α-Mangostin Exhibits Antitumor Activity Against NCI-H1975 Cells via the EGFR/STAT3 Pathway: An Experimental and Molecular Simulation Study
Abstract
1. Introduction
2. Results
2.1. The Antiproliferative Activity of α-Mangostin on NCI-H1975 Cells
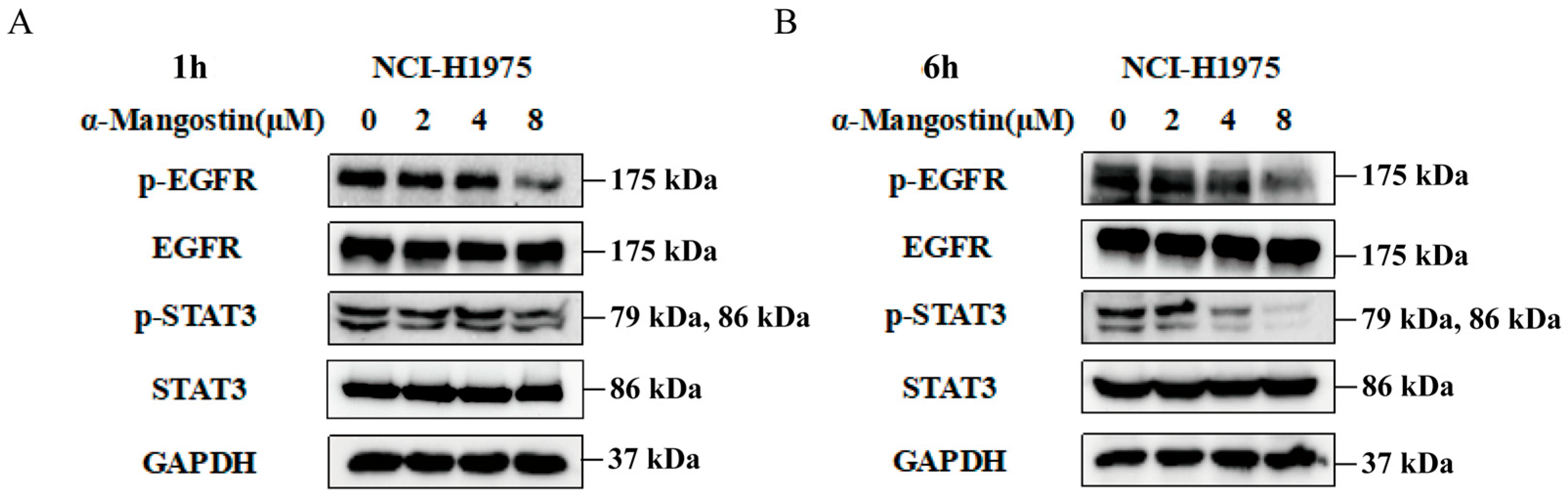
2.2. The Regulation of α-Mangostin on the EGFR/STAT3 Pathway
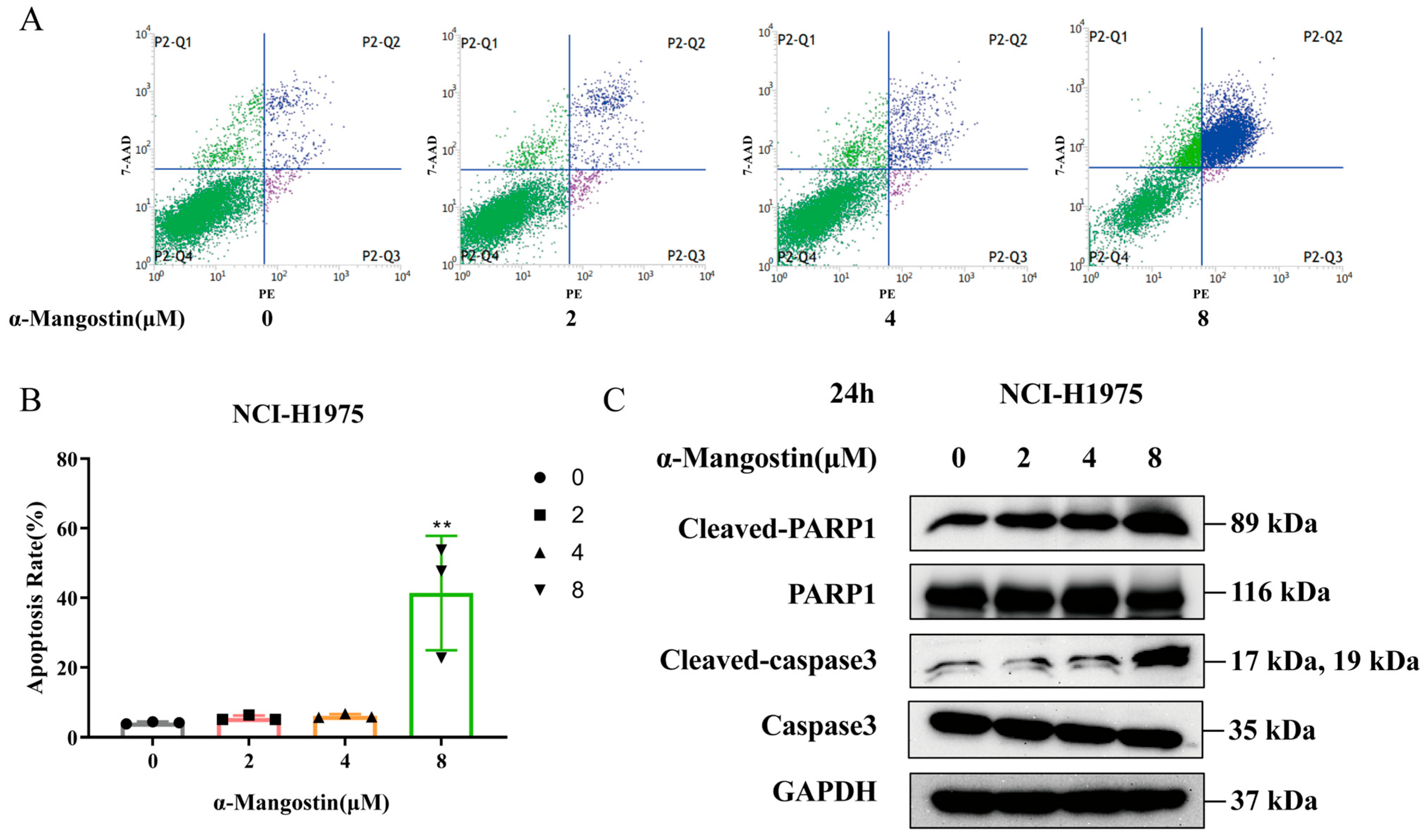
2.3. The Proapoptotic Effects of α-Mangostin on NCI-H1975 Cells
2.4. Induction of α-Mangostin on Cell Cycle Redistribution of NCI-H1975 Cells
2.5. Inhibition of α-Mangostin on the Migration of NCI-H1975 Cells
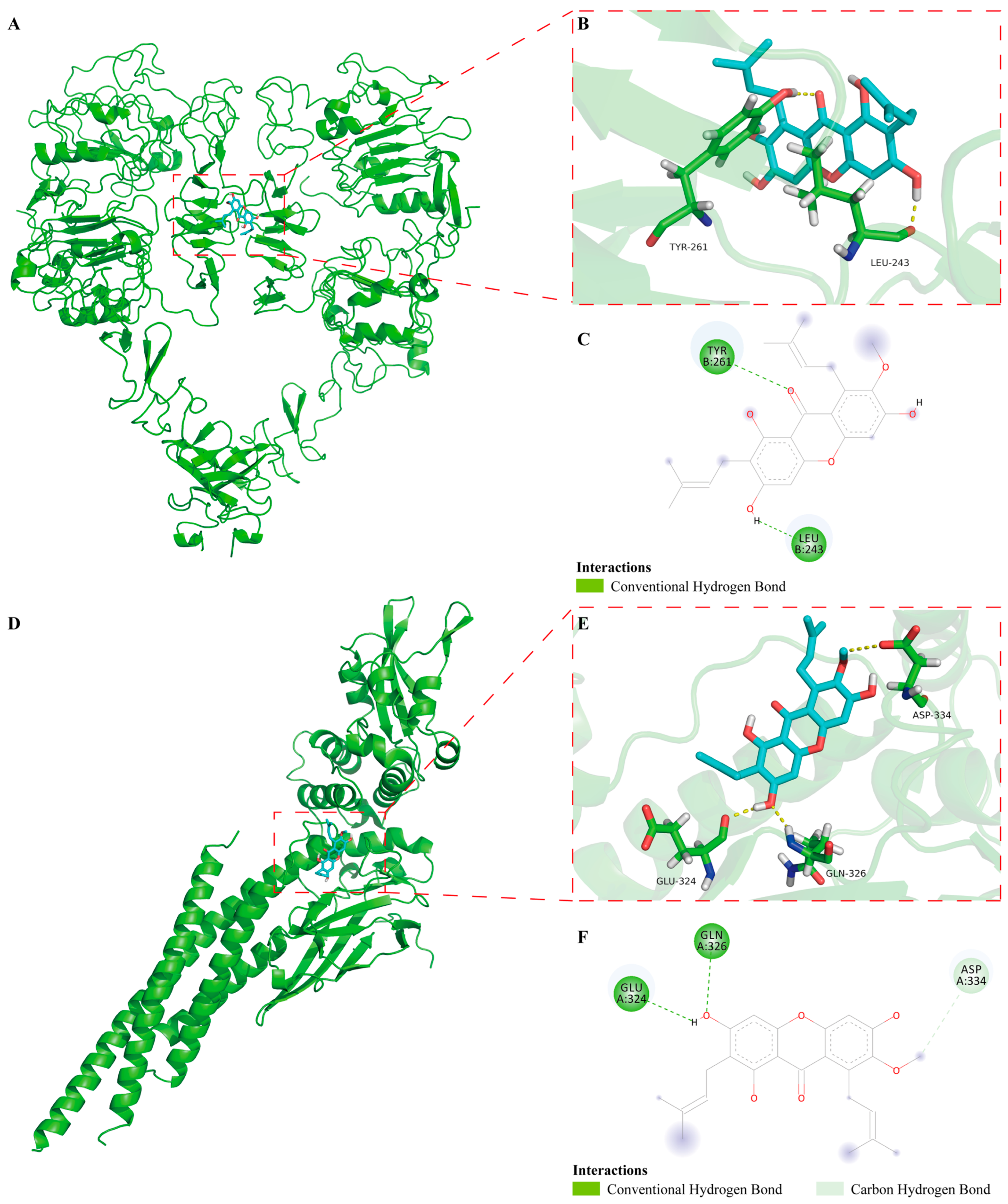
2.6. The Binding Mode of α-Mangostin to EGFR and STAT3 in Molecular Docking
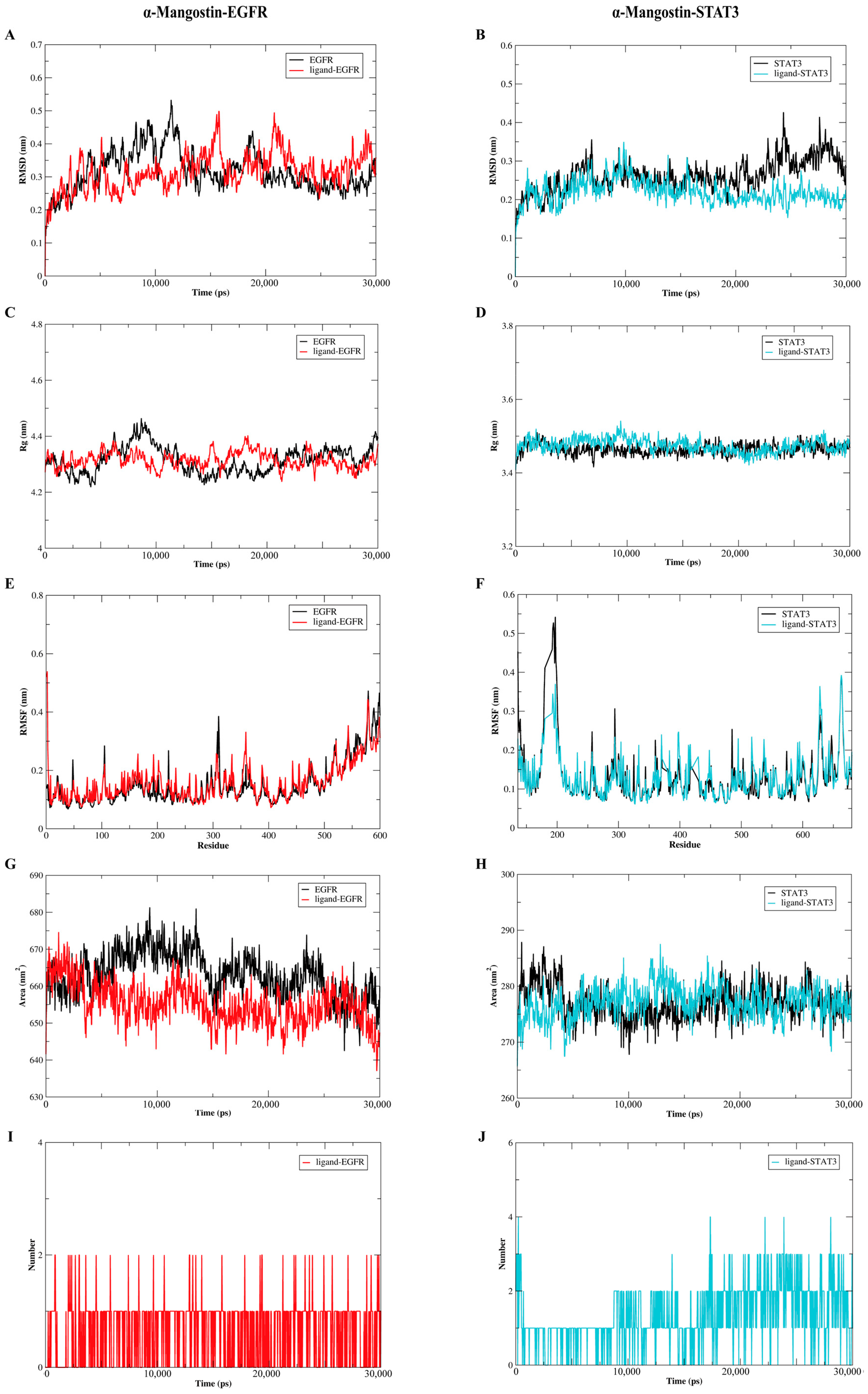
2.7. The Molecular Dynamics Analysis of the Complexes of α-Mangostin and EGFR or STAT3
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. MTT Assay
4.4. Western Blotting
4.5. Cell Apoptosis Assay
4.6. Cell Cycle Assay
4.7. Colony Formation Assay
4.8. Wound Healing Assay
4.9. Molecular Docking
4.10. Molecular Dynamics Simulation
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 10, 815–834. [Google Scholar] [CrossRef]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef]
- Sun, P.Y.; Zhang, S.G.; Qu, Y.N.; Li, X.Y.; Chen, G.R.; Wang, X.J.; Sheng, J.; Wang, J. Coccinic acid exhibits anti-tumor efficacy against NSCLC harboring EGFR L858R/T790M mutation via the EGFR/STAT3 pathway. Bioorg. Chem. 2025, 154, 108038. [Google Scholar] [CrossRef]
- Yin, Z.J.; Jin, F.G.; Liu, T.G.; Fu, E.Q.; Xie, Y.H.; Sun, R.L. Overexpression of STAT3 potentiates growth, survival, and radioresistance of non-small-cell lung cancer (NSCLC) cells. J. Surg. Res. 2011, 171, 675–683. [Google Scholar] [CrossRef]
