Composite ZIF-8 with Cs3Bi2I9 to Enhance the Photodegradation Ability on Methylene Blue
Abstract
1. Introduction
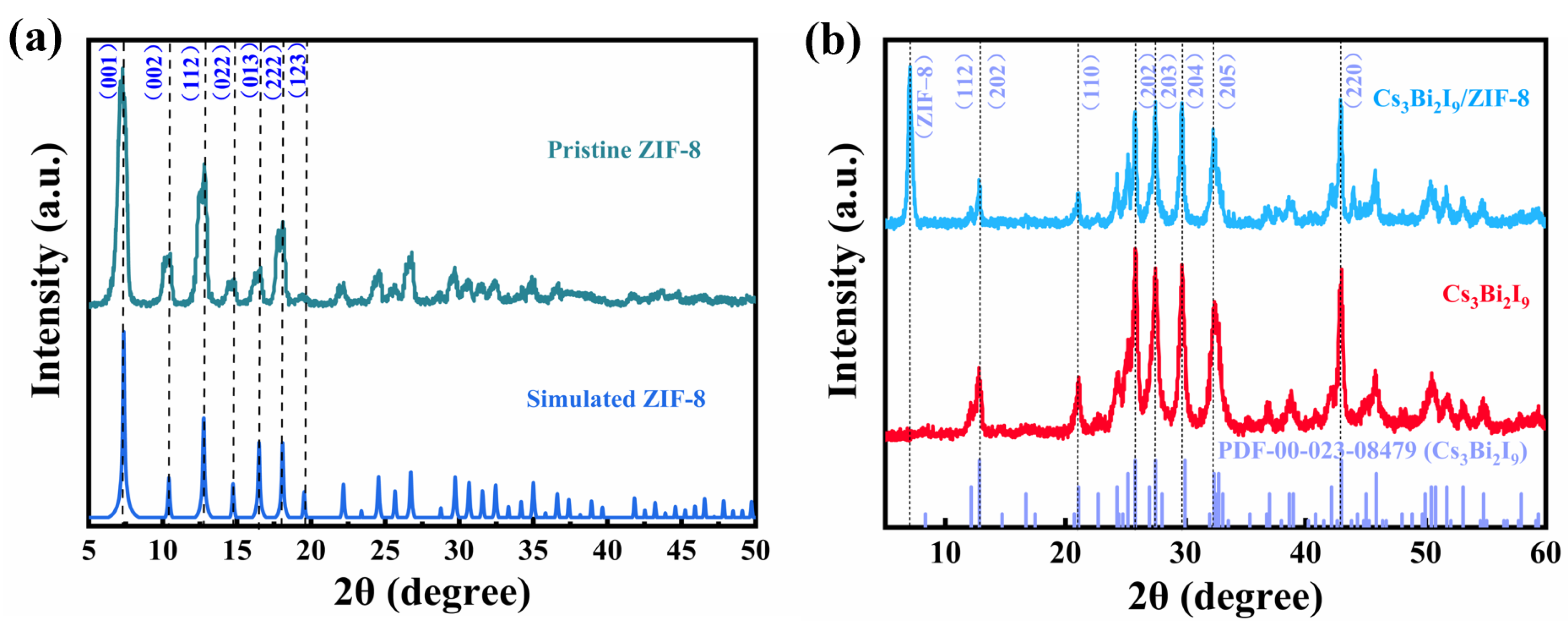
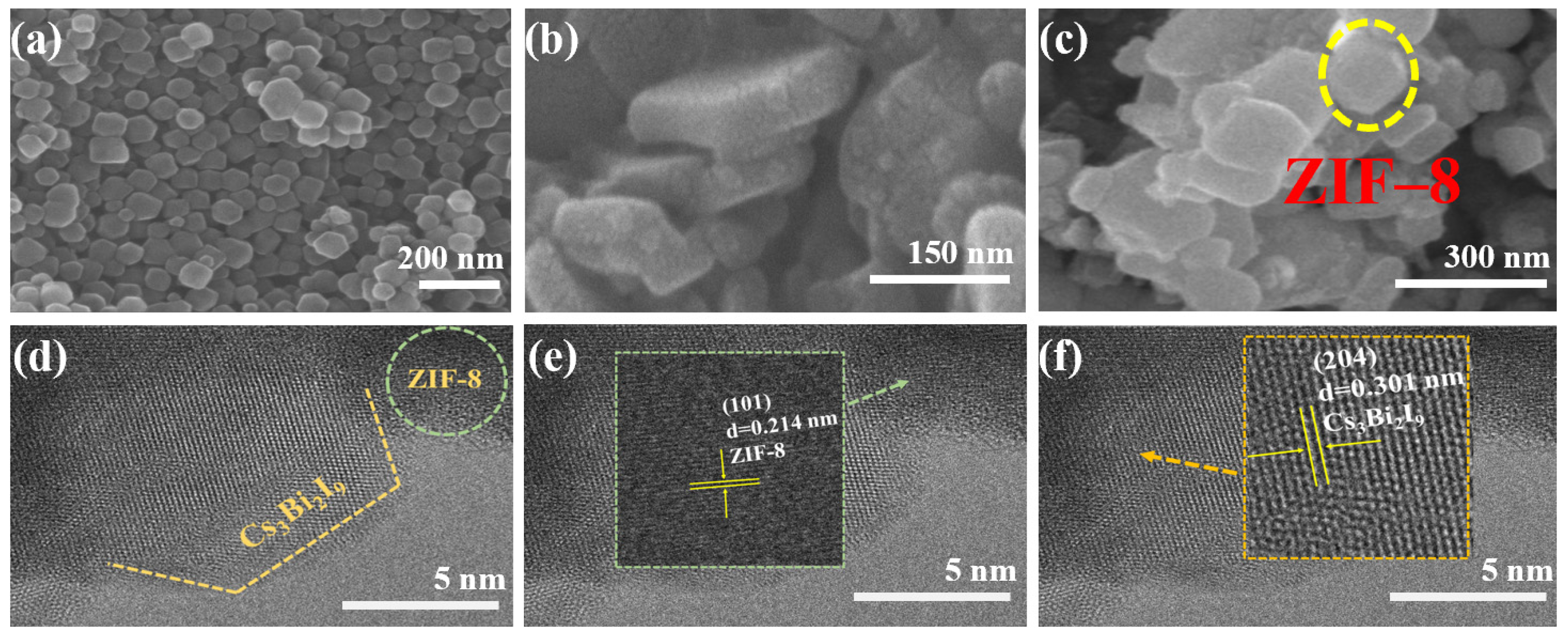
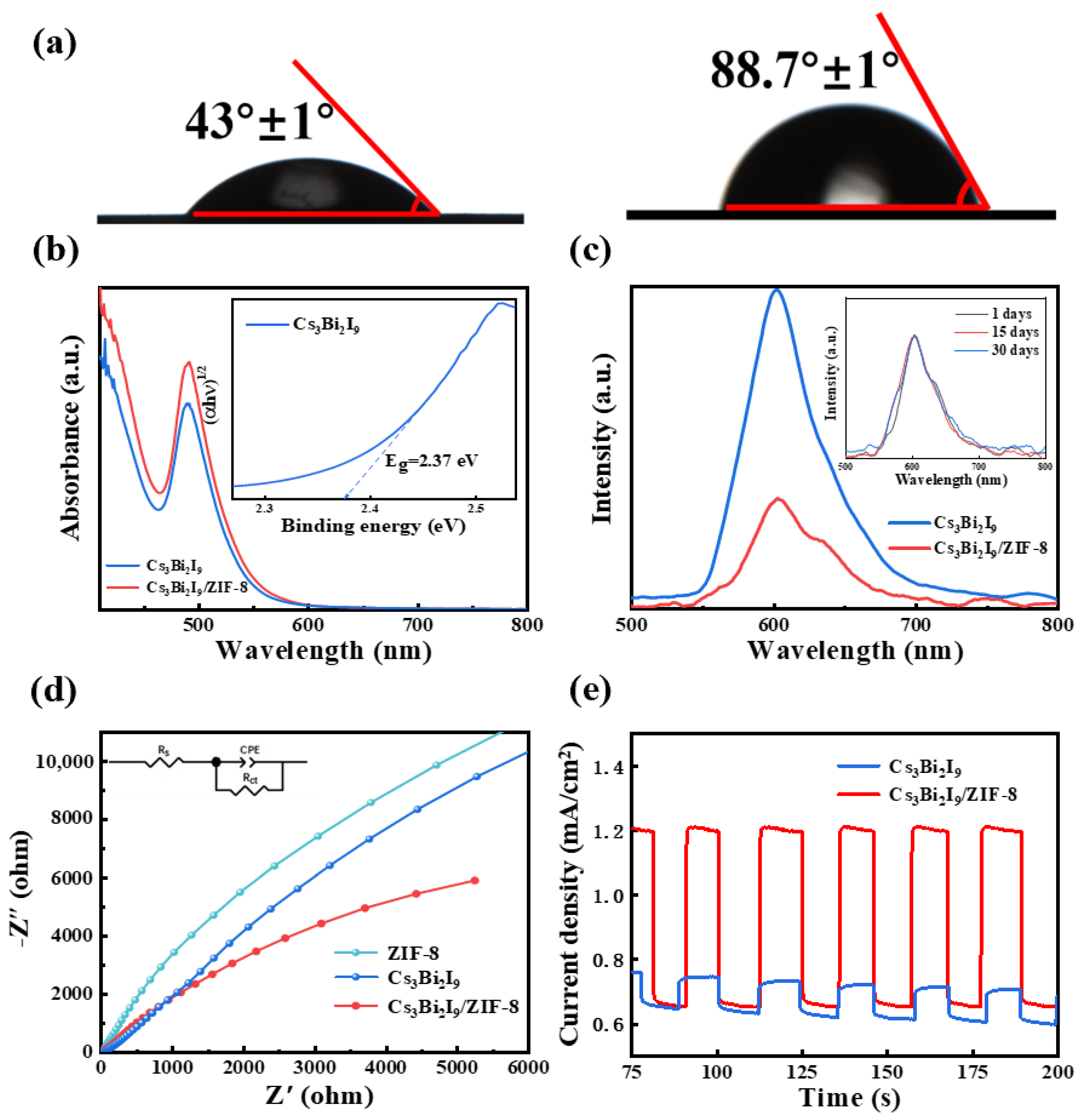
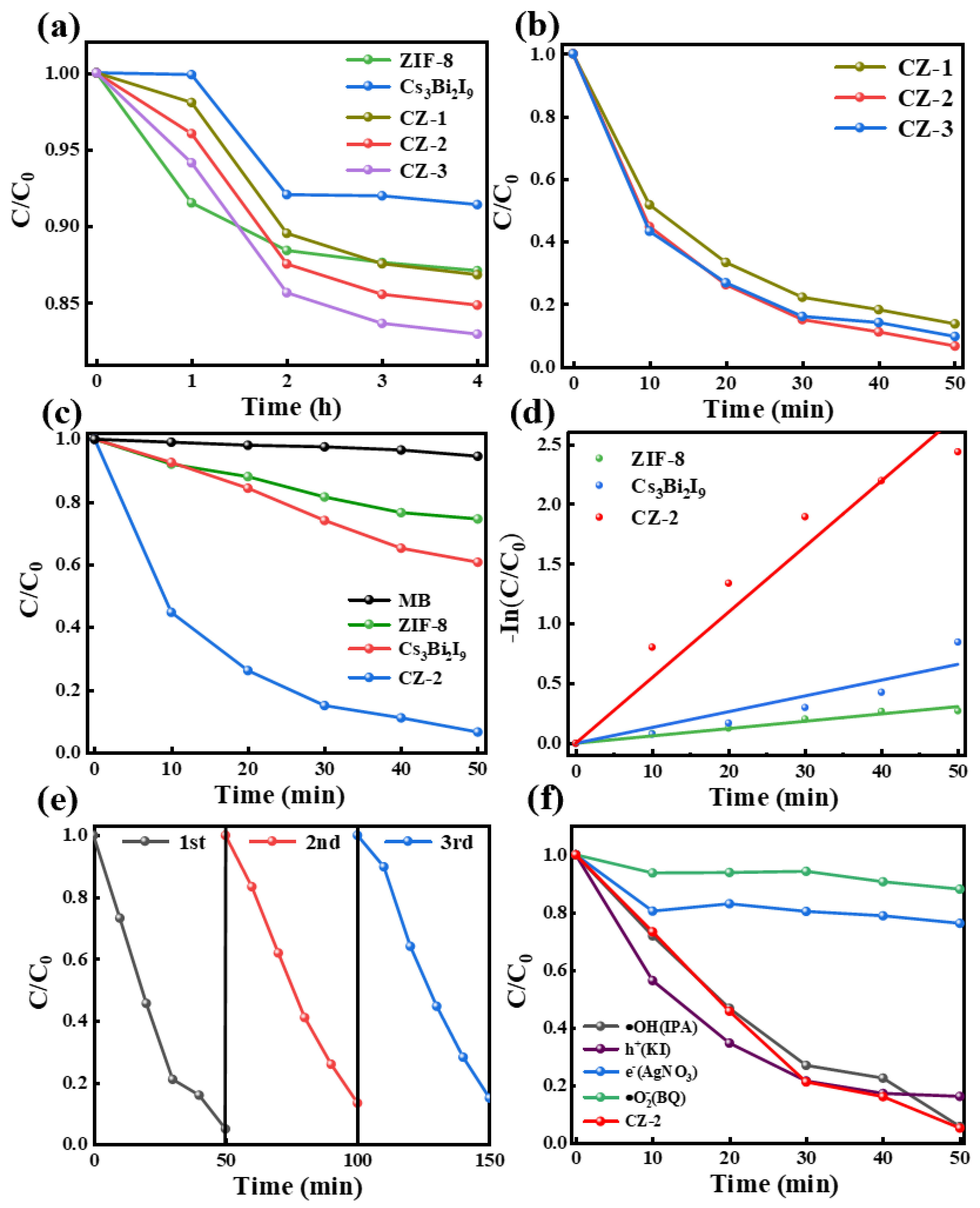
2. Results
3. Experimental Sections
3.1. Chemicals
3.2. Synthesis of ZIF-8, Cs3Bi2I9 and Cs3Bi2I9/ZIF-8
3.3. Adsorption and Photocatalytic Test
3.4. Electrochemical Tests
3.5. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Gayathri, B.; Bharathi, V.D. Photocatalysis for removal of environmental pollutants and fuel production: A review. Environ. Chem. Lett. 2020, 19, 441–463. [Google Scholar] [CrossRef]
- Deng, F.; Shi, H.; Guo, Y.; Luo, X.; Zhou, J. Engineering paths of sustainable and green photocatalytic degradation technology for pharmaceuticals and organic contaminants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100465. [Google Scholar]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar]
- Kumar, M.; Barbhai, M.D.; Puranik, S.; Radha; Natta, S.; Senapathy, M.; Dhumal, S.; Singh, S.; Kumar, S.; Deshmukh, V.P.; et al. Combination of green extraction techniques and smart solvents for bioactives recovery. TrAC Trends Anal. Chem. 2023, 169, 117286. [Google Scholar]
- Yang, K.; Hao, L.; Hou, Y.; Zhang, J.; Yang, J.-H. Summary and application of Ni-based catalysts for electrocatalytic urea oxidation. Int. J. Hydrogen Energy 2024, 51, 966–981. [Google Scholar]
- Yu, Y.; Lin, Z.; Liu, D.; Hou, Y. Exploring the spatially heterogeneous impacts of industrial agglomeration on regional sustainable development capability: Evidence from new energy industries. Environ. Dev. Sustain. 