A Systematic Method for the Identification of Oligosaccharide Constituents in Polygonatum cyrtonema Hua Using UHPLC-Q-Exactive Orbitrap Mass Spectrometry
Abstract
1. Introduction
2. Results
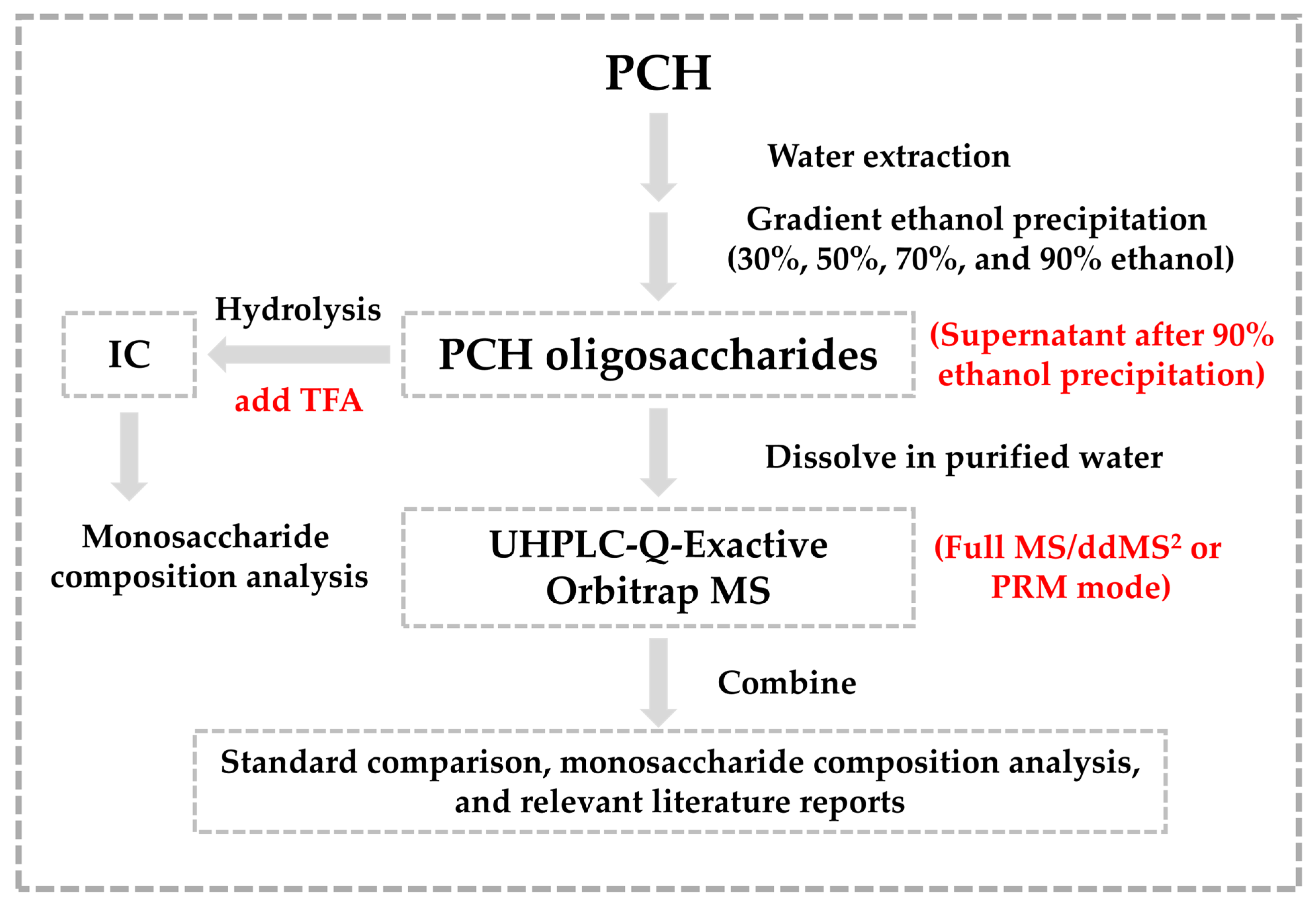
2.1. Analytical Strategy
2.2. Monosaccharide Composition
2.3. Identification and Analysis of PCH Oligosaccharide Components
2.3.1. Identification of FOS
2.3.2. Identification of AOS
2.3.3. Others
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. PCH Oligosaccharide Extraction
4.3. Monosaccharide Composition Analysis
4.3.1. Preparation of Monosaccharide Standards and PCH Solution
4.3.2. Instruments and IC Conditions
4.4. LC-MS Analysis
4.4.1. Preparation of Oligosaccharide Standards and PCH Solution
4.4.2. Instruments and LC-MS Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, X.; Wang, L.; Wang, S.; Yu, N.; Lu, Y.; Lyu, W.; Meng, X. In vitro hypoglycemic and antioxidant activities of steamed Polygonatum cyrtonema Hua with various steaming degrees: Relationship with homoisoflavonoids. Food Biosci. 2023, 53, 102518. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, C.; Li, X.; Gao, Q.; Huang, L.; Xiao, P.; Gao, W. The genus Polygonatum: A review of ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 214, 274–291. [Google Scholar] [CrossRef]
- Liang, J.; Xu, R.; Zong, K.; Yu, N.; Wu, Z.; Wu, H.; Zhou, A. Structural analysis and anti-obesity effect of Polygonatum cyrtonema polysaccharide against obesity induced by high-fat diet in mice. Int. J. Food Sci. Technol. 2021, 56, 4473–4483. [Google Scholar] [CrossRef]
- Shen, W.-D.; Li, X.-Y.; Deng, Y.-Y.; Zha, X.-Q.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P. Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling. Int. J. Biol. Macromol. 2021, 175, 235–241. [Google Scholar] [CrossRef]
- Liu, S.; Jia, Q.-J.; Peng, Y.-Q.; Feng, T.-H.; Hu, S.-T.; Dong, J.-e.; Liang, Z.-S. Advances in Mechanism Research on Polygonatum in Prevention and Treatment of Diabetes. Front. Pharmacol. 2022, 13, 758501. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.; Hu, Y.; Chen, L.; Liu, Y. Integrated comparative metabolomics and network pharmacology approach to uncover the key active ingredients of Polygonati rhizoma and their therapeutic potential for the treatment of Alzheimer’s disease. Front. Pharmacol. 2022, 13, 934947. [Google Scholar] [CrossRef]
