In Situ Growth of Au NPs on Nitrogen-Doped Graphene Quantum Dots Decorated Graphene Composites for the Construction of an Electrochemical Immunosensor and Its Application in CEA Detection
Abstract
1. Introduction
2. Results and Discussion
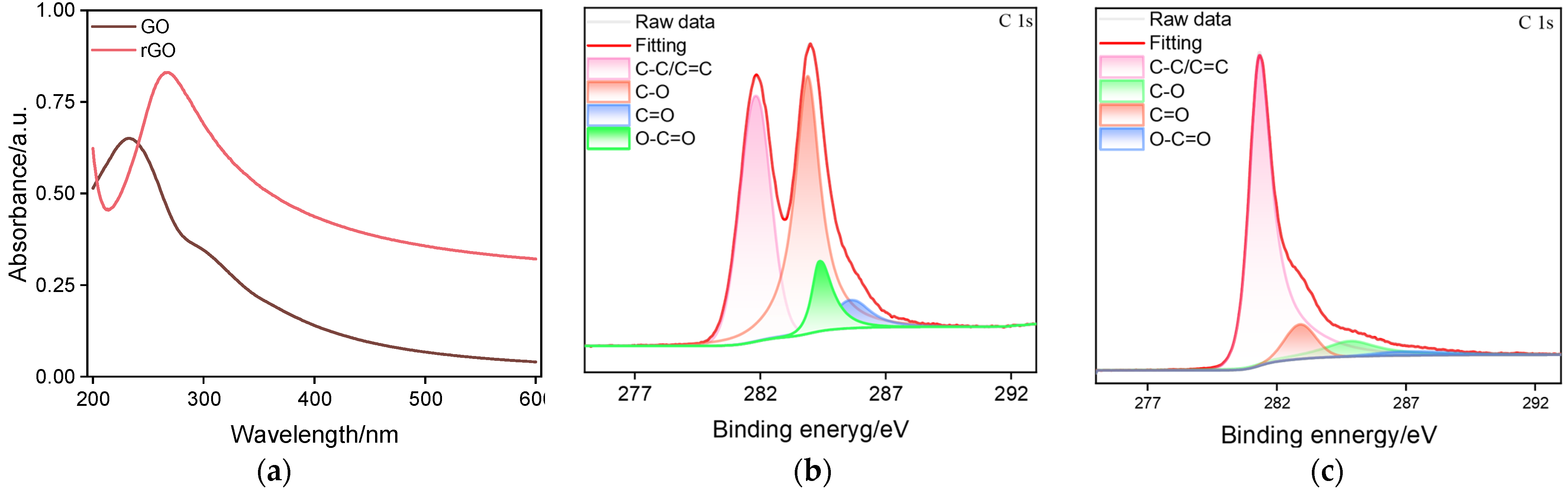
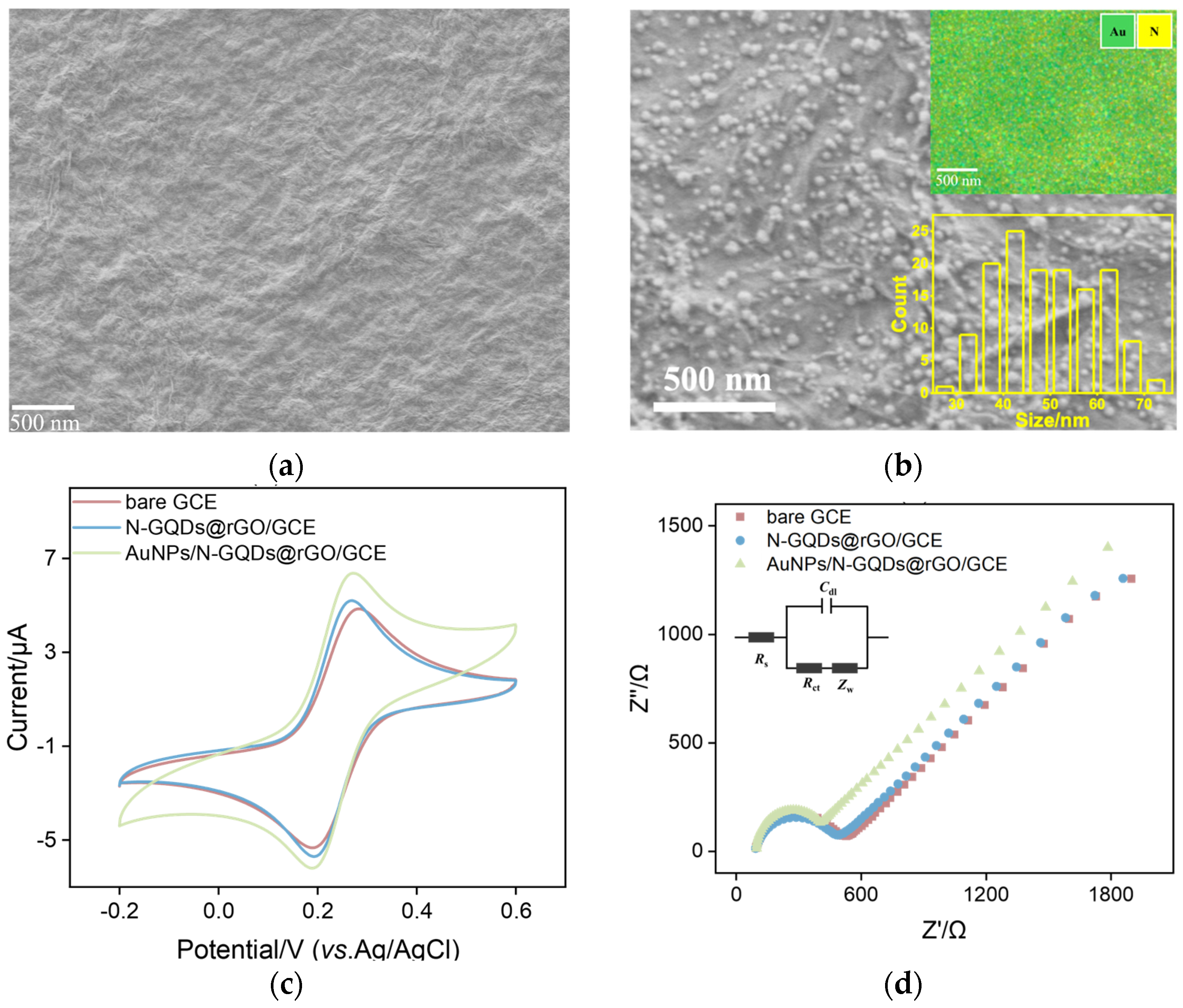
2.1. The Morphology and Structure of AuNPs/N-GQDs@rGO Composites
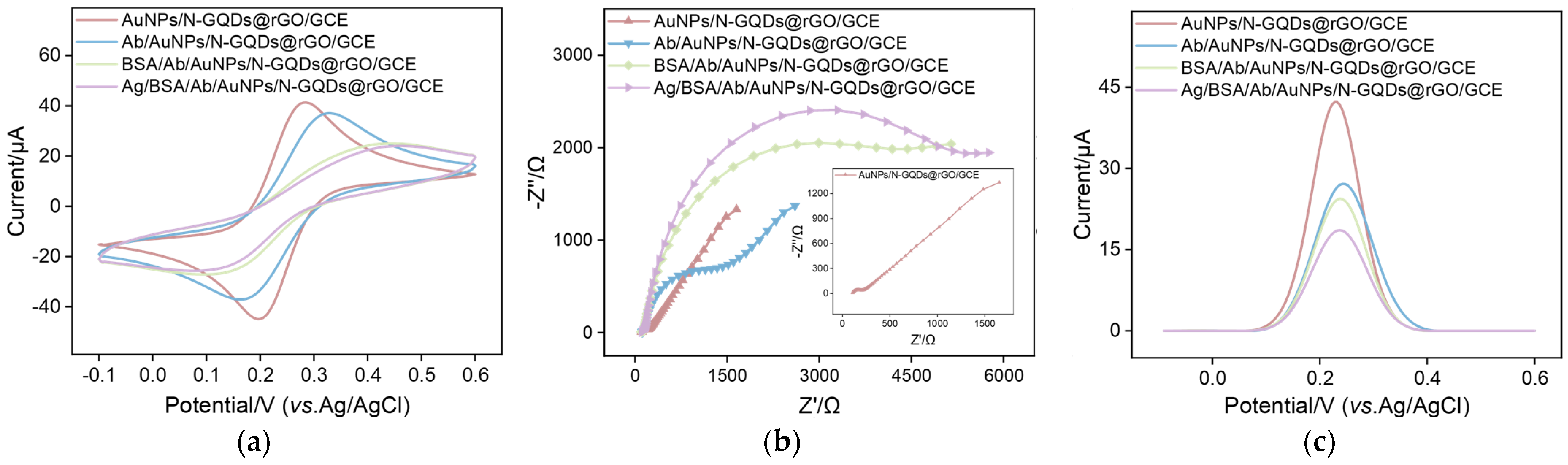
2.2. Electrochemical Characteristics of AuNPs/N-GQDs@rGO-Based Immunosensor
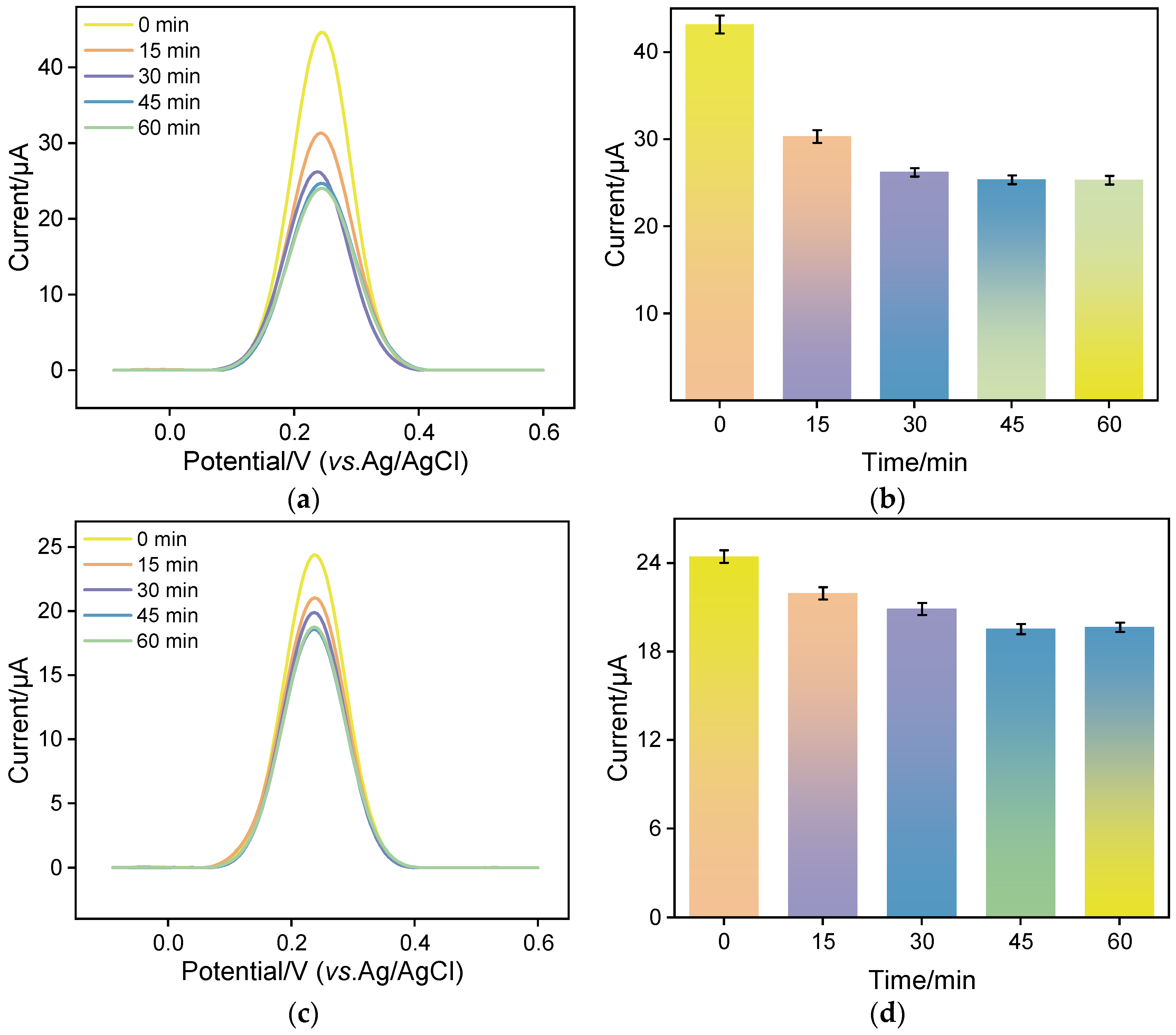
2.3. Optimization of Experiential Conditions of the AuNPs/N-GQDs@rGO/GCE-Based Immunosensor
2.4. DPV Performance of the AuNPs/N-GQDs@rGO/GCE-Based Immunosensor
2.5. Selectivity, Anti-Interference, Reproducibility, and Stability of the AuNPs/N-GQDs@rGO/GCE-Based Immunosensor
2.6. Real Sample Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Characterizations and Instrumentations
3.3. Preparation of rGO
3.4. Preparation of N-GQDs
3.5. Preparation of AuNPs/N-GQDs@rGO/GCE
3.6. Construction of Label-Free Electrochemical Immunosensors BSA/Ab/AuNPs/N-GQDs@rGO/GCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkar, S.; Hazra, S.; Patra, S.; Gogoi, M. Biosensors for cancer detection: A review. TrAC Trends Anal. Chem. 2024, 180, 117978. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Huang, Y.; Zhai, J.; Liao, G.; Wang, Z.; Ning, C. Development of electroactive materials-based immunosensor towards early-stage cancer detection. Coord. Chem. Rev. 2022, 471, 214723. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, S.; Xi, F. Homogeneous aptasensor with electrochemical and electrochemiluminescence dual detection channels enabled by nanochannel-based probe enrichment and DNase I cleavage for tumor biomarker detection. Molecules 2025, 30, 746. [Google Scholar] [CrossRef] [PubMed]
- Afshari Babazad, M.; Foroozandeh, A.; Abdouss, M.; SalarAmoli, H.; Babazad, R.A.; Hasanzadeh, M. Recent progress and challenges in biosensing of carcinoembryonic antigen. TrAC Trends Anal. Chem. 2024, 180, 117964. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Pei, J.; Tian, Y.; Liu, J. A reagentless electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on the interface with redox probe-modified electron transfer wires and effectively immobilized antibody. Front. Chem. 2022, 10, 939736. [Google Scholar] [CrossRef]