2023, 26, 16657–16682. [Google Scholar]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Adsorption behavior of methylene blue by bone char. Int. J. Mod. Phys. B 2017, 31, 1744099. [Google Scholar]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Arulmani, S.; Veeralakshmi, S.; Ashokkumar, M.; Choi, M.Y. Application of advanced materials in sonophotocatalytic processes for the remediation of environmental pollutants. J. Hazard. Mater. 2021, 412, 125245. [Google Scholar]
- Dai, H.; Yuan, X.; Jiang, L.; Wang, H.; Zhang, J.; Zhang, J.; Xiong, T. Recent advances on ZIF-8 composites for adsorption and photocatalytic wastewater pollutant removal: Fabrication, applications and perspective. Coord. Chem. Rev. 2021, 441, 213985. [Google Scholar]
- Rojviroon, T.; Rojviroon, O.; Sirivithayapakorn, S. Photocatalytic decolourisation of dyes using TiO2 thin film photocatalysts. Surf. Eng. 2016, 32, 562–569. [Google Scholar] [CrossRef]
- Yildiz, S.; Canbaz, G.T.; Mihçiokur, H. Photocatalytic degradation of oxytetracycline using ZnO catalyst. Environ. Prog. Sustain. Energy 2024, 43, 14384. [Google Scholar] [CrossRef]
- Sahu, K.; Satpati, B.; Mohapatra, S. Facile fabrication of CuO nanosheets for photocatalytic applications. Appl. Phys. A 2021, 127, 361. [Google Scholar] [CrossRef]
- Uttam Pandit, V.R.; Parshuram Jadhav, G.K.; Sakharam Jawale, V.M.; Dubepatil, R.; Gurao, R.; Late, D.J. Synthesis and characterization of micro-/nano-α-Fe2O3 for photocatalytic dye degradation. RSC Adv. 2024, 14, 29099–29105. [Google Scholar] [CrossRef] [PubMed]
- Maaß, M.C.; Tasch, A.; Jooss, C.; Waitz, T. Photocatalytic hydrogen evolution using ZnS particles and LEDs. J. Chem. Educ. 2022, 99, 2086–2092. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in photocatalysis: A review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.B.; Abd-El-Aziz, A.S.; Mishra, A.K. Advances in graphene-based magnetic and graphene-based/TiO2 nanoparticles in the removal of heavy metals and organic pollutants from industrial wastewater. J. Inorg. Organomet. Polym. Mater. 2020, 31, 463–480. [Google Scholar] [CrossRef]
