A Novel N/P-Doped Carbon Shells/Mn5.64P3 with Hexagonal Crystal Structure Hybrid as a Prospective Anode for Lithium-Ion Batteries
Abstract
1. Introduction
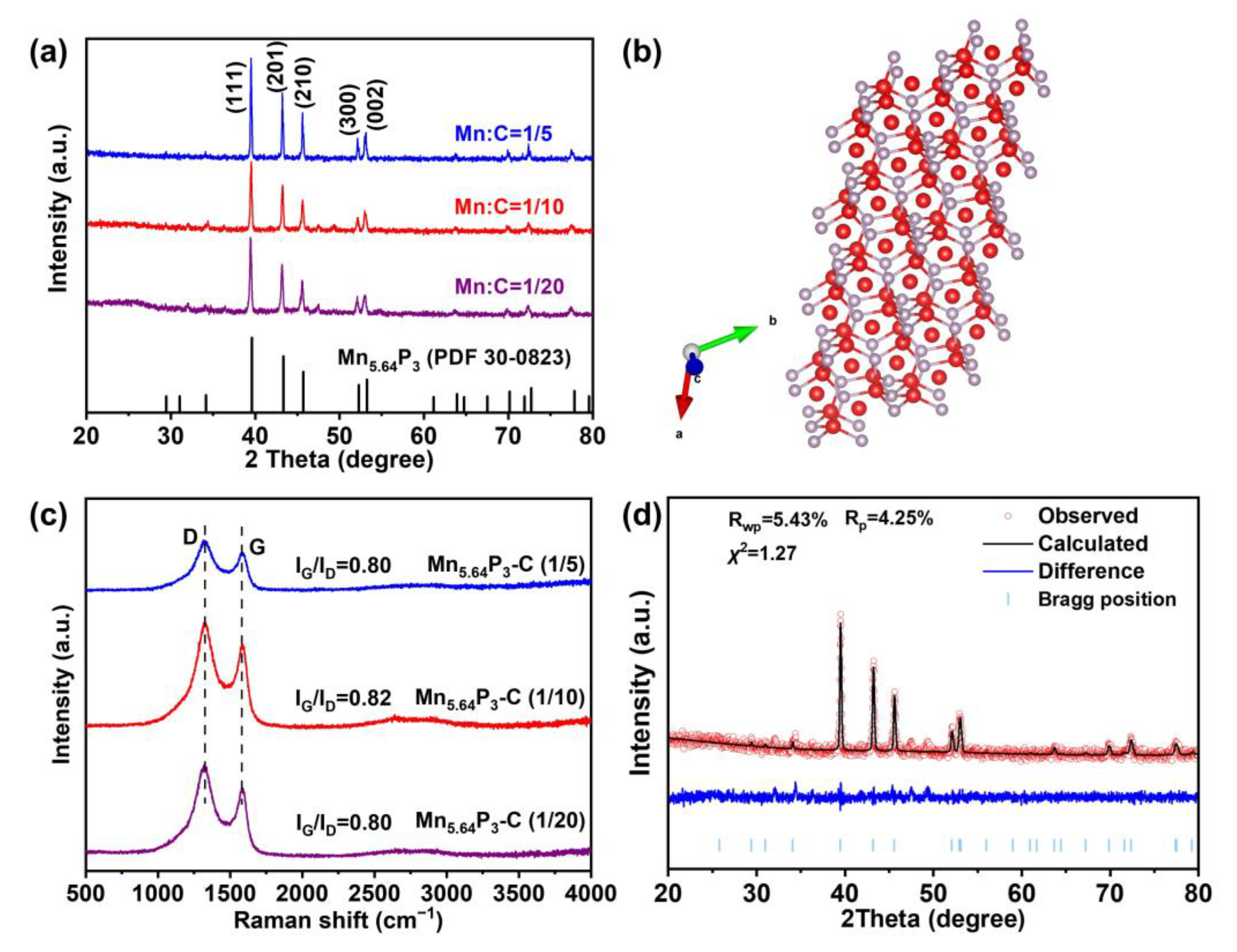
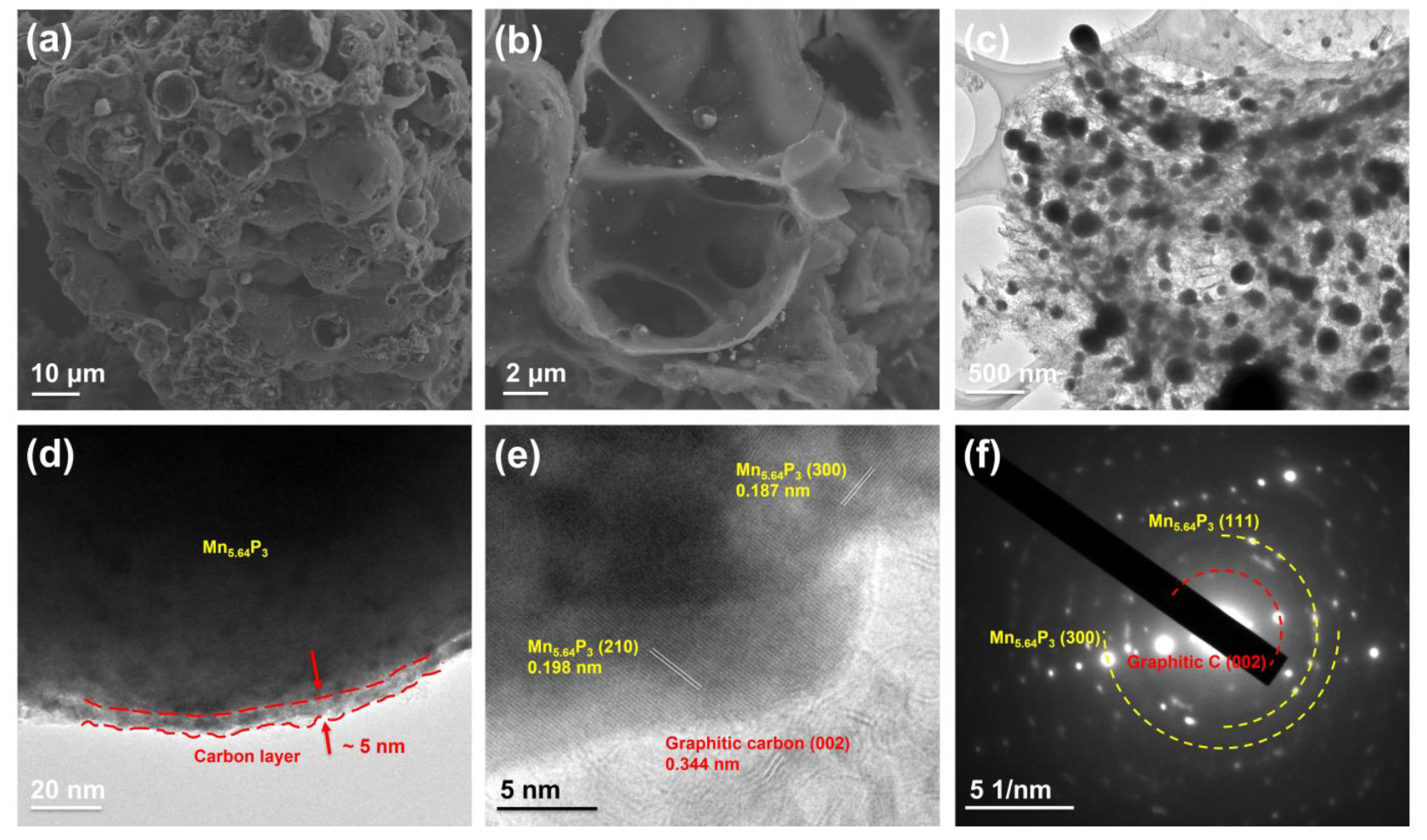
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of the Mn5.64P3-C Hybrid
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Degen, F.; Winter, M.; Bendig, D.; Tübke, J. Energy consumption of current and future production of lithium-ion and post lithium-ion battery cells. Nat. Energy 2023, 8, 1284–1295. [Google Scholar] [CrossRef]
- Li, J.L.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-M.; Liu, Y.; Feng, K.-J.; Ma, J.-Q.; Miao, Y.-J.; Xu, B.-R.; Pan, K.-M.; Akiyoshi, O.; Wang, G.-X.; Zhang, K.-K.; et al. Emerging WS2/WSe2@graphene nanocomposites: Synthesis and electrochemical energy storage applications. Rare Met. 2024, 43, 1–19. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, F.; Lei, Z.; Zhang, W.; Pan, K.; Liu, J.; Chen, M.; Wang, G.; Ren, F.; et al. Facile Synthesis of Antimony Tungstate Nanosheets as Anodes for Lithium-Ion Batteries. Nanomaterials 2019, 9, 1689. [Google Scholar] [CrossRef]
- Liang, S.Z.; Cheng, Y.J.; Zhu, J.; Xia, Y.G.; Müller-Buschbaum, P. A Chronicle Review of Nonsilicon (Sn, Sb, Ge)-Based Lithium/Sodium-Ion Battery Alloying Anodes. Small Methods 2020, 4, 2000218. [Google Scholar] [CrossRef]
- Peng, M.Q.; Shin, K.; Jiang, L.X.; Jin, Y.; Zeng, K.; Zhou, X.L.; Tang, Y.B. Alloy-Type Anodes for High-Performance Rechargeable Batteries. Angew. Chem.-Int. Ed. 2022, 61, e202206770. [Google Scholar] [CrossRef]
- Qian, H.; Liu, Y.; Chen, H.; Feng, K.; Jia, K.; Pan, K.; Wang, G.; Huang, T.; Pang, X.; Zhang, Q. Emerging bismuth-based materials: From fundamentals to electrochemical energy storage applications. Energy Storage Mater. 2023, 58, 232–270. [Google Scholar] [CrossRef]