- Sun, X.L.; Xiang, Z.M.; Xie, Y.R.; Zhang, N.; Wang, L.X.; Wu, Y.L.; Zhang, D.Y.; Wang, X.J.; Sheng, J.; Zi, C.T. Dimeric-(-)-epigallocatechin-3-gallate inhibits the proliferation of lung cancer cells by inhibiting the EGFR signaling pathway. Chem. Biol. Interact. 2022, 365, 110084. [Google Scholar] [CrossRef]
- Baumgartner, U.; Berger, F.; Hashemi Gheinani, A.; Burgener, S.S.; Monastyrskaya, K.; Vassella, E. miR-19b enhances proliferation and apoptosis resistance via the EGFR signaling pathway by targeting PP2A and BIM in non-small cell lung cancer. Mol. Cancer. 2018, 17, 44. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhang, Z.Y.; Zhang, J.L. Matrine increases the inhibitory effects of afatinib on H1975 cells via the IL-6/JAK1/STAT3 signaling pathway. Mol. Med. Rep. 2017, 16, 2733–2739. [Google Scholar] [CrossRef]
- Mao, D.; Feng, L.; Gong, H. The Antitumor and Immunomodulatory Effect of Yanghe Decoction in Breast Cancer Is Related to the Modulation of the JAK/STAT Signaling Pathway. Evid. Based Complement. Altern. Med. 2018, 44, 8460526. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, F.; Zhuang, Y.; Liu, Y.; Xu, L.; Zhao, H.; Xiang, Y.; Dai, X.; Liu, Z.; Huang, X.; et al. Acetyl-bufalin shows potent efficacy against non-small-cell lung cancer by targeting the CDK9/STAT3 signalling pathway. Br. J. Cancer 2021, 124, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Qu, Y.; Wang, Y.; Wang, J.; Wang, X.; Sheng, J. Wighteone exhibits an antitumor effect against EGFR L858R/T790M mutation non-small cell lung cancer. J. Cancer 2021, 12, 3900–3908. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of α-Mangostin from the mangosteen fruit: A review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef]
