Visible-Light-Mediated Synthesis of Anomeric S-Aryl Glycosides via Electron Donor–Acceptor Complex Using Thianthrenium Salts
Abstract
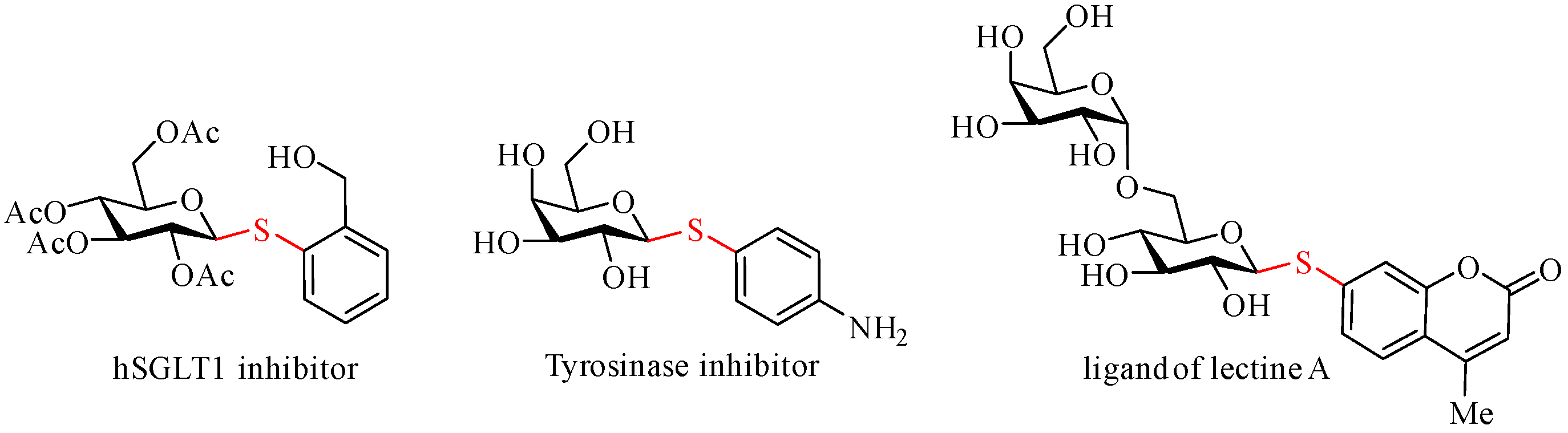
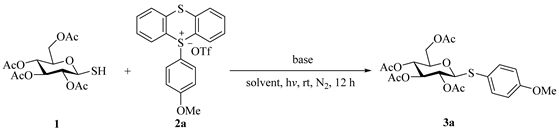
1. Introduction
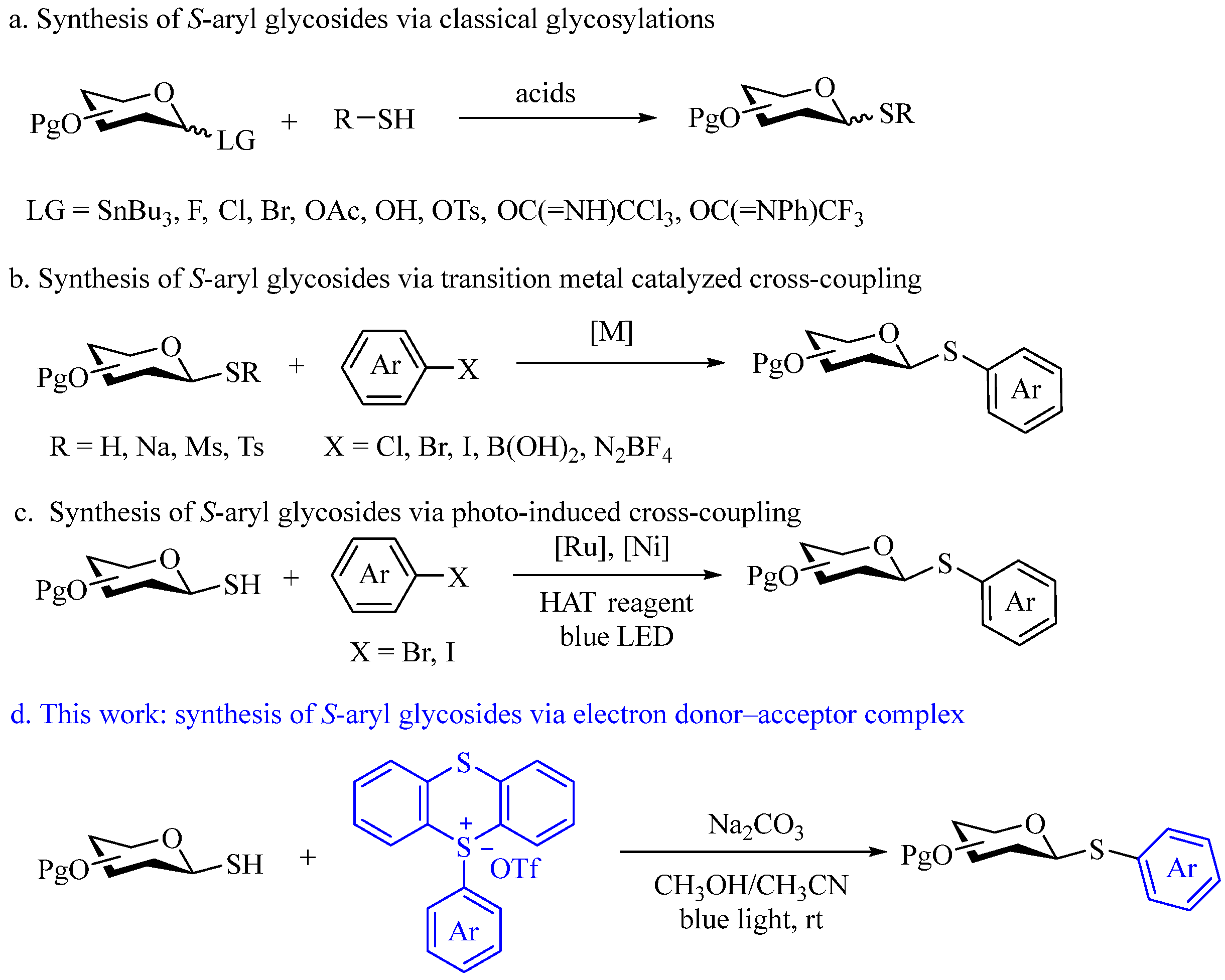
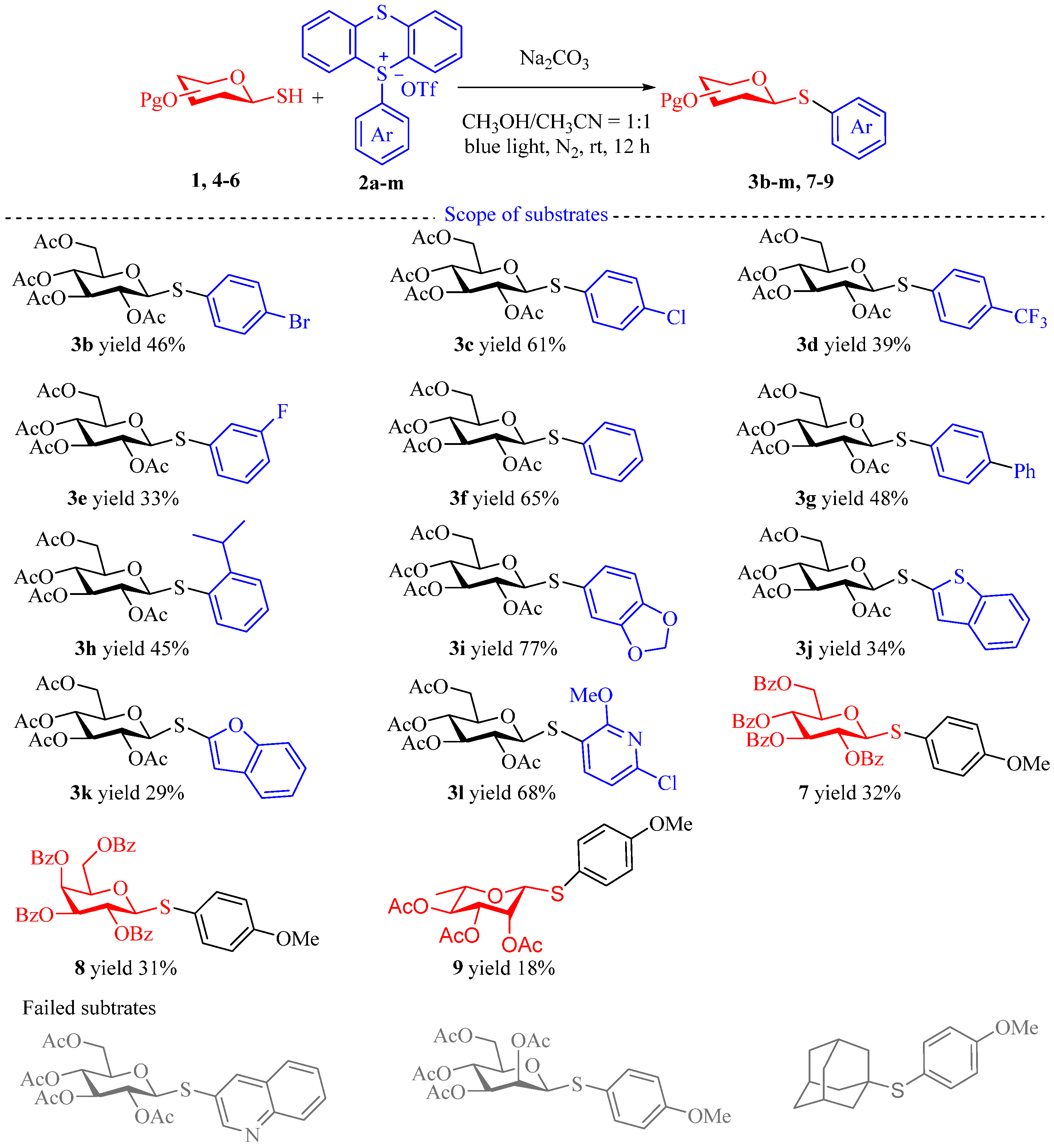
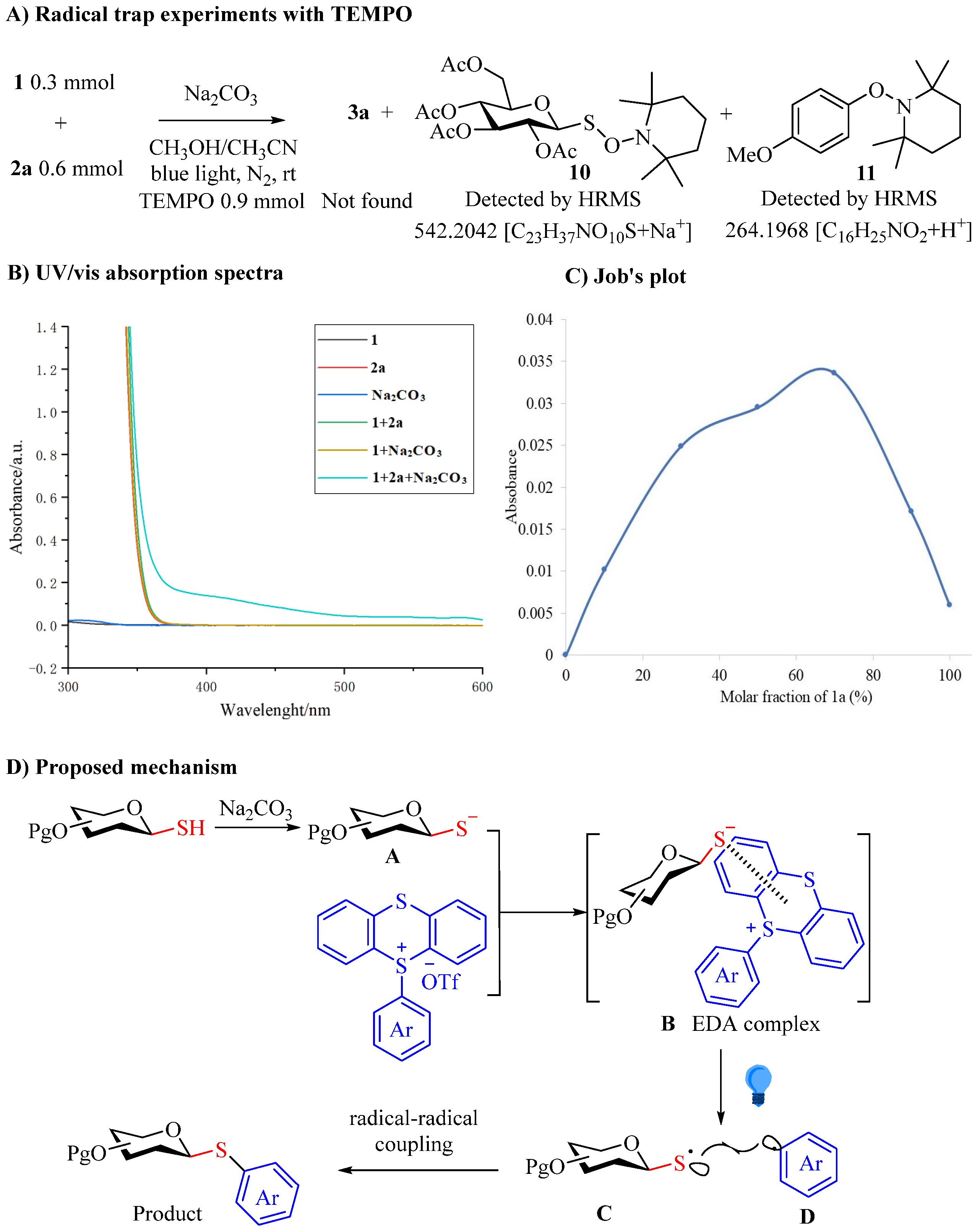
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General Procedure
3.3. Experimental Procedures and Characterization Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.M.; Elliott, T.; Cresswell, P.; Wilson, I.A.; Dwek, R.A. Glycosylation and the Immune System. Science 2001, 291, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Dwek, R.A. Glycobiology: Toward Understanding the Function of Sugars. Chem. Rev. 1996, 96, 683–720. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef]
- Amso, Z.; Bisset, S.W.; Yang, S.-H.; Harris, P.W.R.; Wright, T.H.; Navo, C.D.; Patchett, M.L.; Norris, G.E.; Brimble, M.A. Total chemical synthesis of glycocin F and analogues: S-glycosylation confers improved antimicrobial activity. Chem. Sci. 2018, 9, 1686–1691. [Google Scholar] [CrossRef]
- Lian, G.; Zhang, X.; Yu, B. Thioglycosides in Carbohydrate Research. Carbohydr. Res. 2015, 403, 13–22. [Google Scholar] [CrossRef]
- Witczak, Z.J. Thio sugars: Biological relevance as potential new therapeutics. Curr. Med. Chem. 1999, 6, 165–178. [Google Scholar] [CrossRef]
- Driguez, H. Thiooligosaccharides in glycobiology. In Glycoscience Synthesis of Substrate Analogs and Mimetics; Driguez, H., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 85–116. [Google Scholar]
- Osborn, H.M. Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance. Edited by Alexei V. Demchenko. ChemBioChem 2008, 9, 1671. [Google Scholar] [CrossRef]
- Codée, J.D.C.; Litjens, R.E.J.N.; van den Bos, L.J.; Overkleeft, H.S.; van der Marel, G.A. Thioglycosides in sequential glycosylation strategies. Chem. Soc. Rev. 2005, 34, 769–782. [Google Scholar] [CrossRef]
- Castaneda, F.; Burse, A.; Boland, W.; Kinne, R.K.H. Thioglycosides as inhibitors of hSGLT1 and hSGLT2: Potential therapeutic agents for the control of hyperglycemia in diabetes. Int. J. Med. Sci. 2007, 4, 131–139. [Google Scholar] [CrossRef]
