Ag2S/Zn2+-Decorated g-C3N4 Type-II Heterojunction with Wide-Spectrum Response: Construction and Photocatalytic Performance in Ciprofloxacin Degradation
Abstract
1. Introduction
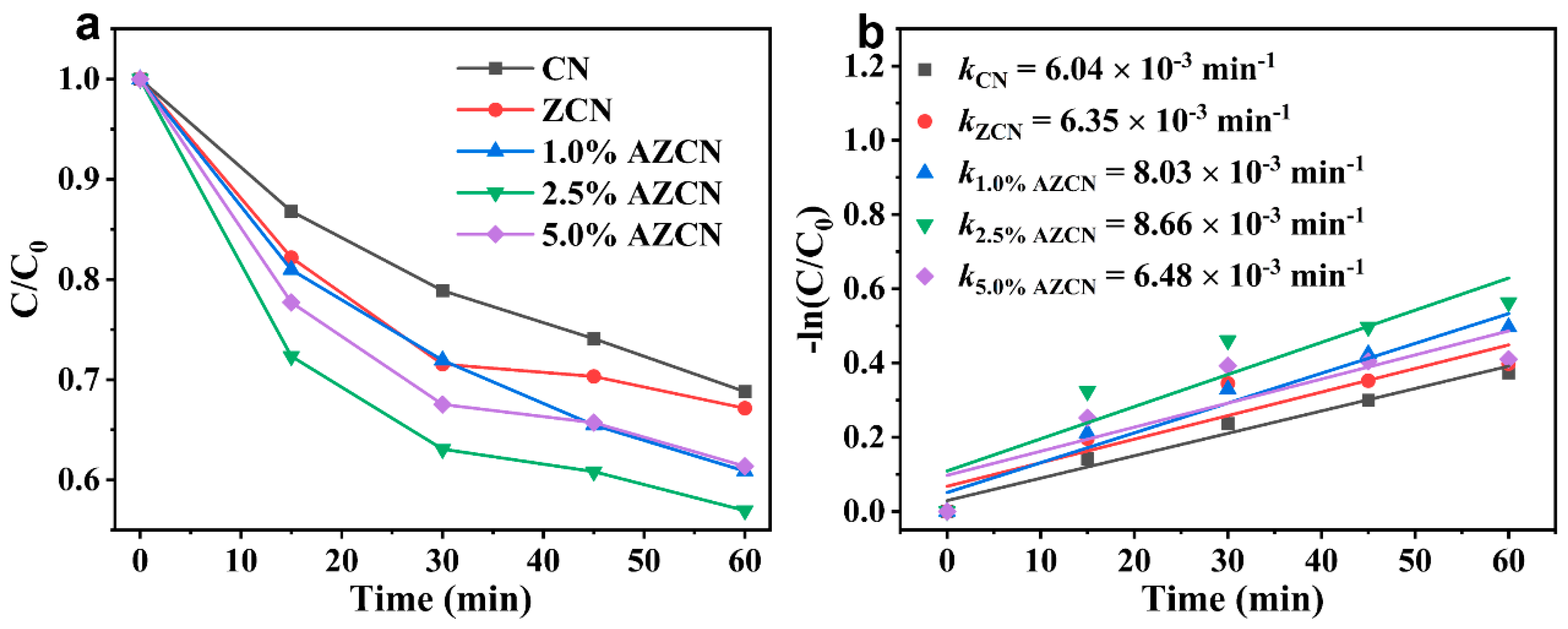
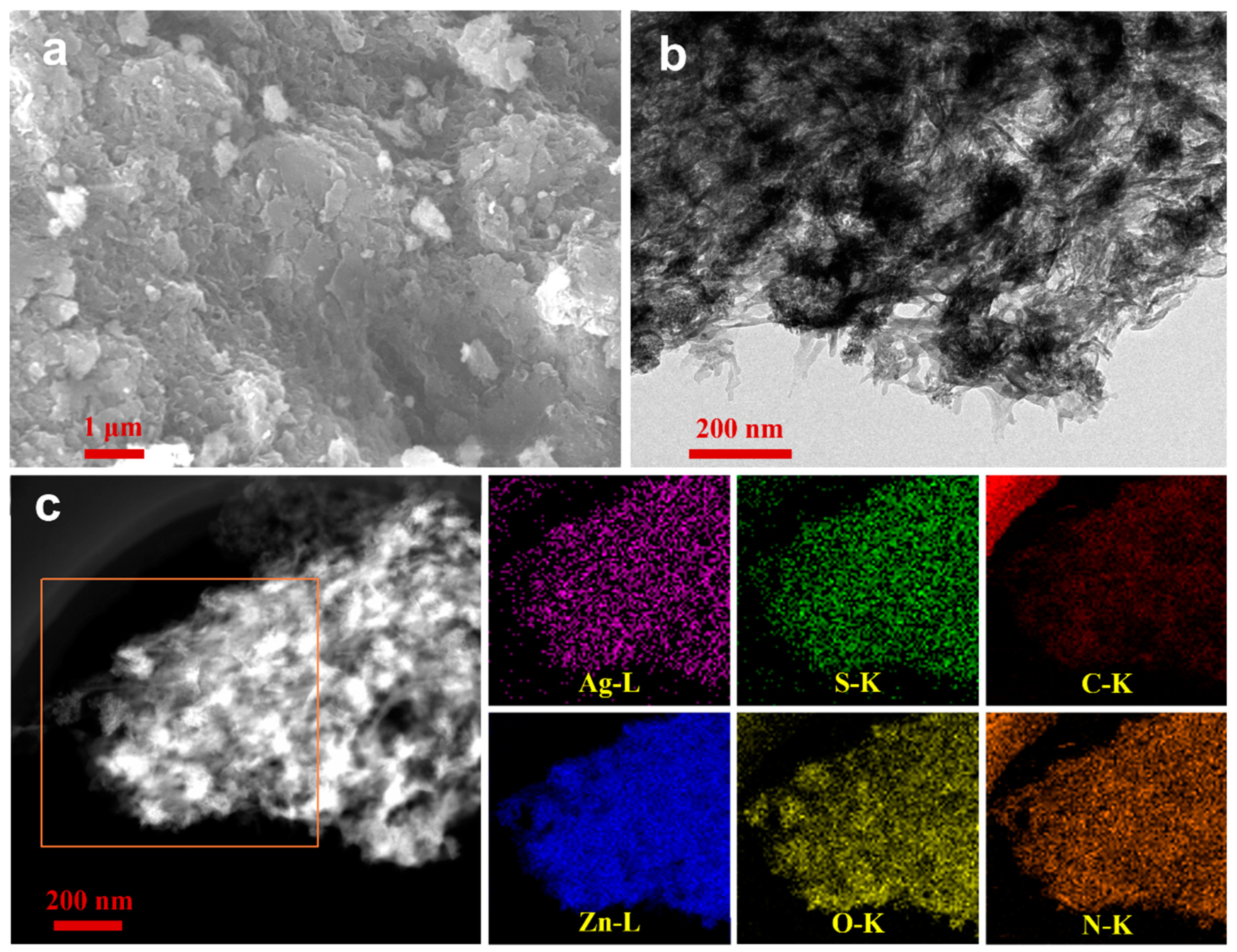
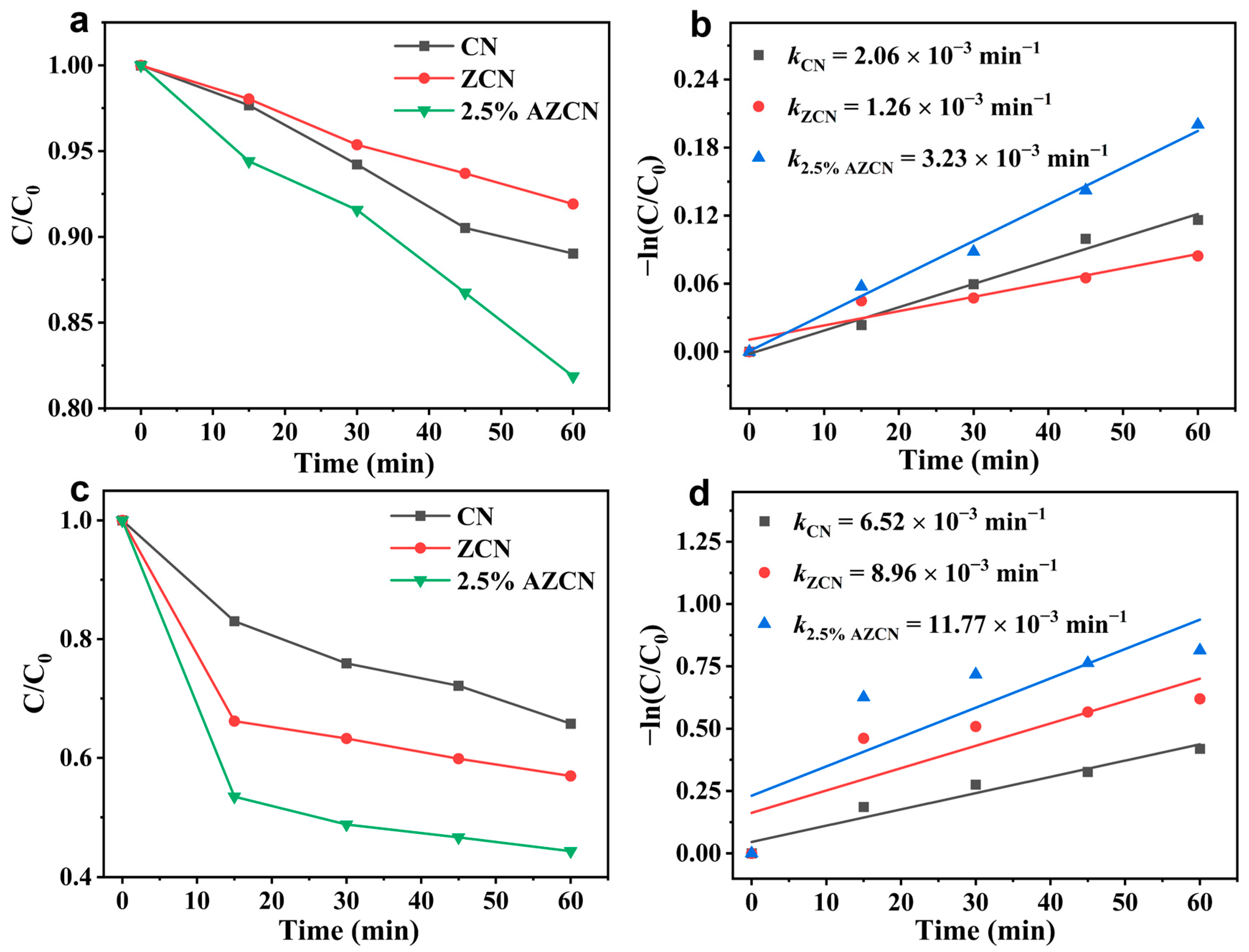
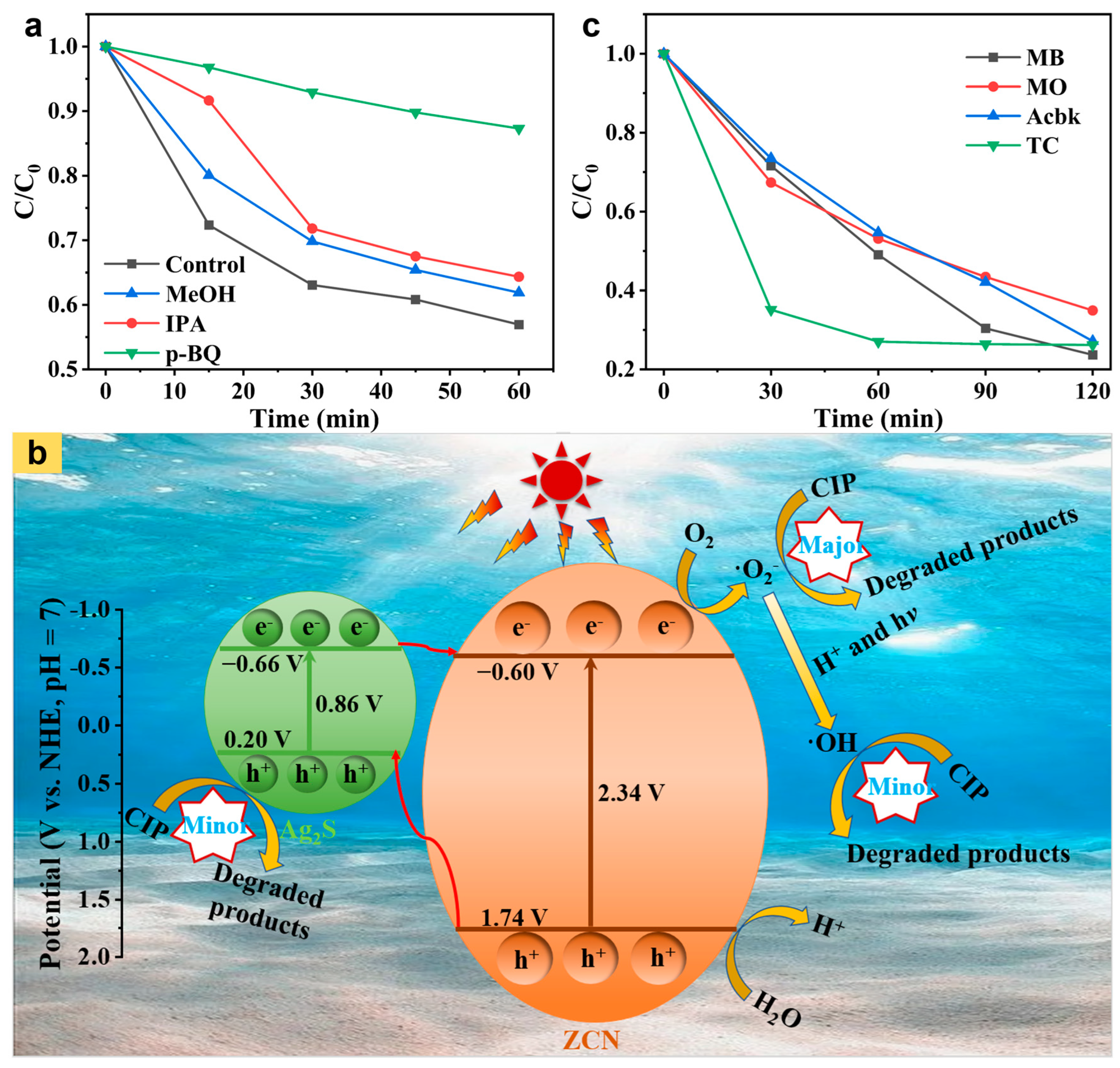
2. Results and Discussion
3. Experimental Methods
3.1. Photocatalyst Synthesis
3.2. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.Q.; Xu, Z.X.; Dong, B. Occurrence, fate, and ecological risk of antibiotics in wastewater treatment plants in China: A review. J. Hazard. Mater. 2024, 469, 133925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, D.; Bai, B.; Ma, Z.Y.; Zong, S.C. Insight into antibiotic removal by advanced oxidation processes (AOPs): Performance, mechanism, degradation pathways, and ecotoxicity assessment. Chem. Eng. J. 2024, 500, 157134. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Routledge, M.; Schlebusch, S.; Lipman, J.; Morris, A.C. Antimicrobial-associated harm in critical care: A narrative review. Intensive Care Med. 2020, 46, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Azqandi, M.; Nateq, K.; Golrizkhatami, F.; Nasseh, N.; Seyedi, N.; Moghaddam, N.S.M.; Fanaei, F. Innovative RGO-bridged S-scheme CuFe2O4@Ag2S heterojunction for efficient sun-light-driven photocatalytic disintegration of ciprofloxacin. Carbon 2025, 231, 119725. [Google Scholar] [CrossRef]
- Peng, Y.J.; Lin, J.L.; Niu, J.L.; Guo, X.L.; Chen, Y.Z.; Hu, T.K.; Cheng, J.H.; Hu, Y.Y. Synergistic effect of ion doping and type-II heterojunction construction and ciprofloxacin degradation by MIL-68(In,Bi)-NH2@BiOBr under visible light. ACS Appl. Mater. Interfaces 2024, 16, 2351–2364. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khalid, A.; Aldhafeeri, Z.M.; Barsoum, I.; Hanna, E.G.; Hasan, M.; Anwar, A.; Aadil, M. Fine-tuning of redox-ability, optical, and electrical properties of Bi2MoO6 ceramics via lanthanide doping and rGO integration for photo-degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2024, 1002, 175466. [Google Scholar] [CrossRef]
