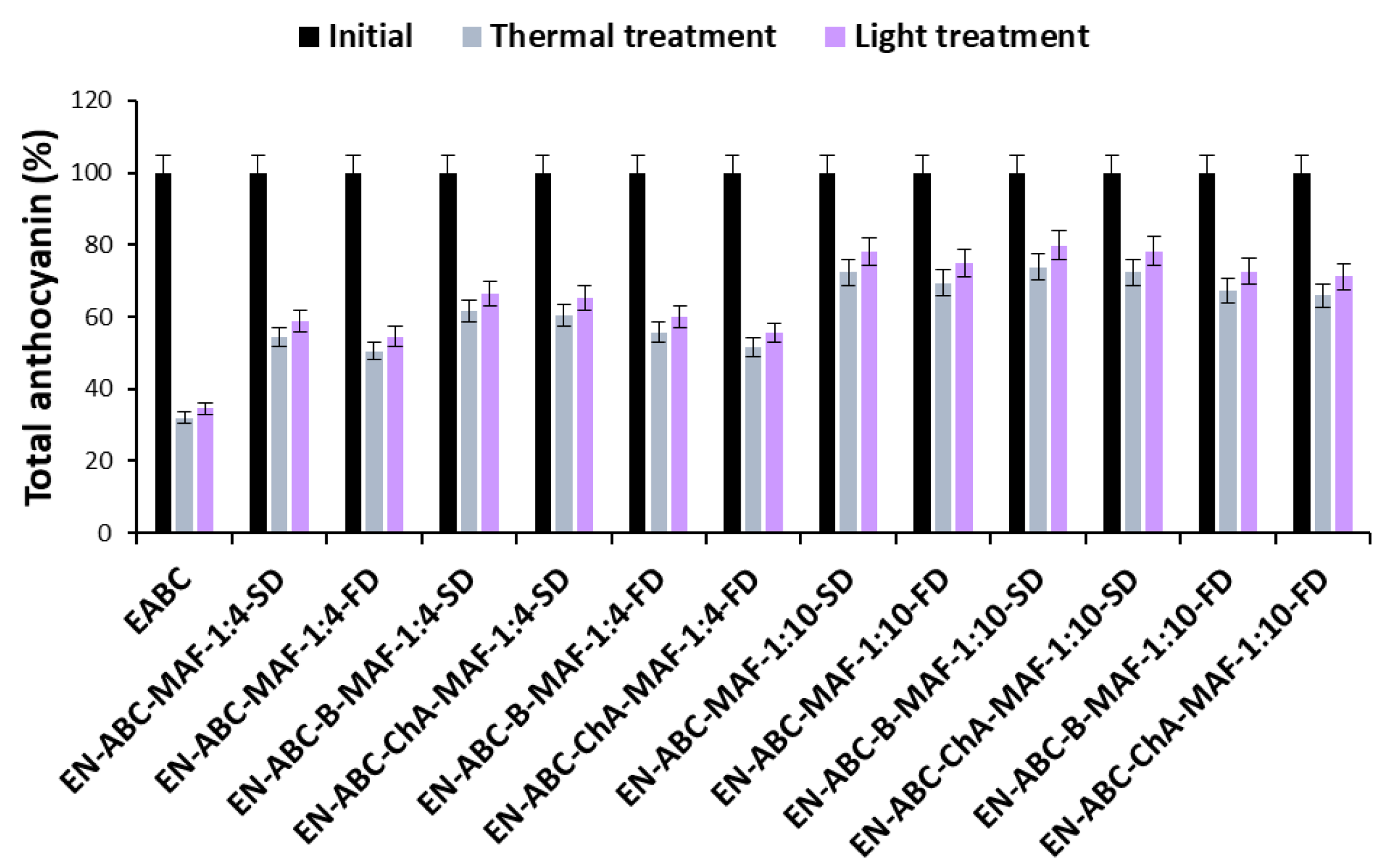
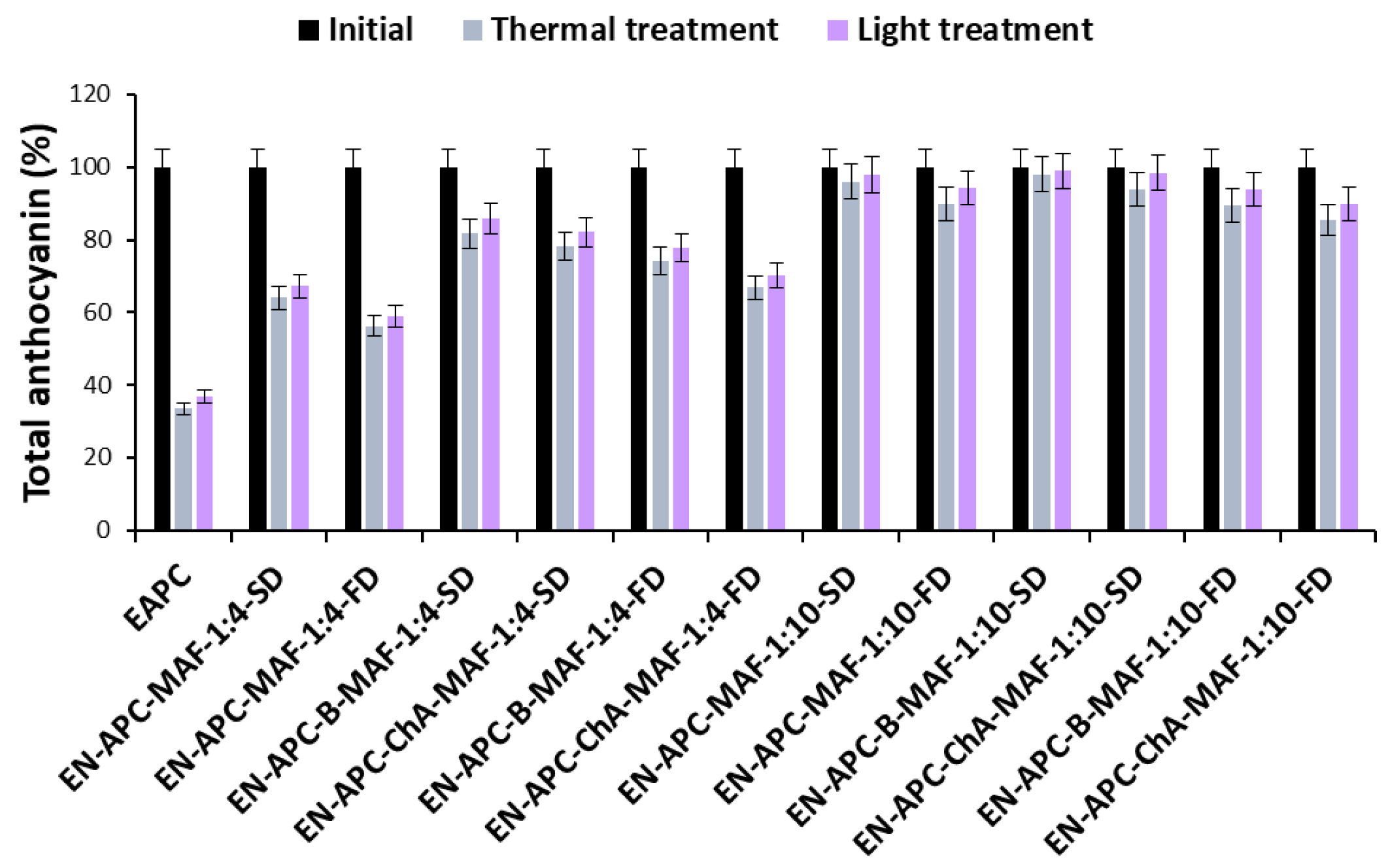
2.3. Stability of Cornflower Anthocyanin Extracts and Encapsulates
3. Materials and Methods
3.1. Materials
3.2. Preparation of Anthocyanin Extracts
3.3. Copigmentation of Anthocyanin Extracts
3.4. Encapsulation of Anthocyanin Extracts
3.4.1. Preparation of Solutions
3.4.2. Spray-Drying Processing
3.4.3. Freeze-Drying Processing
3.5. Qualitative and Quantitative Analysis of Anthocyanins and Other Phenolic Compounds Using UHPLC-DAD-ESI-MS/MS
3.6. Determination of Total Anthocyanins According to the Method of Ronald E. Wrolstade
3.7. Determination of Total Phenolic Compounds by the Folin–Ciocalteu Method
3.8. Thermal Stability of Anthocyanins
3.9. Stability of Anthocyanins Under UV-A Irradiation
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EABC | Extract of anthocyanins from the blue cornflower flowers |
| EAPC | Extract of anthocyanins from the pink cornflower flowers |
| EN-ABC-MAF-1:4-SD | Encapsulate of EABC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by spray-drying |
| EN-ABC-MAF-1:4-FD | Encapsulate of EABC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by freeze-drying |
| EN-ABC-B-MAF-1:4-SD | Encapsulate of EABC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by spray-drying |
| EN-ABC-ChA-MAF-1:4-SD | Encapsulate of EABC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by spray-drying |
| EN-ABC-B-MAF-1:4-FD | Encapsulate of EABC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by freeze-drying |
| EN-ABC-ChA-MAF-1:4-FD | Encapsulate of EABC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by freeze-drying |
| EN-ABC-MAF-1:10-SD | Encapsulate of EABC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by spray-drying |
| EN-ABC-MAF-1:10-FD | Encapsulate of EABC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by freeze-drying |
| EN-ABC-B-MAF-1:10-SD | Encapsulate of EABC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by spray-drying |
| EN-ABC-ChA-MAF-1:10-SD | Encapsulate of EABC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by spray-drying |
