Synthesis and Photophysical Properties of AIE-Type Carbazole-Capped Triphenylmethyl Organic Radicals Featuring Non-Aufbau Electronic Structure and Enhanced Photostability
Abstract
1. Introduction
2. Results and Discussion
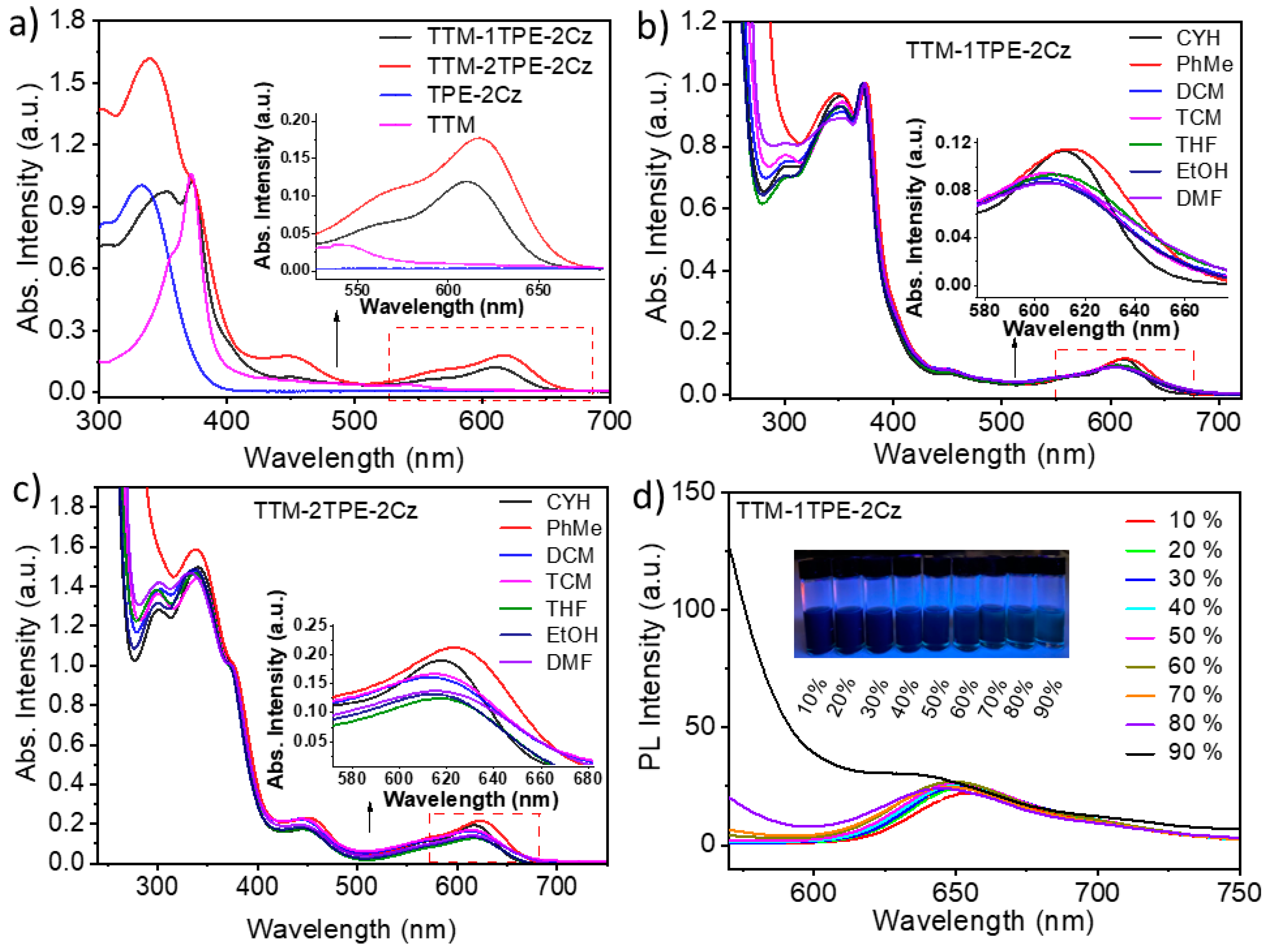
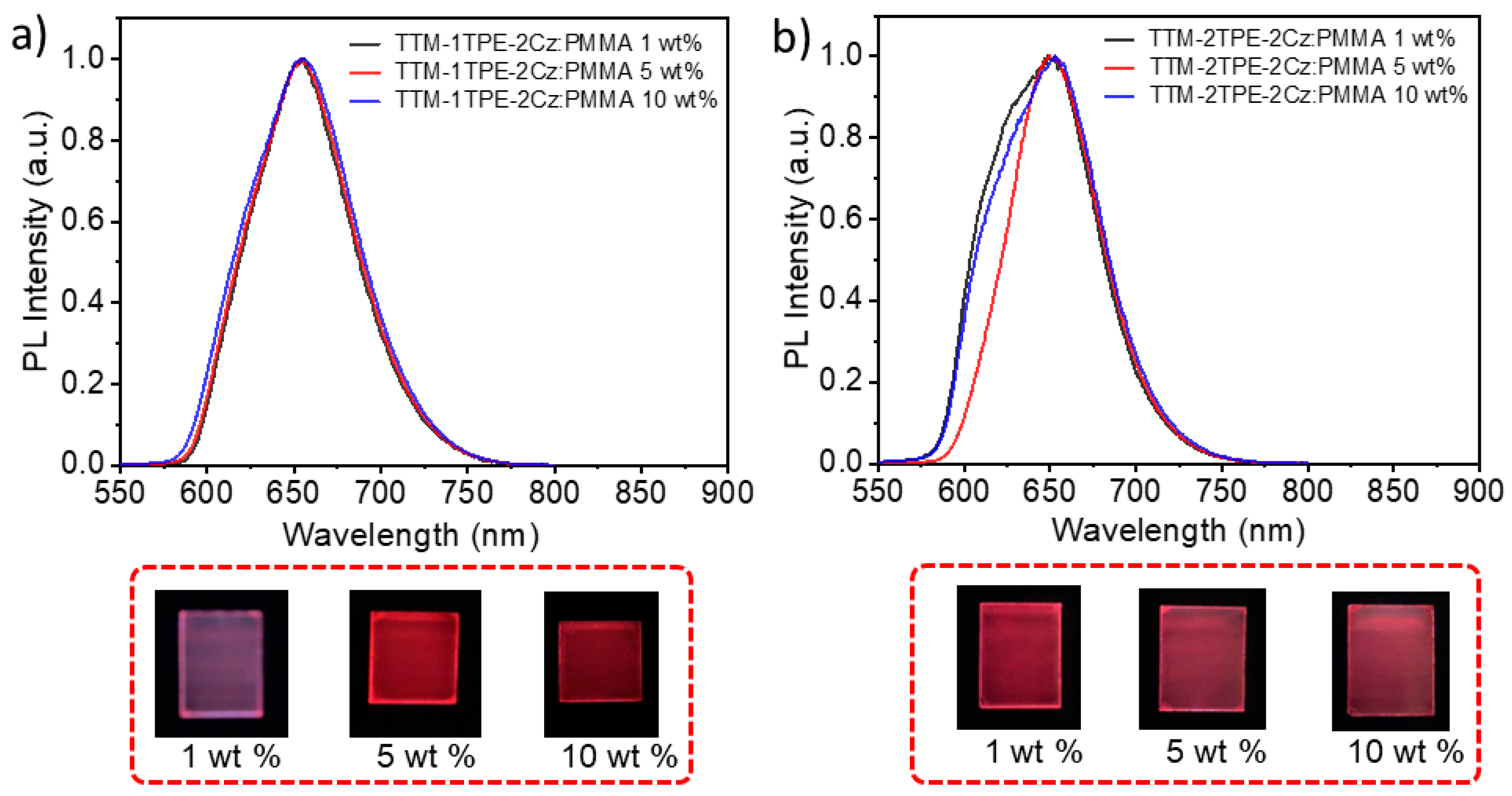
2.1. Fluorescence and UV–Vis Absorption Spectra
2.2. Density Functional Theory (DFT) Calculations
2.3. Cyclic Voltammetry (CV) Measurements
2.4. Stability of Radicals
3. Materials and Methods
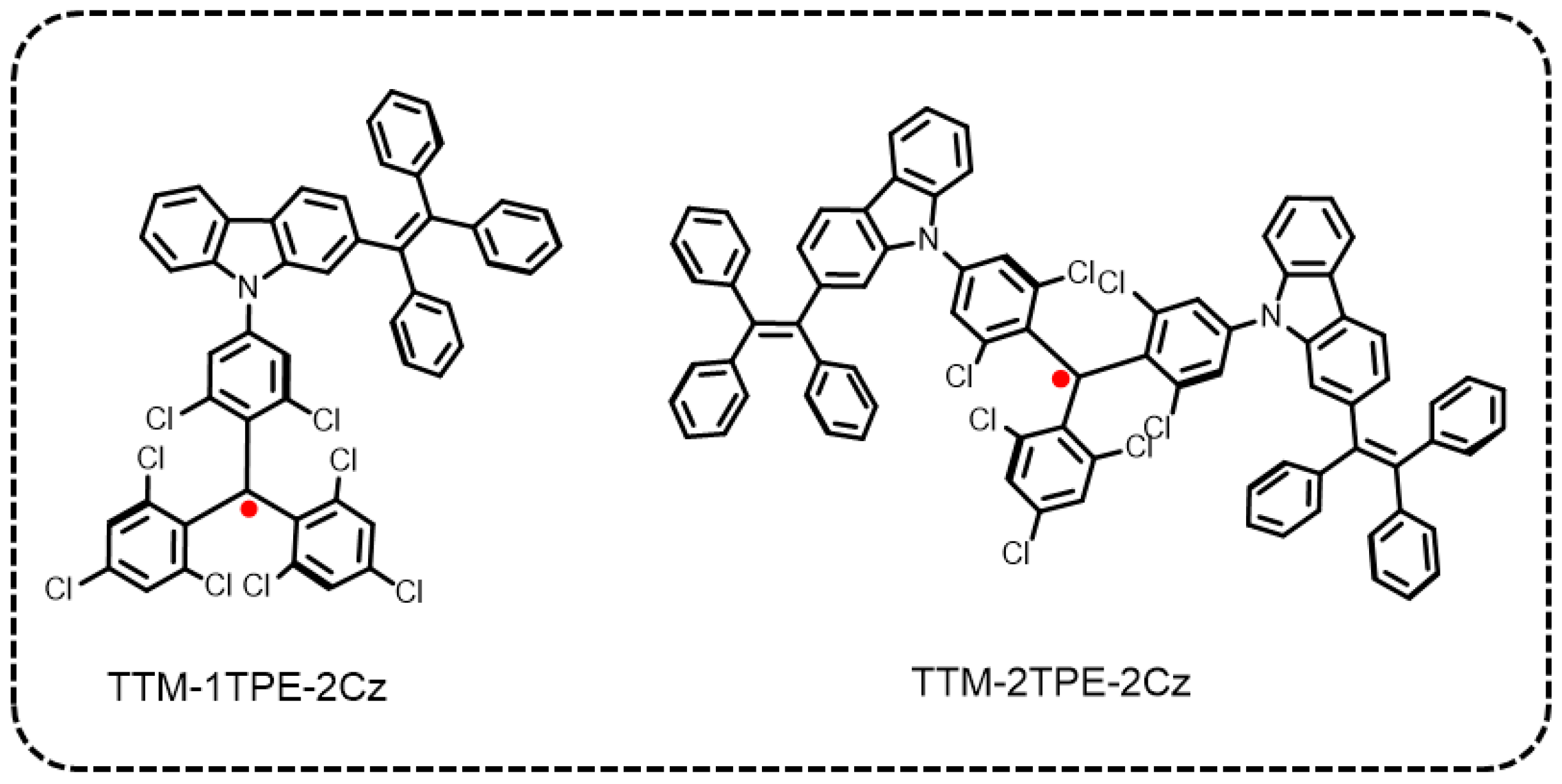
3.1. Preparation of TPE-2Cz, TTM, TTM-1TPE-2Cz, and TTM-2TPE-2Cz
3.2. Materials
3.3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomberg, M. An Instance of Trivalent Carbon: Triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771. [Google Scholar] [CrossRef]
- Shengzhi, D.; Wei, X.; Haoqing, G.; Wenfu, Y.; Ming, Z.; Feng, L. Effects of substituents on luminescent efficiency of stable triaryl methyl radicals. Phys. Chem. Chem. Phys. 2018, 20, 18657–18662. [Google Scholar] [CrossRef]
- Fox, M.A.; Gaillard, E.; Chen, C.C. Photochemistry of stable free radicals: The photolysis of perchlorotriphenylmethyl radicals. J. Am. Chem. Soc. 1987, 109, 7088–7094. [Google Scholar] [CrossRef]
- Carilla, J.; Fajarí, L.; Juliá, L.; Sañé, J.; Rius, J. (2,6-Dichlorophenyl)bis(2,4,6-trichlorophenyl)methyl radical. Synthesis, magnetic behaviour and crystal structure. Tetrahedron 1996, 52, 7013–7024. [Google Scholar] [CrossRef]
- Yan, C.; An, D.; Chen, W.; Zhang, N.; Qiao, Y.; Fang, J.; Lu, X.; Zhou, G.; Liu, Y. Stable Diarylamine-Substituted Tris(2,4,6-trichlorophenyl)methyl Radicals: One-Step Synthesis, Near-Infrared Emission, and Redox Chemistry. CCS Chem. 2022, 4, 3190–3203. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Liu, X.; Gao, P.; Li, G.; He, G.; Rao, B. Air-stable organoradical boron reagents. Angew. Chem. Int. Ed. 2023, 62, e202302835. [Google Scholar] [CrossRef]
