Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications
Abstract
1. Introduction
2. Functionality of the Aromatic Herbs
2.1. Basil (Ocimum basilicum L.)
2.2. Marjoram (Origanum majorana L.)
2.3. Oregano (Origanum vulgare L.)
2.4. Rosemary (Rosmarinus officinalis L.)
2.5. Sage (Salvia officinalis L.)
2.6. Thyme (Thymus vulgaris L.)
2.7. Summer Savory (Satureja hortensis L.)
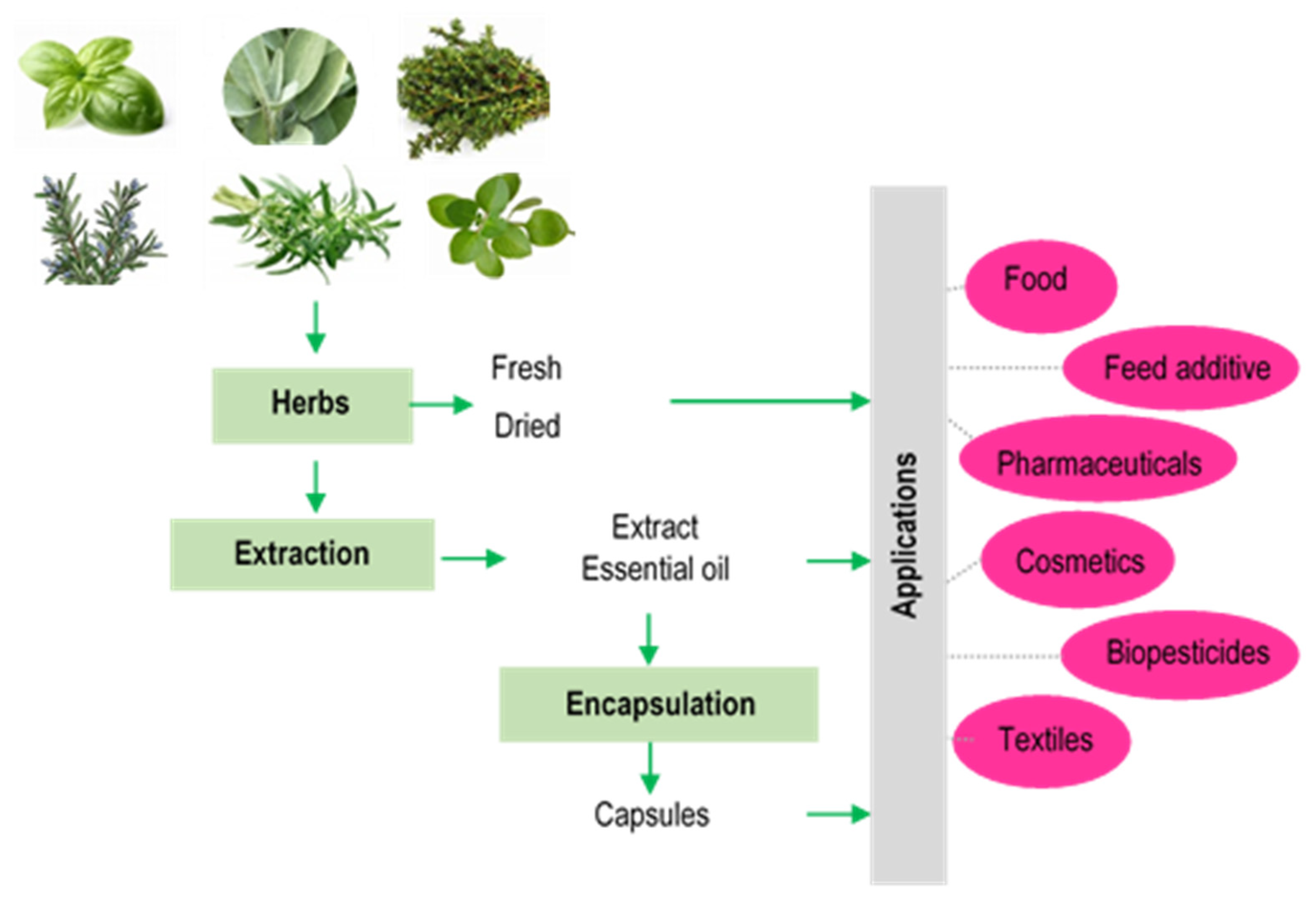
3. Applications
3.1. Applications in Food
3.2. Active Food Packaging
3.3. Feed Additives
3.4. Pharmaceuticals
3.5. Cosmetics
3.6. Biopesticides
3.7. Textiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Ekin Yalcinkaya, I.; Capanoglu, E. Phytochemicals of Herbs and Spices: Health versus Toxicological Effects. Food Chem. Toxicol. 2018, 119, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.V. Introduction. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press: Boca Raton, FL, USA, 2001; Volume 1, pp. 1–12. ISBN 978-1-85573-562-0. [Google Scholar]
- Shylaja, M.R.; Peter, K.V. The Functional Role of Herbal Spices. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 2, pp. 11–21. [Google Scholar]
- Douglas, M.; Heys, J.; Smallfield, B. Herb Spice and Essential Oil: Post-Harvest Operation in Developing Country; UNIDO; FAO: Vienna, Austria, 2005. [Google Scholar]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of Bioactive Properties and Phenolic Compounds in Different Extracts Prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Da Silva, W.M.F.; Kringel, D.H.; De Souza, E.J.D.; Da Rosa Zavareze, E.; Dias, A.R.G. Basil Essential Oil: Methods of Extraction, Chemical Composition, Biological Activities, and Food Applications. Food Bioprocess Technol. 2022, 15, 1–27. [Google Scholar] [CrossRef]
- Shankar, A.; Ali, A.; Abdullah, H.M.; Balaji, J.; Kaur, J.; Saeed, F.; Wasiq, M.; Imran, A.; Jibraeel, H.; Raheem, M.S.; et al. Nutritional Composition, Phytochemical Profile, Therapeutic Potentials, and Food Applications of Rosemary: A Comprehensive Review. J. Food Compos. Anal. 2024, 135, 106688. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Ceramella, J.; Scumaci, D.; Catalano, A.; Sinicropi, M.S.; Tundis, R.; Alcaro, S.; Borges, F. An Update on Recent Studies Focusing on the Antioxidant Properties of Salvia Species. Antioxidants 2023, 12, 2106. [Google Scholar] [CrossRef]
- Bina, F.; Rahimi, R. Sweet Marjoram: A Review of Ethnopharmacology, Phytochemistry, and Biological Activities. J. Evid. Based Complement. Altern. Med. 2017, 22, 175–185. [Google Scholar] [CrossRef]
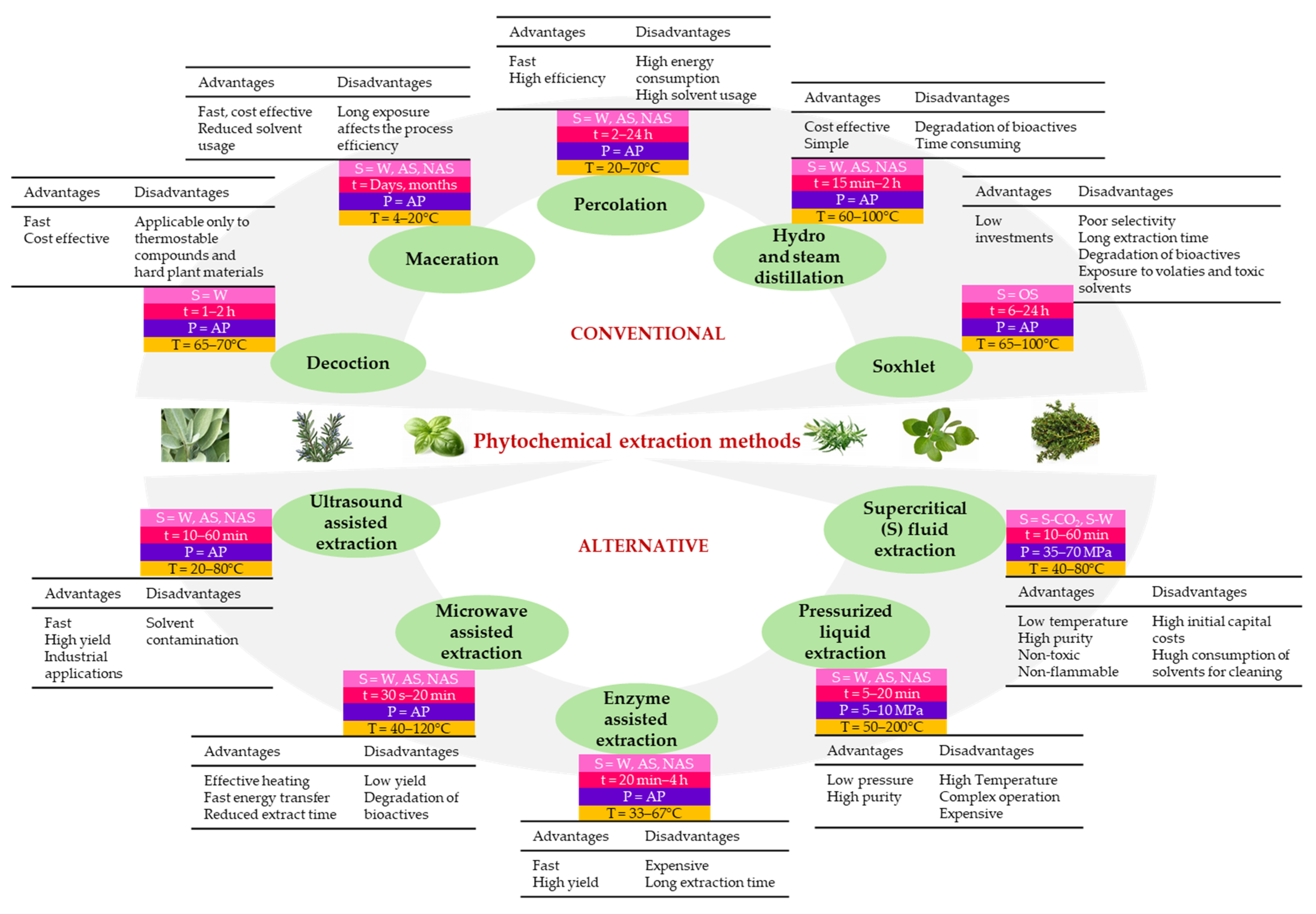
- Patel, K.; Panchal, N.; Ingle, P. Review of Extraction Techniques Extraction Methods: Microwave, Ultrasonic, Pressurized Fluid, Soxhlet Extraction, etc. Int. J. Adv. Res. Chem. Sci. 2019, 6, 6–21. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative Study of Essential Oils Extracted from Egyptian Basil Leaves (Ocimum basilicum L.) Using Hydro-Distillation and Solvent-Free Microwave Extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Yang, J.; Goksen, G.; Zhang, W. Rosemary Essential Oil: Chemical and Biological Properties, with Emphasis on Its Delivery Systems for Food Preservation. Food Control 2023, 154, 110003. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of Ultrasound Assisted Extraction of Antioxidant Compounds from Marjoram (Origanum majorana L.) Using Response Surface Methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Jaime, L.; López De Las Hazas, M.C.; Reglero, G.; Santoyo, S. Supercritical Fluid Extraction as an Alternative Process to Obtain Essential Oils with Anti-Inflammatory Properties from Marjoram and Sweet Basil. Ind. Crops Prod. 2015, 67, 121–129. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Yatoo, M.I.; Thakur, P.; Iqbal, H.M.; Chaicumpa, W.; Michalak, I.; et al. A Comprehensive Review on Chemical Profile and Pharmacological Activities of Ocimum basilicum. Food Rev. Int. 2023, 39, 119–147. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The Effect of Different Solvents and Number of Extraction Steps on the Polyphenol Content and Antioxidant Capacity of Basil Leaves (Ocimum basilicum L.) Extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Saleh, O.; Poudyal, S.; Barka, A.; Qian, Y.; Zheljazkov, V.D. Yield, Composition and Antioxidant Capacity of the Essential Oil of Sweet Basil and Holy Basil as Influenced by Distillation Methods. Chem. Biodivers. 2017, 14, e1600417. [Google Scholar] [CrossRef] [PubMed]
- Güez, C.M.; Souza, R.O.D.; Fischer, P.; Leão, M.F.D.M.; Duarte, J.A.; Boligon, A.A.; Athayde, M.L.; Zuravski, L.; Oliveira, L.F.S.D.; Machado, M.M. Evaluation of Basil Extract (Ocimum basilicum L.) on Oxidative, Anti-Genotoxic and Anti-Inflammatory Effects in Human Leukocytes Cell Cultures Exposed to Challenging Agents. Braz. J. Pharm. Sci. 2017, 53, e15098. [Google Scholar] [CrossRef]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential Production of Phenylpropanoid Metabolites in Callus Cultures of Ocimum basilicum L. with Distinct In Vitro Antioxidant Activities and In Vivo Protective Effects against UV Stress. J. Agric. Food Chem. 2019, 67, 1847–1859. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; Du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Potty, S.N.; Kumar, V.K. Marjoram. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press: Boca Raton FL, USA, 2001; Volume 1, pp. 216–237. ISBN 978-1-85573-562-0. [Google Scholar]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial, Antioxidant, and Sensorial Impacts of Oregano and Rosemary Essential Oils over Broccoli Florets. J. Food Process. Preserv. 2019, 43, e13889. [Google Scholar] [CrossRef]
- Kintzios, S.E.O. Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 2, pp. 215–229. [Google Scholar]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive Two-Dimensional Liquid Chromatography in Analysis of Lamiaceae Herbs: Characterisation and Quantification of Antioxidant Phenolic Acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Akhtar, W.; Shahzad, T.; Malik, A.; Shah, M.A.; Iqbal, S.; Rauf, A.; Zengin, G.; Bouyahya, A.; et al. Rosemary Species: A Review of Phytochemicals, Bioactivities and Industrial Applications. S. Afr. J. Bot. 2022, 151, 3–18. [Google Scholar] [CrossRef]
- Kivilompolo, M.; Obůrka, V.; Hyötyläinen, T. Comparison of GC–MS and LC–MS Methods for the Analysis of Antioxidant Phenolic Acids in Herbs. Anal. Bioanal. Chem. 2007, 388, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Yanishlieva-Maslarova, N.V.; Heinonen, I.M. Rosemary and Sage as Antioxidants. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press: Boca Raton, FL, USA, 2001; Volume 1, pp. 269–275. [Google Scholar]
- Milenković, L.; Ilić, Z.S.; Šunić, L.; Tmušić, N.; Stanojević, L.; Stanojević, J.; Cvetković, D. Modification of Light Intensity Influence Essential Oils Content, Composition and Antioxidant Activity of Thyme, Marjoram and Oregano. Saudi J. Biol. Sci. 2021, 28, 6532–6543. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Biskup, E.; Venskutonis, R.P. Thymes. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 2, pp. 297–321. [Google Scholar]
