Pressure Engineering to Enable Improved Stability and Performance of Metal Halide Perovskite Photovoltaics
Abstract
1. Introduction
2. Results
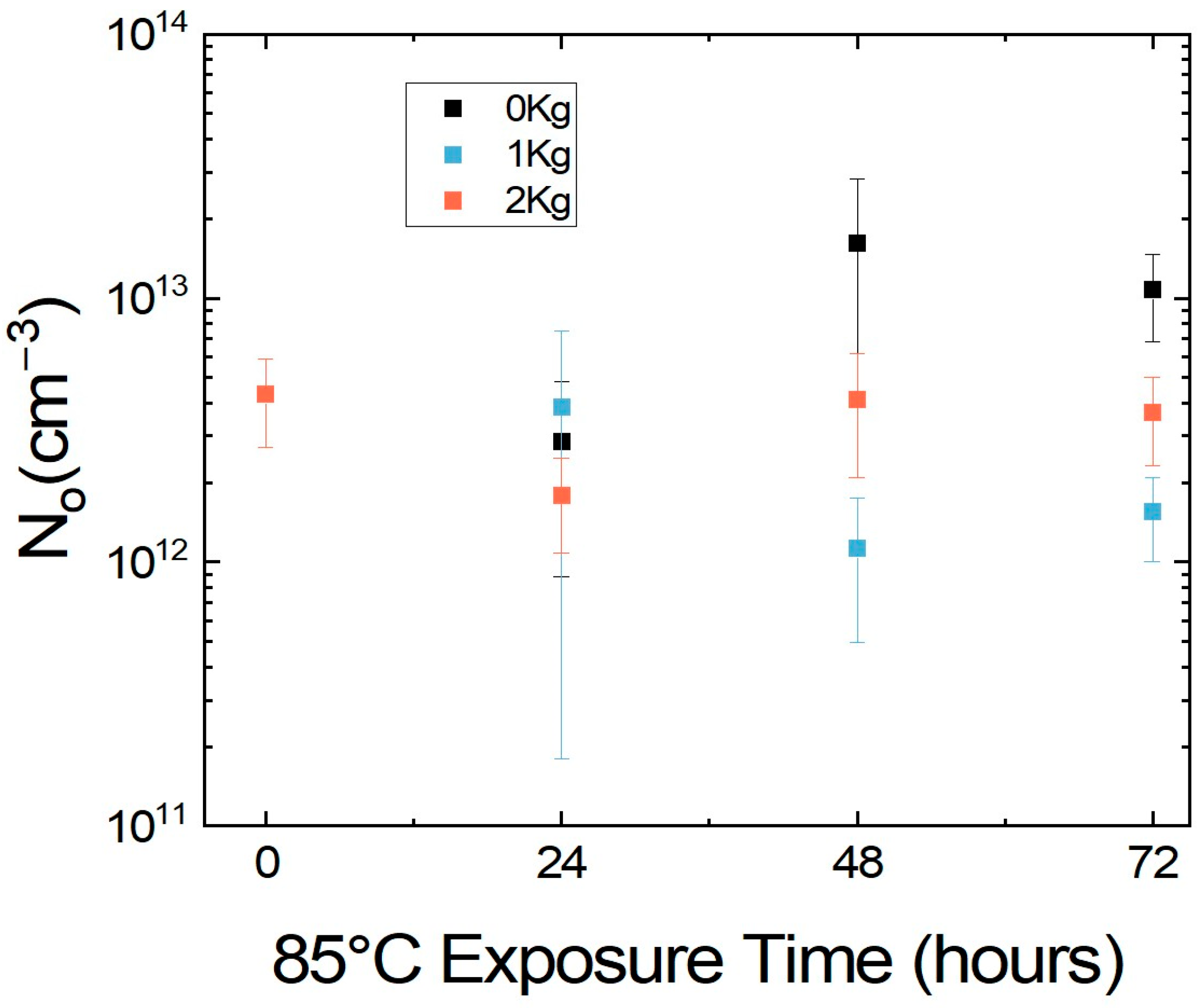
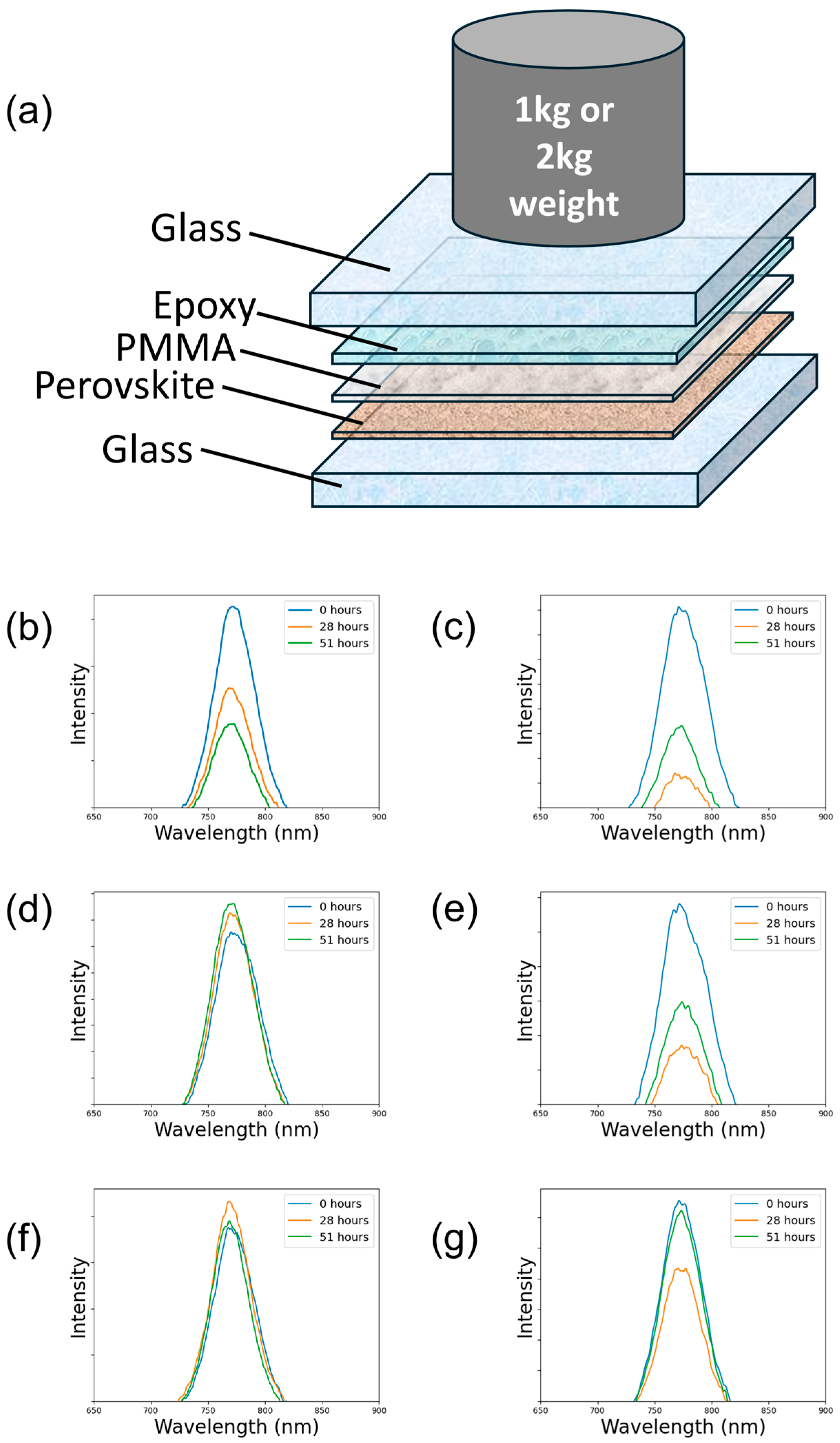
2.1. Continuous Pressure to Simulate Encapsulation
2.2. Tuning Pressure During Encapsulation of MHP Films and Devices
3. Methods
3.1. Fabrication
3.1.1. Methylammonium Lead Iodide (MAPbI3)
3.1.2. NiOx
3.1.3. Cesium Formamidinium Lead Iodide (Cs0.2FA0.8PbI3)
3.1.4. PSC Processing
3.1.5. Encapsulation Approach
3.2. Pressure Application Approach
3.3. Characterization
3.4. Device Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, F.; Shan, Q.; Zeng, H.; Choy, W.C.H. Operational and Spectral Stability of Perovskite Light-Emitting Diodes. ACS Energy Lett. 2021, 6, 3114–3131. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Grätzel, M. The rise of highly efficient and stable perovskite solar cells. Acc. Chem. Res. 2017, 50, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, N.K.; Mahmud, M.A.; Wang, D.; Uddin, A. Perovskite solar cells: Progress and advancements. Energies 2016, 9, 861. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Chen, S.; Wang, S.; Ni, Z.; Van Brackle, C.H.; Yang, S.; Zhao, J.; Yu, Z.; Dai, X.; et al. Revealing defective nanostructured surfaces and their impact on the intrinsic stability of hybrid perovskites. Energy Environ. Sci. 2021, 14, 1563–1572. [Google Scholar] [CrossRef]
- Xie, M.; Tian, J. Operational Stability Issues and Challenges in Metal Halide Perovskite Light-Emitting Diodes. J. Phys. Chem. Lett. 2022, 13, 1962–1971. [Google Scholar] [CrossRef]
