Smart Probes for Ultrasensitive and Highly Selective Sensing of Homocysteine over Cysteine Based on Multi-Cooperative Effects by Using Gold Nanoparticles
Abstract
1. Introduction
2. Experimental Details
2.1. Materials and Apparatus
2.2. Synthesis of AuNPs
2.3. Detection of Hcy
2.4. Real Sample Preparation and Analysis
3. Results and Discussion
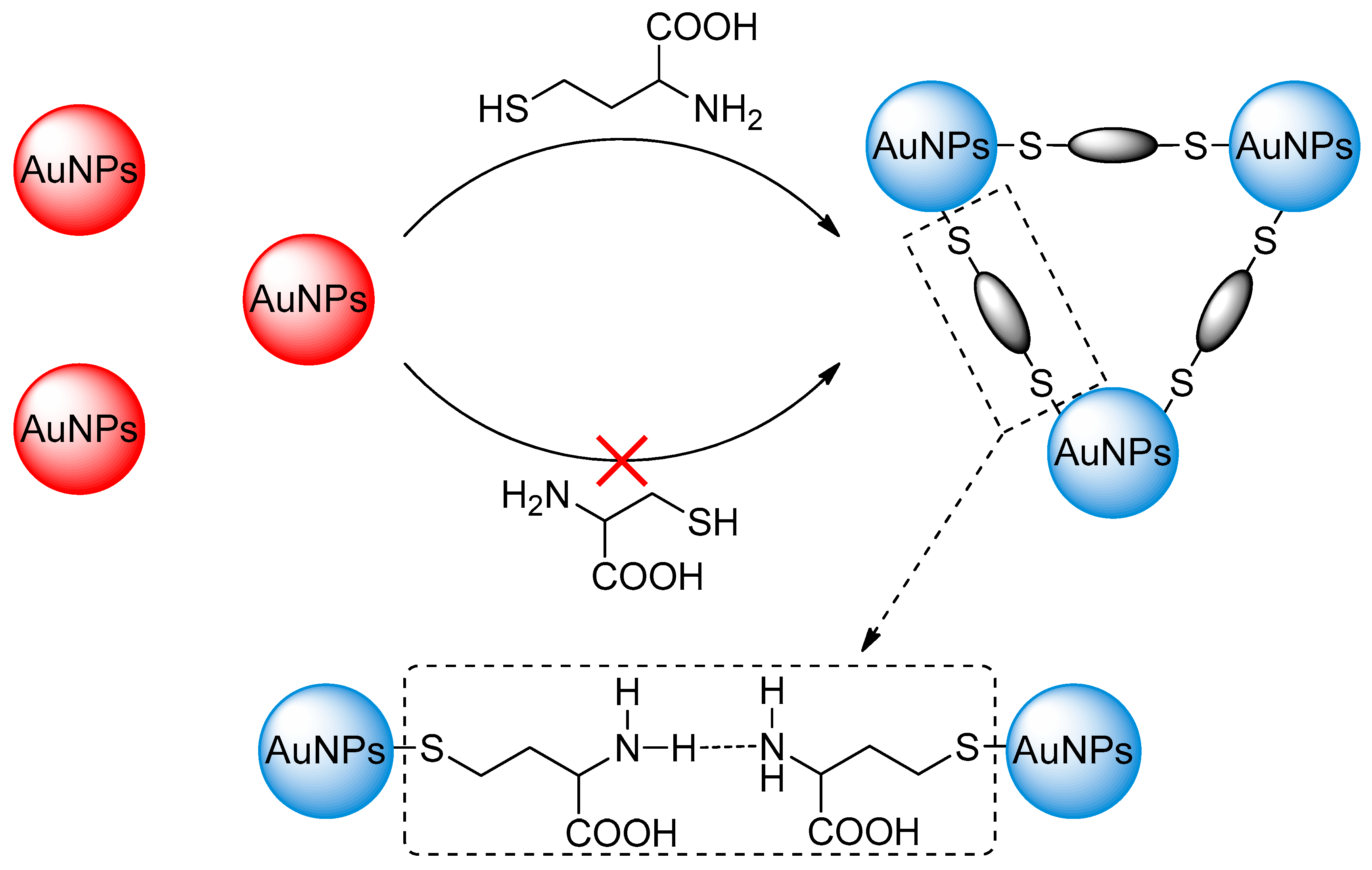
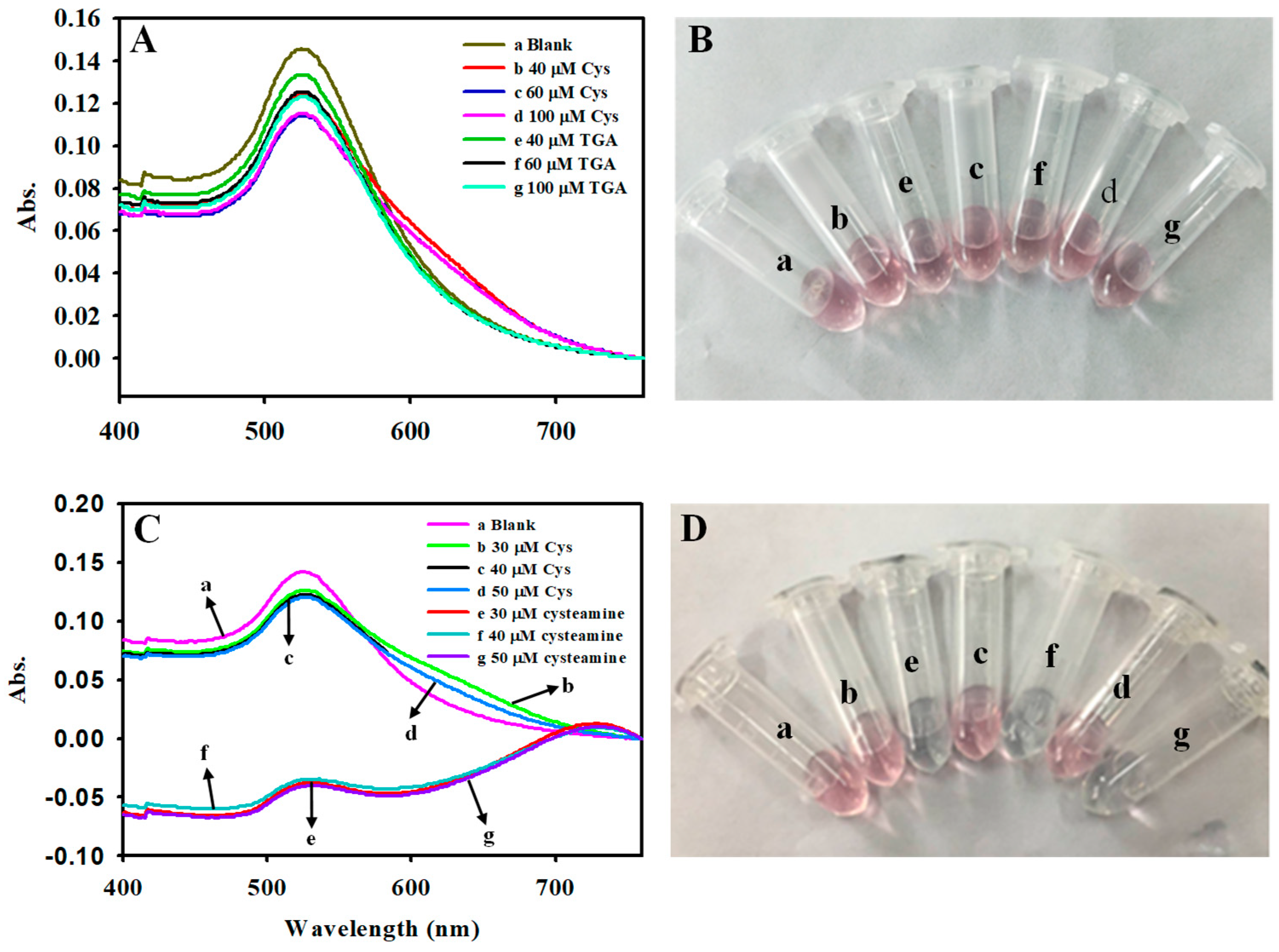
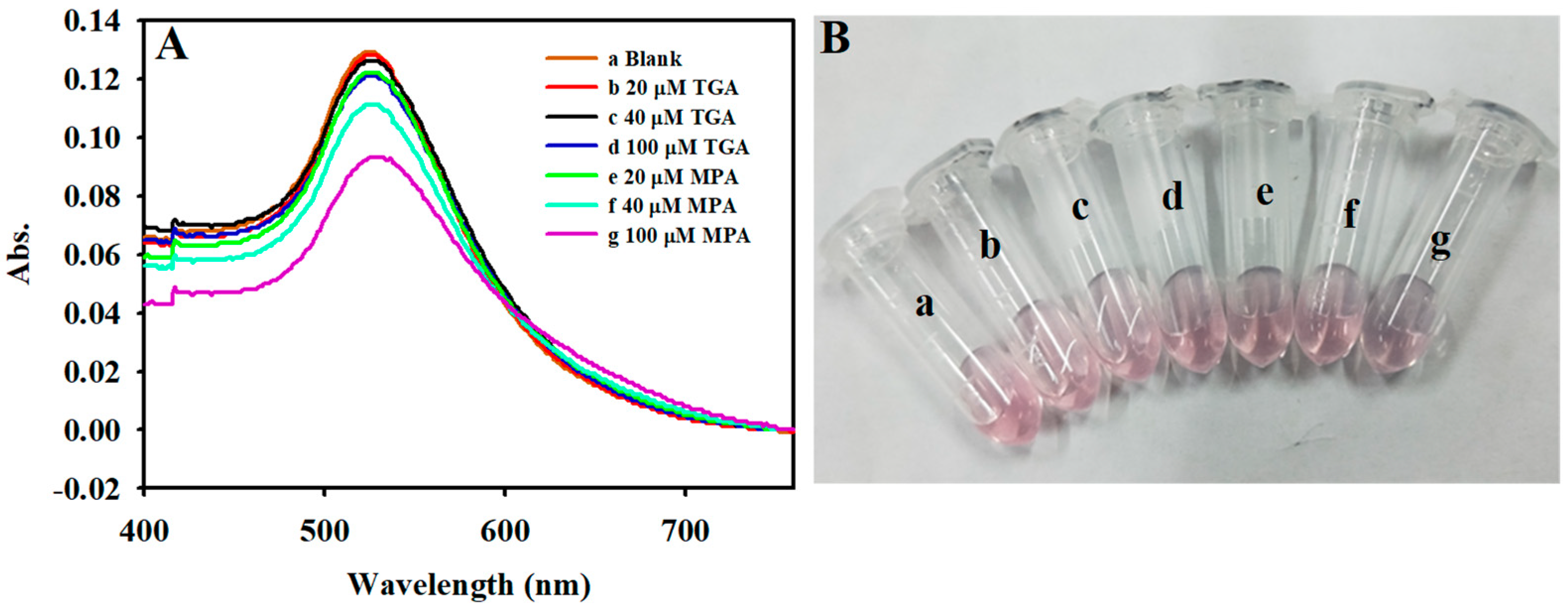
3.1. Choice of Materials
3.2. Characteristics of AuNPs
3.3. The Working Principle of the Proposed Method
3.4. Optimization for Hcy Detection
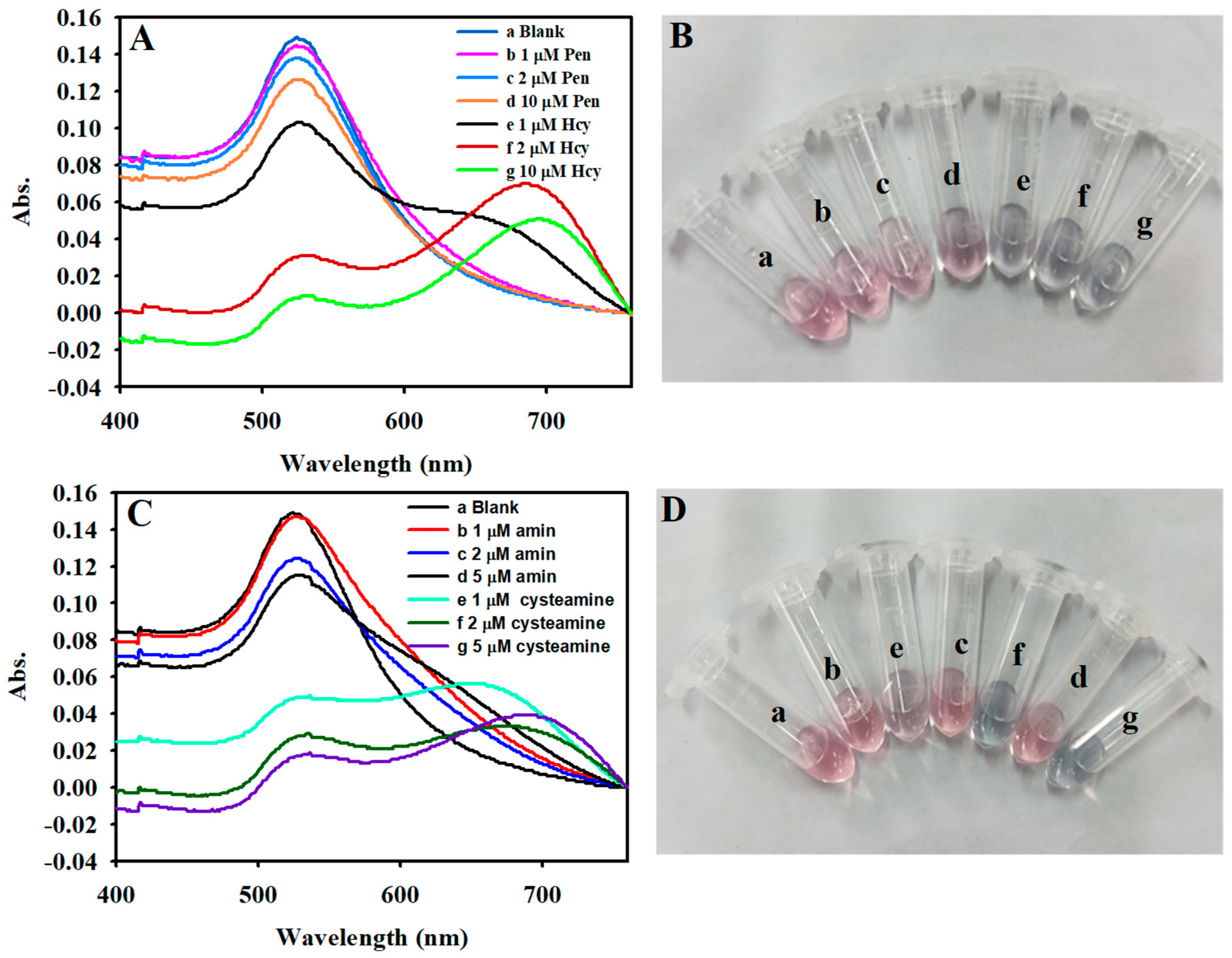
3.5. Sensitive Detection of Hcy
3.6. Specificity for the Detection of Hcy
3.7. Hcy Detection in Human Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Yu, B.; Liu, Z.; Li, J.; Ma, M.; Wang, Y.; Zhu, M.; Yin, H.; Wang, X.; Fu, Y. Homocysteine Directly Interacts and Activates the Angiotensin II Type I Receptor to Aggravate Vascular Injury. Nat. Commun. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, Y.; Wang, B.; Liu, F.; Li, N.; Zhang, D.; Liu, X. Efficient Electrochemical Detection of Homocysteine in Biological Samples Based on Au NPs Multi-Walled Carbon Nanotube Composites. J. Electrochem. Soc. 2023, 170, 127506. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, X.; Li, W.; Chen, W.; Wang, Y.; Li, X.; Ye, Y.; Song, X. Selective and Discriminative Fluorescence Sensing of Cys, Hcy, GSH and H2S with Concise and Distinct Signals. Sens. Actuators B Chem. 2021, 331, 129394. [Google Scholar] [CrossRef]