- Lin, H.; Wang, W.; Peng, M.; Kong, Y.; Zhang, X.; Wei, X.; Shang, H. Pharmacological properties of Polygonatum and its active ingredients for the prevention and treatment of cardiovascular diseases. Chin Med. 2024, 19, 1. [Google Scholar] [CrossRef]
- Pan, M.; Wu, Y.; Sun, C.; Ma, H.; Ye, X.; Li, X. Polygonati Rhizoma: A review on the extraction, purification, structural characterization, biosynthesis of the main secondary metabolites and anti-aging effects. J. Ethnopharmacol. 2024, 327, 118002. [Google Scholar] [CrossRef]
- He, L.; Yan, B.; Yao, C.; Chen, X.; Li, L.; Wu, Y.; Song, Z.; Song, S.; Zhang, Z.; Luo, P. Oligosaccharides from Polygonatum cyrtonema Hua: Structural characterization and treatment of LPS-induced peritonitis in mice. Carbohydr. Polym. 2021, 255, 117392. [Google Scholar] [CrossRef]
- Xu, J.; Tang, C.; Din, A.U.; Lu, Y.; Ma, X.; Zhang, T.; Wu, J.; Zuoqin, D.; Luo, P.; Wu, J. Oligosaccharides of Polygonatum cyrtonema Hua ameliorates dextran sulfate sodium-induced colitis and regulates the gut microbiota. Biomed. Pharmacother. 2023, 161, 114562. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.; Jiang, Y.; Yang, B. Structure identification of the oligosaccharides by UPLC-MS/MS. Food Hydrocoll. 2023, 139, 108558. [Google Scholar] [CrossRef]
- Tang, X.-y.; Shu, Z.-h.; Zhao, P.-c.; Wei, W.; Fan, C.-l.; Yao, Z.-h.; Yao, X.-s.; Dai, Y. A novel strategy with in vivo characterization, extraction, isolation and activity evaluation for discovery of absorbed anti-inflammatory oligosaccharides from Zhu-Ling decoction. Carbohydr. Polym. 2024, 342, 122422. [Google Scholar] [CrossRef]
- Tie, C.; Hu, T.; Guo, B.; Zhang, J. Novel strategy for herbal species classification based on UPLC–HRMS oligosaccharide profiling. J. Pharm. Biomed. Anal. 2015, 111, 14–20. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Han, J.; Zhang, J.; Li, B.; Qi, X.; Zhang, Y.; Hu, F.; Liu, H. UPLC-Q-TOF-MS and UPLC-QQQ-MS were used for the qualitative and quantitative analysis of oligosaccharides in Fufang Ejiao Syrup. J. Pharm. Biomed. Anal. 2023, 224, 115193. [Google Scholar] [CrossRef]