- Tu, Q.; Kim, D.; Shyikh, M.; Kanatzidis, M.G. Mechanics-coupled stability of metal-halide perovskites. Matter 2021, 4, 2765–2809. [Google Scholar] [CrossRef]
- Azmi, R.; Zhumagali, S.; Bristow, H.; Zhang, S.; Yazmaciyan, A.; Pininti, A.R.; Utomo, D.S.; Subbiah, A.S.; De Wolf, S. Moisture-Resilient Perovskite Solar Cells for Enhanced Stability. Adv. Mater. 2024, 36, e2211317. [Google Scholar] [CrossRef]
- Liu, X.; Yu, D.; Song, X.; Zeng, H. Metal Halide Perovskites: Synthesis, Ion Migration, and Application in Field-Effect Transistors. Small 2018, 14, e1801460. [Google Scholar] [CrossRef]
- Oyelade, O.V.; Oyewole, O.K.; Oyewole, D.O.; Adeniji, S.A.; Ichwani, R.; Sanni, D.M.; Soboyejo, W.O. Pressure-Assisted Fabrication of Perovskite Solar Cells. Sci. Rep. 2020, 10, 461. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, M.; Cho, Y.; Young, T.L.; Wang, D.; Yi, H.; Kim, J.; Huang, S.; Ho-Baillie, A.W.Y. Effect of Pressing Pressure on the Performance of Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 2358–2363. [Google Scholar] [CrossRef]
- Szafrański, M.; Katrusiak, A. Photovoltaic Hybrid Perovskites under Pressure. J. Phys. Chem. Lett. 2017, 8, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Hutter, E.M.; Muscarella, L.A.; Wittmann, F.; Versluis, J.; McGovern, L.; Bakker, H.J.; Woo, Y.-W.; Jung, Y.-K.; Walsh, A.; Ehrler, B. Thermodynamic Stabilization of Mixed-Halide Perovskites against Phase Segregation. Cell Rep. Phys. Sci. 2020, 1, 10012. [Google Scholar] [CrossRef]