- Rodrigue, J.; Ganne, G.; Blanchard, B.; Saucier, C.; Giguère, D.; Shiao, T.C.; Varrot, A.; Imberty, A.; Roy, R. Aromatic thioglycoside inhibitors against the virulence factor LecA from Pseudomonas aeruginosa. Org. Biomol. Chem. 2013, 11, 6906–6918. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.-Q.; Zhang, X.; Zou, Y.; Shi, R.; Cao, J.-G.; Xu, S.-Y.; Deng, L.-F.; Zhou, L.; Gong, Y.; Shu, X.; et al. Nonenzymatic Stereoselective S-Glycosylation of Polypeptides and Proteins. J. Am. Chem. Soc. 2021, 143, 11919–11926. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Zhang, L.; Jiao, R.; Zhang, S.; Li, B.; Zhang, X. Chemical and enzymatic synthesis of S-linked sugars and glycoconjugates. Tetrahedron 2021, 81, 131920. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, Y.; Gao, F.; Bi, F.; Wang, W. Direct, stereoselective thioglycosylation enabled by an organophotoredox radical strategy. Chem. Sci. 2020, 11, 13079–13084. [Google Scholar] [CrossRef]
- Zhu, F.; Miller, E.; Zhang, S.-Q.; Yi, D.; O’Neill, S.; Hong, X.; Walczak, M.A. Stereoretentive C(sp3)–S Cross-Coupling. J. Am. Chem. Soc. 2018, 140, 18140–18150. [Google Scholar] [CrossRef]
- Baryal, K.N.; Zhu, D.; Li, X.; Zhu, J. Umpolung reactivity in the stereoselective synthesis of S-linked 2-deoxyglycosides. Angew. Chem. Int. Ed. 2013, 52, 8012–8016. [Google Scholar] [CrossRef]
- Thayer, D.A.; Yu, H.N.; Galan, M.C.; Wong, C.-H. A General Strategy toward S-Linked Glycopeptides. Angew. Chem. Int. Ed. 2005, 44, 4596–4599. [Google Scholar] [CrossRef]
- Zhu, X.; Schmidt, R.R. Synthesis of α-S-linked glycopeptides in water containing solution. Tetrahedron Lett. 2003, 44, 6063–6067. [Google Scholar] [CrossRef]
- Ibrahim, N.; Alami, M.; Messaoudi, S. Recent Advances in Transition-Metal-Catalyzed Functionalization of 1-Thiosugars. Asian J. Org. Chem. 2018, 7, 2026–2038. [Google Scholar] [CrossRef]
- Montoir, D.; Amoura, M.; Ababsa, Z.E.A.; Vishwanatha, T.M.; Yen-Pon, E.; Robert, V.; Beltramo, M.; Piller, V.; Alami, M.; Aucagne, V.; et al. Synthesis of aryl-thioglycopeptides through chemoselective Pd-mediated conjugation. Chem. Sci. 2018, 9, 8753–8759. [Google Scholar] [CrossRef]
- Brachet, E.; Brion, J.-D.; Alami, M.; Messaoudi, S. Nickel-Catalyzed Arylation, Alkenylation, and Alkynylation of Unprotected Thioglycosides at Room Temperature. Chem. Eur. J. 2013, 19, 15276–15280. [Google Scholar] [CrossRef] [PubMed]