- Beldjoudi, Y.; Nascimento, M.A.; Cho, Y.J.; Yu, H.; Aziz, H.; Tonouchi, D.; Eguchi, K.; Matsushita, M.M.; Awaga, K.; Osorio-Roman, I.; et al. Multifunctional dithiadiazolyl radicals: Fluorescence, electrolumines-cence, and photoconducting behavior in Pyren-1′-yl-dithiadiazolyl. J. Am. Chem. Soc. 2018, 140, 6260–6270. [Google Scholar] [CrossRef]
- Mizuno, A.; Matsuoka, R.; Mibu, T.; Kusamoto, T. Luminescent radicals. Chem. Rev. 2024, 124, 1034–1121. [Google Scholar] [CrossRef]
- Shaikh, A.C.; Moutet, J.; Veleta, J.M.; Hossain, M.; Bloch, J.; Astashkin, A.V.; Gianetti, T.L. Persistent, highly localized, and tunable [4]helicene radicals. Chem. Sci. 2020, 11, 11060–11067. [Google Scholar] [CrossRef]
- Fabri, B.; Funaioli, T.; Frédéric, L.; Elsner, C.; Bordignon, E.; Zinna, F.; Di Bari, L.; Pescitelli, G.; Lacour, J. Triple parafunctionalized cations and neutral radicals of enantiopure diaza [4]helicenes. J. Am. Chem. Soc. 2024, 146, 8308–8319. [Google Scholar] [CrossRef]
- Guo, H.; Peng, Q.; Chen, X.-K.; Gu, Q.; Dong, S.; Evans, E.W.; Gillett, A.J.; Ai, X.; Zhang, M.; Credgington, D.; et al. High stability and luminescence efficiency in donor–acceptor neutral radicals not following the Aufbau principle. Nat. Mater. 2019, 18, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Obolda, A.; Ai, X.; Zhang, M.; Li, F. Up to 100% Formation Ratio of Doublet Exciton in Deep-Red Organic Light-Emitting Diodes Based on Neutral π-Radical. ACS Appl. Mater. Interfaces 2016, 8, 35472–35478. [Google Scholar] [CrossRef]
- Cui, Z.; Ye, S.; Wang, L.; Guo, H.; Obolda, A.; Dong, S.; Chen, Y.; Ai, X.; Abdurahman, A.; Zhang, M.; et al. Radical-Based Organic Light-Emitting Diodes with Maximum External Quantum Efficiency of 10.6%. J. Phys. Chem. Lett. 2018, 9, 6644–6648. [Google Scholar] [CrossRef] [PubMed]