- Li, W.; Yu, R.; Xi, F. Enhanced electrochemiluminescence of luminol and-dissolved oxygen by nanochannel-confined Au nanomaterials for sensitive immunoassay of carcinoembryonic antigen. Molecules 2024, 29, 4880. [Google Scholar] [CrossRef]
- Zhou, X.; Han, Q.; Zhou, J.; Liu, C.; Liu, J. Reagentless electrochemical detection of tumor biomarker based on stable confinement of electrochemical probe in bipolar silica nanochannel film. Nanomaterials 2023, 13, 1645. [Google Scholar] [CrossRef]
- Manaf, B.A.A.; Hong, S.P.; Rizwan, M.; Arshad, F.; Gwenin, C.; Ahmed, M.U. Recent advancement in sensitive detection of carcinoembryonic antigen using nanomaterials based immunosensors. Surf. Interfaces 2023, 36, 102596. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Xi, F.; Lu, C. Sensitive electrochemical detection of carcinoembryonic antigen based on biofunctionalized nanochannel modified carbonaceous electrode. Molecules 2024, 29, 858. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Sadighbayan, K.; Tohid-kia, M.R.; Khosroushahi, A.Y.; Hasanzadeh, M. Development of electrochemical biosensors for tumor marker determination towards cancer diagnosis: Recent progress. TrAC Trends Anal. Chem. 2019, 118, 73–88. [Google Scholar] [CrossRef]
- Fan, X.; Wu, J.; Zhang, T.; Liu, J. Electrochemical/electrochemiluminescence sensors based on vertically-ordered mesoporous silica films for biomedical analytical applications. ChemBioChem 2024, 25, e202400320. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, W.; Liang, X.; Yang, Y.; Zhou, Y. Carbon nanotubes in electrochemical, colorimetric, and fluorimetric immunosensors and immunoassays: A review. Microchim. Acta 2020, 187, 206. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Qian, Y.F.; Zhou, J.R.; Zheng, L.; Wang, Y.B. Fluorescence-based quantitative platform for ultrasensitive food allergen detection: From immunoassays to DNA sensors. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3343–3364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, D.; Zhang, G.; Zhang, D.; Shi, X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 2021, 223, 121722. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Y.; Liu, J. Sensitive detection of biomarker in gingival crevicular fluid based on enhanced electrochemiluminescence by nanochannel-confined Co3O4 nanocatalyst. Biosensors 2025, 15, 63. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, C.; Qu, H.; Xi, F. Immunosensor with enhanced electrochemiluminescence signal using platinum nanoparticles confined within nanochannels for highly sensitive detection of carcinoembryonic antigen. Molecules 2023, 28, 6559. [Google Scholar] [CrossRef]