- Fatima, R.; Warsi, M.F.; Zulfiqar, S.; Ragab, S.A.; Shakir, I.; Sarwar, M.I. Nanocrystalline transition metal oxides and their composites with reduced graphene oxide and carbon nanotubes for photocatalytic applications. Ceram. Int. 2020, 46, 16480–16492. [Google Scholar] [CrossRef]
- Hong, X.; Wang, X.; Li, Y.; Fu, J.; Liang, B. Progress in graphene/metal oxide composite photocatalysts for degradation of organic pollutants. Catalysts 2020, 10, 921. [Google Scholar] [CrossRef]
- Mehra, S.; Mamta; Tawale, J.; Gupta, G.; Singh, V.N.; Srivastava, A.K.; Sharma, S.N. Evaluating Pb-based and Pb-free halide perovskites for solar-cell applications: A simulation study. Heliyon 2024, 10, 33243. [Google Scholar] [CrossRef]
- Thien, G.S.H.; Ab Rahman, M.; Yap, B.K.; Tan, N.M.L.; He, Z.; Low, P.L.; Devaraj, N.K.; Ahmad Osman, A.F.; Sin, Y.K.; Chan, K.Y. Recent advances in halide perovskite resistive switching memory devices: A transformation from lead-based to lead-free perovskites. ACS Omega 2022, 7, 39472–39481. [Google Scholar] [CrossRef]
- Walker, B.; Kim, G.H.; Kim, J.Y. Pseudohalides in lead-based perovskite semiconductors. Adv. Mater. 2019, 31, 1807029. [Google Scholar]
- Kwak, J.I.; Lee, T.-Y.; An, Y.-J. Assessing the potential toxicity of hazardous material released from Pb-based perovskite solar cells to crop plants. J. Clean. Prod. 2023, 423, 138856. [Google Scholar]
- Zhu, Y.; Kang, Y.; Huang, H.; Zhuang, D.; Li, M.; Ling, Z.; Peng, K.; Zhai, L.; Zou, C. Systematic evaluation of the biotoxicity of Pb-based perovskite materials and perovskite solar cells. J. Mater. Chem. A 2024, 12, 2916–2923. [Google Scholar]