| EN-ABC-B-MAF-1:10-FD | Encapsulate of EABC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by freeze-drying |
| EN-ABC-ChA-MAF-1:10-FD | Encapsulate of EAPC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by freeze-drying |
| EN-APC-MAF-1:4-SD | Encapsulate of EAPC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by spray-drying |
| EN-APC-MAF-1:4-FD | Encapsulate of EAPC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by freeze-drying |
| EN-APC-B-MAF-1:4-SD | Encapsulate of EAPC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by spray-drying |
| EN-APC-ChA-MAF-1:4-SD | Encapsulate of EAPC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by spray-drying |
| EN-APC-B-MAF-1:4-FD | Encapsulate of EAPC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by freeze-drying |
| EN-APC-ChA-MAF-1:4-FD | Encapsulate of EAPC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:4 obtained by freeze-drying |
| EN-APC-MAF-1:10-SD | Encapsulate of EAPC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by spray-drying |
| EN-APC-MAF-1:10-FD | Encapsulate of EAPC with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by freeze-drying |
| EN-APC-B-MAF-1:10-SD | Encapsulate of EAPC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by spray-drying |
| EN-APC-ChA-MAF-1:10-SD | Encapsulate of EAPC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by spray-drying |
| EN-APC-B-MAF-1:10-FD | Encapsulate of EAPC and baicalin with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by freeze-drying |
| EN-APC-ChA-MAF-1:10-FD | Encapsulate of EAPC and chlorogenic acid with maltodextrin and acacia fiber (1:1) in a weight ratio of 1:10 obtained by freeze-drying |
References
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.; Barouh, N.; Barea, B.; Fernandes, A.; de Freitas, V.; Salas, E. Isolation and characterization of anthocyanins from Hibiscus sabdariffa flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Gamage, G.; Lim, Y.; Choo, W. Anthocyanins from Clitoria ternatea Flower: Biosynthesis, Extraction, Stability, Antioxidant Activity and Applications. Front. Plant Sci. 2021, 12, 792303. [Google Scholar]