- Głowacki, R.; Stachniuk, J.; Borowczyk, K.; Jakubowski, H. Quantification of Homocysteine and Cysteine by Derivatization with Pyridoxal 5′-Phosphate and Hydrophilic Interaction Liquid Chromatography. Anal. Bioanal. Chem. 2016, 408, 1935–1941. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Q.; An, P.; Du, Y.; Zhao, J.; Song, A.; Huang, G. Relationship Between Folate, Vitamin B12, Homocysteine, Transaminase and Mild Cognitive Impairment in China: A Case-Control Study. Int. J. Food Sci. Nutr. 2020, 71, 315–324. [Google Scholar] [CrossRef]
- Liu, F.; Han, L.; Yang, Y.; Xue, Z.; Lu, X.; Liu, X. Designable Synthesis of A Novel Layered MXene Loaded Gold Nanocluster Composite for Efficient Electrochemical Sensing of Homocysteine in Biological Samples. Chem. Eng. J. 2023, 461, 141928. [Google Scholar] [CrossRef]
- Wang, W.; Rusin, O.; Xu, X.; Kim, K.K.; Escobedo, J.O.; Fakayode, S.O.; Fletcher, K.A.; Lowry, M.; Schowalter, C.M.; Lawrence, C.M. Detection of Homocysteine and Cysteine. J. Am. Chem. Soc. 2005, 127, 15949–15958. [Google Scholar] [CrossRef]
- Jiang, Y.; Mistretta, B.; Elsea, S.; Sun, Q. Simultaneous Determination of Plasma Total Homocysteine and Methionine by Liquid Chromatography-Tandem Mass Spectrometry. Clin. Chim. Acta 2017, 464, 93–97. [Google Scholar] [CrossRef]
- Lačná, J.; Foret, F.; Kubáň, P. Capillary Electrophoresis in the Analysis of Biologically Important Thiols. Electrophoresis 2017, 38, 203–222. [Google Scholar] [CrossRef]