- Peng, P.; Yan, X.Y.; Zhou, X.D.; Chen, L.X.; Li, X.; Miao, Y.J.; Zhao, F. Enhancing degradation of antibiotic-combined pollutants by a hybrid system containing advanced oxidation and microbial treatment, a review. J. Hazard. Mater. 2024, 480, 136300. [Google Scholar] [CrossRef]
- Yang, J.M.; Tian, S.F.; Song, Z.; Hao, Y.G.; Lu, M.H. Recent advances in sorption-based photocatalytic materials for the degradation of antibiotics. Coord. Chem. Rev. 2025, 523, 216257. [Google Scholar] [CrossRef]
- Samy, M.; Tang, S.R.; Zhang, Y.G.; Leung, D.Y.C. Understanding the variations in degradation pathways and generated by-products of antibiotics in modified TiO2 and ZnO photodegradation systems: A comprehensive review. J. Environ. Manag. 2024, 370, 122402. [Google Scholar] [CrossRef]
- Xie, Q.J.; Huang, H.M.; Zhang, C.L.; Shi, H.F. Regulating spin polarization of Mn-doped ZnFe2O4 for boosting antibiotic degradation: Intermediate, toxicity assessment and mechanism. J. Clean. Prod. 2024, 466, 142886. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Chen, Y.W.; Huang, Y.Z.; Yang, Z.Y.; Guan, G.Q.; Yan, K. Recent advances on the spherical metal oxides for sustainable degradation of antibiotics. Coord. Chem. Rev. 2024, 510, 215813. [Google Scholar] [CrossRef]
- Liang, X.L.; Gu, S.R.; Xie, B.; Xia, S.J. Short review on photoactivation of peroxysulfate for degradation of antibiotics by layered double hydroxides based Z-scheme heterojunctions. Mater. Res. Bull. 2024, 180, 113006. [Google Scholar] [CrossRef]
- Liu, J.F.; Dong, Y.B.; Liu, Q.J.; Liu, W.; Lin, H. MoS2-based nanocomposites and aerogels for antibiotic pollutants removal from wastewater by photocatalytic degradation process: A review. Chemosphere 2024, 354, 141582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wei, J.; Wang, C.; Wang, L.J.; Guo, Z.; Song, Y.H. Recent advance of Fe-based bimetallic persulfate activation catalysts for antibiotics removal: Performance, mechanism, contribution of the key ROSs and degradation pathways. Chem. Eng. J. 2024, 487, 150514. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.J.; Liu, R.J.; Sun, Y.; Ren, B.Q.; Tong, Y.H.; Tao, Y. Advancing antibiotic detection and degradation: Recent innovations in graphitic carbon nitride (g-C3N4) applications. J. Environ. Sci. 2025, 150, 657–675. [Google Scholar] [CrossRef]
