Antiviral Activity of Halogenated Compounds Derived from L-Tyrosine Against SARS-CoV-2
Abstract
1. Introduction
2. Results
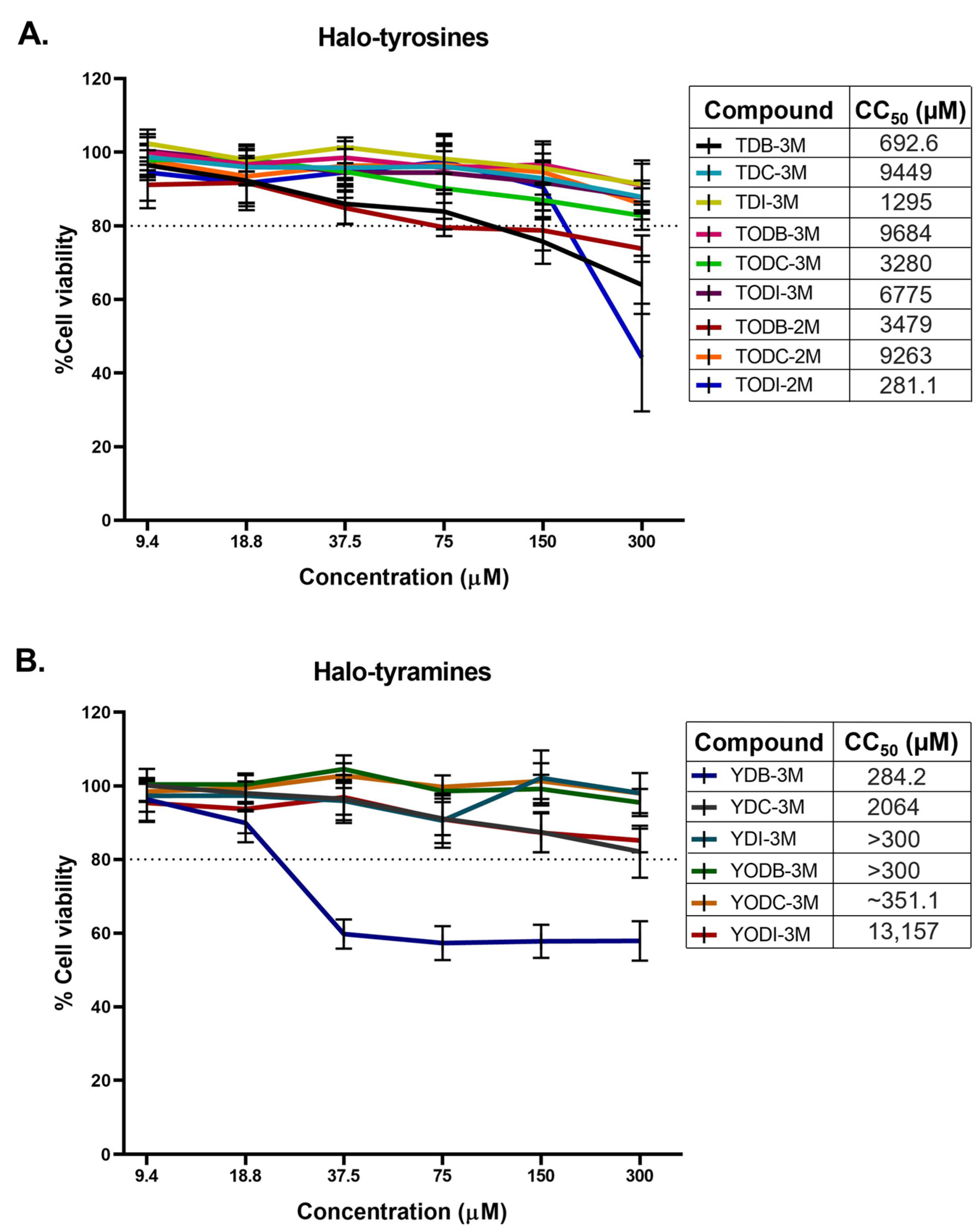
2.1. Halogenated Compounds Derived from L-Tyrosine Do Not Have Significant Cytotoxic Effects on Vero-E6 Cells
2.2. Two Halotyrosine and Three Halotyramine Derivatives Reduce the Viral Titer of SARS-CoV-2
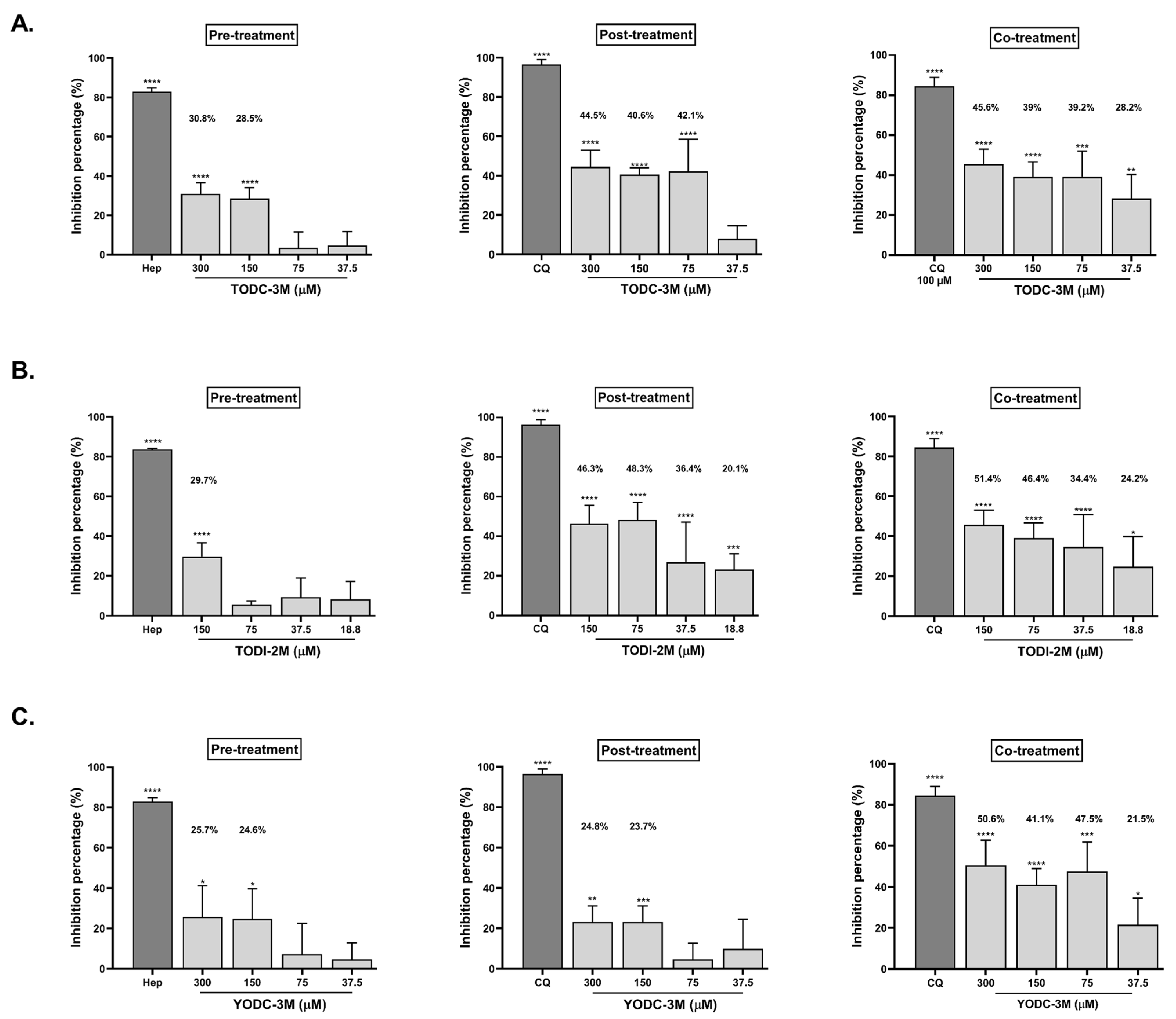
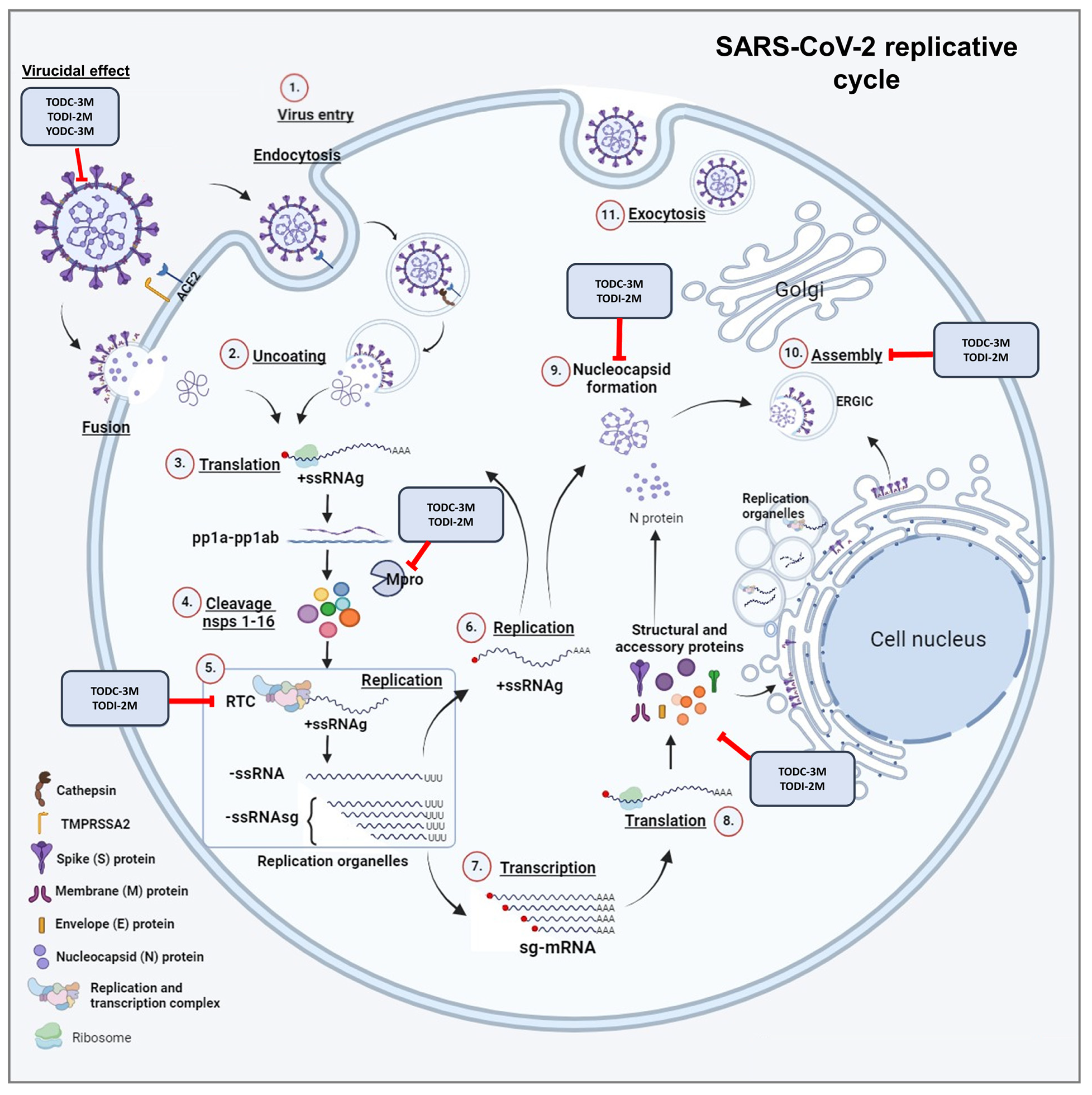
2.3. TODC-3M, TODI-2M, and YODC-3M Showed Significant Effectiveness Against SARS-CoV-2 Through Individual Strategies
2.4. Promising Compounds Inhibit SARS-CoV-2 RNA Replication
2.5. According to In Silico Predictions, the Compounds Presented a Low Probability of Toxicity in Organs and Tissues
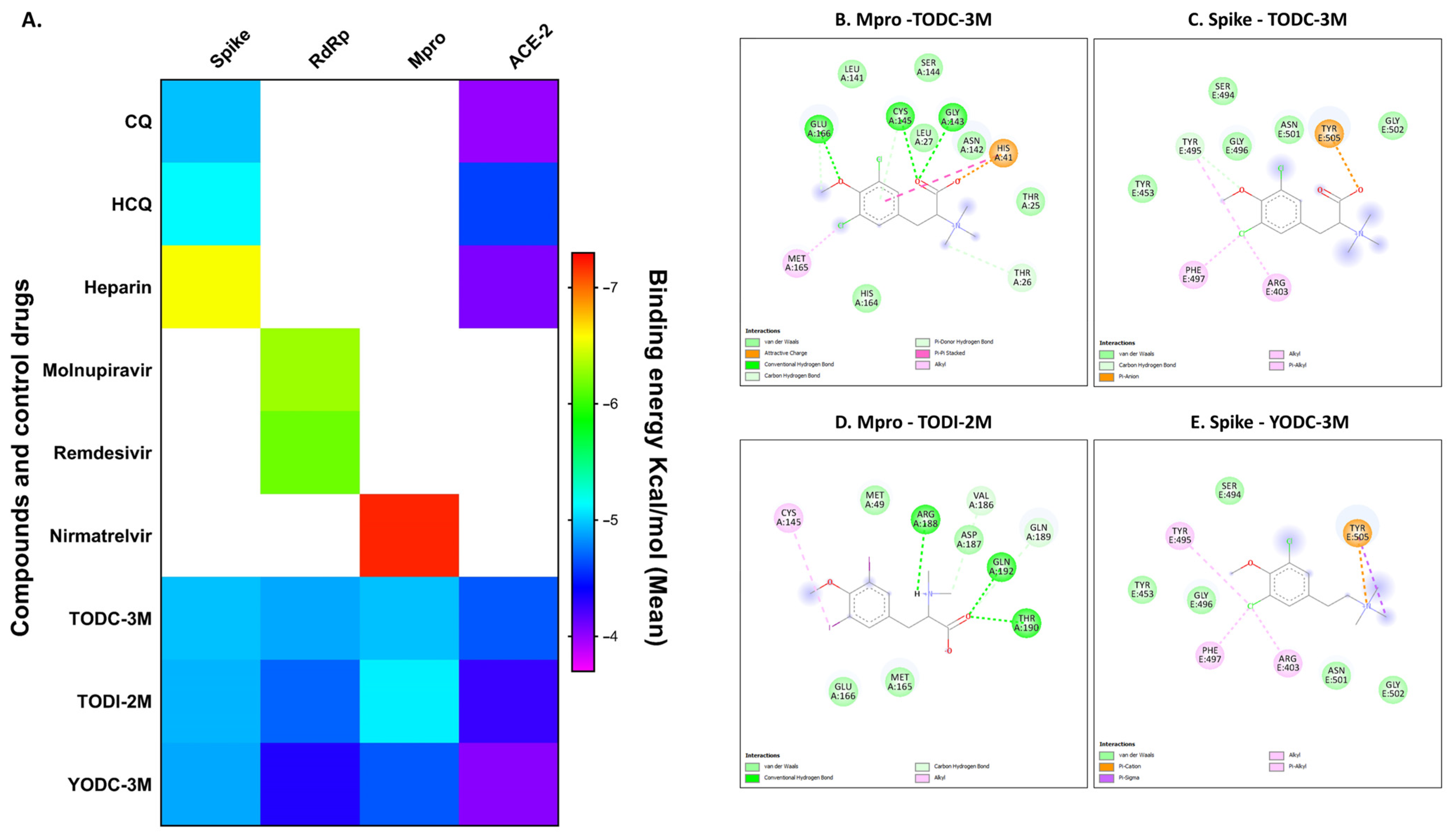