- Kanha, N.; Regenstein, J.M.; Surawang, S.; Pitchakarn, P.; Laokuldilok, T. Properties and kinetics of the in vitro release of anthocyanin-rich microcapsules produced through spray and freeze-drying complex coacervated double emulsions. Food Chem. 2021, 15, 340. [Google Scholar]
- Robert, P.; Fredes, C. The Encapsulation of Anthocyanins from Berry-Type Fruits. Trends in Foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/microencapsulation of anthocyanins; a systematic review and meta-analysis. Food Res. Int. 2020, 132, 109077. [Google Scholar]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar]
- Fan, L.; Wang, Y.; Xie, P.; Zhang, L.; Li, Y.; Zhou, J. Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation. Food Chem. 2019, 275, 299–308. [Google Scholar]
- Deng, C.; Li, S.; Feng, C.; Hong, Y.; Huang, H.; Wang, J.; Wang, L.; Dai, S. Metabolite and gene expression analysis reveal the molecular mechanism for petal colour variation in six Centaurea cyanus cultivars. Plant Physiol. Biochem. 2019, 142, 22–33. [Google Scholar]
- Różyło, R.; Szymańska-Chargot, M.; Gawlik-Dziki, U.; Dziki, D. Spectroscopic, mineral, and antioxidant characteristics of blue colored powders prepared from cornflower aqueous extracts. Food Chem. 2021, 346, 128889. [Google Scholar] [PubMed]
- Różyło, R.; Szymańska-Chargot, M.; Zdunek, A.; Gawlik-Dziki, U.; Dziki, D. Microencapsulated Red Powders from Cornflower Extract—Spectral (FT-IR and FT-Raman) and Antioxidant Characteristics. Molecules 2022, 27, 3094. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kwon, R.H.; Kim, S.A.; Na, H.; Cho, J.-Y.; Kim, H.-W. Characterization of Anthocyanins Including Acetylated Glycosides from Highbush Blueberry (Vaccinium corymbosum L.) Cultivated in Korea Based on UPLC-DAD-QToF/MS and UPLC-Qtrap-MS/MS. Foods 2025, 14, 188. [Google Scholar] [CrossRef]
- Brenes, C.H.; Del Pozo-Insfran, D.; Talcott, S.T. Stability of copigmented anthocyanins and ascorbic acid in a grape juice model system. J. Agric. Food Chem. 2005, 53, 49–56. [Google Scholar] [PubMed]