- Ravindran, P.N.; PiIlai, G.S.; Babu, K.N. Under-Utilized Herbs and Spices. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 2, pp. 53–104. [Google Scholar]
- Tepe, B.; Cilkiz, M. A Pharmacological and Phytochemical Overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef]
- Boroja, T.; Katanić, J.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Jovičić, N.; Mihailović, V. Summer Savory (Satureja hortensis L.) Extract: Phytochemical Profile and Modulation of Cisplatin-Induced Liver, Renal and Testicular Toxicity. Food Chem. Toxicol. 2018, 118, 252–263. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.J.; Romero, M.P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef]
- McCance, K.R.; Flanigan, P.M.; Quick, M.M.; Niemeyer, E.D. Influence of Plant Maturity on Anthocyanin Concentrations, Phenolic Composition, and Antioxidant Properties of 3 Purple Basil (Ocimum basilicum L.) Cultivars. J. Food Compos. Anal. 2016, 53, 30–39. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum basilicum) Essential Oils Depends on Seasonal Variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- He, R.; Chen, W.; Chen, H.; Zhong, Q.; Zhang, H.; Zhang, M.; Chen, W. Antibacterial Mechanism of Linalool against L. Monocytogenes, a Metabolomic Study. Food Control 2022, 132, 108533. [Google Scholar] [CrossRef]
- Zdolec, N.; Franičević, M.; Klanac, L.; Kavain, I.; Batinić, J.; Zadravec, M.; Pleadin, J.; Čobanov, D.; Kiš, M. Antimicrobial Properties of Basil (Ocimum basilicum L.), Sage (Salvia officinalis L.), Lavender (Lavandula officinalis L.), Immortelle (Helichrysum italicum (Roth) G. Don), and Savory (Satureja montana L.) and Their Application in Hard Cheese Production. Hygiene 2024, 4, 135–145. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Tmušić, N.; Mastilović, J.; Kevrešan, Ž.; Stanojević, L.; Danilović, B.; Stanojević, J. Efficiency of Basil Essential Oil Antimicrobial Agents under Different Shading Treatments and Harvest Times. Agronomy 2021, 11, 1574. [Google Scholar] [CrossRef]
- Yaldiz, G.; Camlica, M.; Erdonmez, D. Investigation of Some Basil Genotypes in Terms of Their Effect on Bacterial Communication System, and Antimicrobial Activity. Microb. Pathog. 2023, 182, 106247. [Google Scholar] [CrossRef]
- Gebrehiwot, H.; Bachetti, R.; Dekebo, A. Chemical Composition and Antimicrobial Activities of Leaves of Sweet Basil (Ocimum basilicum L.) Herb. Int. J. Basic Clin. Pharmacol. 2015, 4, 869–875. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, W.; Hao, J.; Wang, Y.; Wei, S.; Zhang, S.; Hu, Y.; Lv, Y. Estragole Inhibits Growth and Aflatoxin Biosynthesis of Aspergillus Flavus by Affecting Reactive Oxygen Species Homeostasis. Microbiol. Spectr. 2023, 11, e01348-23. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, M.; Bai, Y.; Ge, F.; Wang, S. Antioxidant-related Catalase CTA1 Regulates Development, Aflatoxin Biosynthesis, and Virulence in Pathogenic Fungus Aspergillus Flavus. Environ. Microbiol. 2020, 22, 2792–2810. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Stasiak-Różańska, L.; Pluta, A.; Garbowska, M. Antibacterial Activities of Plant-Derived Compounds and Essential Oils against Cronobacter Strains. Eur. Food Res. Technol. 2019, 245, 1137–1147. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of Eugenol against Escherichia coli. J. Herb. Med. 2020, 26, 100406. [Google Scholar] [CrossRef]
- Ghazal, T.S.A.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum Majorana Extracts, Essential Oil and Monoterpenes. Plants 2022, 11, 1432. [Google Scholar] [CrossRef]
- Paudel, P.N.; Satyal, P.; Satyal, R.; Setzer, W.N.; Gyawali, R. Chemical Composition, Enantiomeric Distribution, Antimicrobial and Antioxidant Activities of Origanum majorana L. Essential Oil from Nepal. Molecules 2022, 27, 6136. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Muñoz, S.; Cortez, D.; Rascón, J.; Chavez, S.G.; Caetano, A.C.; Díaz-Manchay, R.J.; Sandoval-Bances, J.; Huyhua-Gutierrez, S.; Gonzales, L.; Chenet, S.M.; et al. Antimicrobial Activity of Origanum Vulgare Essential Oil against Staphylococcus Aureus and Escherichia Coli. Pharmaceuticals 2024, 17, 1430. [Google Scholar] [CrossRef]
- D’Amato, S.; Rossi, C.; Maggio, F.; Valbonetti, L.; Savini, V.; Paparella, A.; Serio, A. Antilisterial Effectiveness of Origanum Vulgare Var. Hirtum and Coridothymus Capitatus Essential Oils and Hydrolates Alone and in Combination. Foods 2024, 13, 860. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Waśkiewicz, A.; Juś, K.; Marchwińska, K.; Frąk, S.; Popowski, D.; Pawlak-Lemańska, K.; Uwineza, P.A.; Gwiazdowski, R.; Padewska, D.; et al. Antimicrobial and Antibiofilm Activity of Origanum Vulgare Extracts Obtained by Supercritical Fluid Extraction Under Various Extraction Conditions. Molecules 2024, 29, 5823. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, J.; Misic, D.; Zizovic, I.; Ristic, M. In vitro Control of Multiplication of Some Food-Associated Bacteria by Thyme, Rosemary and Sage Isolates. Food Control 2012, 25, 110–116. [Google Scholar] [CrossRef]
- Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A.; Bach, H. Antimicrobial, Cytotoxic, and Anti-Inflammatory Activities of Pimenta Dioica and Rosmarinus officinalis Essential Oils. BioMed Res. Int. 2019, 2019, 14038. [Google Scholar] [CrossRef]
- Macedo, L.M.D.; Santos, É.M.D.; Ataide, J.A.; Silva, G.T.D.S.E.; Guarnieri, J.P.D.O.; Lancellotti, M.; Jozala, A.F.; Rosa, P.C.P.; Mazzola, P.G. Development and Evaluation of an Antimicrobial Formulation Containing Rosmarinus officinalis. Molecules 2022, 27, 5049. [Google Scholar] [CrossRef]
- Sidiropoulou, E.; Marugán-Hernández, V.; Skoufos, I.; Giannenas, I.; Bonos, E.; Aguiar-Martins, K.; Lazari, D.; Papagrigoriou, T.; Fotou, K.; Grigoriadou, K.; et al. In vitro Antioxidant, Antimicrobial, Anticoccidial, and Anti-Inflammatory Study of Essential Oils of Oregano, Thyme, and Sage from Epirus, Greece. Life 2022, 12, 1783. [Google Scholar] [CrossRef]
- Ersanli, C.; Tzora, A.; Skoufos, I.; Fotou, K.; Maloupa, E.; Grigoriadou, K.; Voidarou, C.; Zeugolis, D.I. The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus Aureus Strains. Antibiotics 2023, 12, 384. [Google Scholar] [CrossRef]
- Maache, S.; Zbadi, L.; Ghouizi, A.E.; Soulo, N.; Saghrouchni, H.; Siddique, F.; Sitotaw, B.; Salamatullah, A.M.; Nafidi, H.-A.; Bourhia, M.; et al. Antioxidant and Antimicrobial Effects of Essential Oils from Two Salvia Species with in vitro and in silico Analysis Targeting 1AJ6 and 1R4U Proteins. Sci. Rep. 2023, 13, 14038. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated Healing by Topical Administration of Salvia officinalis Essential Oil on Pseudomonas Aeruginosa and Staphylococcus Aureus Infected Wound Model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Essential Oils from Origanum Vulgare and Salvia officinalis Exhibit Antibacterial and Anti-Biofilm Activities against Streptococcus Pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.D.S.A.; Meccatti, V.M.; Pereira, T.C.; Marcucci, M.C.; Hasna, A.A.; Valera, M.C.; De Oliveira, L.D.; Carvalho, C.A.T. Antibacterial Effect of Combinations of Salvia officinalis and Glycyrrhiza Glabra Hydroalcoholic Extracts against Enterococcus Spp. Coatings 2023, 13, 1579. [Google Scholar] [CrossRef]