- Hu, Y.W.; Yang, H.; Balogun, M.S.; Chen, J. Understanding the lithium storage mechanism of free-standing Fe2N nanoparticles. Inorg. Chem. Commun. 2023, 153, 110744. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.Y.; Xu, H.S.; Huo, Z.; Shi, X.Y.; Liu, X.D.; Liu, G.Y.; Yu, C. Facilely Fabricating F-Doped Fe3N Nanoellipsoids Grown on 3D N-Doped Porous Carbon Framework as a Preeminent Negative Material. Molecules 2024, 29, 959. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, J.Z.; Hong, Y.R.; Yang, Y.S.; Tan, L.L.; Li, N.; Ma, C.; Wang, J.W.; Fan, X.L.; Zhu, Y.J. Uncovering the untapped potential of copper(I) sulphide toward lithium-ion storage under ultra-low temperatures. J. Mater. Chem. A 2023, 11, 6168–6180. [Google Scholar] [CrossRef]
- Lenus, S.; Yaoda, L.; Thakur, P.; Lal, A.; Samantaray, S.S.; Dai, Z.F.; Narayanan, T.N. Hydrated LiOH modified Ni0.1Fe0.9PS3 anodes towards safer high-performance lithium-ion batteries. Electrochim. Acta 2024, 483, 144010. [Google Scholar] [CrossRef]
- Chen, F.Z.; Xu, J.; Wang, S.Y.; Lv, Y.H.; Li, Y.; Chen, X.; Xia, A.L.; Li, Y.T.; Wu, J.X.; Ma, L.B. Phosphorus/Phosphide-Based Materials for Alkali Metal-Ion Batteries. Adv. Sci. 2022, 9, 2200740. [Google Scholar] [CrossRef]
- Lan, X.X.; Li, Z.; Zeng, Y.; Han, C.P.; Peng, J.; Cheng, H.M. Phosphorus-based anodes for fast-charging alkali metal ion batteries. Ecomat 2024, 6, e12452. [Google Scholar] [CrossRef]
- He, R.X.; Wang, X.X.; Li, J.H.; Yao, F.; Wang, H.R.; Nie, P.; Chang, L.M. Three-dimensional porous structure CoP/N-doped carbon nanospheres as anode for enhanced lithium storage performance. J. Energy Storage 2024, 101, 113852. [Google Scholar] [CrossRef]
- Mei, P.; Pramanik, M.; Young, C.; Huang, Z.G.; Hossain, M.S.A.; Sugahara, Y.; Yamauchi, Y. Synthesis of mesostructured manganese phosphonate and its promising energy storage application. J. Mater. Chem. A 2017, 5, 23259–23266. [Google Scholar] [CrossRef]
- Bi, W.C.; Zhang, L.F.; Chen, J.; Tian, R.X.; Huang, H.; Yao, M. Lithiation Mechanism and Performance of Monoclinic ZnP2 Anode Materials. Acta Chim. Sin. 2022, 80, 756–764. [Google Scholar] [CrossRef]