- Deng, K.; Ouyang, J.; Hu, N.; Dong, Q.; Chen, C.; Wang, H. Improved Stability of Blue Colour of Anthocyanins from Lycium ruthenicum Murr. Based on Copigmentation. Molecules 2022, 27, 608. [Google Scholar] [CrossRef]
- Gençdağ, E.; Özdemir, E.; Demirci, K.; Görgüç, A.; Yilmaz, F. Copigmentation and stabilization of anthocyanins using organiz molecules and encapsulation techniques. Curr. Plant Biol. 2022, 29, 00238. [Google Scholar]
- Zhang, B.; He, F.; Zhou, P.P.; Liu, Y.; Duan, C.Q. The color expression of copigmentation between malvidin-3-O-glucoside and three phenolic aldehydes in model solutions: The effects of pH and molar ratio. Food Chem. 2016, 199, 220–228. [Google Scholar]
- Vergara, C.; Pino, M.T.; Zamora, O.; Parada, J.; Pérez, R.; Uribe, M.; Kalazich, J. Microencapsulation of Anthocyanin Extracted from Purple Flesh Cultivated Potatoes by Spray Drying and Its Effects on In Vitro Gastrointestinal Digestion. Molecules 2020, 25, 722. [Google Scholar] [CrossRef]
- Troise, A.D.; Colantuono, A.; Fiore, A. Spray-dried olive mill wastewater reduces Maillard reaction in cookies model system. Food Chem. 2020, 323, 126793. [Google Scholar]
- Yawadio, R.; Tanimori, S.; Morita, N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007, 101, 1616–1625. [Google Scholar]
- Ab Rashid, S.; Tong, W.; Leong, C.; Ghazali, N.M.A.; Taher, M.A.; Ahmad, N.; Tan, W.-N.; Teo, S.H. Anthocyanin Microcapsule from Clitoria ternatea: Potential Bio-preservative and Blue Colorant for Baked Food Products. Arab. J. Sci. Eng. 2021, 46, 65–72. [Google Scholar]
- Laleh, G.H.; Frydoonfar, H.; Jameei, R.; Zare, S. The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pak. J. Nutr. 2006, 5, 90–92. [Google Scholar]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, F.; Wang, Z.; Feng, Y.; Han, Y. Advances in the Preparation, Stability, Metabolism, and Physiological Roles of Anthocyanins: A Review. Foods 2023, 12, 3969. [Google Scholar] [CrossRef]
- Fang, J.-L.; Luo, Y.; Yuan, K.; Guo, Y.; Jin, S.-H. Preparation and evaluation of an encapsulated anthocyanin complex for enhancing the stability of anthocyanin. LWT 2020, 117, 108543. [Google Scholar]
- Mahdavi, S.A.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. J. Food Eng. 2016, 181, 59–66. [Google Scholar]