- Yin, C.X.; Xiong, K.M.; Huo, F.J.; Salamanca, J.C.; Strongin, R.M. Fluorescent Probes with Multiple Binding Sites for the Discrimination of Cys, Hcy, and GSH. Angew. Chem. Int. Ed. 2017, 56, 13188–13198. [Google Scholar] [CrossRef]
- Pervez, W.; Yin, C.; Huo, F. Homocysteine Fluorescent Probes: Sensing Mechanisms and Biological Applications. Coord. Chem. Rev. 2025, 522, 216202. [Google Scholar] [CrossRef]
- Wang, W.; Ji, M.; Chen, J.; Wang, P. A Novel Turn-on Type AIE Fluorescent Probe for Highly Selective Detection of Cysteine/Homocysteine and Its Application in Living Cells. Talanta 2022, 239, 123091. [Google Scholar] [CrossRef]
- Chen, W.; Luo, H.; Liu, X.; Foley, J.W.; Song, X. Broadly Applicable Strategy for the Fluorescence Based Detection and Differentiation of Glutathione and Cysteine/Homocysteine: Demonstration in Vitro and in Vivo. Anal. Chem. 2016, 88, 3638–3646. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; He, S.; Zeng, X.; Zhang, J.; Gong, J. A New Lysosome-Targeted NIR Fluorescent Probe for Specific Detection of Cysteine over Homocysteine and Glutathione. Molecules 2023, 28, 6189. [Google Scholar] [CrossRef]
- Huang, W.; He, M.; Chen, S.; Yin, G.; Gan, Y.; Li, H.; Wu, C.; Yin, P. Dual-Channel Fluorescent Detection of Biothiols: A Novel Probe for Distinguishing Cysteine, Homocysteine, Glutathione, and N-Acetylcysteine in Cellular Environments. Spectrochim. Acta Part A 2025, 326, 125221. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, H.; He, W.; Wang, J.; Muddassir, M.; Liu, X.; Wang, Y. Eu/Tb-MOF as Fluorescence Sensors for the Detection Homocysteine in Human Serum Performance and Mechanistic Investigation. Talanta 2024, 280, 126715. [Google Scholar] [CrossRef]
- Guo, T.; Chen, X.; Qu, W.; Yang, B.; Tian, R.; Geng, Z.; Wang, Z. Red and Near-Infrared Fluorescent Probe for Distinguishing Cysteine and Homocysteine Through Single-Wavelength Excitation with Distinctly Dual Emissions. Anal. Chem. 2022, 94, 5006–5013. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Strongin, R.M. Conjugate Addition/Cyclization Sequence Enables Selective and Simultaneous Fluorescence Detection of Cysteine and Homocysteine. Angew. Chem. Int. Ed. 2011, 50, 10690. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.D.; Shao, X.; Zhao, J.; Su, Y.; Xi, D.; Yu, H.; Yue, S.; Xiao, L.j.; Zhao, W. A Turn-on NIR Fluorescent Probe for the Detection of Homocysteine over Cysteine. RSC. Adv. 2014, 4, 54080–54083. [Google Scholar] [CrossRef]
- Yin, G.X.; Niu, T.T.; Gan, Y.B.; Yu, T.; Yin, P.; Chen, H.M.; Zhang, Y.Y.; Li, H.T.; Yao, S.Z. A Multi-Signal Fluorescent Probe with Multiple Binding Sites for Simultaneous Sensing of Cysteine, Homocysteine, and Glutathione. Angew. Chem. 2018, 130, 5085–5088. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Wang, Y.; Zhao, L.; Zhao, T.; Chen, X. Europium-Decorated Graphene Quantum Dots As a Fluorescent Probe for Label-Free, Rapid and Sensitive Detection of Cu2+ and L-Cysteine. Anal. Chim. Acta 2015, 891, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Chang, C.W.; Tseng, W.L. Fluorescent Sensing of Homocysteine in Urine: Using Fluorosurfactant-Capped Gold Nanoparticles and O-Phthaldialdehyde. Analyst 2010, 135, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Jiang, M.; Li, Y.; Xia, L.; Wu, P. Aldehyde-Functionalized Metal-Organic Frameworks for Selective Sensing of Homocysteine over Cys, GSH and Other Natural Amino Acids. Chem. Commun. 2018, 54, 1004–1007. [Google Scholar] [CrossRef]
- Hao, P.; Liu, Y.; Wang, L.; Zhu, X.; Liu, Q. Nanozyme Sensor Array Based on Porphyrin-Modified CoMoO4 to Detect and Distinguish Biologically Important Thiols. ACS Appl. Nano Mater. 2023, 6, 1937–1947. [Google Scholar] [CrossRef]
- Sener, G.; Uzun, L.; Denizli, A. Lysine-Promoted Colorimetric Response of Gold Nanoparticles: A Simple Assay for Ultrasensitive Mercury (II) Detection. Anal. Chem. 2014, 86, 514–520. [Google Scholar] [CrossRef]
- Chen, H.; He, K.; Li, H.; Zhang, Y.; Yao, S. Analyte-Triggered Cyclic Autocatalytic Oxidation Amplification Combined with an Upconversion Nanoparticle Probe for Fluorometric Detection of Copper (II). Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, H.; Cao, S.; Shi, Z.; Lan, M. An Electrochemical Aptasensor Based on MOF-Derived Bimetallic Carbon Nanocomposites Au@ Cu-C for Ultrasensitive Detection of Homocysteine on Portable Potentiostat. Electrochim. Acta 2024, 493, 144442. [Google Scholar] [CrossRef]