2.6. In Silico Analyses of Compounds with Antiviral Activity Revealed Favorable Binding Energies Against Viral and Cellular Proteins
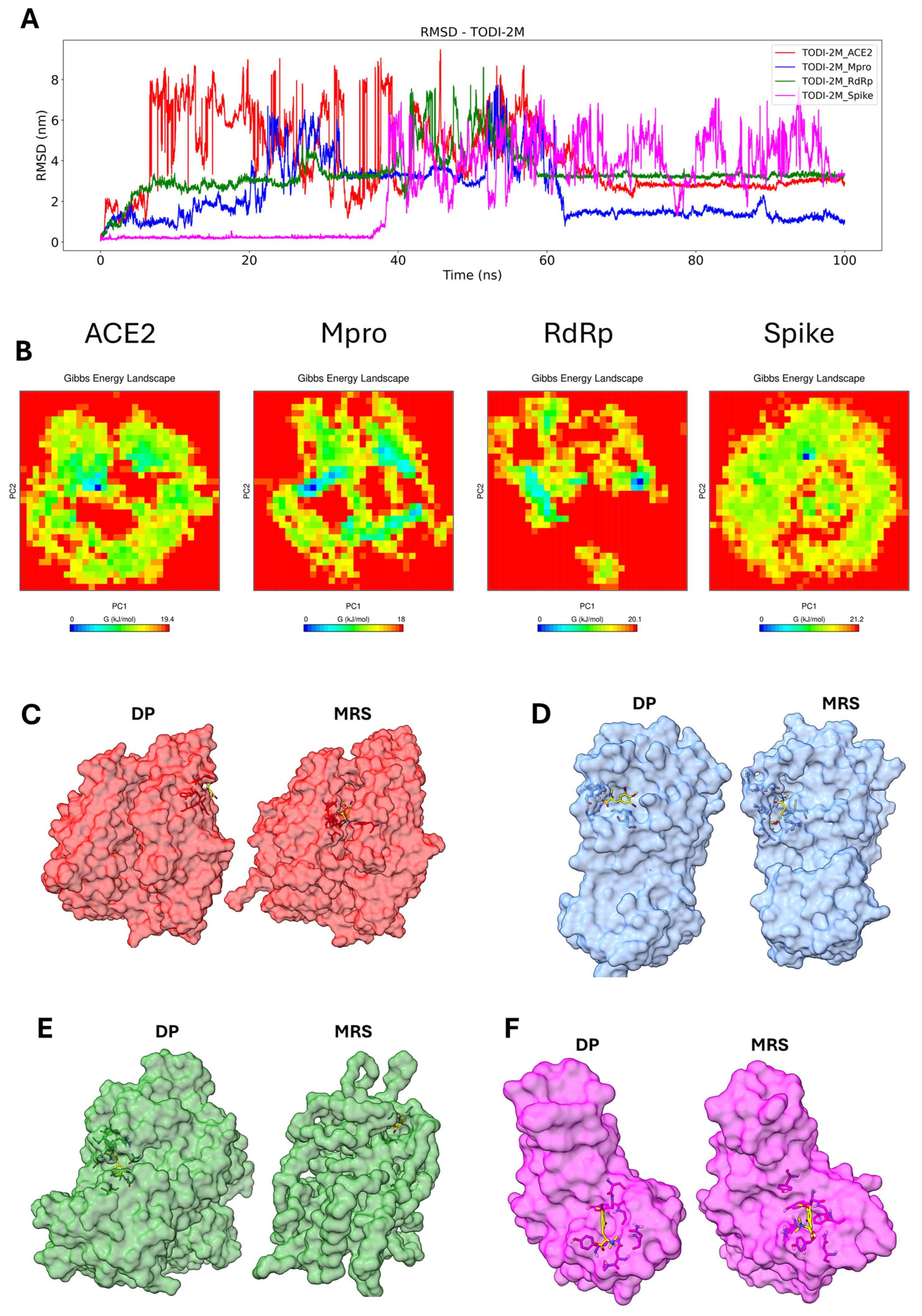
2.7. Molecular Dynamics Simulation of the Compounds with the Tested Proteins
3. Discussion
4. Materials and Methods
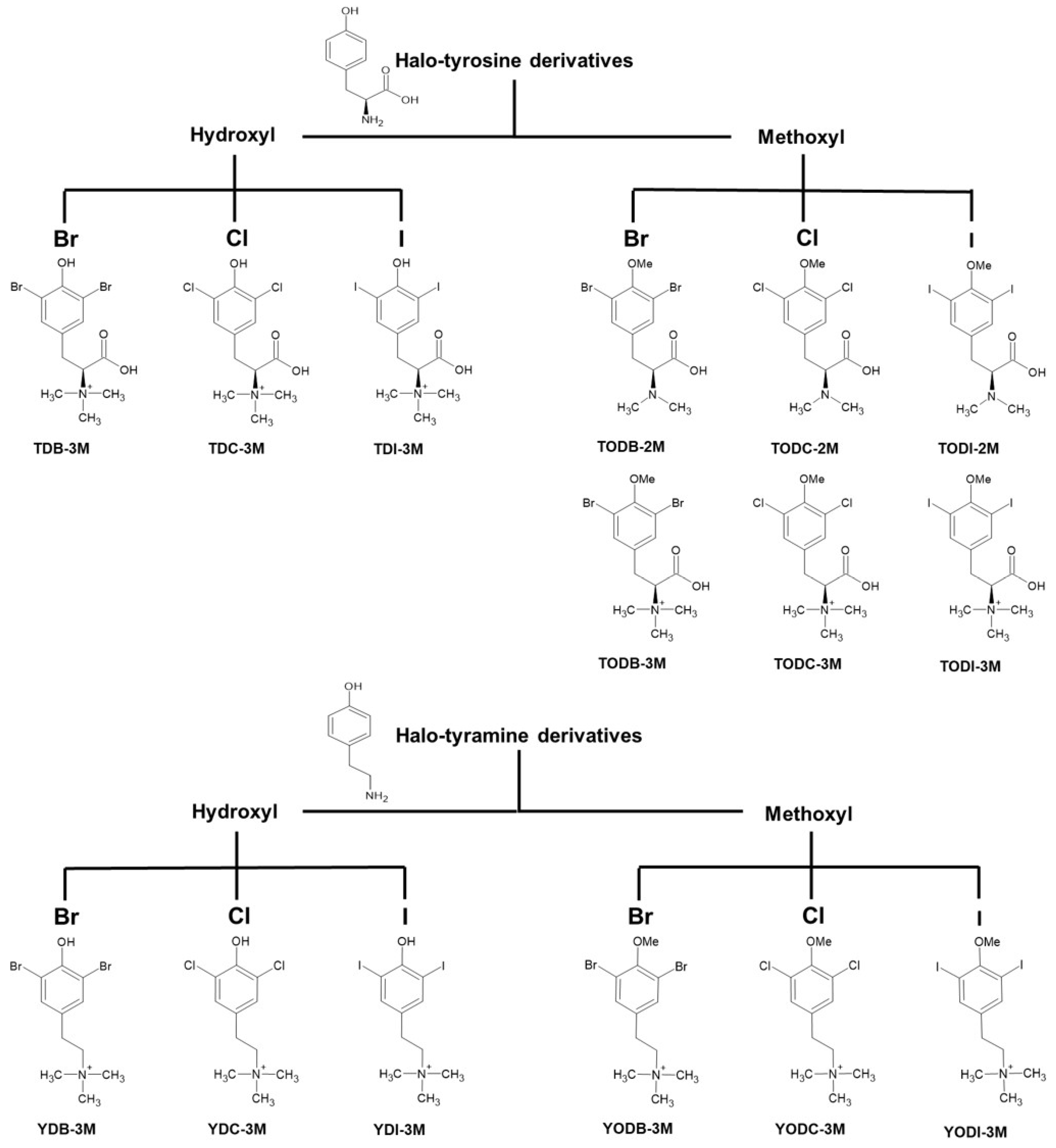
4.1. Halogenated Compounds Derived from L-Tyrosine
4.2. Cell Maintenance and Viral Stock
4.3. Determination of Cytotoxicity
4.4. Evaluation of the Antiviral Activity of Compounds Against SARS-CoV-2 Using Four Treatment Strategies
4.5. Quantification of Viral Particles by Plaque Assay
4.6. Viral Quantification by Real-Time RT-PCR
4.7. In Silico Toxicological Modeling
4.8. Evaluation of the Interactions of Halogenated Compounds with Viral and Cellular Proteins by Molecular Docking
4.8.1. Ligand Preparation
4.8.2. Protein Preparation
