Indocyanine Green-Loaded Quenched Nanoliposomes as Activatable Theranostics for Cancer
Abstract
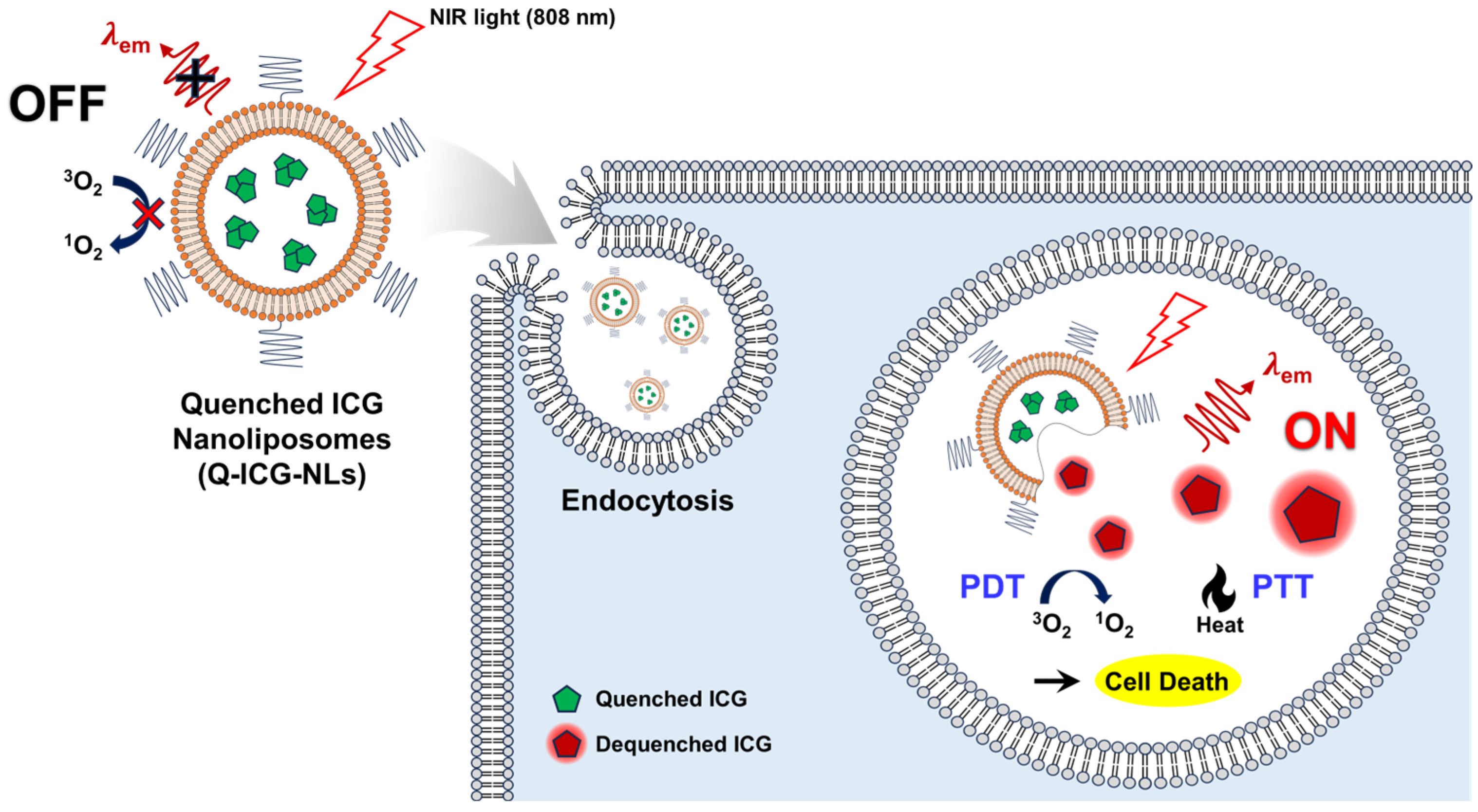
1. Introduction
2. Results and Discussion
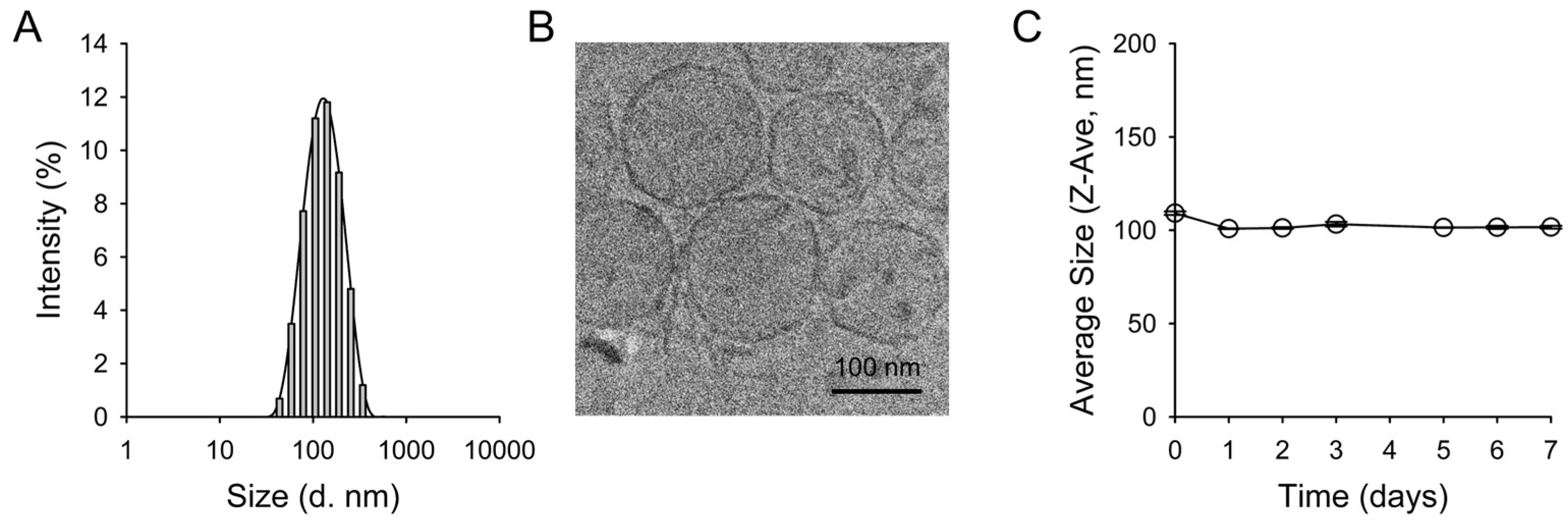
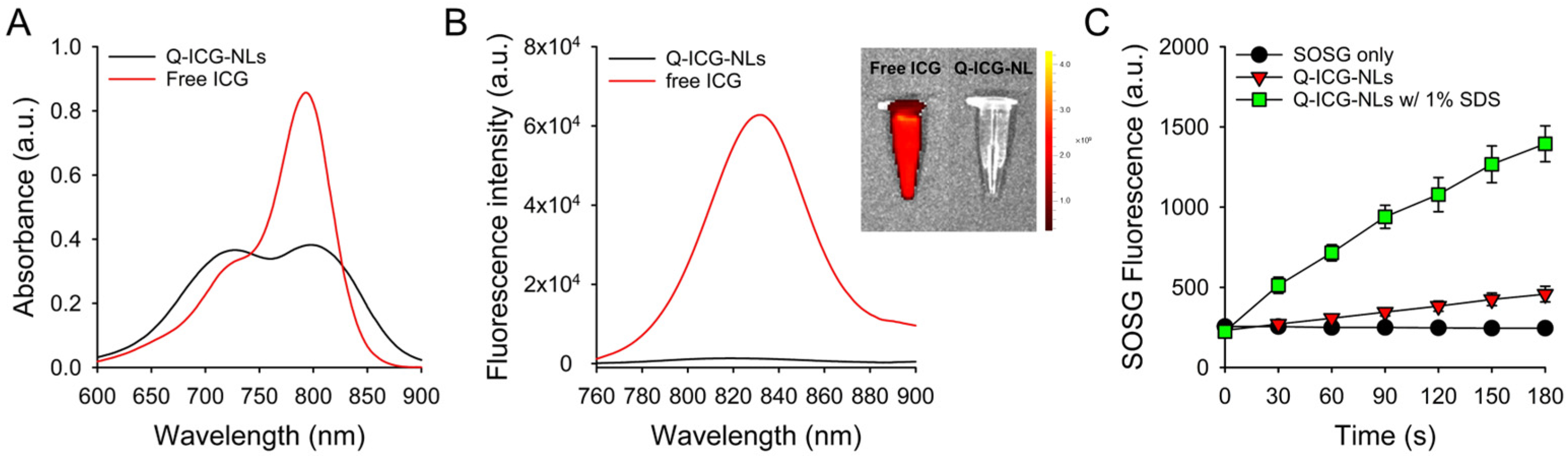
2.1. Preparation and Characterization of Q-ICG-NLs
2.2. Photothermal Effects of Q-ICG-NLs and ICG
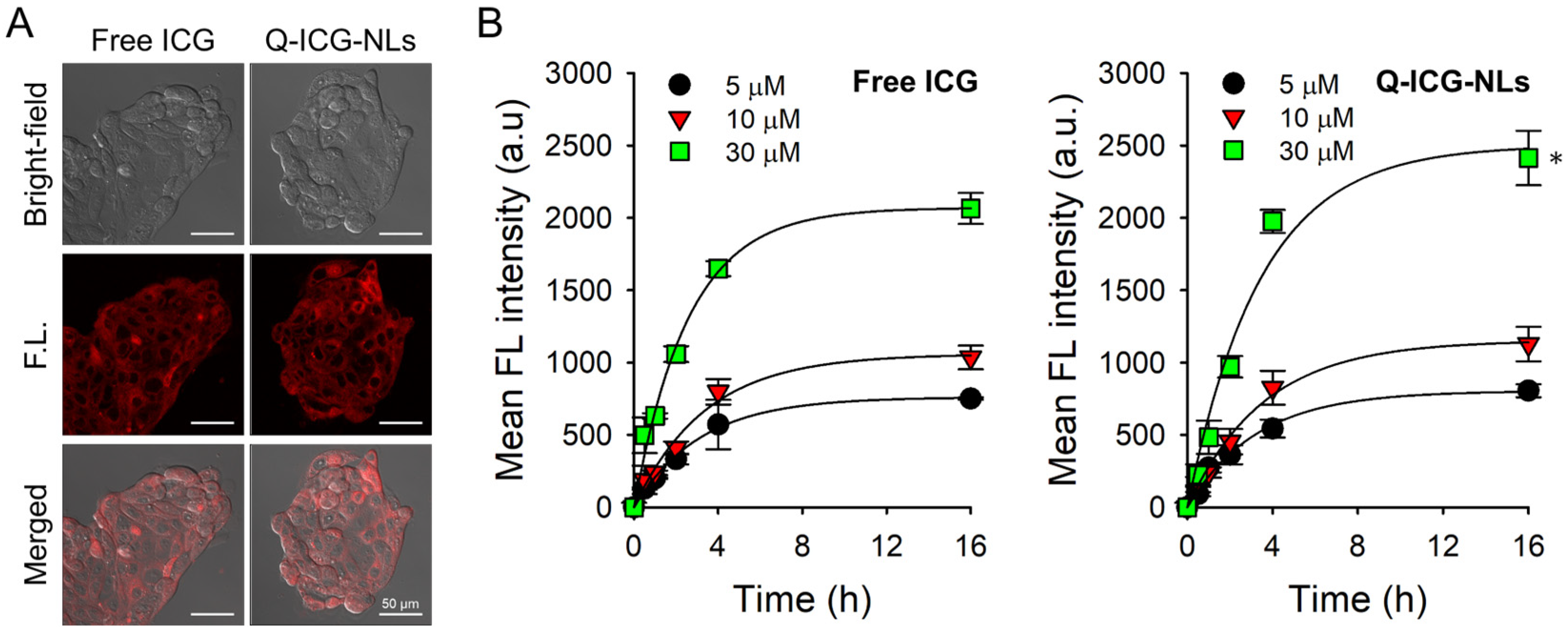
2.3. Cellular Uptake of Q-ICG-NLs and the Subsequent Recovery of Their NIR Fluorescence
2.4. In Vitro Dark Toxicity and Phototoxicity Testing
3. Materials and Methods
3.1. Materials
3.2. Preparation of Quenched ICG-Loaded Nanoliposomes (Q-ICG-NLs)
3.3. Characterization of Q-ICG-NLs
3.4. Measurement of Singlet Oxygen Generation (SOG) During Laser Irradiation
3.5. Measurement of Photothermal Effect During Laser Irradiation
3.6. Cell Culture
3.7. Confocal Fluorescence Microscopy for Intracellular Uptake
3.8. In Vitro Dark Toxicity and Phototoxicity Testing
3.9. Statisitcal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, H.; Kong, Q.; Ma, Y.; Xiong, T.; Qin, T.; Li, S.; Zhu, X. Recent progress in photothermal, photodynamic and sonodynamic cancer therapy: Through the cGAS-STING Pathway to Efficacy-Enhancing Strategies. Molecules 2024, 29, 3704. [Google Scholar] [CrossRef]