- Wu, N.; Peng, B.; Li, T.; Tu, P.; Wang, S.; Li, B.; Liu, W.; Song, Y. Rapid Simultaneous Determination of Four Ganoderic Acids in Ganoderma (Chinese Name: Lingzhi) by Direct Infusion–Multiple Reaction Monitoring Cubed. J. Anal. Test. 2024, 8, 52–62. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, S.; Li, K.; Xiong, P.; Qin, S.; Cai, W. Systematic Screening of Chemical Constituents in the Traditional Chinese Medicine Arnebiae Radix by UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Molecules 2022, 27, 2631. [Google Scholar] [CrossRef]
- Ali, Z.; Frank, R.; Körmöczi, T.; Ilisz, I.; Domoki, F.; Weiczner, R.; Bari, F.; Farkas, E.; Berkecz, R. UHPLC-MS/MS Approach for Following Nimodipine Saturation Kinetics in Acute Rat Brain Slice. J. Anal. Test. 2024, 8, 466–477. [Google Scholar] [CrossRef]
- Tian, T.; Rumachik, N.; Sinrod, A.J.G.; Barile, D.; Liu, Y. Coupling an ion chromatography to high resolution mass spectrometry (IC-MS) for the discovery of potentially prebiotic oligosaccharides in Chardonnay grape marc. J. Chromatogr. B 2023, 1214, 123540. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Liu, G.; Xu, H.; Zhang, X. Chemical elucidation of an arabinogalactan from rhizome of Polygonatum sibiricum with antioxidant activities. Int. J. Biol. Macromol. 2021, 190, 730–738. [Google Scholar] [CrossRef]
- Jin, J.; Lao, J.; Zhou, R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S.; et al. Simultaneous Identification and Dynamic Analysis of Saccharides during Steam Processing of Rhizomes of Polygonatum cyrtonema by HPLC–QTOF–MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, T.; Gao, P.; Wang, L.; Yang, X.; Chen, X.; Chen, Y.; Yue, C.; Liang, K.; Tang, L.; et al. Research on Processing-Induced Chemical Variations in Polygonatum cyrtonema Rhizome by Integrating Metabolomics and Glycomics. Molecules 2022, 27, 5869. [Google Scholar] [CrossRef]
- Liu, N.; Shu, Y.; Yan, Y.-y.; Peng, G.-p.; Wen, H.-m.; Shan, C.-x.; Cui, X.-b.; Wang, X.-z.; Zuo, C.-b.; Li, X.-y. Oligosaccharide Profile Analysis and Quality Control of Atractylodes macrocephala Koidz. Using HPLC-HRMS/MS and a Simple HPLC-ELSD Method. Chromatographia 2022, 85, 47–54. [Google Scholar] [CrossRef]
- Shi, H.; Xu, J.; Wang, W.; Jia, M.; Zhou, Y.; Sun, L. An efficient protocol for the preparation of linear arabino-oligosaccharides. Carbohydr. Res. 2020, 496, 108131. [Google Scholar] [CrossRef]
- Westphal, Y.; Kühnel, S.; Schols, H.A.; Voragen, A.G.J.; Gruppen, H. LC/CE–MS tools for the analysis of complex arabino-oligosaccharides. Carbohydr. Res. 2010, 345, 2239–2251. [Google Scholar] [CrossRef]
