The Advancement of Targeted Alpha Therapy and the Role of Click Chemistry Therein
Abstract
1. Introduction
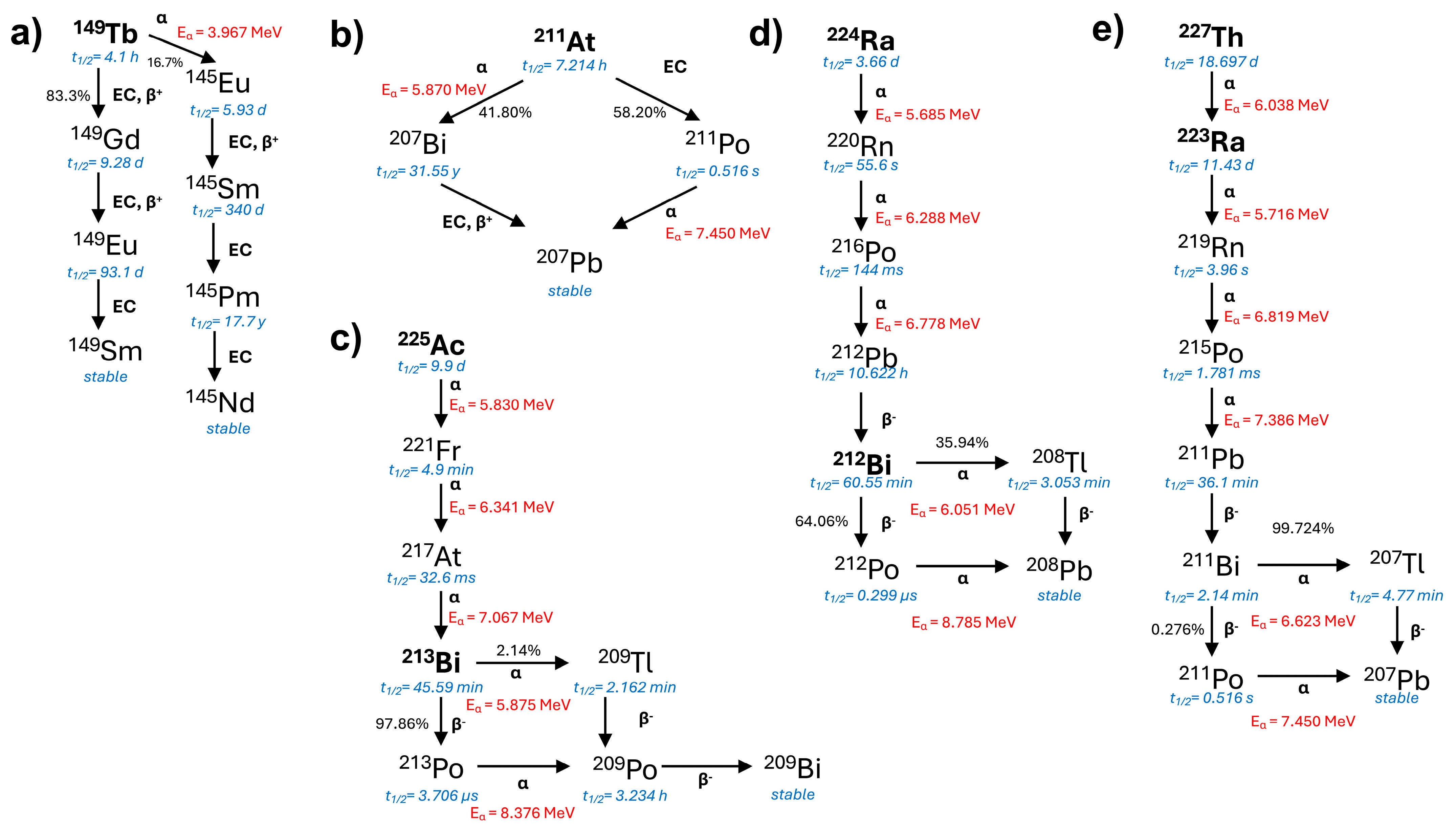
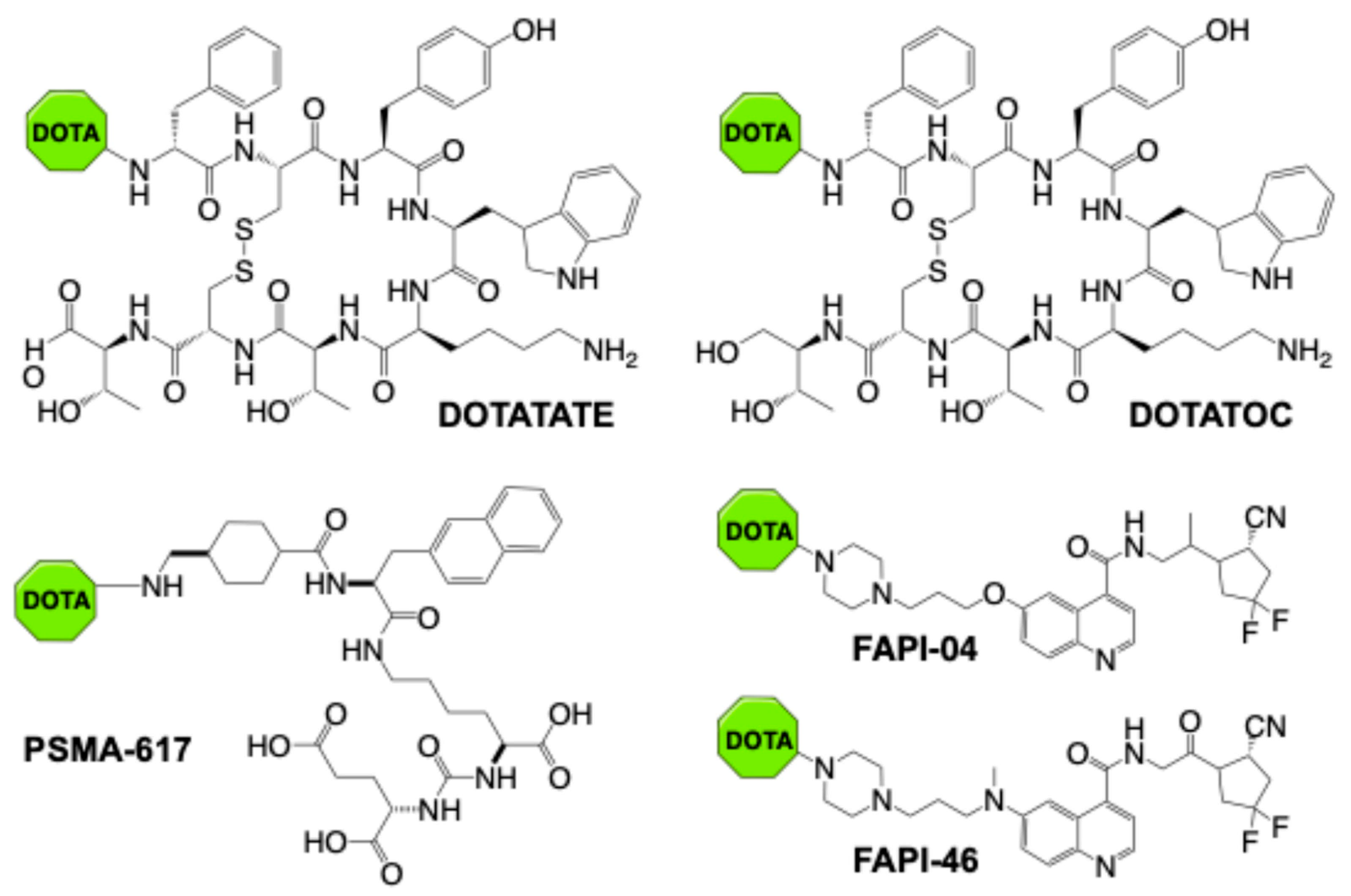
2. Targeted Alpha Therapy in Clinical Application
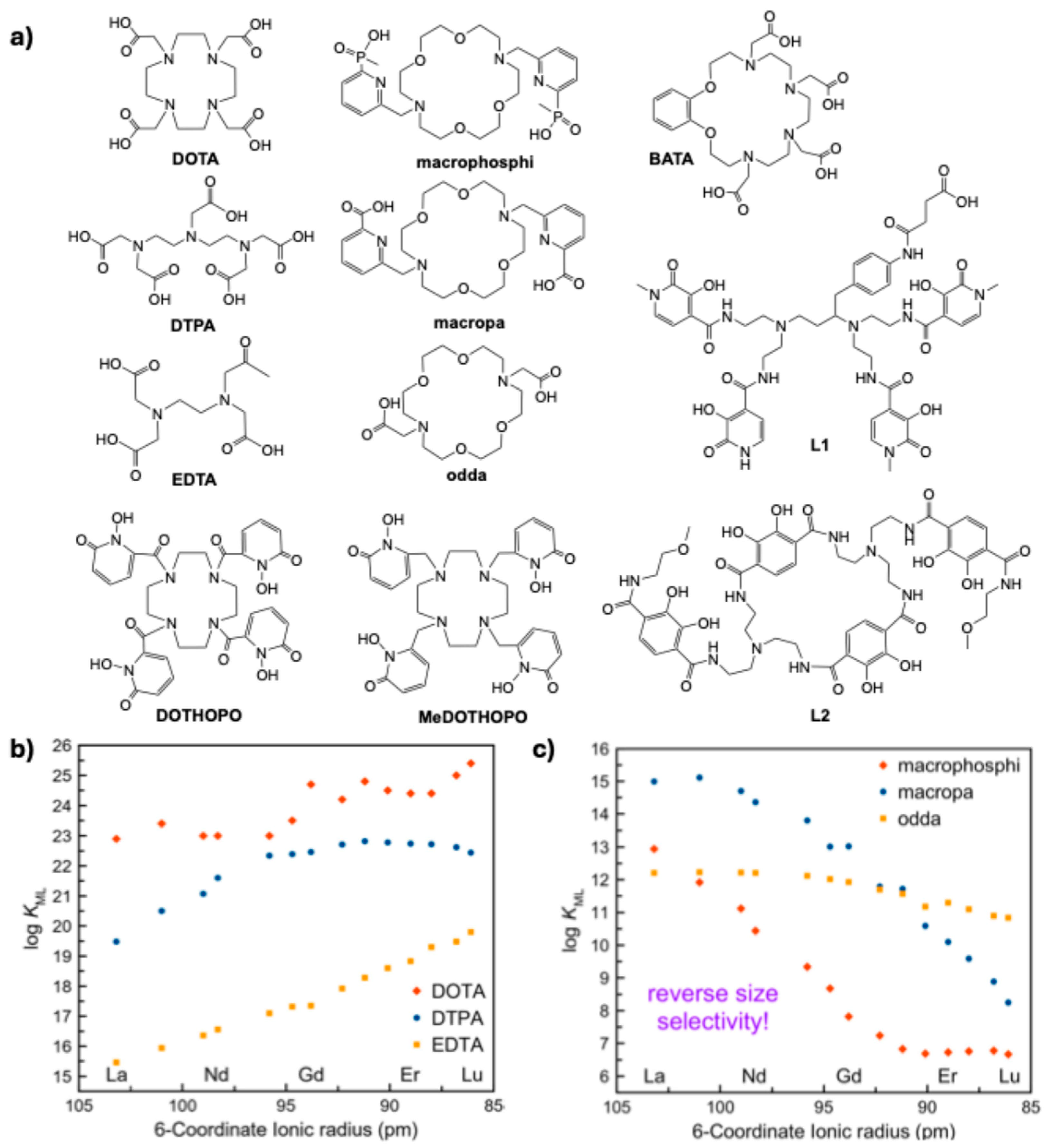
3. Considerations in Bifunctional Chelator Design for α-Emitters
4. Bioconjugation Strategies and the Role of Click Chemistry
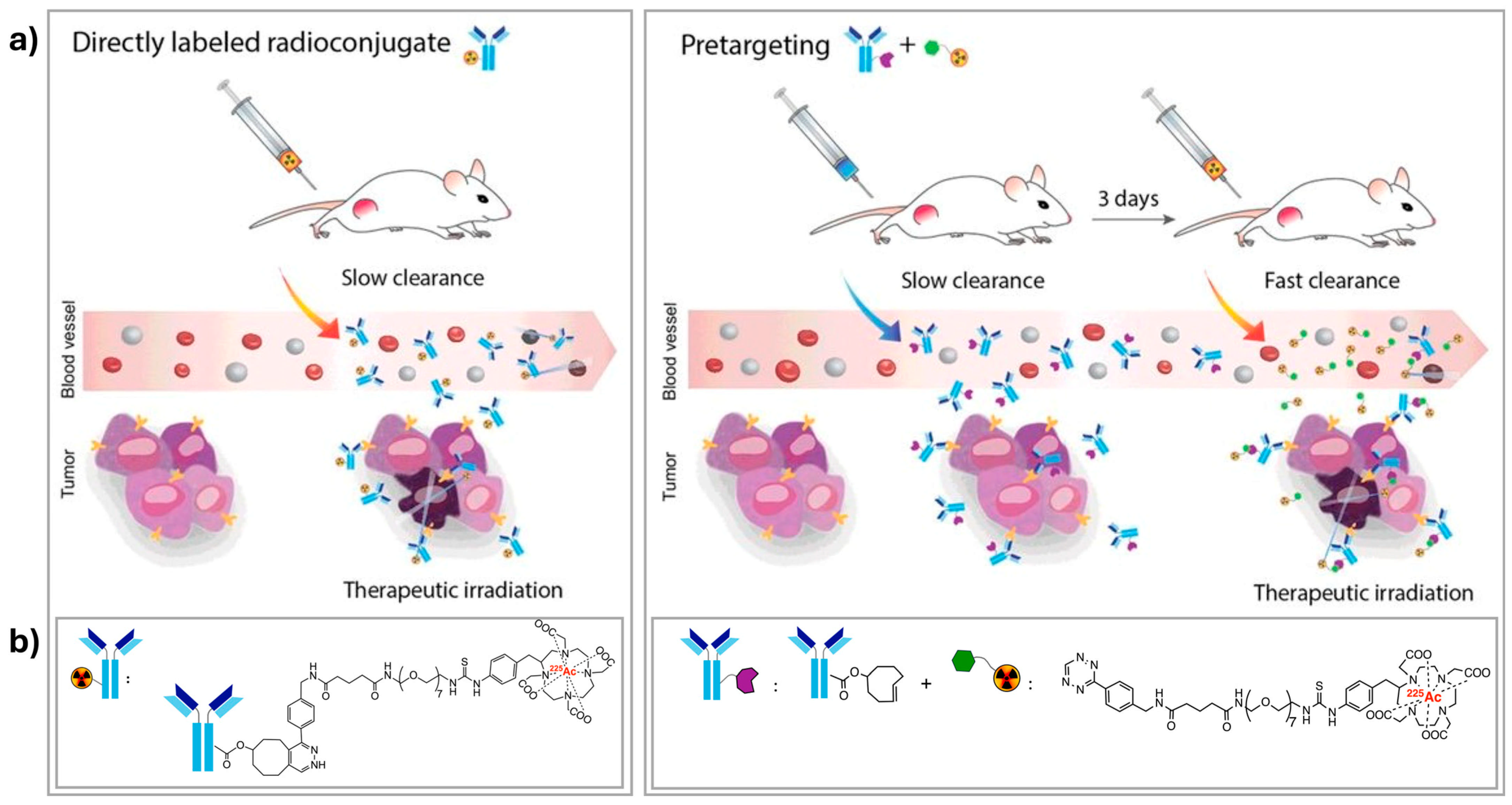
5. Pre-Targeting as a Delivering Strategy in TAT
6. Conclusions and Further Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Artigas, C.; Mileva, M.; Flamen, P.; Karfis, I. Targeted radionuclide therapy: An emerging field in solid tumours. Curr. Opin. Oncol. 