- Guo, S.; Gu, D.; Yang, Y.; Tian, J.; Chen, X. Near-infrared photodynamic and photothermal co-therapy based on organic small molecular dyes. J. Nanobiotechnol. 2023, 21, 348. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.S.; Sohn, D.K.; Yoon, H.M.; Park, K.L.; Park, S.J.; Choi, Y. Multiporous PMMA microballs as a novel fluorescence tissue marker. Chem. Eng. J. 2024, 496, 154267. [Google Scholar] [CrossRef]
- Han, H.H.; Wang, H.M.; Jangili, P.; Li, M.; Wu, L.; Zang, Y.; Sedgwick, A.C.; Li, J.; He, X.P.; James, T.D.; et al. The design of small-molecule prodrugs and activatable phototherapeutics for cancer therapy. Chem. Soc. Rev. 2023, 52, 879–920. [Google Scholar] [CrossRef]
- Cheng, P.; Pu, K. Activatable phototheranostic materials for imaging-guided cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 5286–5299. [Google Scholar] [CrossRef]
- Liu, M.; Li, C. Recent advances in activatable organic photosensitizers for specific photodynamic therapy. Chempluschem 2020, 85, 948–957. [Google Scholar] [CrossRef]
- Hong, S.; Kim, H.; Choi, Y. Indocyanine green-loaded hollow mesoporous silica nanoparticles as an activatable theranostic agent. Nanotechology 2017, 28, 185102. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Shen, C.; Mi, X.Q.; Pu, Q. The primary application of indocyanine green fluorescence imaging in surgical oncology. Front. Surg. 2023, 10, 1077492. [Google Scholar] [CrossRef]