- Hu, D.; Han, B.; Chen, C.; Chen, N.; Zhu, B.; Zhao, J.; Li, S. Determination of seven oligosaccharides and sucrose in Pseudostellaria heterophylla by pressurized liquid extraction and ultra-high performance liquid chromatography with charged aerosol detector and tandem mass spectrometry. J. Chromatogr. A 2020, 1609, 460441. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Li, X.; Bhattacharya, M.; Paviani, B.; Wang, S.C.; Barile, D. A second life for wine grapes: Discovering potentially bioactive oligosaccharides and phenolics in chardonnay marc and its processing fractions. LWT 2021, 144, 111192. [Google Scholar] [CrossRef]
- Yang, C.; Du, Y.; Li, Q.; Liu, L.; Zhao, L.; Gao, C.; Tang, Z.; Zhang, X.; Zhao, Y.; Yang, X. Fructo-oligosaccharides Alleviated Ulcerative Colitis via Gut Microbiota-Dependent Tryptophan Metabolism in Association with Aromatic Hydrocarbon Receptor Activation in Mice. J. Agric. Food Chem. 2024, 72, 27912–27922. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, W.; Wu, H.; Yang, X.; Li, H.; Chen, Y.; Shui, M.; Ding, Y.; Wang, S. Fructans with various molecular weights from Polygonatum cyrtonema Hua differentially ameliorate intestinal inflammation by regulating the gut microbiota and maintaining intestinal barrier. Int. J. Biol. Macromol. 2025, 285, 138359. [Google Scholar] [CrossRef]
- Vigsns Louise, K.; Holck, J.; Meyer Anne, S.; Licht Tine, R. In Vitro Fermentation of Sugar Beet Arabino-Oligosaccharides by Fecal Microbiota Obtained from Patients with Ulcerative Colitis To Selectively Stimulate the Growth of Bifidobacterium spp. and Lactobacillus spp. Appl. Environ. Microbiol. 2011, 77, 8336–8344. [Google Scholar] [CrossRef]
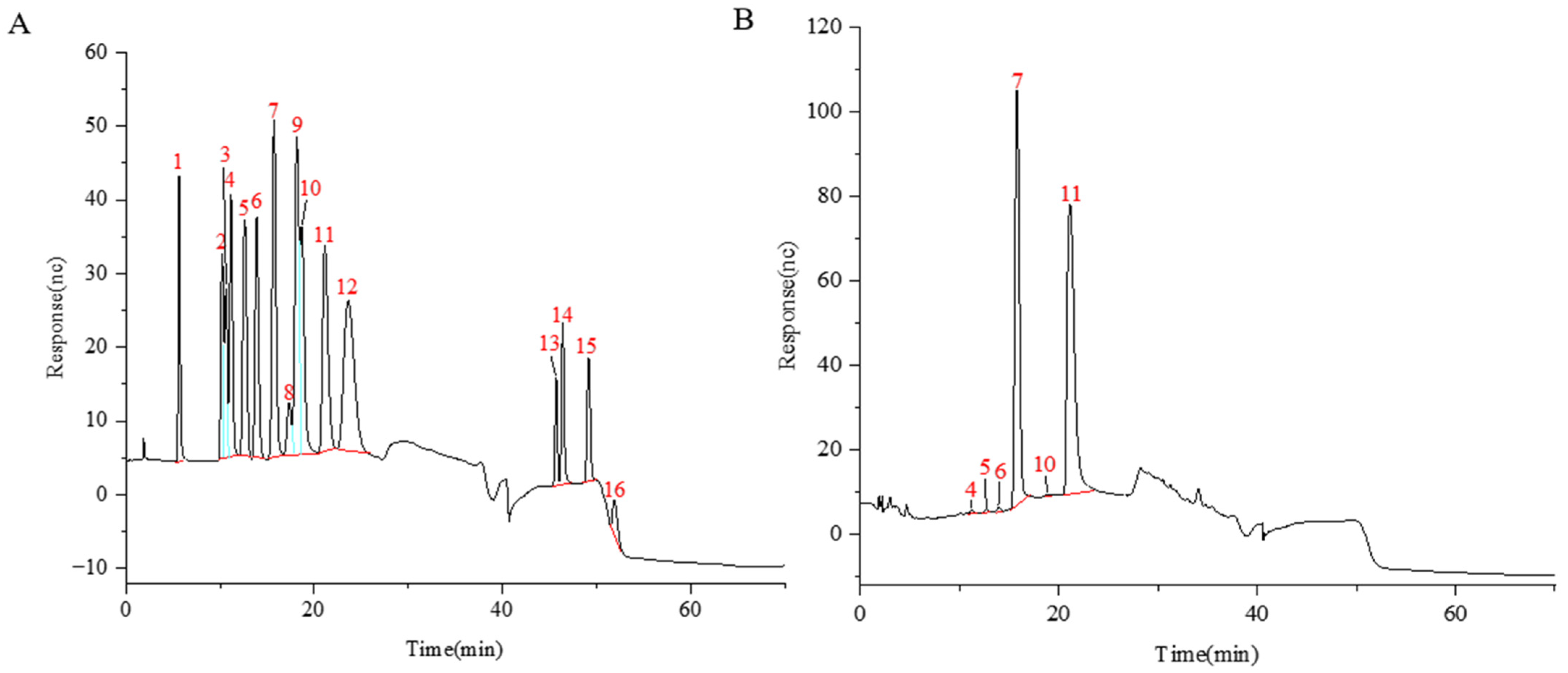

| Peak | tR (min) | Theoretical Mass m/z | Experimental Mass m/z | Error (ppm) | Formula [M − H]− | Identification |
|---|---|---|---|---|---|---|
| 1 **** | 14.06 | 473.1512 | 473.1493 | −3.96 | C17H30O15 | 2Fru:1Ara |
| 2 *** | 14.08 | 413.1301 | 413.1289 | −2.87 | C15H26O13 | arabinotriose |
| 3 **** | 14.81 | 473.1512 | 473.1517 | 1.13 | C17H30O15 | 2Fru:1Ara |
| 4 *** | 15.22 | 413.1301 | 413.1291 | −2.29 | C15H26O13 | arabinotriose |
| 5 *** | 18.03 | 827.2674 | 827.2657 | −2.07 | C30H52O26 | 1F-fructofuranosyl nystose isomer |