2021, 33, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Liepe, K.; Runge, R.; Kotzerke, J. Systemic radionuclide therapy in pain palliation. Am. J. Hosp. Palliat. Med. 2005, 22, 457–464. [Google Scholar] [CrossRef]
- Helal, M.; Dadachova, E. Radioimmunotherapy as a Novel Approach in HIV, Bacterial, and Fungal Infectious Diseases. Cancer Biother. Radiopharm. 2018, 33, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’huyvetter, M.; Devoogdt, N. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert. Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2021, 13, 49. [Google Scholar] [CrossRef]
- Ferrier, M.G.; Radchenko, V.; Wilbur, D.S. Radiochemical aspects of alpha emitting radionuclides for medical application. Radiochim. Acta 2019, 107, 1065–1085. [Google Scholar] [CrossRef]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. alpha-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef]
- Ostuni, E.; Taylor, M.R.G. Commercial and business aspects of alpha radioligand therapeutics. Front. Med. 2022, 9, 1070497. [Google Scholar] [CrossRef]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for Targeted Therapy: Physical Properties. Molecules 2022, 27, 5429. [Google Scholar] [CrossRef]
- Pallares, R.M.; Abergel, R.J. Development of radiopharmaceuticals for targeted alpha therapy: Where do we stand? Front. Med. 2022, 9, 1020188. [Google Scholar] [CrossRef] [PubMed]
- De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Fedorchenko, D.; Alani, S. Simulation of particle release for Diffusing Alpha-Emitters Radiation Therapy. Appl. Radiat. Isot. 2023, 197, 110825. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Delaney, S.; Sarrett, S.M.; Keinänen, O.M.; Zeglis, B.M. Antibody Engineering for Nuclear Imaging and Radioimmunotherapy. J. Nucl. Med. 2022, 63, 1316–1322. [Google Scholar] [CrossRef]
- Verhoeven, M.; Seimbille, Y.; Dalm, S.U. Therapeutic Applications of Pretargeting. Pharmaceutics 2019, 11, 434. [Google Scholar] [CrossRef]
- Boerman, O.C.; van Schaijk, F.G.; Oyen, W.J.G.; Corstens, F.H.M. Pretargeted Radioimmunotherapy of Cancer: Progress Step by Step. J. Nucl. Med. 2003, 44, 400. [Google Scholar]
- Beishenaliev, A.; Loke, Y.L.; Goh, S.J.; Geo, H.N.; Mugila, M.; Misran, M.; Chung, L.Y.; Kiew, L.V.; Roffler, S.; Teo, Y.Y. Bispecific antibodies for targeted delivery of anti-cancer therapeutic agents: A review. J. Control. Release 2023, 359, 268–286. [Google Scholar] [CrossRef]
- Edelmann, M.R.; Sladojevich, F.; Husbands, S.M.; Otteneder, M.B.; Blagbrough, I.S. A Brief Review of Radiolabelling Nucleic Acid-Based Molecules for Tracking and Monitoring. J. Label. Compd. Radiopharm. 2024, 67, 410–424. [Google Scholar] [CrossRef]
- Mitry, M.M.A.; Greco, F.; Osborn, H.M.I. In Vivo Applications of Bioorthogonal Reactions: Chemistry and Targeting Mechanisms. Chem. Eur. J. 2023, 29, e202203942. [Google Scholar] [CrossRef]
- Live Chart of Nuclides. Available online: https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html (accessed on 19 February 2025).