- Carvalho, M.; Barbosa, J.; Da Silva, M.B.R.; Albano, H.; Teixeira, P. Impact of Polysorbate 80 on the Antimicrobial Activity of Oregano and Thyme. Molecules 2024, 30, 81. [Google Scholar] [CrossRef] [PubMed]
- Hofmeisterová, L.; Bajer, T.; Walczak, M.; Šilha, D. Chemical Composition and Antibacterial Effect of Clove and Thyme Essential Oils on Growth Inhibition and Biofilm Formation of Arcobacter Spp. and Other Bacteria. Antibiotics 2024, 13, 1232. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Mousavi, S.M.; Nazem, H. Anti-Bacterial/Fungal and Anti-Cancer Performance of Green Synthesized Ag Nanoparticles Using Summer Savory Extract. J. Exp. Nanosci. 2020, 15, 363–380. [Google Scholar] [CrossRef]
- Macari, A.; Sturza, R.; Lung, I.; Soran, M.-L.; Opriş, O.; Balan, G.; Ghendov-Mosanu, A.; Vodnar, D.C.; Cojocari, D. Antimicrobial Effects of Basil, Summer Savory and Tarragon Lyophilized Extracts in Cold Storage Sausages. Molecules 2021, 26, 6678. [Google Scholar] [CrossRef]
- Prerna; Chadha, J.; Khullar, L.; Mudgil, U.; Harjai, K. A Comprehensive Review on the Pharmacological Prospects of Terpinen-4-Ol: From Nature to Medicine and Beyond. Fitoterapia 2024, 176, 106051. [Google Scholar] [CrossRef]
- Hamed, A.M.; Abd El-Maksoud, A.A.; Hassan, M.A.; Tsakali, E.; Van Impe, J.F.M.; Ahmed, H.A.; Nassrallah, A.A. Enhancing Functional Buffalo Yogurt: Improving Physicochemical Properties, Biological Activities, and Shelf Life Using Marjoram and Geranium Essential Oils. J. Dairy Sci. 2024, 107, 6437–6450. [Google Scholar] [CrossRef]
- Deen, J.I.; Zawad, A.N.M.S.; Uddin, M.; Chowdhury, M.A.H.; Al Araby, S.Q.; Rahman, M.A. Terpinen-4-Ol, A Volatile Terpene Molecule, Extensively Electrifies the Biological Systems against the Oxidative Stress-Linked Pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Johansen, B.; Duval, R.; Sergere, J.-C. First Evidence of a Combination of Terpinen-4-Ol and α-Terpineol as a Promising Tool against ESKAPE Pathogens. Molecules 2022, 27, 7472. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-Ol as an Antibacterial and Antibiofilm Agent against Staphylococcus Aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.-A.W.; Dokla, E.M.; Serya, R.; Abouzid, K.A. Penicillin Binding Protein 2a: An Overview and a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 199, 112312. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial Mechanism of Oregano Essential Oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Piasecki, B.; Balázs, V.L.; Kieltyka-Dadasiewicz, A.; Szabó, P.; Kocsis, B.; Horváth, G.; Ludwiczuk, A. Microbiological Studies on the Influence of Essential Oils from Several Origanum Species on Respiratory Pathogens. Molecules 2023, 28, 3044. [Google Scholar] [CrossRef]
- Chen, J.; Tang, C.; Zhang, R.; Ye, S.; Zhao, Z.; Huang, Y.; Xu, X.; Lan, W.; Yang, D. Metabolomics Analysis to Evaluate the Antibacterial Activity of the Essential Oil from the Leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol. 2020, 253, 112652. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 1–30. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Ghasemi Pirbalouti, A.; Khaghani, S.; Malekpoor, F. Essential Oil Composition and Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Sage (Salvia officinalis L.) Extract under Chitosan Application and Irrigation Frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Ciobanu, S.C.; Predoi, D.; Chifiriuc, M.C.; Iconaru, S.L.; Predoi, M.V.; Popa, M.; Rokosz, K.; Raaen, S.; Marinas, I.C. Salvia officinalis–Hydroxyapatite Nanocomposites with Antibacterial Properties. Polymers 2023, 15, 4484. [Google Scholar] [CrossRef]
- Speranza, B.; Guerrieri, A.; Racioppo, A.; Bevilacqua, A.; Campaniello, D.; Corbo, M.R. Sage and Lavender Essential Oils as Potential Antimicrobial Agents for Foods. Microbiol. Res. 2023, 14, 1089–1113. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Mirzaie, S.; Pirmahalle, F.R.; Yahyaraeyat, R.; Razmyar, J. Effects of Thyme (Thymus vulgaris) Essential Oil on Bacterial Growth and Expression of Some Virulence Genes in Salmonella Enterica Serovar Enteritidis. Vet. Med. Sci. 2024, 10, e70088. [Google Scholar] [CrossRef]
- Giovagnoni, G.; Rossi, B.; Tugnoli, B.; Ghiselli, F.; Bonetti, A.; Piva, A.; Grilli, E. Thymol and Carvacrol Downregulate the Expression of Salmonella Typhimurium Virulence Genes during an In Vitro Infection on Caco-2 Cells. Microorganisms 2020, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Morshdy, A.E.M.A.; El-tahlawy, A.S.; Qari, S.H.; Qumsani, A.T.; Bay, D.H.; Sami, R.; Althubaiti, E.H.; Mansour, A.M.A.; Aljahani, A.H.; Hafez, A.E.-S.E.; et al. Anti-Biofilms’ Activity of Garlic and Thyme Essential Oils against Salmonella Typhimurium. Molecules 2022, 27, 2182. [Google Scholar] [CrossRef] [PubMed]
- Chkhikvishvili, I.; Sanikidze, T.; Gogia, N.; Mchedlishvili, T.; Enukidze, M.; Machavariani, M.; Vinokur, Y.; Rodov, V. Rosmarinic Acid-Rich Extracts of Summer Savory (Satureja Hortensis L.) Protect Jurkat T Cells against Oxidative Stress. Oxidative Med. Cell. Longev. 2013, 2013, 456253. [Google Scholar] [CrossRef]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal Activity of Thyme, Summer Savory and Clove Essential Oils against Aspergillus Flavus in Liquid Medium and Tomato Paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Sharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical Composition and Antifungal Activity of Satureja hortensis L. Essentiall Oil against Planktonic and Biofilm Growth of Candida albicans Isolates from Buccal Lesions of HIV+ Individuals. Microb. Pathog. 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Christaki, S.; Moschakis, T.; Kyriakoudi, A.; Biliaderis, C.G.; Mourtzinos, I. Recent Advances in Plant Essential Oils and Extracts: Delivery Systems and Potential Uses as Preservatives and Antioxidants in Cheese. Trends Food Sci. Technol. 2021, 116, 264–278. [Google Scholar] [CrossRef]
- Tassou, C.C.; Nychas, G.-J.E.; Skandamis, P.N. Herbs and Spices and Antimicrobials. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 2, pp. 22–40. [Google Scholar]
- Kunová, S.; Taglieri, I.; Haščík, P.; Ben Hsouna, A.; Mnif, W.; Venturi, F.; Sanmartin, C.; Čmiková, N.; Kluz, M.I.; Kačániová, M. Dried Herbs as an Easy-to-Use and Cost-Effective Alternative to Essential Oils to Extend the Shelf Life of Sheep Lump Cheese. Foods 2023, 12, 4487. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of Rosemary (Rosmarinus officinalis L.) Extract as Antioxidant in Jelly Candies Made with Fructan Fibres and Stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Atlar, G.C.; Kutlu, G.; Tornuk, F. Design and Characterization of Chitosan-Based Films Incorporated with Summer Savory (Satureja hortensis L.) Essential Oil for Active Packaging. Int. J. Biol. Macromol. 2024, 254, 127732. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, M.K.; Tayarani Najaran, Z.; Nasery, M.; Emami, S.A. Summer Savory (Satureja hortensis L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 757–764. ISBN 978-0-12-416641-7. [Google Scholar]