| 6 *** | 18.38 | 503.1618 | 503.1604 | −2.64 | C18H32O16 | 1-kestose isomer |
| 7 *** | 18.41 | 827.2674 | 827.2655 | −2.30 | C30H52O26 | 1F-fructofuranosyl nystose isomer |
| 8 **** | 20.11 | 473.1512 | 473.1489 | −4.78 | C17H30O15 | 2Fru:1Ara |
| 9 *** | 20.35 | 1151.3730 | 1151.3700 | −2.65 | C42H72O36 | fructoheptasaccharide isomer |
| 10 **** | 20.46 | 473.1512 | 473.1493 | −4.02 | C17H30O15 | 2Fru:1Ara |
| 11 *** | 20.51 | 413.1301 | 413.1286 | −3.62 | C15H26O13 | arabinotriose |
| 12 *** | 20.76 | 503.1618 | 503.1603 | −2.88 | C18H32O16 | 1-kestose isomer |
| 13 *** | 20.89 | 1151.3730 | 1151.3701 | −2.55 | C42H72O36 | fructoheptasaccharide isomer |
| 14 *** | 21.11 | 413.1301 | 413.1289 | −2.87 | C15H26O13 | arabinotriose |
| 15 * | 21.16 | 503.1618 | 503.1603 | −2.88 | C18H32O16 | 1-kestose |
| 16 *** | 21.43 | 809.2568 | 809.2546 | −2.79 | C30H50O25 | arabinohexaose |
| 17 *** | 21.73 | 503.1618 | 503.1602 | −3.00 | C18H32O16 | 1-kestose isomer |
| 18 *** | 21.76 | 1313.4258 | 1313.4222 | −2.77 | C48H82O41 | fructo-oligosaccharide DP8/GF7 |
| 19 *** | 21.95 | 809.2568 | 809.2551 | −2.11 | C30H50O25 | arabinohexaose |
| 20 *** | 22.14 | 545.1723 | 545.1708 | −2.83 | C20H34O17 | arabinotetraose |
| 21 **** | 22.16 | 575.1828 | 575.1813 | −2.81 | C21H36O18 | 3Ara:1Fru |
| 22 *** | 22.16 | 665.2146 | 665.2130 | −2.36 | C24H42O21 | nystose isomer |
| 23 *** | 22.33 | 809.2568 | 809.2545 | −2.94 | C30H50O25 | arabinohexaose |
| 24 *** | 22.33 | 1475.4787 | 1475.4745 | −2.85 | C54H92O46 | fructo-oligosaccharide DP9/GF8 |
| 25 **** | 22.60 | 575.1828 | 575.1813 | −2.69 | C21H36O18 | 3Ara:1Fru |
| 26 *** | 22.62 | 545.1723 | 545.1707 | −3.07 | C20H34O17 | arabinotetraose |
| 27 * | 22.73 | 665.2146 | 665.2129 | −2.54 | C24H42O21 | nystose |
| 28 *** | 23.14 | 1637.5315 | 1637.5239 | −4.64 | C60H102O51 | fructo-oligosaccharide DP10/GF9 |
| 29 **** | 23.17 | 575.1828 | 575.1841 | 2.07 | C21H36O18 | 3Ara:1Fru |
| 30 *** | 23.17 | 665.2146 | 665.2127 | −2.81 | C24H42O21 | nystose isomer |
| 31 *** | 23.41 | 1799.5843 | 1799.5798 | −2.51 | C66H112O56 | fructo-oligosaccharide DP11/GF10 |
| 32 *** | 23.63 | 545.1723 | 545.1078 | −2.83 | C20H34O17 | arabinotetraose |
| 33 * | 24.15 | 827.2674 | 827.2652 | −2.67 | C30H52O26 | 1F-fructofuranosyl nystose |
| 34 *** | 24.28 | 1961.6371 | 1961.6267 | −5.33 | C72H122O61 | fructo-oligosaccharide DP12/GF11 |