4.8.3. Protein–Ligand Interactions
4.9. Molecular Dynamics
4.10. Analysis of Data
4.10.1. Multivariate Analysis
4.10.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- WHO. COVID-19: Cronología de la Actuación de la OMS. 2020. Available online: https://www.who.int/es/news/item/27-04-2020-who-timeline---covid-19 (accessed on 30 January 2024).
- WHO. COVID-19 Epidemiological Update. 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---19-january-2024 (accessed on 30 January 2024).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Veiga, V.C.; Cavalcanti, A.B. Age, host response, and mortality in COVID-19. Eur. Respir. J. 2023, 62, 2300796. [Google Scholar] [CrossRef]
- Lopera, T.J.; Chvatal-Medina, M.; Flórez-Álvarez, L.; Zapata-Cardona, M.I.; Taborda, N.A.; Rugeles, M.T.; Hernandez, J.C. Humoral Response to BNT162b2 Vaccine Against SARS-CoV-2 Variants Decays After Six Months. Front. Immunol. 2022, 13, 879036. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Shoham, S.; Batista, C.; Ben Amor, Y.; Ergonul, O.; Hassanain, M.; Hotez, P.; Kang, G.; Kim, J.H.; Lall, B.; Larson, H.J.; et al. Vaccines and therapeutics for immunocompromised patients with COVID-19. eClinicalMedicine 2023, 59, 101965. [Google Scholar] [CrossRef]
- Zapata-Cardona, M.I.; Flórez-Álvarez, L.; Lopera, T.J.; Chvatal-Medina, M.; Zapata-Builes, W.; Diaz, F.J.; Aguilar-Jimenez, W.; Taborda, N.; Hernandez, J.C.; Rugeles, M.T. Neutralizing antibody titers to Omicron six months after vaccination with BNT162b2 in Colombia. Front. Immunol. 2022, 13, 1102384. [Google Scholar] [CrossRef]
- Zaeck, L.M.; GeurtsvanKessel, C.H.; de Vries, R.D. COVID-19 vaccine effectiveness and evolving variants: Understanding the immunological footprint. Lancet Respir. Med. 2023, 11, 395–396. [Google Scholar] [CrossRef]
- Yaamika, H.; Muralidas, D.; Elumalai, K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J. Taibah Univ. Med. Sci. 2023, 18, 1646–1661. [Google Scholar] [CrossRef]
- Aboul-Fotouh, S.; Mahmoud, A.N.; Elnahas, E.M.; Habib, M.Z.; Abdelraouf, S.M. What are the current anti-COVID-19 drugs? From traditional to smart molecular mechanisms. Virol. J. 2023, 20, 241. [Google Scholar] [CrossRef]
- FDA. USFaDA, Know Your Treatment Options for COVID-19. 2024. Available online: https://www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19#:~:text=If%20you%20are%20infected%20but,has%20approved%20Veklury%20(remdesivir) (accessed on 2 December 2024).