- Brachet, E.; Brion, J.-D.; Messaoudi, S.; Alami, M. Palladium-Catalyzed Cross-Coupling Reaction of Thioglycosides with (Hetero)aryl Halides. Adv. Synth. Catal. 2013, 355, 477–490. [Google Scholar] [CrossRef]
- Bruneau, A.; Roche, M.; Hamze, A.; Brion, J.-D.; Alami, M.; Messaoudi, S. Stereoretentive Palladium-Catalyzed Arylation, Alkenylation, and Alkynylation of 1-Thiosugars and Thiols Using Aminobiphenyl Palladacycle Precatalyst at Room Temperature. Chem. Eur. J. 2015, 21, 8375–8379. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Kou, Y.; Yu, L.; Zhang, Z.-X.; Xue, W. 2′-Cyanoethyl thioglycosides: Effective nucleophiles for synthesis of (hetero)aryl thioglycosides under the catalysis of Cu. Org. Chem. Front. 2015, 2, 1604–1607. [Google Scholar] [CrossRef]
- Chabrier, A.; Bruneau, A.; Benmahdjoub, S.; Benmerad, B.; Belaid, S.; Brion, J.-D.; Alami, M.; Messaoudi, S. Stereoretentive Copper-Catalyzed Directed Thioglycosylation of C(sp2)−H Bonds of Benzamides. Chem. Eur. J. 2016, 22, 15006–15010. [Google Scholar] [CrossRef]
- Benmahdjoub, S.; Ibrahim, N.; Benmerad, B.; Alami, M.; Messaoudi, S. One-Pot Assembly of Unsymmetrical Biaryl Thioglycosides through Chemoselective Palladium-Catalyzed Three-Component Tandem Reaction. Org. Lett. 2018, 20, 4067–4071. [Google Scholar] [CrossRef]
- Bennai, N.; Ibrahim, N.; Marrot, J.; Belkadi, M.; Alami, M.; Magnier, E.; Anselmi, E.; Messaoudi, S. Synthesis of S-Trifluoromethyl S-Arylsulfoximine Thioglycosides through Pd-Catalyzed Migita Cross-Coupling. Eur. J. Org. Chem. 2020, 2020, 4972–4981. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, J.; Wang, N.; Huang, N.; Yao, H. Stereoselective Synthesis of β-S-Glycosides via Palladium Catalysis. J. Org. Chem. 2024, 89, 8815–8827. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, W.; Xie, L.; Liu, W.; Xu, C.; Chen, F.-E. A Robust Copper-Catalyzed Cross-Coupling of Glycosyl Thiosulfonate and Boronic Acids Enables the Construction of Thioglycosides. Org. Lett. 2023, 25, 5714–5718. [Google Scholar] [CrossRef]
- Venkatesh, R.; Tiwari, V.; Kandasamy, J. Copper(I)-Catalyzed Sandmeyer-Type S-Arylation of 1-Thiosugars with Aryldiazonium Salts under Mild Conditions. J. Org. Chem. 2022, 87, 11414–11432. [Google Scholar] [CrossRef]
- Nauš, P.; Lešetický, L.; Smrček, S.; Tišlerová, I.; Štícha, M. Copper-Assisted Arylation of 1-Thiosugars: Efficient Route to Triazene Substituted Arylthioglycosides. Synlett 2003, 2003, 2117–2122. [Google Scholar] [CrossRef]