- Lu, S.; Wu, J.; Luo, T.; Liu, J.; Xi, F.; Zhang, W. Solid-phase electrochemiluminescence immunosensing platform based on bipolar nanochannel array film for sensitive detection of carbohydrate antigen 125. Front. Chem. 2024, 12, 1493368. [Google Scholar] [CrossRef]
- Cancelliere, R.; Paialunga, E.; Grattagliano, A.; Micheli, L. Label-free electrochemical immunosensors: A practical guide. TrAC Trends Anal. Chem. 2024, 180, 117949. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, T.; Zheng, Y.; Liu, J. Dual-mode sensing platform for cancer antigen 15-3 determination based on a silica nanochannel array using electrochemiluminescence and electrochemistry. Biosensors 2023, 13, 317. [Google Scholar] [CrossRef]
- Chen, D.; Luo, X.; Xi, F. Probe-integrated electrochemical immunosensor based on electrostatic nanocage array for reagentless and sensitive detection of tumor biomarker. Front. Chem. 2023, 11, 1121450. [Google Scholar] [CrossRef]
- Yan, L.; Xu, S.; Xi, F. Disposal immunosensor for sensitive electrochemical detection of prostate-specific antigen based on amino-rich nanochannels array-modified patterned indium tin oxide electrode. Nanomaterials 2022, 12, 3810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zou, Y.; Ru, H.; Yan, F.; Liu, J. Silica nanochannels as nanoreactors for the confined synthesis of Ag NPs to boost electrochemical stripping chemiluminescence of the luminol-O2 system for the sensitive aptasensor. Anal. Chem. 2024, 96, 10264–10273. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Wang, H.; Gu, X.; Zhou, Y.; Xi, F. Enhanced electrochemiluminescence of luminol at neutral medium using nanochannel-confined Co3O4 nanozyme for highly sensitive detection of tumor biomarker. Microchem. J. 2025, 209, 112903. [Google Scholar] [CrossRef]
- Deng, X.; Lin, X.; Zhou, H.; Liu, J.; Tang, H. Equipment of vertically-ordered mesoporous silica film on electrochemically pretreated three-dimensional graphene electrodes for sensitive detection of methidazine in urine. Nanomaterials 2023, 13, 239. [Google Scholar] [CrossRef]
- Su, R.; Tang, H.; Xi, F. Sensitive electrochemical detection of p-nitrophenol by pre-activated glassy carbon electrode integrated with silica nanochannel array film. Front. Chem. 2022, 10, 954748. [Google Scholar] [CrossRef]