- He, R.X.; Wang, X.X.; Li, J.H.; Chang, L.M.; Wang, H.R.; Nie, P. Engineering ultra-small tin phosphide encapsulated in 3D phosphorous-doped porous carbon nanosheets as high-performance anodes for lithium-ion batteries. Appl. Surf. Sci. 2024, 654, 159532. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, B.; Peng, Y.H.; Li, Q.; Chen, M.; Liu, X.G.; Wei, Y.Y.; Zuo, X.J. Elastic restraint induced by mesoporous carbon tubes of ultrafine Ni2P nanoparticles for enhanced potassium and lithium storage. Appl. Surf. Sci. 2025, 679, 161184. [Google Scholar] [CrossRef]
- Ou, H.; Li, P.; Jiang, C.Y.; Liu, Y.Q.; Luo, Y.H.; Xing, Z.Y.; Zeb, A.; Wu, Y.B.; Lin, X.M. Synergistic enhancement of Ni2P anode for high lithium/sodium storage by N, P, S triply-doping and soft template-assisted strategy. J. Colloid Interface Sci. 2025, 678, 365–377. [Google Scholar] [CrossRef]
- Zhong, J.J.; Li, J.L. Copper Phosphide Nanostructures Covalently Modified Ti3C2Tx for Fast Lithium-Ion Storage by Enhanced Kinetics and Pesudocapacitance. Small 2024, 20, 2306241. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Jiang, Y.L.; Yang, Z.Z.; Dong, J.; Yang, W.; An, Q.Y.; Mai, L.Q. Electronic Structure Modulation in MoO2/MoP Heterostructure to Induce Fast Electronic/Ionic Diffusion Kinetics for Lithium Storage. Adv. Sci. 2022, 9, 2104504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Gao, S.Y.; Zhang, D.Y.; Niu, X.H.; Li, H.X.; Wang, K.J. In-suit synthesis of FeP/C/CNT composites with excellent performance in supercapacitors and lithium-ion batteries. Chemistryselect 2024, 9, e202304088. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Yang, H. Phase structure changes of MnP anode material during electrochemical lithiation and delithiation process. Electrochim. Acta 2013, 95, 230–236. [Google Scholar] [CrossRef]
- Sim, S.; Cho, J. Li Reaction Mechanism of MnP Nanoparticles. J. Electrochem. Soc. 2012, 159, A669–A672. [Google Scholar] [CrossRef]