- Diniz-Silva, H.T.; Brandão, L.R.; De Sousa Galvão, M.; Madruga, M.S.; Maciel, J.F.; Leite De Souza, E.; Magnani, M. Survival of Lactobacillus Acidophilus LA-5 and Escherichia Coli O157:H7 in Minas Frescal Cheese Made with Oregano and Rosemary Essential Oils. Food Microbiol. 2020, 86, 103348. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Caleja, C.; Barros, L.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. Rosemary Extracts in Functional Foods: Extraction, Chemical Characterization and Incorporation of Free and Microencapsulated Forms in Cottage Cheese. Food Funct. 2016, 7, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Nabigol, A. Chemical Composition and Anti-Fungal Activities of Three Essential Oils from Satureja Spp. on Four Post-Harvest Pathogens of Strawberry Fruit. J. Hortic. Sci. Biotechnol. 2011, 86, 371–376. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Tornuk, F. Inactivation of Non-Toxigenic and Toxigenic Escherichia Coli O157:H7 Inoculated on Minimally Processed Tomatoes and Cucumbers: Utilization of Hydrosols of Lamiaceae Spices as Natural Food Sanitizers. Food Control 2013, 30, 7–14. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.; Dimić, G.; Đerić, N.; Mojović, L.; Tomović, V.; Šojić, B.; Đukić-Vuković, A.; Pejin, J. Growth Control of Molds Isolated from Smoked Fermented Sausages Using Basil and Caraway Essential Oils, in vitro and in vivo. LWT 2020, 123, 109095. [Google Scholar] [CrossRef]
- Hasneen, D.F.; Zaki, N.L.; Abbas, M.S.; Soliman, A.S.; Ashoush, I.S.; Fayed, A.E. Comparative Evaluation of Some Herbs and Their Suitability for Skimmed Milk Yoghurt and Cast Kariesh Cheese Fortification as Functional Foods. Ann. Agric. Sci. 2020, 65, 6–12. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Zynudheen, A.A.; Parvathy, U.; Binsi, P.K. Impact of Chitosan and Oregano Extract on the Physicochemical Properties of Microencapsulated Fish Oil Stored at Different Temperature. Int. J. Food Prop. 2018, 21, 943–956. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.-Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. Essential Oils: Their Chemical Compositions and Their Preservative Effects against Salmonella Inoculated in Minced Beef Meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- Maleš, I.; Dobrinčić, A.; Zorić, Z.; Vladimir-Knežević, S.; Elez Garofulić, I.; Repajić, M.; Skroza, D.; Jerković, I.; Dragović-Uzelac, V. Phenolic, Headspace and Sensory Profile, and Antioxidant Capacity of Fruit Juice Enriched with Salvia officinalis L. and Thymus serpyllum L. Extract: A Potential for a Novel Herbal-Based Functional Beverages. Molecules 2023, 28, 3656. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, A.; Shaarawy, S.; Motawe, F.H.; Refaei, R. Herbal Extract as an Ecofriendly Antibacterial Finishing of Cotton Fabric. Egypt. J. Chem. 2018, 61, 317–327. [Google Scholar] [CrossRef]
- Kramar, A.; Petrović, M.; Mihajlovski, K.; Mandić, B.; Vuković, G.; Blagojević, S.; Kostić, M. Selected Aromatic Plants Extracts as an Antimicrobial and Antioxidant Finish for Cellulose Fabric- Direct Impregnation Method. Fibers Polym. 2021, 22, 3317–3325. [Google Scholar] [CrossRef]
- Begum, A.; Sandhya, S.; Kumar, A.N.; Ali, S.S. Evaluation of Herbal Hair Lotion Loaded with Rosemary for Possible Hair Growth in C57BL/6 Mice. Adv. Biomed. Res. 2023, 12, 60. [Google Scholar] [CrossRef]
- Ferreira-Anta, T.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A Rheological Approach of Seawater-Based Natural Cosmetics with Extracts from Sonicated Medicinal Herbs. Sustain. Chem. Pharm. 2023, 36, 101263. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential Oils as Feed Additives—Future Perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef]
- Ghasemi, R.; Zarei, M.; Torki, M. Adding Medicinal Herbs Including Garlic (Allium sativum) and Thyme (Thymus vulgaris) to Diet of Laying Hens and Evaluating Productive Performance and Egg Quality Characteristics. Am. J. Anim. Vet. Sci. 2010, 5, 151–154. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Efficacy and Persistence of Rosemary Oil as an Acaricide Against Twospotted Spider Mite (Acari: Tetranychidae) on Greenhouse Tomato. J. Econ. Entomol. 2006, 99, 2015–2023. [Google Scholar] [CrossRef]
- Goharrostami, M.; Sendi, J.J.; Hosseini, R.; Allah Mahmoodi, N.O. Effect of Thyme Essential Oil and Its Two Components on Toxicity and Some Physiological Parameters in Mulberry Pyralid Glyphodes Pyloalis Walker. Pestic. Biochem. Physiol. 2022, 188, 105220. [Google Scholar] [CrossRef]
- Altinier, G.; Sosa, S.; Aquino, R.P.; Mencherini, T.; Loggia, R.D.; Tubaro, A. Characterization of Topical Antiinflammatory Compounds in Rosmarinus officinalis L. J. Agric. Food Chem. 2007, 55, 1718–1723. [Google Scholar] [CrossRef]
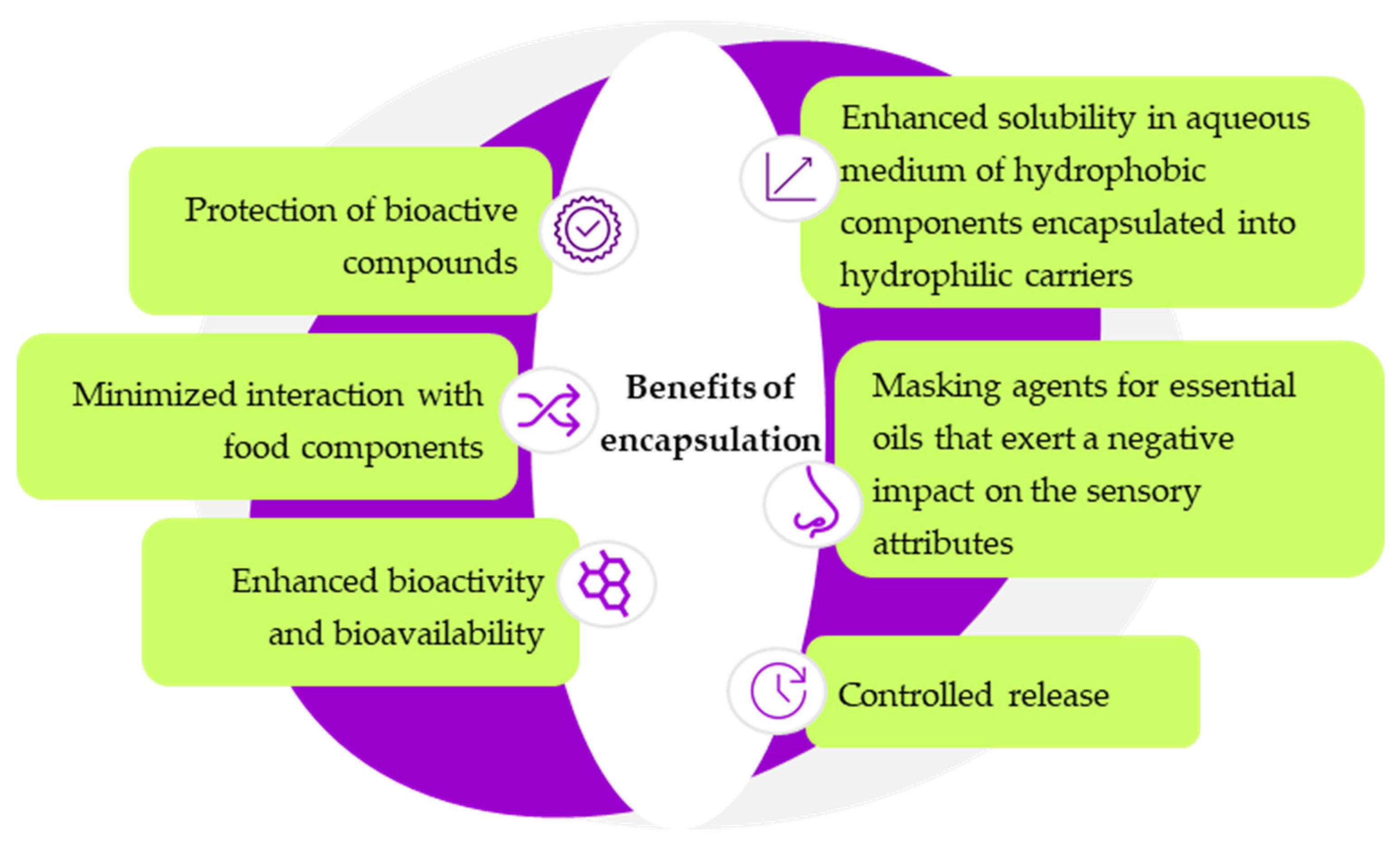
- Hossen, M.A.; Shimul, I.M.; Sameen, D.E.; Rasheed, Z.; Dai, J.; Li, S.; Qin, W.; Tang, W.; Chen, M.; Liu, Y. Essential Oil–Loaded Biopolymeric Particles on Food Industry and Packaging: A Review. Int. J. Biol. Macromol. 2024, 265, 130765. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of Essential Oils to Enhance Their Antimicrobial Activity in Foods. LWT-Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Souza, E.J.D.D.; Pacheco, C.D.O.; Costa, I.H.D.L.; Dias, A.R.G.; Zavareze, E.D.R. Applications of Nanotechnology in Essential Oil Protection to Extend the Shelf Life of Fruits and Vegetables: A Review. Food Control 2025, 170, 111044. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Guimarães, I.C.; Ferreira, C.L.R.; Botrel, D.A.; Borges, S.V.; De Souza, A.U. Microencapsulated Rosemary (Rosmarinus officinalis) Essential Oil as a Biopreservative in Minas Frescal Cheese: Rosemary Oil as a Biopreservative in Cheese. J. Food Process. Preserv. 2017, 41, e12759. [Google Scholar] [CrossRef]
- Rashidaie Abandansarie, S.S.; Ariaii, P.; Charmchian Langerodi, M. Effects of Encapsulated Rosemary Extract on Oxidative and Microbiological Stability of Beef Meat during Refrigerated Storage. Food Sci. Nutr. 2019, 7, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Radünz, M.; Dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; De Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; Da Rosa Zavareze, E. Antimicrobial Potential of Spray Drying Encapsulated Thyme (Thymus vulgaris) Essential Oil on the Conservation of Hamburger-like Meat Products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martín-Belloso, O. Improving the Shelf Life of Low-Fat Cut Cheese Using Nanoemulsion-Based Edible Coatings Containing Oregano Essential Oil and Mandarin Fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Salinas, C.; Lis, M.J.; Coderch, L.; Martí, M. Formation and Characterization of Oregano Essential Oil Nanocapsules Applied onto Polyester Textile. Polymers 2022, 14, 5188. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Erkan, G.; Karavana, S.Y.; Sarıışık, A.M.; Çetmeli Bakadur, A.; Ütebay, B.; Popescu, A. Spray-Dried Oregano Oil and Lavender Oil Microcapsules for Antibacterial Sports and Leisurewear. AATCC J. Res. 2023, 10, 332–345. [Google Scholar] [CrossRef]