- Diao, W.; Zhou, C.; Zhang, Z.; Cao, Y.; Li, Y.; Tang, J.; Liu, G. EGaIn-Modified ePADs for Simultaneous Detection of Homocysteine and C-Reactive Protein in Saliva toward Early Diagnosis of Cardiovascular Disease. ACS Sens. 2024, 9, 4265–4276. [Google Scholar] [CrossRef]
- Cheng, C.; Qiao, J.; Zhao, Z.; Zhang, H.; Qi, L. Poly (N-2-Hydroxypropylmethacrylamide)-Capped Gold Nanoparticles as Nanozymes with Peroxidase-Mimicking Performance for the Colorimetric Monitoring of Serum Homocysteine. Anal. Bioanal. Chem. 2023, 415, 953–960. [Google Scholar] [CrossRef]
- Yang, H.; He, Q.; Pan, J.; Lin, M.; Lao, Z.; Li, Q.; Cui, X.; Zhao, S. PtCu NNanocages with Superior Tetra-Enzyme Mimics for Colorimetric Sensing and Fluorescent Sensing Dehydroepiandrosterone. Sens. Actuators B Chem. 2022, 351, 130905. [Google Scholar] [CrossRef]

| Methods | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|
| Electrochemistry | 0.02–20 | 0.017 | [27] |
| Electrochemistry | 1–50 | 0.22 | [28] |
| Fluorometry | 0–50 | 0.06 | [12] |
| Fluorometry | 0–500 | 2.7 | [13] |
| Fluorometry | 0–25 | 0.11 | [15] |
| Fluorometry | 0–90 | 1.9 | [16] |
| Colorimetry | 3.0–20 | 0.4 | [29] |
| Colorimetry | 2.5–50 | 1.48 | [30] |
| Fluorometry | 0.02–0.6 | 0.008 | Present work |
| Samples | Medical Examination (μM) | Measured (μM) | RSD (n = 3, %) |
|---|---|---|---|
| 1 | 14.1 | 13.5 | 0.17% |
| 2 | 16.1 | 13.5 | 0.50% |