- Liao, H.G.; Huang, K.; Hou, W.D.; Guo, H.Z.; Lian, C.; Zhang, J.Y.; Liu, Z.; Wang, L. Atmosphere engineering of metal-free Te/C3N4 p-n heterojunction for nearly 100% photocatalytic converting CO2 to CO. Adv. Powder Mater. 2024, 3, 100243. [Google Scholar] [CrossRef]
- Yan, W.S.; Hu, P.P.; Jiang, Y.G.; Xu, X.Q.; Ma, X.F.; Wang, M.R.; Bao, X.Y. Ag2S nanoparticles anchored on P-doped g-C3N4: A novel 0D/2D p-n 2 heterojunction for superior photocatalytic inactivation of 3 multidrug-resistant E. coli. Mater. Res. Express. 2022, 9, 095001. [Google Scholar] [CrossRef]
- Zhang, H.T.; Liang, Y.X.; Huang, Y.B.; Zhang, J.; Zhang, J.S.; Hu, B.X.; Ge, G.X.; Liu, J.C.; Bao, F.X. Cd-doped g-C3N4/Ag2S/Ag Z-scheme heterojunction for efficient photocatalytic hydrogen evolution. Fuel 2025, 389, 134549. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.C.; Kamali, M.; Rossi, B.; Appels, L.; Dewil, R. Unraveling the presence and positions of nitrogen defects in defective g-C3N4 for improved organic photocatalytic degradation: Insights from experiments and theoretical calculations. Adv. Funct. Mater. 2024, 34, 2405741. [Google Scholar] [CrossRef]
- Ma, Y.X.; He, D.; Liu, Q.S.; Le, S.K.; Wang, X.J. A S-type 2D/2D heterojunction via intercalating ultrathin g-C3N4 into NH4V4O10 nanosheets and the boosted removal of ciprofloxacin. Appl. Catal. B Environ. Energy 2024, 344, 123642. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Johnson, B.; Bushby, R.J.; Butburee, T.; Khemthong, P.; Nanan, S. One-pot hydrothermal synthesis of g-C3N4/BiOBr/Bi2MoO6 as a Z-scheme heterojunction for efficient photocatalytic degradation of ciprofloxacin (CIP) antibiotic and Rhodamine B (RhB) dye. J. Alloys Compd. 2024, 1008, 176764. [Google Scholar] [CrossRef]
- Sohaimi, K.S.A.; Jaafar, J.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Kurniawan, S.B.; Abdullah, F.; Shakhih, M.F.M. A novel approach of photo-charging and dark-discharging mechanisms by using V2O5/g-C3N4 photocatalysts for ciprofloxacin degradation. Appl. Catal. B Environ. Energy 2024, 357, 124233. [Google Scholar] [CrossRef]