- Devrnja, N.; Anđelković, B.; Ljujić, J.; Ćosić, T.; Stupar, S.; Milutinović, M.; Savić, J. Encapsulation of Fennel and Basil Essential Oils in β-Cyclodextrin for Novel Biopesticide Formulation. Biomolecules 2024, 14, 353. [Google Scholar] [CrossRef]
- Thuekeaw, S.; Angkanaporn, K.; Nuengjamnong, C. Microencapsulated Basil Oil (Ocimum basilicum Linn.) Enhances Growth Performance, Intestinal Morphology, and Antioxidant Capacity of Broiler Chickens in the Tropics. Anim. Biosci. 2022, 35, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible Films for Food Packaging: A Review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey Protein Isolate/Cellulose Nanofibre/TiO2 Nanoparticle/Rosemary Essential Oil Nanocomposite Film: Its Effect on Microbial and Sensory Quality of Lamb Meat and Growth of Common Foodborne Pathogenic Bacteria during Refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Application of Pregelatinized Corn Starch and Basil Essential Oil Edible Coating with Cellulose Nanofiber as Pickering Emulsion Agent to Prevent Quality-Quantity Loss of Mandarin Orange. Food Packag. Shelf Life 2023, 35, 101010. [Google Scholar] [CrossRef]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Fechtali, T.; Mauriello, G. Basil Essential Oil: Composition, Antimicrobial Properties, and Microencapsulation to Produce Active Chitosan Films for Food Packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Xylia, P.; Chrysargyris, A.; Tzortzakis, N. The Combined and Single Effect of Marjoram Essential Oil, Ascorbic Acid, and Chitosan on Fresh-Cut Lettuce Preservation. Foods 2021, 10, 575. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Omar-Aziz, M.; Gharaghani, M.; Khodaiyan, F.; Hosseini, S.S.; Kennedy, J.F. Effect of Mung Bean Protein Isolate/Pullulan Films Containing Marjoram (Origanum majorana L.) Essential Oil on Chemical and Microbial Properties of Minced Beef Meat. Int. J. Biol. Macromol. 2022, 201, 318–329. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodriguez, M.R.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia Graveolens) Essential Oil Added within Pectin Edible Coatings Prevents Fungal Decay and Increases the Antioxidant Capacity of Treated Tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef]
- Ríos-de-Benito, L.F.; Escamilla-García, M.; García-Almendárez, B.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Design of an Active Edible Coating Based on Sodium Caseinate, Chitosan and Oregano Essential Oil Reinforced with Silica Particles and Its Application on Panela Cheese. Coatings 2021, 11, 1212. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Unsalan, O.; Altunayar-Unsalan, C.; Xiong, S.; Manyande, A.; Chen, H. Development and Characterization of Fish Myofibrillar Protein/Chitosan/Rosemary Extract Composite Edible Films and the Improvement of Lipid Oxidation Stability during the Grass Carp Fillets Storage. Int. J. Biol. Macromol. 2021, 184, 463–475. [Google Scholar] [CrossRef]
- Quintana, S.E.; Llalla, O.; García-Risco, M.R.; Fornari, T. Comparison between Essential Oils and Supercritical Extracts into Chitosan-Based Edible Coatings on Strawberry Quality during Cold Storage. J. Supercrit. Fluids 2021, 171, 105198. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Aminzare, M.; Raeisi, M.; Afshari, A.; Mirza Alizadeh, A.; Rezaeigolestani, M. Comparative Evaluation of Edible Films Impregnated with Sage Essential Oil or Lactoperoxidase System: Impact on Chemical and Sensory Quality of Carp Burgers. J. Food Process. Preserv. 2019, 43, e14070. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma Gutiérrez Castañeda, C.; Valencia, M.E.; Grande Tovar, C.D. The Effect of Edible Chitosan Coatings Incorporated with Thymus Capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria x ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.Á.; Hernández-López, M. The Effect of Nanostructured Chitosan and Chitosan-thyme Essential Oil Coatings on Colletotrichum Gloeosporioides Growth in vitro and on Cv Hass Avocado and Fruit Quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Amraie, M.; Salehi, M.; Mohseni, M.; Aloui, H. Effect of Chitosan-based Coatings Enriched with Savory and/or Tarragon Essential Oils on Postharvest Maintenance of Kumquat (Fortunella Sp.) Fruit. Food Sci. Nutr. 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Adetunji, C.O.; Olaniyan, O.T.; Ojo, S.K.; Samuel, M.O.; Temitayo, B.T.; Roli, O.I.; Nimota, O.O.; Oluwabunmi, B.T.; Adetunji, J.B.; et al. Antimicrobial, Antioxidant and Other Pharmacological Activities of Ocimum Species: Potential to Be Used as Food Preservatives and Functional Ingredients. Food Rev. Int. 2023, 39, 1547–1577. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Ozgul Onal, M.; Ozturk, F. Comparison of Ultrastructural Changes and the Anticarcinogenic Effects of Thymol and Carvacrol on Ovarian Cancer Cells: Which Is More Effective? Ultrastruct. Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Mari, A.; Mani, G.; Nagabhishek, S.N.; Balaraman, G.; Subramanian, N.; Mirza, F.B.; Sundaram, J.; Thiruvengadam, D. Carvacrol Promotes Cell Cycle Arrest and Apoptosis through PI3K/AKT Signaling Pathway in MCF-7 Breast Cancer Cells. Chin. J. Integr. Med. 2021, 27, 680–687. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological Activity of Plant-Based Carvacrol and Thymol and Their Impact on Human Health and Food Quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Hajibonabi, A.; Yekani, M.; Sharifi, S.; Nahad, J.S.; Dizaj, S.M.; Memar, M.Y. Antimicrobial Activity of Nanoformulations of Carvacrol and Thymol: New Trend and Applications. OpenNano 2023, 13, 100170. [Google Scholar] [CrossRef]
- De Macedo, L.M.; Santos, É.M.D.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Reglero, G.; Ramírez De Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1223–1231. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer Activity of Rosmarinus officinalis L.: Mechanisms of Action and Therapeutic Potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef]
- Wang, Q.L.; Li, H.; Li, X.X.; Cui, C.Y.; Wang, R.; Yu, N.X.; Chen, L.X. Acute and 30-Day Oral Toxicity Studies of Administered Carnosic Acid. Food Chem. Toxicol. 2012, 50, 4348–4355. [Google Scholar] [CrossRef]
- El-Naggar, S.A.; Abdel-Farid, I.B.; Germoush, M.O.; Elgebaly, H.A.; Alm-Eldeen, A.A. Efficacy of Rosmarinus officinalis Leaves Extract against Cyclophosphamide-Induced Hepatotoxicity. Pharm. Biol. 2016, 54, 2007–2016. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic Effects of Rosemary (Rosmarinus officinalis L.) and Its Active Constituents on Nervous System Disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef]
- Lalićević, S.; Djordjević, I. Comparison of Benzydamine Hydrochloride and Salvia officinalis as an Adjuvant Local Treatment to Systemic Nonsteroidal Anti-Inflammatory Drug in Controlling Pain after Tonsillectomy, Adenoidectomy, or Both: An Open-Label, Single-Blind, Randomized Clinical Trial. Curr. Ther. Res. 2004, 65, 360–372. [Google Scholar] [CrossRef]
- Venkatasalu, M.R.; Murang, Z.R.; Ramasamy, D.T.R.; Dhaliwal, J.S. Oral Health Problems among Palliative and Terminally Ill Patients: An Integrated Systematic Review. BMC Oral Health 2020, 20, 79. [Google Scholar] [CrossRef]