- Van Laere, C.; Koole, M.; Deroose, C.M.; de Voorde, M.V.; Baete, K.; Cocolios, T.E.; Duchemin, C.; Ooms, M.; Cleeren, F. Terbium radionuclides for theranostic applications in nuclear medicine: From atom to bedside. Theranostics 2024, 14, 1720–1743. [Google Scholar] [CrossRef]
- Munekane, M.; Fuchigami, T.; Ogawa, K. Recent advances in the development of 225Ac- and 211At-labeled radioligands for radiotheranostics. Anal. Sci. 2024, 40, 803–826. [Google Scholar] [CrossRef] [PubMed]
- Mourtada, F.; Tomiyoshi, K.; Sims-Mourtada, J.; Mukai-Sasaki, Y.; Yagihashi, T.; Namiki, Y.; Murai, T.; Yang, D.J.; Inoue, T. Actinium-225 Targeted Agents: Where Are We Now? Brachytherapy 2023, 22, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, D.; Vatsa, R.; Sood, A. Challenges and opportunities in developing Actinium-225 radiopharmaceuticals. Nuclear Med. Commun. 2022, 43, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef]
- Juzeniene, A.; Stenberg, V.Y.; Bruland, Ø.S.; Revheim, M.-E.; Larsen, R.H. Dual targeting with 224Ra/212Pb-conjugates for targeted alpha therapy of disseminated cancers: A conceptual approach. Front. Med. 2023, 9, 1051825. [Google Scholar] [CrossRef]
- Trencsényi, G.; Csikos, C.; Képes, Z. Targeted Radium Alpha Therapy in the Era of Nanomedicine: In Vivo Results. Int. J. Mol. Sci. 2024, 25, 664. [Google Scholar] [CrossRef]
- Wick, R.R.; Gössner, W. History and current uses of 224Ra in ankylosing spondylitis and other diseases. Environ. Int. 1993, 19, 467–473. [Google Scholar] [CrossRef]
- Gupta, N.; Devgan, A.; Bansal, I.; Olsavsky, T.D.; Li, S.; Abdelbaki, A.; Kumar, Y. Usefulness of radium-223 in patients with bone metastases. Bayl. Univ. Med. Cent. Proc. 2017, 30, 424–426. [Google Scholar] [CrossRef][Green Version]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Mdlophane, A.; Maes, A.; Mokoala, K.; et al. 3mCRPC Patients Receiving 225Ac-PSMA-617 Therapy in the Post-Androgen Deprivation Therapy Setting: Response to Treatment and Survival Analysis. J. Nucl. Med. 2022, 63, 1496–1502. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: First clinical experience on the efficacy and safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Królicki, L.; Kunikowska, J.; Bruchertseifer, F.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Rola, R.; Morgenstern, A.; Rosiak, E.; et al. 225Ac- and 213Bi-Substance P Analogues for Glioma Therapy. Semin. Nucl. Med. 2020, 50, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Krolicki, L.; Morgenstern, A.; Merlo, A. Targeted Radiolabeled Compounds in Glioma Therapy. Semin. Nucl. Med. 2016, 46, 243–249. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64Cu- and 225Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef]
- Assadi, M.; Rekabpour, S.J.; Jafari, E.; Divband, G.; Nikkholgh, B.; Amini, H.; Kamali, H.; Ebrahimi, S.; Shakibazad, N.; Jokar, N.; et al. Feasibility and Therapeutic Potential of 177Lu–Fibroblast Activation Protein Inhibitor–46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 871–880. [Google Scholar] [CrossRef]
- Lepareur, N.; Ramée, B.; Mougin-Degraef, M.; Bourgeois, M. Clinical Advances and Perspectives in Targeted Radionuclide Therapy. Pharmaceutics 2023, 15, 1733. [Google Scholar] [CrossRef] [PubMed]
- Mdanda, S.; Mdlophane, A.; Ndlovu, H.; Ramonaheng, K.; Qebetu, M.; Mahapane, J.; Kgatle, M.; Mzizi, Y.; Sebatana, R.; Cele, Z.E.D.; et al. Targeted Alpha Therapy in Cancer Management: Therapeutic Prospects of Nuclear Medicine in Oncology. In Interdisciplinary Cancer Research; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–26. [Google Scholar] [CrossRef]