- Niu, S.M.; Li, X.H.; Ding, H.Y.; Wang, W.L.; Li, P.F.; Zheng, X.C.; Cui, M.H. Enhancement of photocatalytic degradation of ciprofloxacin via constructing conductive matrix. Appl. Surf. Sci. 2025, 679, 161259. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Sun, X.F.; Zheng, X.C.; Zhang, X.L.; Guan, X.X. Enhanced photocatalytic performance and mechanism of N-deficiently porous g-C3N4 in organic pollutant degradation. Mater. Res. Bull. 2024, 169, 112510. [Google Scholar] [CrossRef]
- Ma, R.X.; Zheng, H.; Wang, J.; Zheng, X.C.; Zhang, X.L.; Guan, X.X. Zn2+-decorated porous g-C3N4 with nitrogen vacancies: Synthesis, enhanced photocatalytic performance and mechanism in degrading organic contaminants. Mater. Res. Bull. 2025, 183, 113193. [Google Scholar] [CrossRef]
- Yang, Z.H.; Yang, J.K.; Li, L.X.; Cao, W.N.; Zhang, J.; Zhao, H.L.; Wang, L.Q. A novel F-BiVO4/g-C3N4/CdS dual S-scheme heterojunction for high efficient photocatalytic removal of multiple pollutants. Appl. Surf. Sci. 2024, 675, 160738. [Google Scholar] [CrossRef]
- Liang, Y.T.; Chen, R.Y.; Liu, X.D.; Sun, J.Y.; Sun, J.H.; Dong, S.Y.; Feng, J.L. Designing dual-functional mesoporous g-C3N4 nanosheets doped with KPF6 for H2O2 production and antibiotic degradation. J. Alloys Compd. 2024, 996, 174704. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C.J.; Wu, D.N.; Zhao, X.L.; Zhou, L.M.; Lu, X.Y.; Liang, J.; Wang, W.L. A novel LaFeO3/g-C3N4/Ag3PO4 dual Z-scheme heterojunction with enhanced light absorption, charge carrier separation and transfer capacity for photodegradation of antibiotics. J. Alloys Compd. 2024, 1002, 175211. [Google Scholar] [CrossRef]
- Zhu, H.W.; Shi, H.F.; Li, J.P.; Qu, X.S.; Zhao, S.S.; Wang, H.S.; Rong, A.; Chen, Z. Construction of S-scheme Cs3PMo12O40/MnIn2S4 heterojunction for efficient photocatalytic removal of antibiotics: Degradation pathways, toxicity evaluation and mechanism insight. J. Alloys Compd. 2024, 976, 173072. [Google Scholar] [CrossRef]
- Luo, H.K.; Feng, M.H.; Wang, W.S.; Li, X.J.; Li, X.; Yang, S.J. CdS QDs interspersed onto MOF-808 as adsorptive photocatalyst for efficient visible-light driven degradation of ciprofloxacin. J. Alloy. Compd. 2024, 988, 174247. [Google Scholar] [CrossRef]
- Sun, X.F.; Zheng, Z.K.; Ma, J.Y.; Xian, T.; Liu, G.R.; Yang, H. Development of ternary Pt/BaTiO3/Bi2O3 heterostructured piezo-photocatalysts for antibiotic degradation. Appl. Surf. Sci. 2024, 653, 159421. [Google Scholar] [CrossRef]