- Zhang, K.J.; Gu, Q.L.; Yang, K.; Ming, X.J.; Wang, J.X. Anticarcinogenic Effects of α-Mangostin: A Review. Planta Med. 2017, 83, 188–202. [Google Scholar] [CrossRef]
- Yang, A.; Liu, C.; Wu, J.; Kou, X.; Shen, R. A review on α-Mangostin as a potential multi-target-directed ligand for Alzheimers disease. Eur. J. Pharmacol. 2021, 897, 173950. [Google Scholar] [CrossRef]
- Wang, F.; Ma, H.; Liu, Z.; Huang, W.; Xu, X.; Zhang, X. α-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed. Pharmacother. 2017, 92, 672–680. [Google Scholar] [CrossRef]
- Lee, C.H.; Ying, T.H.; Chiou, H.L.; Hsieh, S.C.; Wen, S.H.; Chou, R.H.; Hsieh, Y.H. Alpha-Mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget 2017, 8, 47425–47439. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Ning, H.; Yuan, Z.; Zhong, Y.; Wu, S.; Zeng, J.Z. α-Mangostin Induces Apoptosis and Inhibits Metastasis of Breast Cancer Cells via Regulating RXRα-AKT Signaling Pathway. Front. Pharmacol. 2021, 12, 739658. [Google Scholar] [CrossRef]
- Ittiudomrak, T.; Puthong, S.; Roytrakul, S.; Chanchao, C. α-Mangostin and Apigenin Induced Cell Cycle Arrest and Programmed Cell Death in SKOV-3 Ovarian Cancer Cells. Toxicol. Res. 2019, 35, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, F.; Dong, Y.; Ren, F. α-Mangostin Induces Apoptosis in Human Osteosarcoma Cells Through ROS-Mediated Endoplasmic Reticulum Stress via the WNT Pathway. Cell Transplant. 2021, 30, 9636897211035080. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Shahbazzadeh, F.; Kihara, T. Alpha-Mangostin reduces mechanical stiffness of various cells. Hum. Cell 2020, 33, 347–355. [Google Scholar] [CrossRef]