- Fang, Y.; Liang, Q.; Shi, L.; Wen, J.; Liu, X.; Hong, X.; Zha, X.; Ji, F. Site-Selective S-Arylation of 1-Thiosugars with Aryl Thianthrenium Salts through Copper(I)-Mediated, Photoredox- Catalyzed Reactions. Adv. Synth. Catal. 2024, 366, 2344–2351. [Google Scholar] [CrossRef]
- Zhu, M.; Dagousset, G.; Alami, M.; Magnier, E.; Messaoudi, S. Ni/Photoredox-Dual-Catalyzed Functionalization of 1-Thiosugars. Org. Lett. 2019, 21, 5132–5137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Alami, M.; Messaoudi, S. Electrochemical nickel-catalyzed Migita cross-coupling of 1-thiosugars with aryl, alkenyl and alkynyl bromides. Chem. Commun. 2020, 56, 4464–4467. [Google Scholar] [CrossRef]
- Wang, R.-Q.; Jiang, Q.-H.; Wang, H.-X.; Zhang, X.-W.; Yan, N. Electrochemically Mediated S-Glycosylation of 1-Thiosugars with Xanthene Derivatives. Org. Lett. 2023, 25, 4252–4257. [Google Scholar] [CrossRef]
- Li, F.; Liu, H.; Xing, W.; Zhang, Q.-J.; Wang, L. Electrochemical Nickel-Catalyzed Cross-Coupling of Glycosyl Thiols with Preactivated Phenols and Ketones. Org. Biomol. Chem. 2024, 22, 3597–3601. [Google Scholar] [CrossRef]
- Zhu, M.; Ghouilem, J.; Messaoudi, S. Visible-Light-Mediated Stadler–Ziegler Arylation of Thiosugars with Anilines. ACS Org. Inorg. Au 2022, 2, 351–358. [Google Scholar] [CrossRef]
- Saxena, B.; Patel, R.I.; Sharma, A. Recent Advances in Electron Donor-Acceptor (EDA)-Complex Reactions involving Quaternary Pyridinium Derivatives. Adv. Synth. Catal. 2023, 365, 1538–1564. [Google Scholar] [CrossRef]
- van Dalsen, L.; Brown, R.E.; Rossi-Ashton, J.A.; Procter, D.J. Sulfonium Salts as Acceptors in Electron Donor-Acceptor Complexes. Angew. Chem. Int. Ed. 2023, 62, e202303104. [Google Scholar] [CrossRef]
- Volkov, A.A.; Bugaenko, D.I.; Karchava, A.V. Transition Metal and Photocatalyst Free Arylation via Photoexcitable Electron Donor Acceptor Complexes:Mediation and Catalysis. ChemCatChem 2024, 16, e202301526. [Google Scholar] [CrossRef]
- Tasnim, T.; Ayodele, M.J.; Pitre, S.P. Recent Advances in Employing Catalytic Donors and Acceptors in Electron Donor–Acceptor Complex Photochemistry. J. Org. Chem. 2022, 87, 10555–10563. [Google Scholar] [CrossRef] [PubMed]
- Crisenza, G.E.M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor–Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476. [Google Scholar] [CrossRef] [PubMed]