- Guo, L.; Zhang, C.; Tan, T.; Li, L.; Qian, Q.; Mo, Y.; Lei, X.; Cai, W.; Wang, Z. Effect of band structure on the photocatalytic performance of CsPbBr3/UiO-66 composites. J. Solid State Chem. 2024, 339, 124962. [Google Scholar]
- Gu, Y.; Du, X.; Hua, F.; Wen, J.; Li, M.; Tang, T. Nitrogen-doped graphene quantum dot-passivated δ-phase CsPbI3: A water-stable photocatalytic adjuvant to degrade rhodamine B. Molecules 2023, 28, 7310. [Google Scholar] [CrossRef]
- Saparov, B.; Hong, F.; Sun, J.-P.; Duan, H.-S.; Meng, W.; Cameron, S.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-film preparation and characterization of Cs3Sb2I9: A lead-free layered perovskite semiconductor. Chem. Mater. 2015, 27, 5622–5632. [Google Scholar]
- Lee, L.C.; Huq, T.N.; MacManus-Driscoll, J.L.; Hoye, R.L.Z. Research update: Bismuth-based perovskite-inspired photovoltaic materials. APL Mater. 2018, 6, 084502. [Google Scholar]
- Jain, S.M.; Edvinsson, T.; Durrant, J.R. Green fabrication of stable lead-free bismuth based perovskite solar cells using a non-toxic solvent. Commun. Chem. 2019, 2, 91. [Google Scholar]
- Ghosh, B.; Wu, B.; Mulmudi, H.K.; Guet, C.; Weber, K.; Sum, T.C.; Mhaisalkar, S.; Mathews, N. Limitations of Cs3Bi2I9 as lead-free photovoltaic absorber materials. ACS Appl. Mater. Interfaces 2018, 10, 35000–35007. [Google Scholar]
- Liu, J.; Nie, W.; Yan, L.; Hu, H.; Zhang, G.; Lin, P.; Hu, H.; Xu, L.; Wang, P.; Cui, C. Anisotropic optoelectronic properties of lead-free Cs3Bi2I9 single-crystal photodetectors. J. Phys. D Appl. Phys. 2024, 57, 335101. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Z.; Ma, J.; Zhang, P.; Liu, M.; Li, X.; Zhao, R.; Tang, J.; Ren, Z.; Li, S. An all-solid-state photo-rechargeable battery based on Cs3Bi2I9. Chem. Commun. 2023, 59, 2911–2914. [Google Scholar]