- Li, Q.; Yan, X.T.; Zhao, L.C.; Ren, S.; He, Y.F.; Liu, W.C.; Wang, Z.; Li, X.D.; Jiang, S.; Li, W. α-Mangostin, a Dietary Xanthone, Exerts Protective Effects on Cisplatin-Induced Renal Injury via PI3K/Akt and JNK Signaling Pathways in HEK293 Cells. ACS Omega 2020, 5, 19960–19967. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rojas, J.M.; Cruz, C.; García-López, P.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Ceballos, G.; Espinosa, M.; Meléndez-Zajgla, J.; Pedraza-Chaverri, J. Renoprotection by alpha-Mangostin is related to the attenuation in renal oxidative/nitrosative stress induced by cisplatin nephrotoxicity. Free Radic. Res. 2009, 43, 1122–1132. [Google Scholar] [CrossRef]
- Fei, X.; Jo, M.; Lee, B.; Han, S.B.; Lee, K.; Jung, J.K.; Seo, S.Y.; Kwak, Y.S. Synthesis of xanthone derivatives based on α-Mangostin and their biological evaluation for anti-cancer agents. Bioorg. Med. Chem. Lett. 2014, 9, 2062–2065. [Google Scholar] [CrossRef]
- Jung, H.A.; Su, B.N.; Keller, W.J.; Mehta, R.G.; Kinghorn, D. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food. Chem. 1970, 6, 2077–2082. [Google Scholar] [CrossRef]
- Kim, H.J.; Fei, X.; Cho, S.C.; Choi, B.Y.; Ahn, H.C.; Lee, K.; Seo, S.Y.; Keum, Y.S. Discovery of α-Mangostin as a novel competitive inhibitor against mutant isocitrate dehydrogenase-1. Bioorg. Med. Chem. Lett. 2015, 25, 5625–5631. [Google Scholar] [CrossRef]
- Phan, T.; Shahbazzadeh, F.; Pham, T.; Kihara, T. Alpha-Mangostin inhibits the migration and invasion of A549 lung cancer cells. PeerJ. 2018, 6, e5027. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Aydın, S.G. Review on Molecular Modeling and Docking. SAR J. Sci. Res. 2022, 5, 206–210. [Google Scholar] [CrossRef]