- Monsen, R.E.; Herlofson, B.B.; Gay, C.; Fjeld, K.G.; Hove, L.H.; Malterud, K.E.; Saghaug, E.; Slaaen, J.; Sundal, T.; Tollisen, A.; et al. A Mouth Rinse Based on a Tea Solution of Salvia officinalis for Oral Discomfort in Palliative Cancer Care: A Randomized Controlled Trial. Support Care Cancer 2021, 29, 4997–5007. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Alves Borges Leal, A.L.; et al. Salvia Spp. Plants-from Farm to Food Applications and Phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Ejaz, A.; Waliat, S.; Arshad, M.S.; Khalid, W.; Khalid, M.Z.; Rasul Suleria, H.A.; Luca, M.-I.; Mironeasa, C.; Batariuc, A.; Ungureanu-Iuga, M.; et al. A Comprehensive Review of Summer Savory (Satureja hortensis L.): Promising Ingredient for Production of Functional Foods. Front. Pharmacol. 2023, 14, 1198970. [Google Scholar] [CrossRef] [PubMed]
- Sharmeen, J.; Mahomoodally, F.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Rosmarinus officinalis (Rosemary)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 12S–50S. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Neggaz, S.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Solvent Free Microwave Extraction Followed by Encapsulation of O. Basilicum L. Essential Oil for Insecticide Purpose. J. Stored Prod. Res. 2020, 86, 101575. [Google Scholar] [CrossRef]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a Bacteriophage in Reducing Listeria Monocytogenes on Fresh-Cut Fruits and Fruit Juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant Based Natural Products as Potential Ecofriendly and Safer Biopesticides: A Comprehensive Overview of Their Advantages over Conventional Pesticides, Limitations and Regulatory Aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Savoia, D. Plant-Derived Antimicrobial Compounds: Alternatives to Antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Walentowska, J.; Foksowicz-Flaczyk, J. Thyme Essential Oil for Antimicrobial Protection of Natural Textiles. Int. Biodeterior. Biodegrad. 2013, 84, 407–411. [Google Scholar] [CrossRef]
| Common Name (Botanical Name) | Plant Part Used | Growth Habit and Duration | Climate Zone | |
|---|---|---|---|---|
| Basil (Ocimum basilicum L.) |  | Leaves and terminal shoot | Annual herb. Needs full sun. Grows well in rich and well-drained soil. | Tropical wet and dry, subtropical humid, subtropical dry, or temperate oceanic |
| Marjoram (Origanum majorana L.) |  | Leaves and floral buds | Perennial herb. Needs full sun. Grows on saline alkaline soil. | Subtropical dry summer, temperate continental, or temperate oceanic |
| Oregano (Origanum vulgare L.) |  | Leaves and flower | Perennial herb. Needs full sun. Grows well in alkaline and well-drained soil. | Steppe or semiarid, subtropical dry summer, or temperate oceanic |
| Rosemary (Rosemarinus officinalis L.) |  | Leaves and terminal shoot | Perennial shrub. Needs full sun. Grows well in sandy and well-limed soil. | Steppe or semiarid, subtropical dry summer, or temperate |
| Sage (Salvia officinalis L.) |  | Leaves and terminal shoot | Perennial shrub. | Steppe or semiarid, tropical wet and dry, subtropical dry summer, or temperate |
| Thyme (Thymus vulgaris L.) |  | Leaves and terminal shoot | Perennial shrub. Grows well in dry hillsides. | Steppe or semiarid, tropical wet and dry, subtropical dry summer, or temperate |
| Summer savory (Satureja hortensis L.) |  | Leaves and terminal shoot | Annual herb. Grows in dry and rocky hillsides. | Steppe or semiarid, subtropical dry summer, temperate |
| Aromatic Herb | Details on Nutritive Value (Composition per 100 g) | Main Flavor Compounds | Compounds with Antioxidant Activity | References |
|---|---|---|---|---|
| Basil | Proteins 14.4 g; Fats 4.0 g; Fiber 17.8 g; Ash 14.3 g Main vitamins: C, B1, B2, A, niacin Main minerals: K, Ca, P, Mg, Fe, Na, Zn | D-Linalool, methyl chavicol, eugenol, cineole | Phenolic acids, flavonoids, especially anthocyanins, as well as eugenol, linalool, estragole, α-cadinol, methyl cinnamate, and α-bergamotene Major *: rosmarinic acid, eugenol, linalool Polyphenol content **: 4318 mg/100 g d.w. | [3,15,16,17,18,19,20,21] |
| Marjoram | Proteins 12.7 g; Fats 7.0 g; Fiber 18.1 g; Ash 12.1 g Main vitamins: C, A, niacin Main minerals: Ca, K, Mg, P, Fe, Na, Zn | Carvacrol, D-linalool, eugenol, chavicol, methyl chavicol, D-terpineol, caryophyllene limonene, cineol | Phenolic acids, flavonoids (flavanones, flavonols, flavones) Major: ferulic acid, caffeic acid, carnosic acid, carnosol, luteolin-7-O-glucoside and apigenin-7-O-glucoside Polyphenol content: 3846 mg/100 g d.w. | [3,14,15,21,22] |
| Oregano | Proteins 11.0 g; Fats 10.3 g; Fiber 15.0 g; Ash 7.2 g Main vitamins: vitamins E, B6, B3, B2, B9, B7, A, B1, carotene Main minerals: Ca, K, Mg, P, Fe, Na, Zn, Mn, Cu, Se | Thymol, carvacrol, α-pinene, cineole, linalyl acetate, linalool, dipentene, p-cymene, β-caryophyllene | Derivatives of phenolic acids, flavonoids, tocopherols Major: rosmarinic acid, carvacrol, thymol Polyphenol content: 3117 mg/100 g d.w. | [3,21,23,24,25] |
| Rosemary | Proteins 4.9 g; Fats 15.2 g; Fiber 17.7 g; Ash 6.5 g Main vitamins: vitamins C, A and B3 Main minerals: Ca, K, Mg, P, Na, Fe, Zn | Cineole, borneol, linalool, eucalyptol, camphor, bornyl acetate, α-pinene, camphene, sabinene, phellandrene, α-terpinene | Phenolic acids, flavonoids (flavanols, flavones), and phenolic terpenes Major: carnosic acid, rosmanol, rosemarinic acid, naringin, carnosol Polyphenol content: 2519 mg/100 g d.w. | [3,21,25,26,27] |
| Sage | Proteins 10.6 g; Fats 12.7 g; Fiber 18.1 g; Ash 8.0 g Main vitamins: vitamins C and A, niacin Main minerals: Ca, K, Mg, P, Fe, Na, Zn | Thujone, borneol, cineole, bornylesters, α-pinene, salvene, D-camphor phellandrene, ocimene | Phenolic acids and phenolic terpenes Major: rosmarinic acid, carnosic acid, carnosol, rosmanol Polyphenol content: 2920 mg/100 g d.w. | [3,21,28] |
| Thyme | Proteins 9.1 g; Fats 7.4 g; Fiber 18.6 g; Ash 11.7 g Main vitamins: vitamin A, niacin Main minerals: Ca, K, Mg, P, Fe, Na, Zn | Thymol, carvacrol, linalool, L-borneol, geraniol, amyl alcohol, β-pinene, camphene, p-cymene, caryophyllene, 1,8-cineole | Phenolic acids, flavonoids, as well as terpenes: carvacrol, thymol, p-cymene, caryophyllene, carvone, borneol Major: rosmarinic acid, thymol Polyphenol content: 1815 mg/100 g d.w. | [3,21,29,30] |
| Summer savory | Proteins 15.0 g; Fats 5.9 g; Fiber 30.7 g; Ash 7.5 g Main vitamins: vitamin A, niacin Main minerals: Ca, K, Mg, P, Fe, Na, Zn | carvacrol, γ-terpinene, p-cymene, pinene, myrcene, α-terpinene, eugenol, β-caryophyllene | Phenolic acids, flavonoids, as well as terpenes: carnosol, carvacrol, thymol Major: rosmarinic acid, thymol and carvacrol Polyphenol content: 4512 mg/100 g d.w. | [21,31,32,33] |
| Herbs/ Extract or Essential Oils | Data on Antimicrobial Activity | References | |||
|---|---|---|---|---|---|
| Strains | Inhibition Zone Diameter (mm) | MIC | MBC | ||
| Basil | |||||
| Essential oil | S. aureus ATCC 25923 | 24.4 ± 1.1 | 1.5 mg/mL | nd | [36] |