- Mei, P.; Lee, J.; Pramanik, M.; Alshehri, A.; Kim, J.; Henzie, J.; Kim, J.H.; Yamauchi, Y. Mesoporous Manganese Phosphonate Nanorods as a Prospective Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 19739–19745. [Google Scholar] [CrossRef]
- Gillot, F.; Monconduit, L.; Morcrette, M.; Doublet, M.L.; Dupont, L.; Tarascon, J.M. On the Reactivity of Li8-yMnyP4 toward Lithium. Chem. Mater. 2005, 17, 3627–3635. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, W.-S.; Hong, S.-H. Solid solution phosphide (Mn1−xFexP) as a tunable conversion/alloying hybrid anode for lithium-ion batteries. Nanoscale 2019, 11, 13494–13501. [Google Scholar] [CrossRef]
- Kim, K.-H.; Oh, J.; Jung, C.-H.; Kim, M.; Gallant, B.M.; Hong, S.-H. A Novel Solid Solution Mn1-xVxP Anode with Tunable Alloying/Insertion Hybrid Electrochemical Reaction for High Performance Lithium Ion Batteries. Energy Storage Mater. 2021, 41, 310–320. [Google Scholar] [CrossRef]
- Bai, J.; Xi, B.; Mao, H.; Lin, Y.; Ma, X.; Feng, J.; Xiong, S. One-Step Construction of N,P-Codoped Porous Carbon Sheets/CoP Hybrids with Enhanced Lithium and Potassium Storage. Adv. Mater. 2018, 30, 1802310. [Google Scholar] [CrossRef]
- Hong, X.; Deng, C.; Wang, G.; Wang, X.; Dong, W. Composition and morphology transition of NF/MnP/NiCoP composite electrode induced by charge/discharge activation. Chem. Eng. J. 2023, 451, 139036. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Sun, S.; Wei, K.; Liu, H.; Li, G.; Sun, Y.; Li, X.; Qian, J.; Du, S.; et al. Boosting oxygen reduction via MnP nanoparticles encapsulated by N, P-doped carbon to Mn single atoms sites for Zn-air batteries. J. Colloid Interface Sci. 2024, 657, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Forney, M.W.; Ganter, M.J.; Staub, J.W.; Ridgley, R.D.; Landi, B.J. Prelithiation of Silicon–Carbon Nanotube Anodes for Lithium Ion Batteries by Stabilized Lithium Metal Powder (SLMP). Nano Lett. 2013, 13, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, W.; Xie, W.; Cui, H.; Li, Y.; Zhang, C.; Ji, Z.; Liu, F.; Chen, R.; Sun, H.; et al. Synthesis of three-dimensional honeycomb-like Fe3N@NC composites with enhanced lithium storage properties. Carbon 2022, 192, 162–169. [Google Scholar] [CrossRef]