- Zhu, X.; Xuan, L.; Gong, J.; Liu, J.; Wang, X.; Xi, F.; Chen, J. Three-dimensional macroscopic graphene supported vertically-ordered mesoporous silica-nanochannel film for direct and ultrasensitive detection of uric acid in serum. Talanta 2022, 238, 123027. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Yan, F.; Liu, J. Vertical silica nanochannels and o-phenanthroline chelator for the detection of trace Fe(II). ACS Appl. Nano Mater. 2024, 7, 7743–7752. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Zheng, Y.; Liu, J. Solid-phase electrochemiluminescence enzyme electrodes based on nanocage arrays for highly sensitive detection of cholesterol. Biosensors 2024, 14, 403. [Google Scholar] [CrossRef]
- Yu, R.; Zhao, Y.; Liu, J. Solid electrochemiluminescence sensor by immobilization of emitter ruthenium(II)tris(bipyridine) in bipolar silica nanochannel film for sensitive detection of oxalate in serum and urine. Nanomaterials 2024, 14, 390. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, T.; Tang, H.; Liu, J. Novel electrochemical and electrochemiluminescence dual-modality sensing platform for sensitive determination of antimicrobial peptides based on probe encapsulated liposome and nanochannel array electrode. Front. Nutr. 2022, 9, 962736. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gong, J.; Han, Q.; Hu, W.; Yan, F.; Liu, J. Nanogold amplified electrochemiluminescence/electrochemistry in bipolar silica nanochannel array for ultrasensitive detection of SARS-CoV-2 pseudoviruses. Talanta 2024, 277, 126319. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Su, R.; Xi, F. Sensitive detection of noradrenaline in human whole blood based on Au nanoparticles embedded vertically-ordered silica nanochannels modified pre-activated glassy carbon electrodes. Front. Chem. 2023, 11, 1126213. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Gu, X.; He, L.; Xi, F. A highly sensitive immunosensor based on nanochannel-confined nano-gold enhanced electrochemiluminescence for procalcitonin detection. Front. Chem. 2023, 11, 1274424. [Google Scholar] [CrossRef]