- Carrera, C.; Aliaño-González, M.J.; Rodríguez-López, J.; Ferreiro-González, M.; Ojeda-Copete, F.; Barbero, G.F.; Palma, M. Optimization of an Ultrasound-Assisted Extraction Method for the Analysis of Major Anthocyanin Content in Erica australis Flowers. Molecules 2021, 26, 2884. [Google Scholar] [CrossRef]
- Oracz, J.; Żyżelewicz, D.; Pacholczyk-Sienicka, B. UHPLC-DAD-ESI-HRMS/MS profile of phenolic compounds in northern red oak (Quercus rubra L., syn. Q. borealis F. Michx) seeds and its transformation during thermal processing. Ind. Crops Prod. 2022, 189, 115860. [Google Scholar]
- Escher, G.B.; Wen, M.; Zhang, L.; Rosso, N.D.; Granato, D. Phenolic composition by uhplc-q-tof-ms/ms and stability of anthocyanins from Clitoria ternatea L. (Butterfly pea) blue petals. Food Chem. 2020, 331, 127341. [Google Scholar]
- Thuy, N.M.; Minh, V.Q.; Ben, T.C.; Thi Nguyen, M.T.; Ha, H.T.N.; Tai, N.V. Identification of Anthocyanin Compounds in Butterfly Pea Flowers (Clitoria ternatea L.) by Ultra Performance Liquid Chromatography/Ultraviolet Coupled to Mass Spectrometry. Molecules 2021, 26, 4539. [Google Scholar] [CrossRef]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N.Q. Optimization of Total Anthocyanin Content, Stability and Antioxidant Evaluation of the Anthocyanin Extract from Vietnamese Carissa carandas L. Fruits. Processes 2019, 7, 468. [Google Scholar] [CrossRef]
- Oracz, J.; Prejzner, M.; Grzelczyk, J.; Kowalska, G.; Żyżelewicz, D. Bioactive Compounds, Antioxidant Activity and Sensory Properties of Northern Red Oak (Quercus rubra L., syn. Q. borealis F. Michx) Seeds Affected by Roasting Conditions. Molecules 2023, 28, 2299. [Google Scholar] [PubMed]
- Bakowska, A.; Kucharska, A.Z.; Oszmianski, J. The effect of heating, UV irradiation and storage on stability of anthocyanin-polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
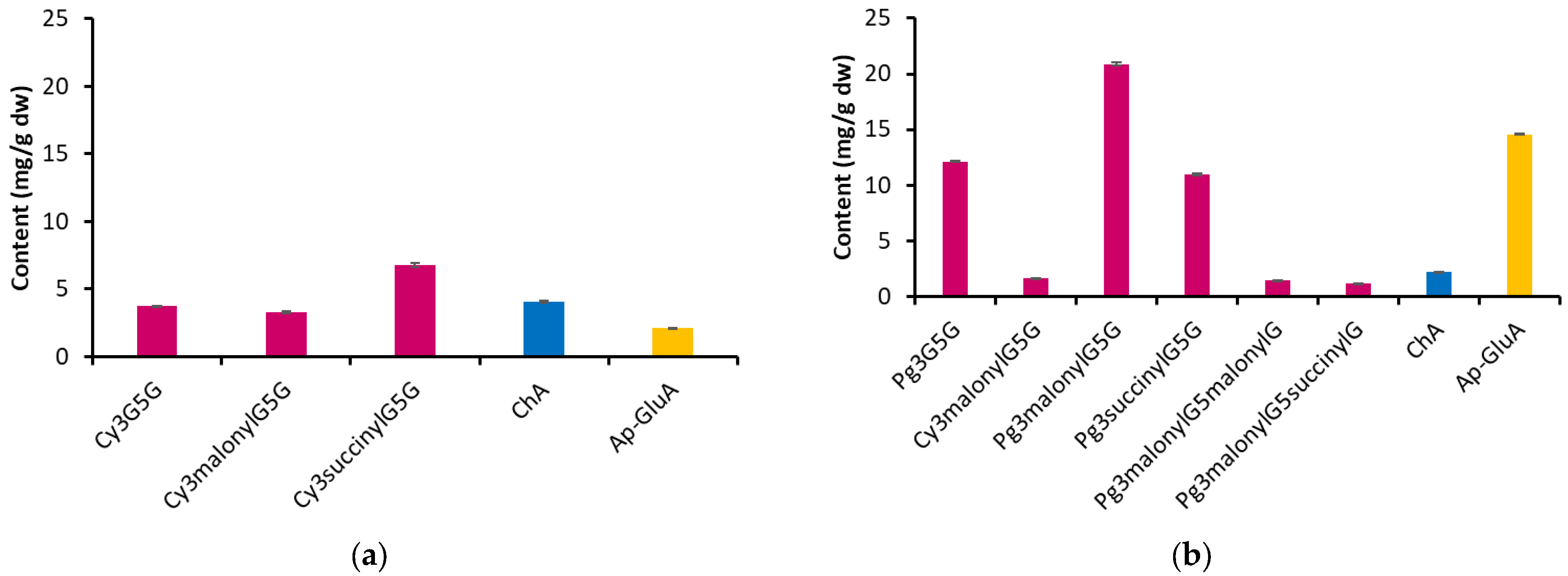



| Peak No. | Rt (min) | [M+H]+ (m/z) | Fragment Ions (m/z) | Identified Compound | Abbreviation | EABC | EAPC |
|---|---|---|---|---|---|---|---|
| 1 | 12.41 | 611.15 | 449.10, 287.05 | Cyanidin 3,5-O-diglucoside | Cy3G5G | + | − |