| 24.0 ± 1.0 | 0.9 mg/mL | ||||
| B. subtilis ATCC 10707 | 26.1 ± 1.1 | 0.8 mg/mL | |||
| Dino genotype Essential oil | S. epidermidis | 11.33 17.00 (Ethanol extract) | nd | nd | [42] |
| E. coli | 17.33 | ||||
| P. aeruginosa | 16.67 | ||||
| PI 253157 genotype Essential oil | S. epidermidis | 17.33 | |||
| PI 296391 genotype Essential oil | S. aureus | 17.00 | |||
| E. coli | 22.00 | ||||
| ‘Genovese’, Blue net treatment Essential oil | Bacillus cereus | 26.5 ± 3.21 | nd | nd | [41] |
| S. aureus | 38.7 ± 1.15 | ||||
| P. vulgaris | 28.0 ± 3.79 | ||||
| C. albicans | 33.7 ± 0.58 | ||||
| ‘Genovese’, Pearl net treatment | Bacillus subtilis | 26.5 ± 7.18 | |||
| K. pneumoniae | 34.3 ± 3.44 | ||||
| Ethanolic extract | S. aureus | >20 (+++) | nd | nd | [40] |
| Essential oil | C. sakazakii Lv53 | 10.1 ± 0.4 | 0.2% for the eugenol | 0.05% (MTC) | [46] |
| Marjoram | |||||
| Essential oil | S. aureus ATCC 29213 | 16 ± 0.5 | 0.125% | nd | [48] |
| S. aureus MRSA ATCC 43300 | 14 ± 0.3 | 0.125% | |||
| E. coli ATCC 25922, E. coli AG-100 | 14 ± 0.4 | 0.125% 0.250% | |||
| Essential oil | S. aureus ATCC 29213 | nd | 0.156 mg/mL | nd | [49] |
| A. niger ATCC 16888, C. albicans ATCC 18804 | 0.078 mg/mL | ||||
| Trichophyton mentagrophytes ATCC 18748, Aspergillus fumigatus ATCC 96918 | 0.157 mg/mL | ||||
| Oregano | |||||
| Essential oil | E. coli ATCC 25922 | 20.7 | 0.49 mg/mL | 0.99 mg/mL | [50] |
| S. aureus ATCC 25923 | 16.7 | 1.9 mg/mL | 7.9 mg/mL | ||
| S. aureus isolated from a patient | 19.7 | 1.9 mg/mL | 3.9 mg/mL | ||
| Commercial essential oil O. vulgare var. hirtum | clinical sources L. monocytogenes L3 | nd | 1.25 µL/mL | nd | [51] |
| L253, L291 | 0.625 µL/mL | ||||
| L239, L317 | 0.3125 µL/mL | ||||
| L315 | 2.5 µL/mL | ||||
| L368 | 10.0 µL/mL | ||||
| SC-CO2 extracts of dried, ground | M. luteus ATCC 10240, S. aureus ATCC 33862, E. faecalis ATCC 19433, | nd | 0.25 mg/mL | 0.25 mg/mL | [52] |
| L. monocytogenes ATCC 19115 | 0.25 mg/mL | 0.5 mg/mL | |||
| E. coli ATCC 8739, S. enterica ser. Enteritidis ATCC 13076 | 0.5 mg/mL | 0.5 mg/mL | |||
| P. aeruginosa ATCC 9027, C. jejuni ATCC 33291 | 0.5 mg/mL | 1.0 mg/mL | |||
| Rosemary | |||||
| SC-CO2 extracts from dried leaves | B. cereus | nd | 0.320 mg/mL | nd | [53] |
| E. faecium | 1.280 mg/mL | ||||
| E. coli, S. enterica ser. Enteritidis | 2.560 mg/mL | ||||
| Commercial essential oil | A. baumannii ATCC BAA-747 | nd | 0.500 mg/mL | nd | [54] |
| C. albicans ATCC 10231 | 0.600 mg/mL | ||||
| Infusion from dried rosemary leaves | S. aureus | nd | ≥1.5 mg/mL | nd | [55] |
| P. aeruginosa | 10 | ≥6.0 mg/mL | nd | ||
| Salvia spp. (sage) | |||||
| S. fructicosa (Greece sage) | S. aureus ATCC 29213 | 18 | 2.25 mg/mL | 4.50 mg/mL | [56] |
| E. coli ATCC 25922 | 10 | 0.563 mg/mL | 2.812 mg/mL | ||
| Aerial parts from S. fruticose (Greece sage) | Methicillin-Sensitive S. aureus | 17.464 ± 0.253 | 2.853 mg/mL | nd | [57] |
| S. aureus MRSA | 11.184 ± 0.209 | ||||
| S. aureus ATCC 29213 | 9.399 ± 0.148 | ||||
| S. officinalis | S. aureus | 33.66 ± 5.68 | 18.75 mg/mL | 37.5 mg/mL | [58] |
| P. mirabilis ATCC 29906 | 12.33 ± 0.57 | 0.29 mg/mL | 1.17 mg/mL | ||
| B. subtilis ATCC 6633 | 14 ± 1.73 | 2.34 mg/mL | 4.69 mg/mL | ||
| C. albicans ATCC 10231 | 25.33 ± 1.15 | 4.69 mg/mL | 9.37 mg/mL | ||
| S. officinalis aerial flowering parts | S. aureus ATCC 25923 | nd | 0.125 mg/mL | 0.125 mg/mL | [59] |
| P. aeruginosa ATCC 27853 | 0.125 mg/mL | 4.00 mg/mL | |||
| S. officinalis flowering period | S. pyogenes ATCC 19615 | nd | 0.5 mg/mL | 0.5 mg/mL | [60] |
| S. officinalis Hydroethanolic extract | S. faecalis clinical strain 2 | nd | nd | 2.1 mg/mL | [61] |
| S. faecalis clinical strain 2 | 1.0 mg/mL | ||||
| S. faecalis clinical strain 3 | 8.7 mg/mL | ||||
| E. faecium clinical strain 1 | 2.1 mg/mL | ||||
| E. faecium clinical strain 2 | 4.3 mg/mL | ||||
| Thyme | |||||
| Commercial essential oil | B. cereus ESB014 | 36.1 ± 2.2 | 0.19% | nd | [62] |
| B. stearothermophilus ESB016 | 41.0 ± 4.9 | 0.39% | |||
| C. perfringens 1.16 | 22.0 ± 1.0 | 0.09% | |||
| E. faecalis ATCC 29212 | 28.9 ± 1.1 | 0.19% | |||
| E. flavescens DSMZ 7370 | 30.1 ± 0.4 | 0.02% | |||
| L. monocytogenes 7946 | 36.0 ± 1.4 | 0.05% | |||
| S. aureus MRSA | 34.7 ± 3.0 | 0.09% | |||
| A. baumannii ESB028 | 32.6 ± 3.7 | 0.05% | |||
| P. mirabilis ESB027 | 36.7 ± 1.8 | 0.05% | |||
| S. Tiphymurium ESB009 | 29.9 ± 1.8 | 0.09% | |||
| Y. enterocolitica ESB024 | 47.8 ± 3.3 | 0.09% | |||
| C. albicans ESB025 | 50.7 ± 2.8 | 0.09% | |||
| Commercial essential oil | A. butzleri CCUG 30484 | 47.5 ± 1.5 | >1.024 mg/mL | nd | [63] |
| S. aureus CCM 4223 | 41.4 ± 3.7 | 1.024 mg/mL | |||
| Summer savory | |||||
| Aqueous extract | S. aureus ATCC 6538, E. faecalis ATCC 6057, S. cerevisiae ATCC 9763 | nd | 0.250 mg/mL | 0.500 mg/mL | [64] |
| K. pneumoniae ATCC 7881, E. coli ATCC 33876 | nd | 0.250 mg/mL | <0.250 mg/mL | ||
| Hydroalcoholic extract | L. monocytogenes ATCC 19114 | nd | 0.31 mg/mL | 0.62 mg/mL | [65] |
| P. aeruginosa ATCC 27853, S. typhimurium ATCC 14028 | 5.0 mg/mL | 10.0 mg/mL | |||
| E. coli ATCC 25922 | 2.5 mg/mL | 5.0 mg/mL | |||
| Plant | Extract (E)/Essential Oil (EO) | Type of Product | Properties in the Products | References |
|---|---|---|---|---|
| Food | ||||
| Basil | EO | Fermented sausages | The addition of EO reduced the mold growth on the surface of sausages indicating its potential for antifungal protection of fermented sausages against Penicillium carneum and Penicillium polonicum. | [97] |
| Marjoram | E | Skimmed yogurt UF-Kariesh cheese | The addition of extract to 1% in yogurt and 2% in cheese contributed to increasing the antioxidant activity while maintaining consumer acceptability in terms of sensorial attributes. | [98] |
| Oregano | EO | Fish oil | The addition of oregano EO before encapsulation of fish oil by spray-drying increased the oxidative stability during storage. | [99] |
| Sage | EO | Minced beef meat | Antimicrobial effect against Salmonella sp. Maintained the quality and extended the shelf life of raw or processed meat during refrigeration. Acceptable consumer acceptability of sensorial attributes. | [100] |
| Thyme, sage | E | Fruit juice | The fruit juices containing 10% aqueous thyme and sage extract showed increased antioxidant activity and superior sensorial attributes. | [101] |
| Summer savory | EO | Strawberries | Fumigation of strawberry surface with an emulsion containing summer savory essential oil exerted ant-fungal activity against post-harvest pathogenic fungi and extended the shelf life of the strawberries. | [95] |
| Textile | ||||
| Sage | E | Cotton fabric | Antibacterial activity against Gram-negative, Gram-positive bacteria and yeasts unicellular fungi. | [102] |
| Sage | E | Viscose fabric | Direct impregnation of extracts onto the fabric represents an eco-friendly, low-cost disposable medical textile for skin wound treatment. | [103] |
| Cosmetics | ||||
| Rosemary | E | Hair lotion | 1% herbal hair lotion is an excellent hair growth promoter. | [104] |