- Ghanizadeh, F.; Shemirani, F. Fe3O4/ZnO/L-lysine functionalized graphene oxide a promising nanocomposite with double Z-scheme visible photocatalytic and dark catalytic activity for water remediation. Appl. Surf. Sci. 2024, 672, 160816. [Google Scholar] [CrossRef]
- Cheng, J.J.; Deng, Z.R.; Zheng, X.Y.; Chu, C.Y.; Guo, Y.F. Construction and actual application of In2O3/BiOBr heterojunction for effective removal of ciprofloxacin under visible light: Photocatalytic mechanism, DFT calculation, degradation pathway and toxicity evaluation. J. Alloys Compd. 2024, 971, 172779. [Google Scholar] [CrossRef]
- Mohamed, M.J.S.; Gondal, M.A. Synthesis of carbon-metal interface ilmenite type nanostructures using ultrasonication-assisted sol-gel process for visible-light-driven hydrogen production from methanol and antibiotic wastewater removal. J. Alloy. Compd. 2024, 1002, 175382. [Google Scholar] [CrossRef]
- Chen, L.J.; Arshad, M. Preparation of MoS2/V2O5 nanocomposites for ciprofloxacin degradation and Cr (VI) reduction. J. Alloys Compd. 2024, 1006, 176224. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wu, L.S.; Liang, L.W.; Liu, D.; Luo, J.J.; Lv, Q.F.; Liang, L.L.; Deng, H.T. Construction of BiOCOOH/Bi2MoO6 Z-scheme heterojunction for visible-light-driven photocatalytic degradation of ciprofloxacin: Performance and mechanistic insights. J. Alloys Compd. 2024, 1008, 176682. [Google Scholar] [CrossRef]
- Rahmana, M.M.; Yasmeena, F.; Tareka, M.; Basith, M.A. Dysprosium chromite nanoparticles: A promising photocatalyst for the remediation of ciprofloxacin and methylene blue from wastewater. J. Alloys Compd. 2024, 1010, 177295. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, W.D.; Huang, K.; Guo, H.Z.; Wang, Z.M.; Lian, C.; Zhang, J.Y.; Wu, D.L.; Lei, Z.D.; Liu, Z.; et al. Engineering built-in electric field microenviroment of CQDs/g-C3N4 heterojunction for efficient photocatalytic CO2 reduction. Adv. Sci. 2024, 11, 2403607. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Y.Z.; Chen, J.; Song, M.X.; Zhang, L.L. Multivalent effect of defect engineered Ag2S/g-C3N4 3D porous floating catalyst with enhanced contaminant removal efficiency. Int. J. Environ. Res. Public Hearth 2023, 20, 1357–1368. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. Synthesis and characterization of g-C3N4 nanosheets decorated Ag2S composites for investigation of catalytic reduction of 4-nitrophenol, antioxidant and antimicrobial activities. J. Mol. Struct. 2019, 1186, 423–433. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Miao, Y.Q.; Wang, G.Q.; Chen, W.X.; Lu, W.Y. Solar-driven zinc-doped graphitic carbon nitride photocatalytic fibre for simultaneous removal of hexavalent chromium and pharmaceuticals. Environ. Technol. 2022, 43, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, R.; Abbastabar, H.A.; Zakaria, A.; Zamiri, G.; Singh, M.B.; Ferreira, J.M.F. The structural and optical constants of Ag2S semiconductor nanostructure in the Far-Infrared. Chem. Cent. J. 2015, 9, 28–34. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements-BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Li, M.; Liu, Y.L.; Ren, T.Z.; Yuan, Z.Y. Carbon-doped ZnO hybridized homogeneously with graphitic carbon nitride nanocomposites for photocatalysis. J. Phys. Chem. C 2014, 118, 10963–10971. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Chen, P.F.; Chen, L.; Wu, M.F.; Dai, X.Q.; Xing, P.X.; Lin, H.J.; Zhao, L.H.; He, Y.M. Facile fabrication of novel Ag2S/K-g-C3N4 composite and its enhanced performance in photocatalytic H2 evolution. J. Colloid Interf. Sci. 2020, 568, 117–129. [Google Scholar] [CrossRef]
- Dascalu, I.; Calderon-Moreno, J.M.; Osiceanu, P.; Bratan, V.; Hornoiu, C.; Somacescu, S. ZnO modified anatase TiO2 thin films templated by tripropylamine and their electrical properties. Thin Solid Films 2021, 729, 138697. [Google Scholar] [CrossRef]
- Basu, M.; Nazir, R.; Mahala, C.; Fageria, P.; Chaudhary, S.; Gangopadhyay, S.; Pande, S. Ag2S/Ag heterostructure: A promising electrocatalyst for the hydrogen evolution reaction. Langmuir 2017, 33, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.K.; Cao, J.H.; Yu, J.H.; Dong, J.X.; Zhao, Y.T.; Zhao, F.P.; Zhang, D.F.; Pu, X.P. Anchoring ZnIn2S4 nanosheets on cross-like FeSe2 to construct photothermal-enhanced S-scheme heterojunction for photocatalytic H2 evolution. J. Colloid. Interf. Sci. 2024, 673, 463–474. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. Facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV-Vis spectroscopy data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Toh, H.S.; Compton, R.G. Nano-impacts’: An electrochemical technique for nanoparticle sizing in optically opaque solutions. ChemistryOpen 2015, 4, 261–263. [Google Scholar] [CrossRef]
- Tian, Y.N.; Li, J.; Zheng, H.; Guan, X.X.; Zhang, X.L.; Zheng, X.C. Synthesis and enhanced photocatalytic performance of Ni2+-doped Bi4O7 nanorods with broad-spectrum photoresponse. Sep. Purif. Technol. 2022, 300, 121898. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhang, H.Y.; Yao, S.; Dong, S.Y.; Chao, C.; Fan, F.J.; Jia, H.Y.; Dong, M.J. Controlled fabrication of flexible graphene aerogels via naturally dried and its photocatalysis-persulfate activation for ciprofloxacin degradation under sunlight. J. Alloys Compd. 2024, 1006, 175603. [Google Scholar] [CrossRef]
- Tian, Y.N.; Ma, R.X.; Zheng, X.C.; Li, J.; Guan, X.X.; Wang, X.F. In-situ construction and exciting photocatalytic performance of broad-spectrum responsive BiO2-x/Bi2O2CO3 heterojunctions in tetracycline degradation. Environ. Res. 2025, 264, 120359. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Jiang, L.S.; Wang, K.; Li, Y.; Zhang, G. Novel AgI/BiSbO4 heterojunction for efficient photocatalytic degradation of organic pollutants under visible light: Interfacial electron transfer pathway, DFT calculation and degradation mechanism study. J. Hazard. Mater. 2021, 410, 124948. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.H.; Wang, Y.Z.; Liu, N.S.; Liu, C.X.; Du, P.; Xia, Y.; He, S.F. Photocatalytic reaction pathways and mechanisms investigation for effective organic pollution degradation via in-situ construction of BiOCl/UiO66-NH2 heterostructure. Appl. Surf. Sci. 2024, 669, 160537. [Google Scholar] [CrossRef]





| Photocatalyst | CIP Concentration (mg L−1) | Photocatalyst Dosage (g L−1) | Light Type | Irradiation Time (min) | CIP Elimination Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Gd@Bi2MoO6/rGO | 5 | 0.40 | simulated solar light | 120 | 81.7 | [6] |
| UCN-1 h | 2.5 | 0.50 | simulated solar light | 90 | 98.8 | [19] |