| 2 | 14.13 | 595.16 | 433.11, 271.06 | Pelargonidin 3,5-di-O-glucoside | Pg3G5G | − | + |
| 3 | 14.25 | 353.09 * | 191.06 | Chlorogenic acid | ChA | + | + |
| 4 | 17.28 | 697.15 | 535.10, 449.10, 287.05 | Cyanidin 3-O-(6″-malonyl-glucoside)-5-O-glucoside | Cy3malonylG5G | + | + |
| 5 | 19.18 | 681.16 | 519.11, 433.11, 271.06 | Pelargonidin 3-O-(6″-malonyl-glucoside)-5-O-glucoside | Pg3malonylG5G | − | + |
| 6 | 19.56 | 711.17 | 549.12, 449.10, 287.05 | Cyanidin 3-O-(6″-O-succinyl-glucoside)-5-O-glucoside | Cy3succinylG5G | + | − |
| 7 | 21.77 | 695.17 | 533.12, 433.11, 271.06 | Pelargonidin 3-O-(6″-O-succinyl-glucoside)-5-O-glucoside | Pg3succinylG5G | − | + |
| 8 | 25.78 | 767.12 | 519.11, 433.11, 271.06 | Pelargonidin 3-O-malonyl-glucoside-5-O-malonyl-glucoside | Pg3malonylG5malonylG | − | + |
| 9 | 26.87 | 781.17 | 533.12, 519.11, 433.11, 271.07 | Pelargonidin 3-O-malonyl-glucoside-5-O-succinyl-glucoside | Pg3malonylG5succinylG | − | + |
| 10 | 31.79 | 447.08 * | 271.06 | Apigenin 7-O-glucuronide | Ap-GluA | + | + |
| EABC | EAPC | |
|---|---|---|
| Total anthocyanins (mg Cy3G/g dw) | 15.78 ± 0.76 a | 60.00 ± 1.60 b |
| Total phenolic compounds (mg GA/g dw) | 96.32 ± 0.55 a | 411.17 ± 2.35 b |
| TEACDPPH (μM TEg dw) | 402.19 ± 2.30 a | 511.88 ± 2.93 b |
| TEACFRAP (μM TE/g dw) | 383.47 ± 1.78 a | 485.81± 1.56 b |
| Sample | TAC (mg Cy3G/g dw) | TPC (mg GA/g dw) | TEACDPPH (μM TE/g dw) | TEACFRAP (μM TE/g dw) |
|---|---|---|---|---|
| EN-ABC-MAF-1:4-SD | 3.93 ± 0.05 b | 80.49 ± 0.15 c | 319.53 ± 1.05 e | 307.70 ± 1.12 g |
| EN-ABC-MAF-1:4-FD | 4.03 ± 0.04 b | 94.46 ± 0.14 d | 375.01 ± 1.13 f | 361.13 ± 1.78 h |
| EN-ABC-B-MAF-1:4-SD | 3.93 ± 0.07 b | 112.68 ± 0.16 f | 447.34 ± 0.95 i | 430.79 ± 1.45 j |
| EN-ABC-ChA-MAF-1:4-SD | 4.03 ± 0.06 b | 109.45 ± 0.13 e | 434.53 ± 0.89 h | 418.45 ± 1.43 i |
| EN-ABC-B-MAF-1:4-FD | 3.91 ± 0.05 b | 125.07 ± 0.12 h | 496.53 ± 0.78 k | 478.16 ± 1.27 l |
| EN-ABC-ChA-MAF-1:4-FD | 4.01 ± 0.07 b | 123.53 ± 0.11 g | 490.42 ± 0.65 j | 472.27 ± 1.39 k |
| EN-ABC-MAF-1:10-SD | 1.57 ± 0.05 a | 51.11 ± 0.08 a | 128.71 ± 1.02 a | 138.32 ± 1.38 a |