- Gamero, V.; Velasco, D.; Latorre, S.; López-Calahorra, F.; Brillas, E.; Juliá, L. [4-(N-Carbazolyl)-2,6-dichlorophenyl]bis(2,4,6-trichlorophenyl)methyl radical an efficient red light-emitting paramagnetic molecule. Tetrahedron Lett. 2006, 47, 2305–2309. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Luminescence, Ssability, and proton response of an open-shell (3,5-Dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical. Angew. Chem. Int. Ed. 2014, 53, 11845–11848. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Chen, Y.; Feng, Y.; Li, F. A Stable Room-Temperature Luminescent Biphenylmethyl Radical. Angew. Chem. Int. Ed. 2018, 57, 2869–2873. [Google Scholar] [CrossRef]
- Abdurahman, A.; Chen, Y.; Ai, X.; Ablikim, O.; Gao, Y.; Dong, S.; Li, B.; Yang, B.; Zhang, M.; Li, F. A pure red luminescent β-carboline-substituted biphenylmethyl radical: Photophysics, stability and OLEDs. J. Mater. Chem. C 2018, 6, 11248–11254. [Google Scholar] [CrossRef]
- Wu, C.; Lu, C.; Yu, S.; Zhang, M.; Zhang, H.; Zhang, M.; Li, F. Highly efficient near-infrared luminescent radicals with emission peaks over 750 nm. Angew. Chem. Int. Ed. 2024, 63, e202412483. [Google Scholar] [CrossRef]
- Kimura, S.; Matsuoka, R.; Kimura, S.; Nishihara, H.; Kusamoto, T. Radical-Based Coordination Polymers as a Platform for Magnetoluminescence. J. Am. Chem. Soc. 2021, 143, 5610–5615. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, D.T.; Nishihara, P.D.H. Enhanced luminescent properties of an open-shell (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical by coordination to gold. Angew. Chem. Int. Ed. 2015, 54, 3731–3734. [Google Scholar] [CrossRef]
- Kimura, S.; Kimura, S.; Kato, K.; Teki, Y.; Nishihara, H.; Kusamoto, T. A ground-state-dominated magnetic field effect on the luminescence of stable organic radicals. Chem. Sci. 2021, 12, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Uejima, M.; Ota, W.; Sato, T.; Kusaka, S.; Matsuda, R.; Nishihara, H.; Kusamoto, T. An Open-shell, Luminescent, Two-Dimensional Coordination Polymer with a Honeycomb Lattice and Triangular Organic Radical. J. Am. Chem. Soc. 2021, 143, 4329–4338. [Google Scholar] [CrossRef]