- Lou, Y.; Fang, M.; Chen, J.; Zhao, Y. Formation of highly luminescent cesium bismuth halide perovskite quantum dots tuned by anion exchange. Chem. Commun. 2018, 54, 3779–3782. [Google Scholar]
- Ji, J.W.; Zhang, L.J.; Yi, W.J.; Wang, Y.; Chen, J.H.; Yue, X.Z.; Yi, S.S. Re3P4@C/TiO2 ohmic junction boosts charge carrier separation for photocatalytic hydrogen evolution. Chem. Eng. J. 2024, 500, 157277. [Google Scholar] [CrossRef]
- Yali, Z.; Rong, W.; Aolin, L.; Jialei, H.; Zhilong, Z.; Shunhang, W. Constructing surface oxygen vacancy in the [Bi2O2]2+ layer defects mediated Bi2MoO6 enhanced visible light responsive photocatalytic activity. J. Chem. Phys. 2024, 161, 184707. [Google Scholar]
- Qiao, Y.N.; Zhang, Y.; Yuan, J.; Xue, H.M.; Jia, B. Construction of NiCo2S4/ZnCdS Schottky junction with unidirectional interfacial electron transfer for boosting photocatalytic hydrogen production. ACS Appl. Energy Mater. 2024, 7, 9921–9929. [Google Scholar] [CrossRef]
- Tang, T.; Dou, X.; Zhang, H.; Wang, H.; Li, M.; Hu, G.; Wen, J.; Jiang, L. Enhancing the photocatalytic activity of lead-free halide perovskite Cs3Bi2I9 by compositing with Ti3C2 MXene. Molecules 2024, 29, 5096. [Google Scholar] [CrossRef]
- Chen, S.; Yin, H.; Liu, P.; Wang, Y.; Zhao, H. Stabilization and performance enhancement strategies for halide perovskite photocatalysts. Adv. Mater. 2022, 35, 2203836. [Google Scholar]
- Fang, Z.; Yue, X.; Xiang, Q. Atomically contacted Cs3Bi2Br9 QDs@UiO-66 composite for photocatalytic CO2 reduction. Small 2024, 20, 2401914. [Google Scholar]
- Zhang, Z.; Luo, X.; Wang, B.; Zhang, J. Electron transport improvement of perovskite solar cells via a ZIF-8-derived porous carbon skeleton. ACS Appl. Energy Mater. 2019, 2, 2760–2768. [Google Scholar]
- Karim, M.R.; Choi, C.H.; Mohammad, A.; Yoon, T. Fabrication of high-performance supercapacitor of surface-engineered ZIF-8 for energy storage applications. J. Energy Storage 2024, 93, 112199. [Google Scholar]