| CNNS/NH4V4O10 | 10 | 0.50 | simulated solar light | 100 | 92.0 | [20] |
| V2O5/g-C3N4 | 10 | 1.00 | visible light | 180 | 91.0 | [22] |
| F-BiVO4/g-C3N4/CdS | 20 | 1.00 | simulated solar light | 30 | 90.0 | [26] |
| KPF6-g-C3N4 | 10 | 0.40 | visible light | 180 | 90.8 | [27] |
| LaFeO3/g-C3N4/Ag3PO4 | 10 | 0.75 | visible light | 120 | 90.2 | [28] |
| Cs3PMo12O40/MnIn2S4 | 20 | 1.00 | visible light | 60 | 61.8 | [29] |
| CdS QDs@MOF-808 | 40 | 0.20 | visible light | 180 | 82.0 | [30] |
| Pt/BaTiO3/Bi2O3 | 10 | 1.00 | simulated solar light | 60 | 63.0 | [31] |
| Fe3O4/ZnO/Lys-rGO | 20 | 0.25 | visible light | 60 | 38.0 | [32] |
| In2O3/BiOBr | 10 | 0.50 | visible light | 90 | 93.5 | [33] |
| BC15@FeTiO | 10 | 0.20 | visible light | 120 | 91.1 | [34] |
| MoS2/V2O5 | 20 | 0.40 | visible light | 120 | 98.7 | [35] |
| BiOCOOH/Bi2MoO6 | 20 | 0.50 | visible light | 120 | 95.6 | [36] |
| DyCrO3 | 12 | 0.80 | simulated solar light | 240 | 83.0 | [37] |
| 2.5% AZCN | 10 | 0.20 | visible light | 60 | 43.1% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zheng, H.; Ma, R.; Zheng, X.; Guan, X. Ag2S/Zn2+-Decorated g-C3N4 Type-II Heterojunction with Wide-Spectrum Response: Construction and Photocatalytic Performance in Ciprofloxacin Degradation. Molecules 2025, 30, 1417. https://doi.org/10.3390/molecules30071417
Wang C, Zheng H, Ma R, Zheng X, Guan X. Ag2S/Zn2+-Decorated g-C3N4 Type-II Heterojunction with Wide-Spectrum Response: Construction and Photocatalytic Performance in Ciprofloxacin Degradation. Molecules. 2025; 30(7):1417. https://doi.org/10.3390/molecules30071417
Chicago/Turabian StyleWang, Chengyang, Han Zheng, Ruxue Ma, Xiucheng Zheng, and Xinxin Guan. 2025. "Ag2S/Zn2+-Decorated g-C3N4 Type-II Heterojunction with Wide-Spectrum Response: Construction and Photocatalytic Performance in Ciprofloxacin Degradation" Molecules 30, no. 7: 1417. https://doi.org/10.3390/molecules30071417
APA StyleWang, C., Zheng, H., Ma, R., Zheng, X., & Guan, X. (2025). Ag2S/Zn2+-Decorated g-C3N4 Type-II Heterojunction with Wide-Spectrum Response: Construction and Photocatalytic Performance in Ciprofloxacin Degradation. Molecules, 30(7), 1417. https://doi.org/10.3390/molecules30071417