- Castellanos, S.; Velasco, D.; López-Calahorra, F.; Brillas, E.; Julia, L. Taking Advantage of the Radical Character of Tris(2,4,6-trichlorophenyl)methyl To Synthesize New Paramagnetic Glassy Molecular Materials. J. Org. Chem. 2008, 73, 3759–3767. [Google Scholar] [CrossRef]
- Abdurahman, A.; Hele, T.J.H.; Gu, Q.; Zhang, J.; Peng, Q.; Zhang, M.; Friend, R.H.; Li, F.; Evans, E.W. Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mater. 2020, 19, 1224–1229. [Google Scholar] [CrossRef]
- Obolda, A.; Li, W.; Abdulahat, M.; Ma, F.; Li, B.; Ai, X.; Zhang, M.; Li, F. High-efficiency deep-red organic radical crystals and OLEDs with solid-state fluorescence and excellent photostability. Org. Electron. 2022, 107, 106564. [Google Scholar] [CrossRef]
- Lu, C.; Cho, E.; Wan, K.; Wu, C.; Gao, Y.; Coropceanu, V.; Brédas, J.; Li, F. Achieving Nearly 100% Photoluminescence Quantum Efficiency in Organic Radical Emitters by Fine-Tuning the Effective Donor-Acceptor Distance. Adv. Funct. Mater. 2024, 34, 2314811. [Google Scholar] [CrossRef]
- Lu, C.; Cho, E.; Cui, Z.; Gao, Y.; Cao, W.; Brédas, J.; Coropceanu, V.; Li, F. Towards Efficient and Stable Donor-Acceptor Luminescent Radicals. Adv. Mater. 2022, 35, e2208190. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and Exciplexes of Conjugated Polymers. Science 1994, 265, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Langenegger, S.M.; Häner, R. Control of aggregation-induced emission by DNA hybridization. Chem. Commun. 2013, 49, 5835–5837. [Google Scholar] [CrossRef]
- Singh, A.; Lim, C.-K.; Lee, Y.-D.; Maeng, J.-H.; Lee, S.; Koh, J.; Kim, S. Tuning Solid-State Fluorescence to the Near-Infrared: A Combinatorial Approach to Discovering Molecular Nanoprobes for Biomedical Imaging. ACS Appl. Mater. Interfaces 2013, 5, 8881–8888. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Jia, X.; Xue, P.; Jia, J.; Lu, R. Synthesis, Mechanochromism and Acid Response of the Fluorescence Dyes Based on Quinoxalines Modified with Tetraphenylethylenes. Acta Chim. Sin. 2016, 74, 165–171. [Google Scholar] [CrossRef]