- Ke, F.; Wang, C.; Jia, C.; Wolf, N.R.; Yan, J.; Niu, S.; Devereaux, T.P.; Karunadasa, H.I.; Mao, W.L.; Lin, Y. Preserving a robust CsPbI3 perovskite phase via pressure-directed octahedral tilt. Nat. Commun. 2021, 12, 7183. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wessel, P.; Zhu, J.; Montiel-Chicharro, D.; Betts, T.R.; Mordvinkin, A.; Gottschalg, R. Influence of Lamination Conditions of EVA Encapsulation on Photovoltaic Module Durability. Materials 2023, 16, 6945. [Google Scholar] [CrossRef]
- Aitola, K.; Sonai, G.G.; Markkanen, M.; Kaschuk, J.J.; Hou, X.; Miettunen, K.; Lund, P.D. Encapsulation of commercial and emerging solar cells with focus on perovskite solar cells. Sol. Energy 2022, 237, 264–283. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, G.-Z.; Zhang, Y.; Yang, N.; Li, Y.; Chen, Q. Development of encapsulation strategies towards the commercialization of perovskite solar cells. Energy Environ. Sci. 2022, 15, 13–55. [Google Scholar] [CrossRef]
- Ocaña, L.; Montes, C.; González-Pérez, S.; González-Díaz, B.; Llarena, E. Characterization of a New Low Temperature Encapsulation Method with Ethylene-Vinyl Acetate under UV Irradiation for Perovskite Solar Cells. Appl. Sci. 2022, 12, 5228. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, F.; Cao, Y.; Li, D.; Liu, Q.; Liu, H.; Li, S. Progress on the stability and encapsulation techniques of perovskite solar cells. Org. Electron. 2022, 106, 106515. [Google Scholar] [CrossRef]
- Toniolo, F.; Bristow, H.; Babics, M.; Loiola, L.M.D.; Liu, J.; Said, A.A.; Xu, L.; Aydin, E.; Allen, T.G.; Meneghetti, M.; et al. Efficient and reliable encapsulation for perovskite/silicon tandem solar modules. Nanoscale 2023, 15, 16984–16991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Guo, S.; Wang, Z.; Sun, X.; Lin, J.; Luo, Q.; Ma, C.-Q. Laminated high-performance semi-transparent perovsktie solar cells: Enabled by sticky polyethyleminine as glue. Org. Electron. 2022, 100, 106352. [Google Scholar] [CrossRef]