- Haghighat-Shishavan, S.; Nazarian-Samani, M.; Nazarian-Samani, M.; Roh, H.-K.; Chung, K.-Y.; Cho, B.-W.; Kashani-Bozorg, S.F.; Kim, K.-B. Strong, persistent superficial oxidation-assisted chemical bonding of black phosphorus with multiwall carbon nanotubes for high-capacity ultradurable storage of lithium and sodium. J. Mater. Chem. A 2018, 6, 10121–10134. [Google Scholar] [CrossRef]
- Chakraborty, D.; Dam, T.; Modak, A.; Pant, K.K.; Chandra, B.K.; Majee, A.; Ghosh, A.; Bhaumik, A. A novel crystalline nanoporous iron phosphonate based metal–organic framework as an efficient anode material for lithium ion batteries. New J. Chem. 2021, 45, 15458–15468. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Gao, J.; Li, H.; Zhang, J.; Jiang, A.; Liu, Y.; Ren, F. A Novel N/P-Doped Carbon Shells/Mn5.64P3 with Hexagonal Crystal Structure Hybrid as a Prospective Anode for Lithium-Ion Batteries. Molecules 2025, 30, 1346. https://doi.org/10.3390/molecules30061346
Wang F, Gao J, Li H, Zhang J, Jiang A, Liu Y, Ren F. A Novel N/P-Doped Carbon Shells/Mn5.64P3 with Hexagonal Crystal Structure Hybrid as a Prospective Anode for Lithium-Ion Batteries. Molecules. 2025; 30(6):1346. https://doi.org/10.3390/molecules30061346
Chicago/Turabian StyleWang, Fei, Jingxia Gao, Hui Li, Junle Zhang, Aiyun Jiang, Yong Liu, and Fengzhang Ren. 2025. "A Novel N/P-Doped Carbon Shells/Mn5.64P3 with Hexagonal Crystal Structure Hybrid as a Prospective Anode for Lithium-Ion Batteries" Molecules 30, no. 6: 1346. https://doi.org/10.3390/molecules30061346
APA StyleWang, F., Gao, J., Li, H., Zhang, J., Jiang, A., Liu, Y., & Ren, F. (2025). A Novel N/P-Doped Carbon Shells/Mn5.64P3 with Hexagonal Crystal Structure Hybrid as a Prospective Anode for Lithium-Ion Batteries. Molecules, 30(6), 1346. https://doi.org/10.3390/molecules30061346