- Aldred, M.P.; Li, C.; Zhang, G.-F.; Gong, W.-L.; Li, A.D.; Dai, Y.; Ma, D.; Zhu, M.-Q. Fluorescence quenching and en-hancement of vitrifiable oligofluorenes end-capped with tetraphenylethene. J. Mater. Chem. 2012, 22, 7515–7528. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef]
- Birks, J.B. Organic Molecular Photophysics; Wiely: London, UK, 1973. [Google Scholar]
- Tanushi, A.; Kimura, S.; Kusamoto, T.; Tominaga, M.; Kitagawa, Y.; Nakano, M.; Nishihara, H. NIR Emission and Acid-Induced Intramolecular Electron Transfer Derived from a SOMO–HOMO Converted Non-Aufbau Electronic Structure. J. Phys. Chem. C 2019, 123, 4417–4423. [Google Scholar] [CrossRef]
- Chen, Z.; Li, F. Proton-induced Conversion from Non-Aufbau to Aufbau Electronic Structure of an Organic Radical with Turn-on Fluorescence. Chem. Res. Chin. Univ. 2022, 38, 798–802. [Google Scholar] [CrossRef]
- Obolda, A.; Li, W.; Ding, Z.; Ma, F.; Abdulahat, M.; Hu, Z.; Wang, J. Synthesis and photophysical properties of me-ta-position-substituted triphenylmethyl-type radicals with non-conjugated donor/accepters. Dye. Pigment. 2024, 226, 112116. [Google Scholar] [CrossRef]
- Wen, L.; Zang, C.; Gao, Y.; Tao, Y.; Li, G.; Shan, G.; Sun, H.; Xie, W.; Su, Z. Engineering of aggregation-induced emission luminogens by isomeric strategy to achieve high-performance optoelectronic device. Dye. Pigment. 2020, 173, 107912. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09 Rev. A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]





| Radical | Doping Ratio (wt%) | λe a (nm) | τ b (ns) | ФF c (%) | kr d (×107 s−1) | knr e (×108 s−1) | knr/kr |
|---|---|---|---|---|---|---|---|