- Penukula, S.; Tippin, F.; Li, M.; Khawaja, K.A.; Yan, F.; Rolston, N. Use of carbon electrodes to reduce mobile ion concentration and improve reliability of metal halide perovskite photovoltaics. Energy Mater. 2024, 4, 400060. [Google Scholar] [CrossRef]
- Penukula, S.; Estrada Torrejon, R.; Rolston, N. Quantifying and Reducing Ion Migration in Metal Halide Perovskites through Control of Mobile Ions. Molecules 2023, 28, 5026. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgard, E.; Penukula, S.; Casareto, M.; Rolston, N. Pressure Engineering to Enable Improved Stability and Performance of Metal Halide Perovskite Photovoltaics. Molecules 2025, 30, 1292. https://doi.org/10.3390/molecules30061292
Burgard E, Penukula S, Casareto M, Rolston N. Pressure Engineering to Enable Improved Stability and Performance of Metal Halide Perovskite Photovoltaics. Molecules. 2025; 30(6):1292. https://doi.org/10.3390/molecules30061292
Chicago/Turabian StyleBurgard, Erin, Saivineeth Penukula, Marco Casareto, and Nicholas Rolston. 2025. "Pressure Engineering to Enable Improved Stability and Performance of Metal Halide Perovskite Photovoltaics" Molecules 30, no. 6: 1292. https://doi.org/10.3390/molecules30061292
APA StyleBurgard, E., Penukula, S., Casareto, M., & Rolston, N. (2025). Pressure Engineering to Enable Improved Stability and Performance of Metal Halide Perovskite Photovoltaics. Molecules, 30(6), 1292. https://doi.org/10.3390/molecules30061292