- Dudhat, K. Panoramic Review on Progress and Development of Molecular Docking. Pharm. Sci. Technol. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Tuo, Y.; Lu, X.; Tao, F.; Tukhvatshin, M.; Xiang, F.; Wang, X.; Shi, Y.; Lin, J.; Hu, Y. The Potential Mechanisms of Catechins in Tea for Anti-Hypertension: An Integration of Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation. Foods 2024, 13, 2685. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, P.W.; Rose, A.S.; Tiemann, J.K.S. Bringing Molecular Dynamics Simulation Data into View. Trends Biochem. Sci. 2019, 44, 902–913. [Google Scholar] [CrossRef]
- Zhang, S.G.; Yang, Y.F.; Zhang, R.H.; Gao, J.; Wu, M.Y.; Wang, J.; Sheng, J.; Sun, P.Y. The Potential Mechanism of Alpiniae oxyphyllae Fructus Against Hyperuricemia: An Integration of Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Nutrients 2024, 17, 71. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Pavan, N.M.; Jones, A.M.; Ximenes, V.F. Solvent-Induced Lag Phase during the Formation of Lysozyme Amyloid Fibrils Triggered by Sodium Dodecyl Sulfate: Biophysical Experimental and In Silico Study of Solvent Effects. Molecules 2023, 28, 6891. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Le, D.C.S.; Tang, B.; Cornelio, M.L.; Jones, A.M. Synthesis and Spectroscopic Analysis of Piperine- and Piperlongumine-Inspired Natural Product Scaffolds and Their Molecular Docking with IL-1β and NF-κB Proteins. Molecules 2020, 25, 2841. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Pao, W.; Miller, V.A.; Politi, K.A.; Riely, G.J.; Somwar, R.; Zakowski, M.F.; Kris, M.G.; Varmus, H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005, 2, e73. [Google Scholar] [CrossRef]