- Modi, S.; Kahwash, R.; Kissling, K. Case Report: Tacrolimus toxicity in the setting of concurrent Paxlovid use in a heart-transplant recipient. Eur. Heart J. Case Rep. 2023, 7, ytad193. [Google Scholar] [CrossRef]
- Prikis, M.; Cameron, A. Paxlovid (Nirmatelvir/Ritonavir) and Tacrolimus Drug-Drug Interaction in a Kidney Transplant Patient with SARS-2-CoV infection: A Case Report. Transplant. Proc. 2022, 54, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Q.-S.; Liu, X.-L.; Wang, H.-L.; Liu, W. Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS. Pharmaceuticals 2022, 15, 1455. [Google Scholar] [CrossRef]
- Zadeh, N.M.; Asl, N.S.M.; Forouharnejad, K.; Ghadimi, K.; Parsa, S.; Mohammadi, S.; Omidi, A. Mechanism and adverse effects of COVID-19 drugs: A basic review. Int. J. Physiol. Pathophysiol. Pharmacol. 2021, 13, 102–109. [Google Scholar]
- Ferranna, M. Causes and costs of global COVID-19 vaccine inequity. Semin. Immunopathol. 2024, 45, 469–480. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs. Clin. Infect. Dis. 2023, 76, 165–171. [Google Scholar] [CrossRef]
- Gómez-Archila, L.G.; Rugeles, M.T.; Zapata, W. Actividad antiviral de compuestos aislados de esponjas marinas. Rev. Biol. Mar. Oceanogr. 2014, 49, 401–412. [Google Scholar]
- Laus, G. Biological activities of natural halogen compounds. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 757–809. [Google Scholar]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated Compounds from Marine Algae. Mar. Drugs 2010, 78, 2301–2317. [Google Scholar] [CrossRef]
- Victoria, C.; Schulz, G.; Klöhn, M.; Weber, S.; Holicki, C.M.; Brüggemann, Y.; Becker, M.; Gerold, G.; Eiden, M.; Groschup, M.H.; et al. Halogenated Rocaglate Derivatives: Pan-antiviral Agents against Hepatitis E Virus and Emerging Viruses. J. Med. Chem. 2024, 67, 289–321. [Google Scholar] [CrossRef]
- Erdélyi, M.; Esterhuysen, C.; Zhu, W. Halogen Bonding: From Fundamentals to Applications. Chempluschem 2021, 86, 1229–1230. [Google Scholar] [CrossRef]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Restrepo, M.P.; Quintero-Gil, D.C.; Muñoz, S.A.P.; Galeano, E.; Zapata, W.; Martinez-Gutierrez, M. In Vitro and In Silico Anti-Arboviral Activities of Dihalogenated Phenolic Derivates of L-Tyrosine. Molecules 2021, 26, 3430. [Google Scholar] [CrossRef]
- Serna-Arbeláez, M.S.; García-Cárcamo, V.; Rincón-Tabares, D.S.; Guerra, D.; Loaiza-Cano, V.; Martinez-Gutierrez, M.; Pereañez, J.A.; Pastrana-Restrepo, M.; Galeano, E.; Zapata, W. In Vitro and In Silico Antiviral Activity of Di-Halogenated Compounds Derived from L-Tyrosine against Human Immunodeficiency Virus 1 (HIV-1). Curr. Issues Mol. Biol. 2023, 45, 8173–8200. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Hassan, A.; El-Aziz, T.M.A.; Stockand, J.D.; Arafa, R.K. Marine Brominated Tyrosine Alkaloids as Promising Inhibitors of SARS-CoV-2. Molecules 2021, 26, 6171. [Google Scholar] [CrossRef]
- Galeano, E.; Thomas, O.P.; Robledo, S.; Munoz, D.; Martinez, A. Antiparasitic bromotyrosine derivatives from the marine sponge Verongula rigida. Mar. Drugs 2011, 9, 1902–1913. [Google Scholar] [CrossRef]
- AlAjmi, M.F.; Azhar, A.; Owais, M.; Rashid, S.; Hasan, S.; Hussain, A.; Rehman, T. Antiviral potential of some novel structural analogs of standard drugs repurposed for the treatment of COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 6676–6688. [Google Scholar] [CrossRef]
- Vique-Sanchez, J.L. Avoiding the Interaction between S-protein of SARS-CoV-2 and ACE2, to Develop an Adjuvant Antiviral by Molecular Docking. Biointerface Res. Appl. Chem. 2021, 12, 5234–5265. [Google Scholar] [CrossRef]
- WHO. Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. 2023. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic#:~:text=The%20WHO%20Director%2DGeneral%20concurs,of%20international%20concern%20(PHEIC) (accessed on 19 March 2024).