- Komal, S.; Kukreti, S.; Kaushik, M. Gold nanoclusters: An ultrasmall platform for multifaceted applications. Talanta 2021, 234, 122623. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, H.; Yadav, D.; Saini, C.; Kumari, R.; Kumar, G.; Kajjam, A.B.; Pandit, V.; Ayoub, M.; Saloni; et al. Synergistic advancements in nanocomposite design: Harnessing the potential of mixed metal oxide/reduced graphene oxide nanocomposites for multifunctional applications. J. Energy Storage 2024, 93, 112317. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A concise review on GO, rGO and metal oxide/rGO composites: Fabrication and their supercapacitor and catalytic applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Adil, S.F.; Ashraf, M.; Khan, M.; Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Tremel, W.; Tahir, M.N. Advances in Graphene/Inorganic Nanoparticle Composites for Catalytic Applications. Chem. Rec. 2022, 22, e202100274. [Google Scholar] [CrossRef]
- Ma, N.; Luo, X.; Wu, W.; Liu, J. Fabrication of a disposable electrochemical immunosensor based on nanochannel array modified electrodes and gated electrochemical signals for sensitive determination of C-reactive protein. Nanomaterials 2022, 12, 3981. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical sensor nanoarchitectonics for sensitive detection of uric acid in human whole blood based on screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef]
- Lv, N.; Qiu, X.; Han, Q.; Xi, F.; Wang, Y.; Chen, J. Anti-biofouling electrochemical sensor based on the binary nanocomposite of silica nanochannel array and graphene for doxorubicin detection in human serum and urine samples. Molecules 2022, 27, 8640. [Google Scholar] [CrossRef] [PubMed]
- Darabdhara, G.; Das, M.R.; Singh, S.P.; Rengan, A.K.; Szunerits, S.; Boukherroub, R. Ag and Au nanoparticles/reduced graphene oxide composite materials: Synthesis and application in diagnostics and therapeutics. Adv. Colloid Interface Sci. 2019, 271, 101991. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, A.; Hasanzadeh, M.; Shadjou, N.; Guardia, M.d.l. Electrochemical biosensing using N-GQDs: Recent advances in analytical approach. TrAC Trends Anal. Chem. 2018, 105, 484–491. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, J.; Xie, L.; Tang, H.; Wang, K.; Liu, J. One-step preparation of nitrogen-doped graphene quantum dots with anodic electrochemiluminescence for sensitive detection of hydrogen peroxide and glucose. Front. Chem. 2021, 9, 688358. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, Z.; Zeng, Z.; Wang, W.; Kong, L.; Liu, J.; Chen, P. Graphene quantum dots assisted exfoliation of atomically-thin 2D materials and as-formed 0D/2D van der Waals heterojunction for HER. Carbon 2021, 184, 554–561. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Li, Y.; Gu, X.; Zhao, J.; Xi, F. Fabrication of a Ratiometric Fluorescence Sensor Based on Carbon Dots as Both Luminophores and Nanozymes for the Sensitive Detection of Hydrogen Peroxide. Molecules 2022, 27, 7379. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile synthesis of iron and nitrogen co-doped carbon dot nanozyme as highly efficient peroxidase mimics for visualized detection of metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, J.; Ding, Y.; Tang, H.; Xi, F. Iron and nitrogen co-doped graphene quantum dots as highly active peroxidases for the sensitive detection of l-cysteine. New J. Chem. 2021, 45, 19056–19064. [Google Scholar] [CrossRef]
- Chaudhary, M.; Doong, R.A.; Kumar, N.; Tseng, T.Y. Ternary Au/ZnO/rGO nanocomposites electrodes for high performance electrochemical storage devices. Appl. Surf. Sci. 2017, 420, 118–128. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel three-dimensional graphene nanomesh prepared by facile electro-etching for improved electroanalytical performance for small biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, G.; Sailjoi, A.; Liu, J. Facile pretreatment of three-dimensional graphene through electrochemical polarization for improved electrocatalytic performance and simultaneous electrochemical detection of catechol and hydroquinone. Nanomaterials 2022, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ding, Y.; Su, R.; Lu, D.; Tang, H.; Xi, F. Silica nanochannel array film supported by ß-cyclodextrin-functionalized graphene modified gold film electrode for sensitive and direct electroanalysis of acetaminophen. Front. Chem. 2022, 9, 812086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosens. Bioelectron. 2020, 165, 112370. [Google Scholar] [CrossRef]
- Purohit, B.; Kumar, A.; Kumari, R.; Mahato, K.; Roy, S.; Srivastava, A.; Chandra, P. 3D gold dendrite and reduced graphene oxide-chitosan nanocomposite-based immunosensor for carcinoembryonic antigen detection in clinical settings. Surf. Interfaces 2024, 47, 104197. [Google Scholar] [CrossRef]
- Lan, Q.; Ren, C.; Lambert, A.; Zhang, G.; Li, J.; Cheng, Q.; Hu, X.; Yang, Z. Platinum Nanoparticle-decorated Graphene Oxide@Polystyrene Nanospheres for Label-free Electrochemical Immunosensing of Tumor Markers. ACS Sustain. Chem. Eng. 2020, 8, 4392–4399. [Google Scholar] [CrossRef]