- Fransvea, P.; Chiarello, M.M.; Fico, V.; Cariati, M.; Brisinda, G. Indocyanine green: The guide to safer and more effective surgery. World J. Gastrointest. Surg. 2024, 16, 641–649. [Google Scholar] [PubMed]
- Shirata, C.; Kaneko, J.; Inagaki, Y.; Kokudo, T.; Sato, M.; Kiritani, S.; Akamatsu, N.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci. Rep. 2017, 7, 13958. [Google Scholar]
- Lee, H.; Li, Y.; Bao, K.; Lee, S.S.; Kang, H.; Choi, H.S.; Choi, Y. Zwitterionic alginate derivative as a new delivery platform for enhanced blood circulation. J. Pharm. Investig. 2025, 55, 143–154. [Google Scholar]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar]
- Díaz Tovar, J.S.; Kassab, G.; Inada, N.M.; Bagnato, V.S.; Kurachi, C. Photobleaching kinetics and effect of solvent in the photophysical properties of indocyanine green for photodynamic therapy. Chemphyschem 2023, 24, e202300381. [Google Scholar]
- Chauhan, N.; Cabrera, M.; Chowdhury, P.; Nagesh, P.K.; Dhasmana, A.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Indocyanine green-based glow nanoparticles probe for cancer imaging. Nanotheranostics 2023, 7, 353–367. [Google Scholar]