- Mulliken, R.S. Molecular Compounds and their Spectra. III. The Interaction of Electron Donors and Acceptors. J. Org. Chem. 1952, 56, 801–822. [Google Scholar] [CrossRef]
- van der Zee, L.J.C.; Pahar, S.; Richards, E.; Melen, R.L.; Slootweg, J.C. Insights into Single-Electron-Transfer Processes in Frustrated Lewis Pair Chemistry and Related Donor–Acceptor Systems in Main Group Chemistry. Chem. Rev. 2023, 123, 9653–9675. [Google Scholar] [CrossRef]
- Rosokha, S.V.; Kochi, J.K. Fresh Look at Electron-Transfer Mechanisms via the Donor/Acceptor Bindings in the Critical Encounter Complex. Acc. Chem. Res. 2008, 41, 641–653. [Google Scholar] [CrossRef]
- Lima, C.G.S.; Lima, T.d.M.; Duarte, M.; Jurberg, I.D.; Paixão, M.W. Organic Synthesis Enabled by Light-Irradiation of EDA Complexes: Theoretical Background and Synthetic Applications. ACS Catal. 2016, 6, 1389–1407. [Google Scholar] [CrossRef]
- Cabrera-Afonso, M.J.; Granados, A.; Molander, G.A. Sustainable Thioetherification via Electron Donor–Acceptor Photoactivation Using Thianthrenium Salts. Angew Chem. Int. Ed. 2022, 61, e202202706. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Y.-X.; Liang, P.-P.; Hu, G.-Q.; Liu, J.-H. S-Arylation of Thioic S-Acid Using Thianthrenium Salts via Photoactivation of Electron Donor–Acceptor Complex. J. Org. Chem. 2024, 89, 12508–12513. [Google Scholar] [CrossRef]
- Shen, J.; Li, J.; Chen, M.; Yue, X.; Shi, X. Photoinduced Radical Desulfurative C(sp3)–C(sp2) Coupling via Electron Donor–Acceptor Complexes. Org. Lett. 2024, 26, 1495–1500. [Google Scholar] [CrossRef]
- Yang, F.; He, G.-C.; Sun, S.-H.; Song, T.-T.; Min, X.-T.; Ji, D.-W.; Guo, S.-Y.; Chen, Q.-A. Selective C–S Bond Constructions Using Inorganic Sulfurs via Photoinduced Electron Donor–Acceptor Activation. J. Org. Chem. 2022, 87, 14241–14249. [Google Scholar] [CrossRef]
- De Kreijger, S.; Troian-Gautier, L. Sulfonylation enabled through the photoactivation of EDA complexes. Chem. Catal. 2022, 2, 653–656. [Google Scholar] [CrossRef]