| EN-ABC-MAF-1:10-FD | 1.61 ± 0.03 a | 51.77 ± 0.11 a | 130.92 ± 0.85 a | 140.53 ± 1.51 b |
| EN-ABC-B-MAF-1:10-SD | 1.57 ± 0.02 a | 77.32 ± 0.12 b | 187.53 ± 0.92 c | 203.73 ± 1.34 d |
| EN-ABC-ChA-MAF-1:10-SD | 1.61 ± 0.05 a | 76.37 ± 0.11 b | 176.34 ± 0.95 b | 194.40 ± 1.41 c |
| EN-ABC-B-MAF-1:10-FD | 1.57 ± 0.04 a | 79.94 ± 0.12 c | 195.79 ± 0.91 d | 212.10 ± 1.30 f |
| EN-ABC-ChA-MAF-1:10-FD | 1.61 ± 0.03 a | 77.98 ± 0.15 b | 189.67± 0.78 c | 205.88 ± 1.29 e |
| EN-APC-MAF-1:4-SD | 14.93 ± 0.09 c | 171.79 ± 0.16 f | 428.95 ± 0.84 g | 316.18 ± 1.27 f |
| EN-APC-MAF-1:4-FD | 15.31 ± 0.05 d | 201.62 ± 0.17 g | 503.44 ± 0.78 h | 371.08 ± 1.23 g |
| EN-APC-B-MAF-1:4-SD | 14.93 ± 0.04 c | 240.50 ± 0.19 i | 600.54 ± 0.65 j | 442.65 ± 1.42 i |
| EN-APC-ChA-MAF-1:4-SD | 15.31 ± 0.08 d | 232.35 ± 0.18 h | 580.18 ± 0.76 i | 427.65 ± 1.28 h |
| EN-APC-B-MAF-1:4-FD | 14.87 ± 0.07 c | 266.95 ± 0.21 k | 666.58 ± 0.98 l | 491.33 ± 1.54 k |
| EN-APC-ChA-MAF-1:4-FD | 15.25 ± 0.05 d | 257.90 ± 0.17 j | 643.98 ± 1.05 k | 474.68 ± 1.34 j |
| EN-APC-MAF-1:10-SD | 5.97 ± 0.02 a | 89.77 ± 0.15 a | 122.66 ± 0.67 a | 111.81 ± 1.16 a |
| EN-APC-MAF-1:10-FD | 6.12 ± 0.03 a,b | 93.37 ± 0.11 b | 128.56 ± 0.98 b | 116.81 ± 1.33 b |
| EN-APC-B-MAF-1:10-SD | 5.97 ± 0.07 a | 112.05 ± 0.13 d | 206.40 ± 0.67 d | 167.61 ± 1.62 c |
| EN-APC-ChA-MAF-1:10-SD | 6.12 ± 0.05 a,b | 113.76 ± 0.12 e | 204.56 ± 0.58 c | 167.54 ± 1.45 c |
| EN-APC-B-MAF-1:10-FD | 5.95 ± 0.06 a | 108.45 ± 0.15 c | 227.63 ± 0.85 f | 176.88 ± 1.12 e |
| EN-APC-ChA-MAF-1:10-FD | 6.10 ± 0.03 a,b | 107.63 ± 0.13 c | 220.98 ± 0.76 e | 172.95 ± 1.23 d |
| Sample | Color Stability Index | |
|---|---|---|
| Thermal Treatment | Light Treatment | |
| EABC | 0.61 ± 0.03 a | 0.72 ± 0.02 a |
| EN-ABC-MAF-1:4-SD | 0.84 ± 0.01 a | 0.85 ± 0.01 b |
| EN-ABC-MAF-1:4-FD | 0.83 ± 0.01 a | 0.84 ± 0.02 b |
| EN-ABC-B-MAF-1:4-SD | 0.86 ± 0.02 b | 0.87 ± 0.02 b,c |
| EN-ABC-ChA-MAF-1:4-SD | 0.86 ± 0.02 b | 0.87 ± 0.01 b,c |
| EN-ABC-B-MAF-1:4-FD | 0.83 ± 0.03 a | 0.85 ± 0.01 b |
| EN-ABC-ChA-MAF-1:4-FD | 0.82 ± 0.02 a | 0.84 ± 0.02 b |
| EN-ABC-MAF-1:10-SD | 0.86 ± 0.02 b | 0.87 ± 0.02 b,c |