- Miederer, M.; Benešová-Schäfer, M.; Mamat, C.; Kästner, D.; Pretze, M.; Michler, E.; Brogsitter, C.; Kotzerke, J.; Kopka, K.; Scheinberg, D.A.; et al. Alpha-Emitting Radionuclides: Current Status and Future Perspectives. Pharmaceuticals 2024, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front. Med. 2023, 9, 1076210. [Google Scholar] [CrossRef]
- Jurcic, J.G. Targeted Alpha-Particle Therapy for Hematologic Malignancies. Semin. Nucl. Med. 2020, 50, 152–161. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Baranyai, Z.; Tircsó, G.; Rösch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020, 2020, 36–56. [Google Scholar] [CrossRef]
- Peters, J.A.; Djanashvili, K.; Geraldes, C.F.G.C.; Platas-Iglesias, C. The chemical consequences of the gradual decrease of the ionic radius along the Ln-series. Coord. Chem. Rev. 2020, 406, 213146. [Google Scholar] [CrossRef]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef]
- Deal, K.A.; Davis, I.A.; Mirzadeh, S.; Kennel, S.J.; Brechbiel, M.W. Improved in Vivo Stability of Actinium-225 Macrocyclic Complexes. J. Med. Chem. 1999, 42, 2988–2992. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Simms, M.E.; Bryantsev, V.S.; Benny, P.D.; Griswold, J.R.; Delmau, L.H.; Thiele, N.A. Elucidating the coordination chemistry of the radium ion for targeted alpha therapy. Chem. Commun. 2022, 58, 9938–9941. [Google Scholar] [CrossRef]
- Matazova, E.V.; Egorova, B.V.; Zubenko, A.D.; Pashanova, A.V.; Mitrofanov, A.A.; Fedorova, O.A.; Ermolaev, S.V.; Vasiliev, A.N.; Kalmykov, S.N. Insights into Actinium Complexes with Tetraacetates—AcBATA versus AcDOTA: Thermodynamic, Structural, and Labeling Properties. Inorg. Chem. 2023, 62, 12223–12236. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.P.; Lohrey, T.D.; Booth, C.H.; Carter, K.P.; Parker, B.F.; Larsen, Å.; Smeets, R.; Ryan, O.B.; Cuthbertson, A.S.; Abergel, R.J. Solution Thermodynamics and Kinetics of Metal Complexation with a Hydroxypyridinone Chelator Designed for Thorium-227 Targeted Alpha Therapy. Inorg. Chem. 2018, 57, 14337–14346. [Google Scholar] [CrossRef]
- Pham, T.A.; Xu, J.; Raymond, K.N. A Macrocyclic Chelator with Unprecedented Th4+ Affinity. J. Am. Chem. Soc. 2014, 136, 9106–9115. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.J.; Cosby, A.G.; Wacker, J.N.; Aguirre Quintana, L.M.; Peterson, A.; Minasian, S.G.; Abergel, R.J. Macrocyclic 1,2-Hydroxypyridinone-Based Chelators as Potential Ligands for Thorium-227 and Zirconium-89 Radiopharmaceuticals. Inorg. Chem. 2023, 62, 20721–20732. [Google Scholar] [CrossRef] [PubMed]
- Szucs, Z.; van Rooyen, J.; Zeevaart, J.R. Recoil effect on beta-decaying in vivo generators, interpreted for 103Pd/103mRh. Appl. Radiat. Isot. 2009, 67, 1401–1404. [Google Scholar] [CrossRef]
- Hu, A.; Wilson, J.J. Advancing Chelation Strategies for Large Metal Ions for Nuclear Medicine Applications. Acc. Chem. Res. 2022, 55, 904–915. [Google Scholar] [CrossRef]
- Spicer, C.D.; Pashuck, E.T.; Stevens, M.M. Achieving Controlled Biomolecule–Biomaterial Conjugation. Chem. Rev. 2018, 118, 7702–7743. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Ma, D.; Simon, J.; Frank, R.K.; Scheinberg, D.A. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002, 57, 841–847. [Google Scholar] [CrossRef]