| Tyme, sage, rosemary | E | Shampoo | Enhanced antioxidant properties of samples containing herb extracts while maintaining the technological properties. | [105] |
| Feed additive | ||||
| Oregano, sage | EO | Eggs | Increased egg production and reduced the incidence of broken–cracked eggs. | [106] |
| Rosemary | Freeze-dried | Pork meat | No effect on the sensorial attributes or carcass properties. | [106] |
| Thyme | Powder | Eggs | Increased the egg weight and yolk color. | [107] |
| Biopesticides | ||||
| Rosemary | EO | Twospotted Spider Mite | Rosemary essential oil caused complete mortality of spider mites on greenhouse tomato plants at concentrations that are not phytotoxic to the host plant. | [108] |
| Thyme | EO | Glyphodes pyloalis W | Thyme EO showed potential to control G. pyloalis larvae in mulberry orchards. | [109] |
| Pharmaceuticals | ||||
| Rosemary | E | Anti-inflammatory product | The extract tested in mice using croton oil ear test showed anti-inflammatory activity comparable to indomethacin. | [110] |
| Plant | Extract (E)/ Essential Oil (EO) | Wall Material Encapsulation Method | Type of Product | Properties in Products | Reference |
|---|---|---|---|---|---|
| Food | |||||
| Oregano | EO | Sodium alginate Mandarin fiber | Low fat cut cheese | Edible coating containing min. 2% EO enhanced the appearance and the microbial stability of cut cheese resulting in increasing the shelf life. | [117] |
| Rosemary | E | Alginate Spray-drying | Cottage cheese | Cheese-containing capsules with rosemary extract presented superior antioxidant activity during storage. | [94] |
| Rosemary | EO | Inulin and whey protein isolate Spray-drying | Minas frescal cheese | The addition of encapsulated essential oil increased the shelf life by controlling the proliferation of mesophilic bacteria. | [114] |
| Rosemary | E | Soybean protein isolate Freeze-drying | Beef meat fillets | Encapsulation of rosemary extract increased the antimicrobial and antioxidant activity. Nano-encapsulation reduced the microbial and lipid oxidation during storage and extended the shelf life to up to 21 days. | [115] |
| Thyme | EO | Sodium casein and maltodextrin spray-drying | Hamburger-like meat products | The encapsulated essential oil exerted high antioxidant and antimicrobial activity with promising potential to be used as a preservative in hamburgers. | [116] |
| Textile | |||||
| Oregano | EO | Poly-ε-caprolactone nanoparticles nanoprecipitation | Polyester textile | Encapsulation of essential oil improved fabric comfort and promoted antibacterial properties. | [118] |
| Oregano | EO | Spray-drying | Sports and leisurewear fabrics | Encapsulation delayed the degradation of EO and showed antibacterial protection against E. coli and S. aureus in terms of oil and washings. | [119] |
| Biopesticide | |||||
| Basil | EO | β-cyclodextrin co-precipitation | Colorado potato beetle | Spraying the potato plant with encapsulated EO altered the growth, dynamics of development, and proteolytic activity of larvae. | [120] |
| Feed additive | |||||
| Basil | EO | Sodium alginate Chitosan | Broiler chicken | Microencapsulation of basil oil showed promising potential for improvement of intestinal integrity and nutrient utilization. | [121] |
| Cosmetics | |||||
| Rosemary | EO | Lipid nanoparticles | Gels | Compared with gels containing nonencapsulated essential oil, gels containing 3% rosemary essential oil-loaded lipid nanoparticles applied on skin surfaces for one week, twice a day exerted positive effects on skin hydration and elasticity of volunteers. | [122] |
| Plant | Polymer | Food | Properties in Food | Reference |
|---|---|---|---|---|
| Basil | Starch, cellulose nanofibers | Mandarin orange | Coating prevented weight loss and maintained the quality of the fruits in terms of surface color and pH for up to 12 days of storage at room temperature | [125] |
| Basil | Chitosan | Cooked ham | Chitosan film was able to reduce the pH increase and the growth of aerobic mesophilic bacteria during storage (10 days, 4 °C) | [126] |
| Marjoram | Chitosan | Fresh-cut lettuce | Coated lettuce presented antimicrobial activity against total viable counts, yeast and mold counts | [127] |
| Marjoram | Mung bean protein isolate/pullulan | Minced beef meat | The incorporation of essential oil into films was able to prevent the oxidative degradation of minced beef samples and presented antimicrobial activity against S. aureus and E. coli | [128] |
| Oregano | Pectin edible coating | Tomatoes | Coating with essential oil inhibited the growth of Alternaria alternate and promoted the increase in antioxidant activity of the tomatoes | [129] |
| Oregano | Sodium caseinate, chitosan | Panela cheese | Coating improved the quality and safety of the Panela cheese in terms of microbial growth delay and moisture loss | [130] |
| Rosemary | Fish myofibrillar protein/chitosan | Fish fillet | The composite film possessed a protective effect on fish muscle by decreasing lipid oxidation during storage | [131] |
| Rosemary | Whey proteins, cellulose, nanofiber, titanium dioxide nanoparticles | Fresh lamb | The nanocomposite film increased the shelf life of fresh lamb up to 15 days (compared to control) Antimicrobial effect against Gram-positive bacteria | [124] |
| Rosemary | Chitosan coating | Strawberry | The fungal decay was lower in coated strawberries during storage at 4 °C for 10 days | [132] |
| Rosemary, oregano | Pectin coating | Broccoli | The use of the two EOs in pectin coating of fresh broccoli showed high antimicrobial activity and antioxidant effect without limiting the sensorial acceptability | [23] |
| Sage | Chitosan, alginate, gelatin | Carp burgers | Minimum spoilage changes during 20 days of storage Superior sensorial attributes in terms of odor and overall acceptability compared with control | [133] |
| Thyme | Chitosan coating | Strawberry | Increased shelf life for at least 15 days at 5 °C High stability of physico-chemical and antioxidant properties | [134] |
| Thyme | Chitosan coating | Avocado | The quality of avocado in terms of color and firmness was protected by coating with chitosan and thyme essential oil Antifungal effect against Colletotrichum gloeosporioides | [135] |
| Savory | Chitosan coating | Kumquat | Coating prevented weight loss, maintained the vitamin C content during storage for 30 days at 7 °C, with minimum changes in the sensorial properties | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore-Gurgu, L.; Dumitrașcu, L.; Aprodu, I. Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules 2025, 30, 1304. https://doi.org/10.3390/molecules30061304
Grigore-Gurgu L, Dumitrașcu L, Aprodu I. Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules. 2025; 30(6):1304. https://doi.org/10.3390/molecules30061304
Chicago/Turabian StyleGrigore-Gurgu, Leontina, Loredana Dumitrașcu, and Iuliana Aprodu. 2025. "Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications" Molecules 30, no. 6: 1304. https://doi.org/10.3390/molecules30061304
APA StyleGrigore-Gurgu, L., Dumitrașcu, L., & Aprodu, I. (2025). Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules, 30(6), 1304. https://doi.org/10.3390/molecules30061304