- Wang, Y.G.; Kim, H.; Mun, S.; Kim, D.; Choi, Y. Indocyanine green-loaded perfluorocarbon nanoemulsions for bimodal (19)F-magnetic resonance/nearinfrared fluorescence imaging and subsequent phototherapy. Quant. Imaging Med. Surg. 2013, 3, 132–140. [Google Scholar]
- Cheung, C.C.L.; Ma, G.; Karatasos, K.; Seitsonen, J.; Ruokolainen, J.; Koffi, C.R.; Hassan, H.A.F.M.; Al-Jamal, W.T. Liposome-templated indocyanine green J-aggregates for in vivo near-infrared imaging and stable photothermal heating. Nanotheranostics 2020, 4, 91–106. [Google Scholar]
- Goldberg, S.N.; Gazelle, G.S.; Halpern, E.F.; Rittman, W.J.; Mueller, P.R.; Rosenthal, D.I. Radiofrequency tissue ablation: Importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad. Radiol. 1996, 3, 212–218. [Google Scholar]
- Thomsen, S. Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem. Photobiol. 1991, 53, 825–835. [Google Scholar]
- Agarwal, S.; Ganesh, S. Perinuclear mitochondrial clustering, increased ROS levels, and HIF1 are required for the activation of HSF1 by heat stress. J. Cell Sci. 2020, 133, jcs245589. [Google Scholar] [PubMed]