| 35 *** | 24.64 | 827.2674 | 827.2650 | −2.90 | C30H52O26 | 1F-fructofuranosyl nystose isomer |
| 36 * | 24.94 | 989.3202 | 989.3172 | −3.06 | C36H62O31 | 1,1,1,1-kestohexaose |
| 37 *** | 24.97 | 1637.5315 | 1637.5234 | −4.94 | C60H102O51 | fructo-oligosaccharide DP10/GF9 |
| 38 *** | 25.41 | 1799.5843 | 1799.5691 | −8.48 | C66H112O56 | fructo-oligosaccharide DP11/GF10 |
| 39 * | 25.66 | 1151.3730 | 1151.3694 | −3.19 | C42H72O36 | fructoheptasaccharide |
| 40 *** | 26.46 | 1313.4258 | 1313.4219 | −3.04 | C48H82O41 | fructo-oligosaccharide DP8/GF7 |
| 41 *** | 27.04 | 1475.4787 | 1475.4747 | −2.69 | C54H92O46 | fructo-oligosaccharide DP9/GF8 |
| 42 *** | 27.65 | 1637.5315 | 1637.5267 | −2.93 | C60H102O51 | fructo-oligosaccharide DP10/GF9 |
| 43 *** | 28.32 | 1799.5843 | 1799.5786 | −3.19 | C66H112O56 | fructo-oligosaccharide DP11/GF10 |
| 44 *** | 28.92 | 1961.6371 | 1961.6313 | −2.97 | C72H122O61 | fructo-oligosaccharide DP12/GF11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Gao, B.; Yang, Q.; Huo, Y.; Li, K.; Shu, L.; Fan, L.; Liu, Y.; Li, H.; Cai, W. A Systematic Method for the Identification of Oligosaccharide Constituents in Polygonatum cyrtonema Hua Using UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Molecules 2025, 30, 1433. https://doi.org/10.3390/molecules30071433
Yang S, Gao B, Yang Q, Huo Y, Li K, Shu L, Fan L, Liu Y, Li H, Cai W. A Systematic Method for the Identification of Oligosaccharide Constituents in Polygonatum cyrtonema Hua Using UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Molecules. 2025; 30(7):1433. https://doi.org/10.3390/molecules30071433
Chicago/Turabian StyleYang, Suyu, Bowen Gao, Qingrui Yang, Yanghui Huo, Kailin Li, Liangyin Shu, Lingxuan Fan, Yiliang Liu, Huanting Li, and Wei Cai. 2025. "A Systematic Method for the Identification of Oligosaccharide Constituents in Polygonatum cyrtonema Hua Using UHPLC-Q-Exactive Orbitrap Mass Spectrometry" Molecules 30, no. 7: 1433. https://doi.org/10.3390/molecules30071433
APA StyleYang, S., Gao, B., Yang, Q., Huo, Y., Li, K., Shu, L., Fan, L., Liu, Y., Li, H., & Cai, W. (2025). A Systematic Method for the Identification of Oligosaccharide Constituents in Polygonatum cyrtonema Hua Using UHPLC-Q-Exactive Orbitrap Mass Spectrometry. Molecules, 30(7), 1433. https://doi.org/10.3390/molecules30071433