- Shi, Y.; Zhou, H.; Dai, L.; Liu, D.; Du, W. Preparation of ordered macroporous ZIF-8-derived magnetic carbon materials and its application for lipase immobilization. Catalysts 2024, 14, 55. [Google Scholar] [CrossRef]
- Wang, Z.; Han, R.; Li, L.; Sun, J.; Yang, J.; Pan, M.; Wang, S. Electrochemical strategy based on the synergistic effect of ZIF-8 and MWCNTs for quantitation of tert-butylhydroquinone in oils and fried chips. Microchem. J. 2023, 185, 108268. [Google Scholar]
- Ayyob, M.; Sun, Z.; Liu, Y.Y.; Wang, Y.; Wang, A. Performance of amine-modified CdS-D@ZIF-8 nanocomposites for enhanced photocatalytic H2 evolution. Int. J. Hydrogen Energy 2023, 48, 25645–25659. [Google Scholar]
- Karthieka, R.R.; Prakash, T. Dose-dependent X-ray sensing behaviour of Cs3Bi2I9: PVDF-HFP nanocomposites. Phys. E Low-Dimens. Syst. Nanostructures 2021, 133, 114823. [Google Scholar]
- Ta, D.N.; Nguyen, H.K.D.; Trinh, B.X.; Le, Q.T.N.; Ta, H.N.; Nguyen, H.T. Preparation of nano-ZIF-8 in methanol with high yield. Can. J. Chem. Eng. 2018, 96, 1518–1531. [Google Scholar]
- Zheng, G.; Chen, Z.; Sentosun, K.; Pérez-Juste, I.; Bals, S.; Liz-Marzán, L.M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Hong, M. Shape control in ZIF-8 nanocrystals and metal nanoparticles@ZIF-8 heterostructures. Nanoscale 2017, 9, 16645–16651. [Google Scholar]
- Pramod, A.K.; Kushvaha, S.S.; Batabyal, S.K. Lead-free Cs3Bi2I9 perovskite hexagonal microplates: A promising material solution-processed for ultraviolet self-powered photodetectors. J. Alloys Compd. 2024, 1006, 176320. [Google Scholar]
- Pambudi, F.I. Electronic properties of heterometallic zeolitic imidazolate framework and its encapsulation with Ni, Pd and Pt. Inorg. Chem. Commun. 2022, 143, 109798. [Google Scholar]