- Li, Y.; Gao, L.; Tan, X.; Li, F.; Zhao, M.; Peng, S. Lipid rafts-mediated endocytosis and physiology-based cell membrane traffic models of doxorubicin liposomes. Biochim. Biophys. Acta 2016, 1858, 1801–1811. [Google Scholar] [PubMed]
- Li, Y.; Wang, J.; Wientjes, M.G.; Au, J.L. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv. Drug Deliv. Rev. 2012, 64, 29–39. [Google Scholar] [PubMed]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in cancer therapy: How did we start and where are we now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Yoo, Y.; Choi, Y. Indocyanine Green-Loaded Quenched Nanoliposomes as Activatable Theranostics for Cancer. Molecules 2025, 30, 1414. https://doi.org/10.3390/molecules30071414
Lim J, Yoo Y, Choi Y. Indocyanine Green-Loaded Quenched Nanoliposomes as Activatable Theranostics for Cancer. Molecules. 2025; 30(7):1414. https://doi.org/10.3390/molecules30071414
Chicago/Turabian StyleLim, Junwoo, Yeojin Yoo, and Yongdoo Choi. 2025. "Indocyanine Green-Loaded Quenched Nanoliposomes as Activatable Theranostics for Cancer" Molecules 30, no. 7: 1414. https://doi.org/10.3390/molecules30071414
APA StyleLim, J., Yoo, Y., & Choi, Y. (2025). Indocyanine Green-Loaded Quenched Nanoliposomes as Activatable Theranostics for Cancer. Molecules, 30(7), 1414. https://doi.org/10.3390/molecules30071414