- Tian, D.; Moe, B.; Huang, G.; Jiang, P.; Ling, Z.-C.; Li, X.-F. Cytotoxicity of Halogenated Tyrosyl Compounds, an Emerging Class of Disinfection Byproducts. Chem. Res. Toxicol. 2020, 33, 1028–1035. [Google Scholar] [CrossRef]
- Horvat, M.; Avbelj, M.; Durán-Alonso, M.B.; Banjanac, M.; Petković, H.; Iskra, J. Antiviral Activities of Halogenated Emodin Derivatives against Human Coronavirus NL63. Molecules 2021, 26, 6825. [Google Scholar] [CrossRef]
- Yang, X.; Srivastava, R.; Howell, S.H.; Bassham, D.C. Activation of autophagy by unfolded proteins during endoplasmic reticulum stress. Plant J. 2016, 85, 83–95. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, H.; Yin, S.; Wang, P.; Ye, X.; Zhang, J. Aryl-, halogenated- and alkyl- organophosphate esters induced oxidative stress, endoplasmic reticulum stress and NLRP3 inflammasome activation in HepG2 cells. Environ. Pollut. 2023, 316, 120559. [Google Scholar] [CrossRef]
- Köseler, A.; Sabirli, R.; Gören, T.; Türkçüer, I.; Kurt, Ö. Endoplasmic Reticulum Stress Markers in SARS-COV-2 Infection and Pneumonia: Case-Control Study. Vivo 2020, 34, 1645–1650. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Samimi, N.; Farjam, M.; Klionsky, D.J.; Rezaei, N. The role of autophagy in the pathogenesis of SARS-CoV-2 infection in different cell types. Autophagy 2022, 18, 1728–1731. [Google Scholar] [CrossRef]
- Shaban, M.S.; Müller, C.; Mayr-Buro, C.; Weiser, H.; Meier-Soelch, J.; Albert, B.V.; Weber, A.; Linne, U.; Hain, T.; Babayev, I.; et al. Multi-level inhibition of coronavirus replication by chemical ER stress. Nat. Commun. 2021, 12, 5536. [Google Scholar] [CrossRef]
- Auld, D.S.; Inglese, J.; Dahlin, J.L. Assay Interference by Aggregation. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK442297/ (accessed on 3 July 2024).
- Popa-Burke, I.; Russell, J. Compound precipitation in high-concentration DMSO solutions. SLAS Discov. Adv. Sci. Drug Discov. 2014, 19, 1302–1308. [Google Scholar] [CrossRef]
- Grigorov, B.; Rabilloud, J.; Lawrence, P.; Gerlier, D. Rapid Titration of Measles and Other Viruses: Optimization with Determination of Replication Cycle Length. PLoS ONE 2011, 6, e24135. [Google Scholar] [CrossRef]
- Xiao, S.; Yuan, Z.; Huang, Y. Disinfectants against SARS-CoV-2: A Review. Viruses 2022, 14, 1721. [Google Scholar] [CrossRef]
- Guerrero Bernal, C.G.; Reyes Uribe, E.; Salazar Flores, J.; Varela Hernández, J.J.; Gómez-Sandoval, J.R.; Martínez Salazar, S.Y.; Gutiérrez Maldonado, A.F.; Aguilar Martínez, J.; Lomelí Martínez, S.M. Oral Antiseptics against SARS-CoV-2: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8768. [Google Scholar] [CrossRef]
- Trujillo-Correa, A.I.; Quintero-Gil, D.C.; Diaz-Castillo, F.; Quiñones, W.; Robledo, S.M.; Martinez-Gutierrez, M. In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting. BMC Complement. Altern. Med. 2019, 19, 298. [Google Scholar] [CrossRef]
- Barducci, A.; Bonomi, M.; Parrinello, M.J.W.I.R.C.M.S. Metadynamics. Wires Comput. Mol. Sci. 2011, 1, 826–843. [Google Scholar]
- Zuckerman, D.M.; Chong, L.T. Weighted Ensemble Simulation: Review of Methodology, Applications, and Software. Annu. Rev. Biophys. 2017, 46, 43–57. [Google Scholar] [CrossRef]
- Wang, J.; Arantes, P.R.; Bhattarai, A.; Hsu, R.V.; Pawnikar, S.; Huang, Y.M.M.; Palermo, G.; Miao, Y. Gaussian accelerated molecular dynamics: Principles and applications. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1521. [Google Scholar] [CrossRef] [PubMed]
- Sgorlon, G.; Roca, T.P.; Passos-Silva, A.M.; Custódio, M.G.F.; Queiroz, J.A.d.S.; da Silva, A.L.F.; Teixeira, K.S.; Batista, F.S.; Salcedo, J.M.V.; Rampazzo, R.d.C.P.; et al. SARS-CoV-2 Spike Protein Mutations in Different Variants: A Comparison Between Vaccinated and Unvaccinated Population in Western Amazonia. Bioinform. Biol. Insights 2023, 17, 11779322231186477. [Google Scholar] [CrossRef] [PubMed]
- Díaz, F.J.; Aguilar-Jiménez, W.; Flórez-Álvarez, L.; Valencia, G.; Laiton-Donato, K.; Franco-Muñoz, C.; Álvarez-Díaz, D.; Mercado-Reyes, M.; Rugeles, M.T. Aislamiento y caracterización de una cepa temprana de SARS-CoV-2 durante la epidemia de 2020 en Medellín, Colombia. Biomédica 2020, 40, 148–158. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Test for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Zapata-Cardona, M.I.; Flórez-Álvarez, L.; Zapata-Builes, W.; Guerra-Sandoval, A.L.; Guerra-Almonacid, C.M.; Hincapié-García, J.; Rugeles, M.T.; Hernandez, J.C. Atorvastatin Effectively Inhibits Ancestral and Two Emerging Variants of SARS-CoV-2 in vitro. Front. Microbiol. 2022, 13, 721103. [Google Scholar] [CrossRef]
- SimulationsPlus. ADMET Predictor®. Available online: https://www.simulations-plus.com/software/admetpredictor/ (accessed on 14 May 2024).