- Chen, X.; Chen, C.; Zang, J. Bi2MoO6 nanoflower-like microsphere photocatalyst modified by boron doped carbon quantum dots: Improving the photocatalytic degradation performance of BPA in all directions. J. Alloys Compd. 2023, 962, 171167. [Google Scholar]
- Chen, X.; Chen, C.; Zang, J. Hybrid of carbon quantum dots modified g-C3N4 nanosheets and MoS2 nanospheres: Indirectly promote hydroxyl group production for efficient degradation of methyl orange. Diam. Relat. Mater. 2023, 139, 110385. [Google Scholar]
- Hong, Y.; Wang, B.; Hu, S.; Lu, S.; Wu, Q.; Fu, M.; Gu, C.; Wang, Y. Preparation and photocatalytic performance of Zn2SnO4/ZIF-8 nanocomposite. Ceram. Int. 2023, 49, 11027–11037. [Google Scholar]
- Jing, H.P.; Wang, C.C.; Zhang, Y.W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Didwal, P.N.; Mulani, S.R.; Patil, M.S.; Devan, R.S. Photocatalytic activity of MnTiO3 perovskite nanodiscs for the removal of organic pollutants. Heliyon 2021, 7, 07297. [Google Scholar]
- Wang, Y.; Wang, L.; Liu, R.; Li, X. Casein templated synthesis of porous perovskite and its application in visible-light photocatalytic degradation of methylene blue. Mater. Sci. Semicond. Process. 2019, 103, 104597. [Google Scholar]
- Bresolin, B.M.; Ben Hammouda, S.; Sillanpää, M. An emerging visible-light organic–inorganic hybrid perovskite for photocatalytic applications. Nanomaterials 2020, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Vaish, R.; Elqahtani, Z.M.; Kebaili, I.; Al-Buriahi, M.S.; Sung, T.H.; Hwang, W.; Kumar, A. Piezo-photocatalytic activity of Bi2VO5.5 for methylene blue dye degradation. J. Mater. Res. Technol. 2022, 21, 1998–2012. [Google Scholar]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102900. [Google Scholar]