- Fan, X.; Deng, D.; Chen, Z.; Qi, J.; Li, Y.; Han, B.; Huan, K.; Luo, L. A sensitive amperometric immunosensor for the detection of carcinoembryonic antigen using ZnMn2O4@reduced graphene oxide composites as signal amplifier. Sens. Actuat. B Chem. 2021, 339, 129852. [Google Scholar] [CrossRef]
- Yang, H.; Bao, J.; Huo, D.; Zeng, Y.; Wang, X.; Samalo, M.; Zhao, J.; Zhang, S.; Shen, C.; Hou, C. Au doped poly-thionine and poly-m-Cresol purple: Synthesis and their application in simultaneously electrochemical detection of two lung cancer markers CEA and CYFRA21-1. Talanta 2021, 224, 121816. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Yang, K.; Wang, L.; Han, B.; Sun, S.; Wen, J. An electrochemiluminescence immunosensor based on functionalized metal organic layers as emitters for sensitive detection of carcinoembryonic antigen. Sens. Actuat. B Chem. 2023, 393, 134317. [Google Scholar] [CrossRef]
- Tian, X.; Cao, P.; Sun, D.; Wang, Z.; Ding, M.; Yang, X.; Li, Y.; Ouyang, R.; Miao, Y. Synthesis of CeBi0.4O3.7 nanofeather for ultrasensitive sandwich-like immunoassay of carcinoembryonic antigen. Appl. Surf. Sci. 2020, 528, 146956. [Google Scholar] [CrossRef]
- Liang, H.; Luo, Y.; Li, Y.; Song, Y.; Wang, L. An immunosensor using electroactive COF as signal probe for electrochemical detection of carcinoembryonic antigen. Anal. Chem. 2022, 94, 5352–5358. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liao, X.; Mei, L.; Zhang, M.; Chen, S.; Qiao, X.; Hong, C. An immunosensor using functionalized Cu2O/Pt NPs as the signal probe for rapid and highly sensitive CEA detection with colorimetry and electrochemistry dual modes. Sens. Actuators B 2021, 341, 130032. [Google Scholar] [CrossRef]
- He, P.; Zhang, Q.; Liu, Q. Impedimetric aptasensor based on MOF based composite for measuring of carcinoembryonic antigen as a tumor biomarker. Chemosphere 2023, 338, 139339. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, Z.; Chen, M.; Xi, F. Highly active nanozyme based on nitrogen-doped graphene quantum dots and iron ion nanocomposite for selective colorimetric detection of hydroquinone. Talanta 2025, 281, 126817. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Liu, J.; Mou, Y. Nanochannel confined graphene quantum dots/platinum nanoparticles boosts electrochemiluminescence of luminal-O2 system for sensitive immunoassay. Talanta 2025, 285, 127223. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, J.; Deng, X.; Chen, J.; Xi, F.; Wang, X. Colorimetric and fluorescent dual-modality sensing platform based on fluorescent nanozyme. Front. Chem. 2021, 9, 774486. [Google Scholar] [CrossRef]