- Uchikura, T.; Hara, Y.; Tsubono, K.; Akiyama, T. Visible-Light-Driven C–S Bond Formation Based on Electron Donor–Acceptor Excitation and Hydrogen Atom Transfer Combined System. ACS Org. Inorg. Au 2021, 1, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Chiba, S. Leveraging of Sulfur Anions in Photoinduced Molecular Transformations. JACS Au 2021, 1, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-P.; Nie, F.-Y.; Song, Q.-H. Visible-Light-Mediated Alkylation of Thiophenols via Electron Donor–Acceptor Complexes Formed between Two Reactants. J. Org. Chem. 2021, 86, 12419–12426. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lim, C.-H.; Miyake, G.M. Visible-Light-Promoted C–S Cross-Coupling via Intermolecular Charge Transfer. J. Am. Chem. Soc. 2017, 139, 13616–13619. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, B.; Cao, Y.; Liu, Y.; Wang, Z.; Wang, Q. Visible-Light-Driven Synthesis of Aryl Xanthates and Aryl Dithiocarbamates via an Electron Donor–Acceptor Complex. Org. Lett. 2022, 24, 8895–8900. [Google Scholar] [CrossRef]
- Le, Z.; He, S.; Hou, J.; Ye, M.; Chen, J.; Lv, G.; Huang, T.; Yang, Z.; Wu, Y. Visible-light-mediated synthesis of non-anomeric S-aryl glycosides via a photoactive electron-donor–acceptor complex. Chem. Commun. 2023, 59, 13759–13762. [Google Scholar]
- Hou, J.-Y.; Zhang, L.; He, S.-Y.; Ye, M.-L.; Chen, J.; Huang, T.-L.; Lv, G.-H.; Hai, L.; Yang, Z.-Z.; Wu, Y. Visible-light-induced electron donor–acceptor (EDA) complex-initiated synthesis of non-anomeric S-aryl glycosides. Org. Chem. Front. 2023, 10, 6200–6204. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, M.; Wang, L.; Hu, K.; Liu, D.; Liu, H.; Sun, J.-S.; Codée, J.D.C.; Zhang, Q. Regio- and Stereoselective Organocatalyzed Relay Glycosylations To Synthesize 2-Amino-2-deoxy-1,3-dithioglycosides. Org. Lett. 2023, 25, 3611–3617. [Google Scholar] [CrossRef]
- Wan, Y.; Deng, L.; Wang, L.; Tu, Y.; Liu, H.; Sun, J.-s.; Zhang, Q. Synthesis of 2-Amino-2-deoxy-1,3-dithioidoglycosides via Organocatalytic relay Glycosylation of 3-O-acetyl-2-Nitrogalactals. Chin. J. Chem. 2023, 41, 2837–2842. [Google Scholar] [CrossRef]
- Wu, X.; Gao, P.; Chen, F. Synthetic Applications of Sulfonium Salts as Aryl Radical Precursors. Eur. J. Org. Chem. 2023, 26, e202300864. [Google Scholar] [CrossRef]