- Yao, M.; Yu, H.; Ding, K.; Du, Z.; Zhang, W.; Xia, Y.; Pan, X.; Wang, W.; Liu, X.; Li, S.; et al. Quantum dot/ZIF-67/ZIF-8 heterostructure for efficient photocatalytic hydrogen production. ACS Appl. Nano Mater. 2024, 7, 11445–11454. [Google Scholar]
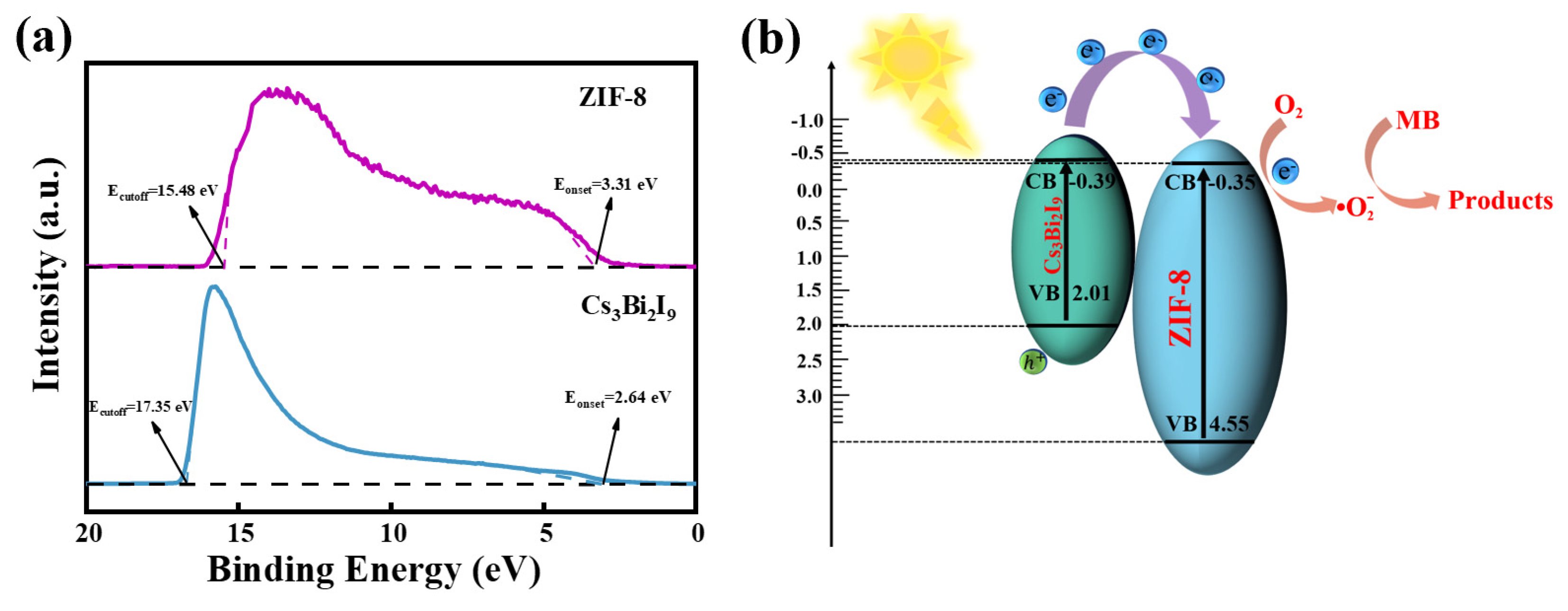
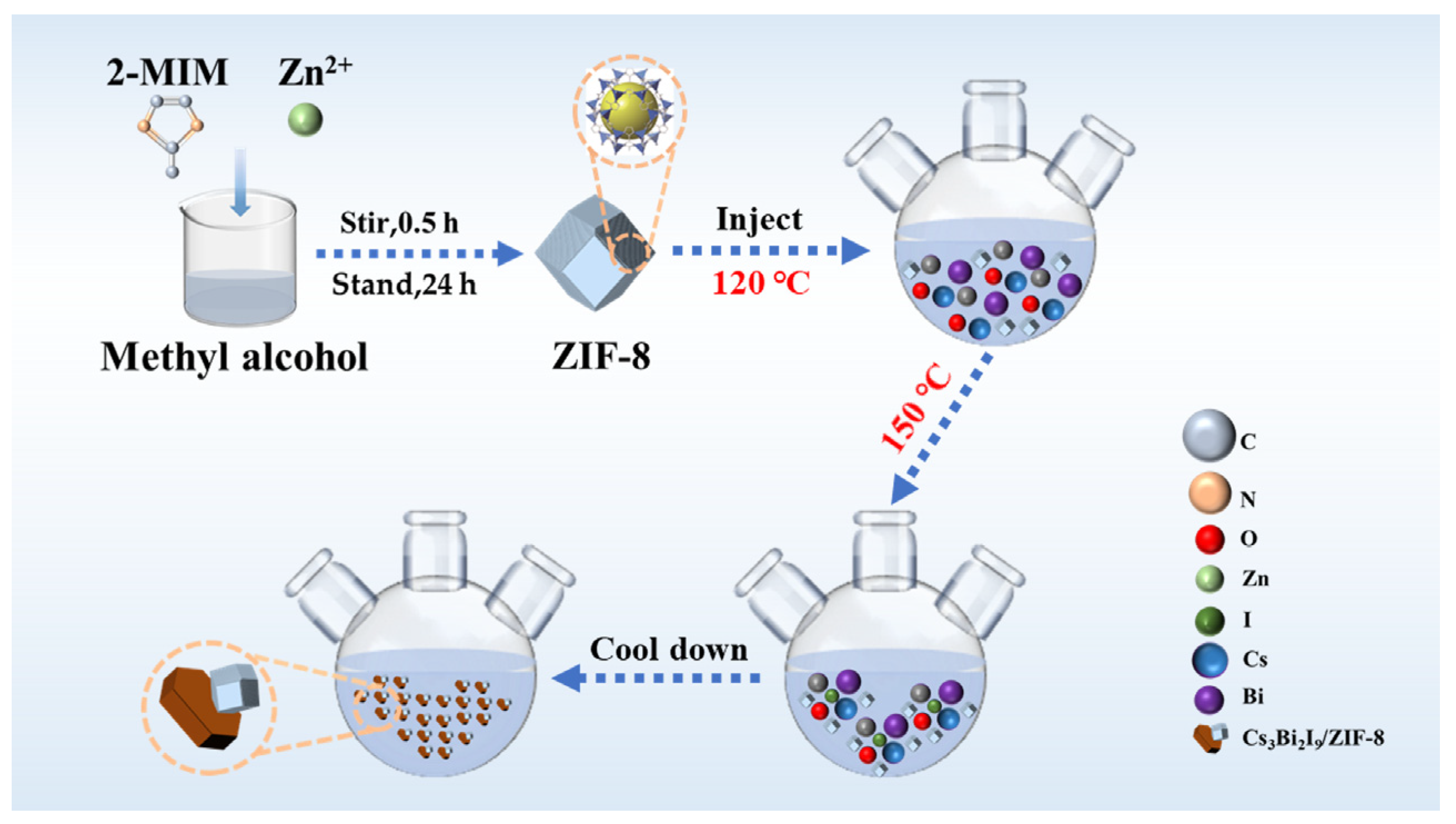
| Catalyst | Illumination | Quantity | C0 | Efficiency | Catalytic Time | Ref. |
|---|---|---|---|---|---|---|
| ZIF-8 | Ultraviolet light | 25 mg | 10 mg/L | 82.3% | 120 min | [53] |
| MnTiO3 | Visible light | 25 mg | 10 mg/L | 89.7% | 180 min | [54] |
| LaMnO3 | Visible light | 5 mg | 5 mg/L | 95% | 120 min | [55] |
| MAIPb | Visible light | 25 mg | 10 mg/L | 90% | 60 min | [56] |
| BiVO4 | Visible light | 0.25 g | 5 mg/L | 81% | 240 min | [57] |
| TiO2 | Ultraviolet light | 0.15 g | 10 mg/L | 81.4% | 100 min | [58] |
| Cs3Bi2I9/ZIF-8 | Visible light | 20 mg | 20 mg/L | 93.4% | 50 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Zhang, H.; Wang, H.; Dou, X.; Wen, J.; Jiang, L. Composite ZIF-8 with Cs3Bi2I9 to Enhance the Photodegradation Ability on Methylene Blue. Molecules 2025, 30, 1413. https://doi.org/10.3390/molecules30071413
Tang T, Zhang H, Wang H, Dou X, Wen J, Jiang L. Composite ZIF-8 with Cs3Bi2I9 to Enhance the Photodegradation Ability on Methylene Blue. Molecules. 2025; 30(7):1413. https://doi.org/10.3390/molecules30071413
Chicago/Turabian StyleTang, Tao, Haoran Zhang, Hexu Wang, Xiaoyu Dou, Jianfeng Wen, and Li Jiang. 2025. "Composite ZIF-8 with Cs3Bi2I9 to Enhance the Photodegradation Ability on Methylene Blue" Molecules 30, no. 7: 1413. https://doi.org/10.3390/molecules30071413
APA StyleTang, T., Zhang, H., Wang, H., Dou, X., Wen, J., & Jiang, L. (2025). Composite ZIF-8 with Cs3Bi2I9 to Enhance the Photodegradation Ability on Methylene Blue. Molecules, 30(7), 1413. https://doi.org/10.3390/molecules30071413