| EN-ABC-MAF-1:10-FD | 0.85 ± 0.01 b | 0.86 ± 0.02 b |
| EN-ABC-B-MAF-1:10-SD | 0.91 ± 0.01 d | 0.93 ± 0.01 d |
| EN-ABC-ChA-MAF-1:10-SD | 0.90 ± 0.02 d | 0.92 ± 0.01 d |
| EN-ABC-B-MAF-1:10-FD | 0.87 ± 0.02 b,c | 0.85 ± 0.02 b |
| EN-ABC-ChA-MAF-1:10-FD | 0.87 ± 0.02 b,c | 0.84 ± 0.02 b |
| EAPC | 0.70 ± 0.01 a | 0.86 ± 0.01 a |
| EN-APC-MAF-1:4-SD | 0.85 ± 0.01 b | 0.87 ± 0.03 a |
| EN-APC-MAF-1:4-FD | 0.84 ± 0.03 b | 0.86 ± 0.01 a |
| EN-APC-B-MAF-1:4-SD | 0.87 ± 0.02 c | 0.89 ± 0.01 b |
| EN-APC-ChA-MAF-1:4-SD | 0.87 ± 0.02 c | 0.89 ± 0.02 b |
| EN-APC-B-MAF-1:4-FD | 0.85 ± 0.01 b | 0.86 ± 0.01 a |
| EN-APC-ChA-MAF-1:4-FD | 0.84 ± 0.01 b | 0.86 ± 0.02 a |
| EN-APC-MAF-1:10-SD | 0.90 ± 0.01 d | 0.91 ± 0.02 b,c |
| EN-APC-MAF-1:10-FD | 0.87 ± 0.02 c | 0.89 ± 0.02 b |
| EN-APC-B-MAF-1:10-SD | 0.96 ± 0.02 f | 0.97 ± 0.03 e |
| EN-APC-ChA-MAF-1:10-SD | 0.94 ± 0.02 e | 0.94 ± 0.02 d |
| EN-APC-B-MAF-1:10-FD | 0.89 ± 0.01 c,d | 0.92 ± 0.02 c |
| EN-APC-ChA-MAF-1:10-FD | 0.87 ± 0.02 c | 0.91 ± 0.02 b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popowska, A.; Oracz, J. Influence of Copigmentation and Encapsulation on Stability and Antioxidant Activity of Anthocyanins from Blue and Pink Cornflower (Centaurea cyanus L.) Flowers. Molecules 2025, 30, 1467. https://doi.org/10.3390/molecules30071467
Popowska A, Oracz J. Influence of Copigmentation and Encapsulation on Stability and Antioxidant Activity of Anthocyanins from Blue and Pink Cornflower (Centaurea cyanus L.) Flowers. Molecules. 2025; 30(7):1467. https://doi.org/10.3390/molecules30071467
Chicago/Turabian StylePopowska, Aleksandra, and Joanna Oracz. 2025. "Influence of Copigmentation and Encapsulation on Stability and Antioxidant Activity of Anthocyanins from Blue and Pink Cornflower (Centaurea cyanus L.) Flowers" Molecules 30, no. 7: 1467. https://doi.org/10.3390/molecules30071467
APA StylePopowska, A., & Oracz, J. (2025). Influence of Copigmentation and Encapsulation on Stability and Antioxidant Activity of Anthocyanins from Blue and Pink Cornflower (Centaurea cyanus L.) Flowers. Molecules, 30(7), 1467. https://doi.org/10.3390/molecules30071467