- Cai, Y.; Chatterjee, S.; Ritter, T. Photoinduced Copper-Catalyzed Late-Stage Azidoarylation of Alkenes via Arylthianthrenium Salts. J. Am. Chem. Soc. 2023, 145, 13542–13548. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.M.; Karl, T.; Berger, F.; Torkowski, L.; Ritter, T. Late-Stage Heteroarylation of Hetero(aryl)sulfonium Salts Activated by α-Amino Alkyl Radicals. Angew Chem. Int. Ed. 2021, 60, 13609–13613. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Sang, R.; Ham, W.-S.; Plutschack, M.B.; Berger, F.; Chabbra, S.; Schnegg, A.; Genicot, C.; Ritter, T. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 2020, 12, 56–62. [Google Scholar] [CrossRef]
- Ye, F.; Berger, F.; Jia, H.; Ford, J.; Wortman, A.; Börgel, J.; Genicot, C.; Ritter, T. Aryl Sulfonium Salts for Site-Selective Late-Stage Trifluoromethylation. Angew Chem. Int. Ed. 2019, 58, 14615–14619. [Google Scholar] [CrossRef]
- Sang, R.; Korkis, S.E.; Su, W.; Ye, F.; Engl, P.S.; Berger, F.; Ritter, T. Site-Selective C−H Oxygenation via Aryl Sulfonium Salts. Angew Chem. Int. Ed. 2019, 58, 16161–16166. [Google Scholar] [CrossRef]
- Engl, P.S.; Häring, A.P.; Berger, F.; Berger, G.; Pérez-Bitrián, A.; Ritter, T. C–N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts. J. Am. Chem. Soc. 2019, 141, 13346–13351. [Google Scholar] [CrossRef]
- Berger, F.; Plutschack, M.B.; Riegger, J.; Yu, W.; Speicher, S.; Ho, M.; Frank, N.; Ritter, T. Site-selective and versatile aromatic C−H functionalization by thianthrenation. Nature 2019, 567, 223–228. [Google Scholar] [CrossRef]
- Meng, H.; Liu, M.-S.; Shu, W. Organothianthrenium salts: Synthesis and utilization. Chem. Sci. 2022, 13, 13690–13707. [Google Scholar] [CrossRef]
- Zhao, D.; Petzold, R.; Yan, J.; Muri, D.; Ritter, T. Tritiation of aryl thianthrenium salts with a molecular palladium catalyst. Nature 2021, 600, 444–449. [Google Scholar] [CrossRef]

| Entry | Aryl Source | hv/nm | Solvent | Base | Yield % b |
|---|---|---|---|---|---|
| 1 | 2a (2 eq) | 365 | DMSO | Et3N (2 eq) | 16 |
| 2 | 2a (2 eq) | 395 | DMSO | Et3N (2 eq) | 32 |
| 3 | 2a (2 eq) | 425 | DMSO | Et3N (2 eq) | 31 |
| 4 | 2a (2 eq) | 455 | DMSO | Et3N (2 eq) | 10 |
| 5 | 2a (2 eq) | 395 | DCM | Et3N (2 eq) | 6 |
| 6 | 2a (2 eq) | 395 | THF | Et3N (2 eq) | 4 |
| 7 | 2a (2 eq) | 395 | CH3CN | Et3N (2 eq) | 25 |
| 8 | 2a (2 eq) | 395 | CH3OH | Et3N (2 eq) | 24 |
| 9 | 2a (2 eq) | 395 | DMA | Et3N (2 eq) | 24 |
| 10 | 2a (2 eq) | 395 | DMF | Et3N (2 eq) | 9 |
| 11 | 2a (2 eq) | 395 | Toluene | Et3N (2 eq) | - |
| 12 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Et3N (2 eq) | 31 |
| 13 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | DIPEA (2 eq) | 21 |