| 1 | 656 | 7.82 | 4.9 | 0.626 | 1.22 | 19.5 | |
| TTM-1TPE-2Cz | 5 | 655 | 5.65 | 5.0 | 0.885 | 1.68 | 19.0 |
| 10 | 655 | 2.36 | 1.3 | 0.551 | 4.20 | 76.2 | |
| 1 | 655 | 7.34 | 3.6 | 0.490 | 1.32 | 26.7 | |
| TTM-2TPE-2Cz | 5 | 656 | 2.01 | 2.8 | 1.39 | 4.84 | 34.8 |
| 10 | 656 | 0.96 | 0.7 | 0.729 | 10.3 | 141.1 |
| Radical | λOnset/ nm | HOMO Cal a/CV b (eV) | SOMO Cal a/CV b (eV) | SUMO Cal a/Eg c (eV) | LUMO Cal a/CV b (eV) | ΔE Cal a/Eg d (eV) |
|---|---|---|---|---|---|---|
| TTM-1TPE-2Cz | 645 | −5.30/−5.26 | −5.40/- | −3.33/−3.34 | −1.44/- | 2.07/1.92 |
| TTM-2TPE-2Cz | 658 | −5.28/−5.20 | −5.29/- | −3.26/−3.32 | −1.40/- | 2.03/1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parida, H.; Ma, F.; Liu, Z.; Ding, Z.; Ablikim, O. Synthesis and Photophysical Properties of AIE-Type Carbazole-Capped Triphenylmethyl Organic Radicals Featuring Non-Aufbau Electronic Structure and Enhanced Photostability. Molecules 2025, 30, 1344. https://doi.org/10.3390/molecules30061344
Parida H, Ma F, Liu Z, Ding Z, Ablikim O. Synthesis and Photophysical Properties of AIE-Type Carbazole-Capped Triphenylmethyl Organic Radicals Featuring Non-Aufbau Electronic Structure and Enhanced Photostability. Molecules. 2025; 30(6):1344. https://doi.org/10.3390/molecules30061344
Chicago/Turabian StyleParida, Hazretomar, Fudong Ma, Zunqi Liu, Zhaoze Ding, and Obolda Ablikim. 2025. "Synthesis and Photophysical Properties of AIE-Type Carbazole-Capped Triphenylmethyl Organic Radicals Featuring Non-Aufbau Electronic Structure and Enhanced Photostability" Molecules 30, no. 6: 1344. https://doi.org/10.3390/molecules30061344
APA StyleParida, H., Ma, F., Liu, Z., Ding, Z., & Ablikim, O. (2025). Synthesis and Photophysical Properties of AIE-Type Carbazole-Capped Triphenylmethyl Organic Radicals Featuring Non-Aufbau Electronic Structure and Enhanced Photostability. Molecules, 30(6), 1344. https://doi.org/10.3390/molecules30061344