| Sensing Interface | Linear Range (ng∙mL−1) | LOD (pg∙mL−1) | Analysis Method | Construction Step | Refs. |
|---|---|---|---|---|---|
| BSA/Ab/CS 1-rGO/AuND 2/AuNPs/GCE | 0.0001–10 | 0.02 | DPV | 6 | [55] |
| BSA/Ab/streptavidin/CS/PtNPs 3@rGO@PS NSs 4/GCE | 0.05–70 | 0.01 | DPV | 6 | [56] |
| BSA/Ab/Au/ZnMn2O4@rGO/GCE | 0.01–50 | 0.019 | DPV | 5 | [57] |
| BSA/Au-pMCP 5-Ab/Au/3D-G 6/GCE | 0.5–200 | 0.31 | SWV 13 | 5 | [58] |
| BSA/Ab/AuNPs@Ir-Zr-MOL 7/GCE | 0.001–100 | 0.2 | ECL 14 | 4 | [59] |
| Au@CeBi0.4O3.7-Ab2NFs 8/CEA/BSA/Ab1/rGO-Au/GCE | 0.01–100 | 0.12 | DPV | 7 | [60] |
| AuNPs/COF 9TFPB-Thi/Ab2/CEA/BSA/Ab1/COF Tab-Dva/GCE | 0.11–80 | 0.03 | DPV | 7 | [61] |
| Cu2O/PtNPs-Ab2/CEA/BSA/Ab1/AuNPs/GCE | 0.0001–80 | 0.03 | CA 15 | 6 | [62] |
| Apt 10/GNPs 11/MOF 12(801)/rGO/GCE | 2.5–250 | 800 | EIS | 4 | [63] |
| BSA/Ab/AuNPs/N-GQDs@rGO/GCE | 0.001–500 | 0.13 | DPV | 5 | This work |
| Sample | Added b (ng/mL) | Found (ng/mL) | RSD (%, n = 3) | Recovery (%) |
|---|---|---|---|---|
| 0.0100 | 0.0103 | 3.2 | 103 | |
| Serum a | 0.100 | 0.101 | 2.8 | 101 |
| 10.0 | 10.1 | 2.5 | 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Wang, L.; Yan, F. In Situ Growth of Au NPs on Nitrogen-Doped Graphene Quantum Dots Decorated Graphene Composites for the Construction of an Electrochemical Immunosensor and Its Application in CEA Detection. Molecules 2025, 30, 1347. https://doi.org/10.3390/molecules30061347
Yan Z, Wang L, Yan F. In Situ Growth of Au NPs on Nitrogen-Doped Graphene Quantum Dots Decorated Graphene Composites for the Construction of an Electrochemical Immunosensor and Its Application in CEA Detection. Molecules. 2025; 30(6):1347. https://doi.org/10.3390/molecules30061347
Chicago/Turabian StyleYan, Zhengzheng, Lujie Wang, and Fei Yan. 2025. "In Situ Growth of Au NPs on Nitrogen-Doped Graphene Quantum Dots Decorated Graphene Composites for the Construction of an Electrochemical Immunosensor and Its Application in CEA Detection" Molecules 30, no. 6: 1347. https://doi.org/10.3390/molecules30061347
APA StyleYan, Z., Wang, L., & Yan, F. (2025). In Situ Growth of Au NPs on Nitrogen-Doped Graphene Quantum Dots Decorated Graphene Composites for the Construction of an Electrochemical Immunosensor and Its Application in CEA Detection. Molecules, 30(6), 1347. https://doi.org/10.3390/molecules30061347