| 14 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Pyridine (2 eq) | 25 |
| 15 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | DMAP (2 eq) | 26 |
| 16 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | K3PO4 (2 eq) | trace |
| 17 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Li2CO3 (2 eq) | 28 |
| 18 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (2 eq) | 38 |
| 19 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | K2CO3 (2 eq) | 30 |
| 20 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Cs2CO3 (2 eq) | - |
| 21 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (1 eq) | 26 |
| 22 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (3 eq) | 50 |
| 23 | 2a (2 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (5 eq) | 48 |
| 24 | 2a (3 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (3 eq) | 73 |
| 25 c | 2a (3 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (3 eq) | 55 |
| 26 d | 2a (3 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (3 eq) | 49 |
| 27 e | 2a (3 eq) | 395 | CH3CN:CH3OH = 1:1 | Na2CO3 (3 eq) | 23 |
| 28 | 2a (3 eq) | 395 | CH3CN:CH3OH = 1:1 | - | 20 |
| 29 | 2a (3 eq) | - | CH3CN:CH3OH = 1:1 | Na2CO3 (3 eq) | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhang, Y.; Yu, Z.; Liu, Y.; Wang, Z.; Zhang, Q.; Wang, L. Visible-Light-Mediated Synthesis of Anomeric S-Aryl Glycosides via Electron Donor–Acceptor Complex Using Thianthrenium Salts. Molecules 2025, 30, 1315. https://doi.org/10.3390/molecules30061315
Zhou Z, Zhang Y, Yu Z, Liu Y, Wang Z, Zhang Q, Wang L. Visible-Light-Mediated Synthesis of Anomeric S-Aryl Glycosides via Electron Donor–Acceptor Complex Using Thianthrenium Salts. Molecules. 2025; 30(6):1315. https://doi.org/10.3390/molecules30061315
Chicago/Turabian StyleZhou, Zhuoyi, Yufeng Zhang, Zhiqiang Yu, Yuping Liu, Zhen Wang, Qingju Zhang, and Liming Wang. 2025. "Visible-Light-Mediated Synthesis of Anomeric S-Aryl Glycosides via Electron Donor–Acceptor Complex Using Thianthrenium Salts" Molecules 30, no. 6: 1315. https://doi.org/10.3390/molecules30061315
APA StyleZhou, Z., Zhang, Y., Yu, Z., Liu, Y., Wang, Z., Zhang, Q., & Wang, L. (2025). Visible-Light-Mediated Synthesis of Anomeric S-Aryl Glycosides via Electron Donor–Acceptor Complex Using Thianthrenium Salts. Molecules, 30(6), 1315. https://doi.org/10.3390/molecules30061315