- Avogadro. Avogadro: An Open-SOURCE Molecular Builder and Visualization Tool. Available online: https://avogadro.cc/ (accessed on 17 May 2024).
- Jász, Á.; Rák, Á.; Ladjánszki, I.; Cserey, G. Optimized GPU implementation of Merck Molecular Force Field and Universal Force Field. J. Mol. Struct. 2019, 1188, 227–233. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera: A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Terefe, E.M.; Ghosh, A. Molecular Docking, Validation, Dynamics Simulations, and Pharmacokinetic Prediction of Phytochemicals Isolated from Croton dichogamus Against the HIV-1 Reverse Transcriptase. Bioinform. Biol. Insights 2022, 16, 11779322221125605. [Google Scholar] [CrossRef]
- Systèmes, D. BIOVIA Discovery Studio Visualizer, v21.2.0.20298; Dassault Systèmes: San Diego, CA, USA.
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Crystal Structure and Pair Potentials: A Molecular-Dynamics Study. Phys. Rev. Lett. 1980, 45, 1196–1199. [Google Scholar] [CrossRef]




| TDB-3M | TDC-3M | TDI-3M | TODB-3M | TODC-3M | TODI-3M | TODB-2M | TODC-2M | TODI-2M | YDB-3M | YDC-3M | YDI-3M | YODB-3M | YODC-3M | YODI-3M | HCQ | CQ | Molnupiravir | Nirmatrelvir | Remdesivir | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin sensitization | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Respiratory sensitization | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Endocrine toxicity (estrogen receptor) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Endocrine toxicity (androgen receptor) | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Cardiac toxicity (hERG K+ channels) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Chromosomal aberrations | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Neuronal toxicity (phospholipidosis) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Reproductive toxicity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| ALP increase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| GGT Increase | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| LDH increase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| SGOT increase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SGPT increase | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| ADMET Risk (0–7) | 1 | 1 | 1.5 | 2 | 1 | 1.8 | 1 | 1.5 | 0.5 | 1.6 | 2.4 | 1.6 | 0.1 | 1.9 | 1 | 4.8 | 4.4 | 3.7 | 5.5 | 5.1 |
| In Vitro | In Silico | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands | Cytotoxicity | Antiviral Activity by Combined Strategy | Selectivity | Molecular Docking (Kcal/mol) | Toxicity | ||||
| CC50 (uM) | Inhibition * (%) | IC50 (μM) | SI | Spike | RdRp | Mpro | ACE2 | ADMET Risk | |
| TODC-3M | 3280 | 73.4% | 47.1 | 69.6 | −5.0 | −4.9 | −5.0 | −4.7 | 1 |
| TODI-2M | 281.1 | 65.7% | 90.1 | 3.1 | −4.9 | −4.7 | −5.2 | −4.3 | 0.5 |
| YODC-3M | ~351.1 | 43.3% | 18.8 | ~18.7 | −4.9 | −4.3 | −4.7 | −4.0 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Bedoya, P.A.; Zapata-Cardona, M.I.; Monsalve-Escudero, L.M.; Pereañez, J.A.; Guerra-Arias, D.; Pastrana-Restrepo, M.; Galeano, E.; Zapata-Builes, W. Antiviral Activity of Halogenated Compounds Derived from L-Tyrosine Against SARS-CoV-2. Molecules 2025, 30, 1419. https://doi.org/10.3390/molecules30071419
Velásquez-Bedoya PA, Zapata-Cardona MI, Monsalve-Escudero LM, Pereañez JA, Guerra-Arias D, Pastrana-Restrepo M, Galeano E, Zapata-Builes W. Antiviral Activity of Halogenated Compounds Derived from L-Tyrosine Against SARS-CoV-2. Molecules. 2025; 30(7):1419. https://doi.org/10.3390/molecules30071419
Chicago/Turabian StyleVelásquez-Bedoya, Paula A., María I. Zapata-Cardona, Laura M. Monsalve-Escudero, Jaime A. Pereañez, Diego Guerra-Arias, Manuel Pastrana-Restrepo, Elkin Galeano, and Wildeman Zapata-Builes. 2025. "Antiviral Activity of Halogenated Compounds Derived from L-Tyrosine Against SARS-CoV-2" Molecules 30, no. 7: 1419. https://doi.org/10.3390/molecules30071419
APA StyleVelásquez-Bedoya, P. A., Zapata-Cardona, M. I., Monsalve-Escudero, L. M., Pereañez, J. A., Guerra-Arias, D., Pastrana-Restrepo, M., Galeano, E., & Zapata-Builes, W. (2025). Antiviral Activity of Halogenated Compounds Derived from L-Tyrosine Against SARS-CoV-2. Molecules, 30(7), 1419. https://doi.org/10.3390/molecules30071419