- Tangsuksan, P.; Chuerduangphui, J.; Takahashi, Y.C.; Srichana, T.; Hitakomate, E.; Pientong, C.; Ekalaksananan, T.; Nittayananta, W. Mucoadhesive film containing α-Mangostin shows potential role in oral cancer treatment. BMC Oral Health 2021, 21, 512. [Google Scholar] [CrossRef] [PubMed]
- Li, R.R.; Zeng, D.Y. The effects and mechanism of α-Mangostin on chemosensitivity of gastric cancer cells. Kaohsiung J. Med. Sci. 2021, 37, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.J.; Ying, T.H.; Hsieh, S.C.; Lin, C.L.; Yu, Y.L.; Kao, S.H.; Hsieh, Y.H. α-Mangostin attenuates stemness and enhances cisplatin-induced cell death in cervical cancer stem-like cells through induction of mitochondrial-mediated apoptosis. J. Cell. Physiol. 2020, 235, 5590–5601. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. α-Mangostin Nanoparticles Cytotoxicity and Cell Death Modalities in Breast Cancer Cell Lines. Molecules 2021, 26, 5119. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Fermín, L.M.; Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Tapia, E.; Rivero, I.; Pedraza-Chaverri, J. The Protective Effect of Alpha-Mangostin against Cisplatin-Induced Cell Death in LLC-PK1 Cells is Associated to Mitochondrial Function Preservation. Antioxidants 2019, 8, 133. [Google Scholar] [CrossRef]
- Marmor, M.D.; Skaria, K.B.; Yarden, Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 903–913. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Weroha, S.J.; Molina, J.R. Oncogenic pathways, molecularly targeted therapies, and highlighted clinical trials in non-small-cell lung cancer (NSCLC). Clin. Lung Cancer. 2012, 13, 252–266. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Wang, X.; Zhao, Z.; Cai, H.; Xie, M.; Jiang, X.; Zhang, L.; Cheng, J.; Yang, L.; et al. Acetylshikonin exerts anti-tumor effects on non-small cell lung cancer through dual inhibition of STAT3 and EGFR. Phytomedicine 2022, 101, 154109. [Google Scholar] [CrossRef]