| 3 | 10.1 | 9.7 | 0.30% |
| 4 | 9.3 | 8.5 | 0.17% |
| 5 | 8.9 | 8.4 | 0.11% |
| 6 | 11 | 11 | 0.60% |
| 7 | 8.7 | 7.2 | 0.20% |
| 8 | 8.2 | 7.6 | 0.21% |
| 9 | 15.1 | 14.8 | 0.17% |
| 10 | 11.5 | 12.2 | 0.49% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Zhang, P.; Xie, Z.; Zhang, P.; Li, Z.; Yang, Z.; Chen, H. Smart Probes for Ultrasensitive and Highly Selective Sensing of Homocysteine over Cysteine Based on Multi-Cooperative Effects by Using Gold Nanoparticles. Molecules 2025, 30, 1309. https://doi.org/10.3390/molecules30061309
Sun M, Zhang P, Xie Z, Zhang P, Li Z, Yang Z, Chen H. Smart Probes for Ultrasensitive and Highly Selective Sensing of Homocysteine over Cysteine Based on Multi-Cooperative Effects by Using Gold Nanoparticles. Molecules. 2025; 30(6):1309. https://doi.org/10.3390/molecules30061309
Chicago/Turabian StyleSun, Manman, Peihao Zhang, Zeze Xie, Pengcheng Zhang, Zhendong Li, Zhiguang Yang, and Hongyu Chen. 2025. "Smart Probes for Ultrasensitive and Highly Selective Sensing of Homocysteine over Cysteine Based on Multi-Cooperative Effects by Using Gold Nanoparticles" Molecules 30, no. 6: 1309. https://doi.org/10.3390/molecules30061309
APA StyleSun, M., Zhang, P., Xie, Z., Zhang, P., Li, Z., Yang, Z., & Chen, H. (2025). Smart Probes for Ultrasensitive and Highly Selective Sensing of Homocysteine over Cysteine Based on Multi-Cooperative Effects by Using Gold Nanoparticles. Molecules, 30(6), 1309. https://doi.org/10.3390/molecules30061309