- Shen, Y.; Cai, H.; Ma, S.; Zhu, W.; Zhao, H.; Li, J.; Ye, H.; Yang, L.; Zhao, C.; Huang, X.; et al. Telocinobufagin Has Antitumor Effects in Non-Small-Cell Lung Cancer by Inhibiting STAT3 Signaling. J. Nat. Prod. 2022, 85, 765–775. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xian, J.; Zhang, R.; Wang, Z.; Zhang, S.; Zhao, D.; Sheng, J.; Sun, P. α-Mangostin Exhibits Antitumor Activity Against NCI-H1975 Cells via the EGFR/STAT3 Pathway: An Experimental and Molecular Simulation Study. Molecules 2025, 30, 1294. https://doi.org/10.3390/molecules30061294
Wang J, Xian J, Zhang R, Wang Z, Zhang S, Zhao D, Sheng J, Sun P. α-Mangostin Exhibits Antitumor Activity Against NCI-H1975 Cells via the EGFR/STAT3 Pathway: An Experimental and Molecular Simulation Study. Molecules. 2025; 30(6):1294. https://doi.org/10.3390/molecules30061294
Chicago/Turabian StyleWang, Jing, Jiamin Xian, Ruohan Zhang, Zhuoyi Wang, Shuanggou Zhang, Die Zhao, Jun Sheng, and Peiyuan Sun. 2025. "α-Mangostin Exhibits Antitumor Activity Against NCI-H1975 Cells via the EGFR/STAT3 Pathway: An Experimental and Molecular Simulation Study" Molecules 30, no. 6: 1294. https://doi.org/10.3390/molecules30061294
APA StyleWang, J., Xian, J., Zhang, R., Wang, Z., Zhang, S., Zhao, D., Sheng, J., & Sun, P. (2025). α-Mangostin Exhibits Antitumor Activity Against NCI-H1975 Cells via the EGFR/STAT3 Pathway: An Experimental and Molecular Simulation Study. Molecules, 30(6), 1294. https://doi.org/10.3390/molecules30061294