- Borchardt, P.E.; Yuan, R.R.; Miederer, M.; McDevitt, M.R.; Scheinberg, D.A. Targeted actinium-225 in vivo generators for therapy of ovarian cancer. Cancer Res. 2003, 63, 5084–5090. [Google Scholar]
- Maguire, W.F.; McDevitt, M.R.; Smith-Jones, P.M.; Scheinberg, D.A. Efficient 1-step radiolabeling of monoclonal antibodies to high specific activity with 225Ac for α-particle radioimmunotherapy of cancer. J. Nucl. Med. 2014, 55, 1492–1498. [Google Scholar] [CrossRef]
- Poty, S.; Membreno, R.; Glaser, J.M.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. The inverse electron-demand Diels–Alder reaction as a new methodology for the synthesis of 225Ac-labelled radioimmunoconjugates. Chem. Commun. 2018, 54, 2599–2602. [Google Scholar] [CrossRef] [PubMed]
- Denk, C.; Wilkovitsch, M.; Aneheim, E.; Herth, M.M.; Jensen, H.; Lindegren, S.; Mikula, H. Multifunctional Clickable Reagents for Rapid Bioorthogonal Astatination and Radio-Crosslinking. ChemPlusChem 2019, 84, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Reissig, F.; Bauer, D.; Zarschler, K.; Novy, Z.; Bendova, K.; Ludik, M.-C.; Kopka, K.; Pietzsch, H.-J.; Petrik, M.; Mamat, C. Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study. Cancers 2021, 13, 1974. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Cornejo, M.A.; Hoang, T.T.; Lewis, J.S.; Zeglis, B.M. Click Chemistry and Radiochemistry: An Update. Bioconj. Chem. 2023, 34, 1925–1950. [Google Scholar] [CrossRef]
- Peppicelli, S.; Calorini, L.; Bianchini, F.; Papucci, L.; Magnelli, L.; Andreucci, E. Acidity and hypoxia of tumor microenvironment, a positive interplay in extracellular vesicle release by tumor cells. Cell. Oncol. 2025, 48, 27–41. [Google Scholar] [CrossRef]
- Lammers, T. Nanomedicine Tumor Targeting. Adv. Mater. 2024, 36, e2312169. [Google Scholar] [CrossRef]
- Reardan, D.T.; Meares, C.F.; Goodwin, D.A.; McTigue, M.; David, G.S.; Stone, M.R.; Leung, J.P.; Bartholomew, R.M.; Frincke, J.M. Antibodies against metal chelates. Nature 1985, 316, 265–268. [Google Scholar] [CrossRef]
- Berton, C.; Klingler, S.; Prytuliak, S.; Holland, J.P. New tactics in the design of theranostic radiotracers. npj Imaging 2024, 2, 23. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, Y.; Yang, Y.; Huang, W.; Wang, Y.; Ye, D. Recent Advances in Pretargeted Imaging of Tumors in Vivo. Anal. Sens. 2022, 2, e202200013. [Google Scholar] [CrossRef]
- Staudt, M.; Herth, M.M. Clearing and Masking Agents in Pretargeting Strategies. Pharmaceuticals 2023, 16, 497. [Google Scholar] [CrossRef]
- Goodwin, D.A.; Mears, C.F.; McTigue, M.; David, G.S. Monoclonal antibody hapten radiopharmaceutical delivery. Nuclear Med. Commun. 1986, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Cheal, S.M.; Chung, S.K.; Vaughn, B.A.; Cheung, N.-K.V.; Larson, S.M. Pretargeting: A Path Forward for Radioimmunotherapy. J. Nucl. Med. 2022, 63, 1302. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Carter, L.M.; Mandleywala, K.; Membreno, R.; Abdel-Atti, D.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. Leveraging Bioorthogonal Click Chemistry to Improve 225Ac-Radioimmunotherapy of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 868–880. [Google Scholar] [CrossRef]
- Rossin, R.; Renart Verkerk, P.; van den Bosch, S.M.; Vulders, R.C.M.; Verel, I.; Lub, J.; Robillard, M.S. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem. Int. Ed. 2010, 49, 3375–3378. [Google Scholar] [CrossRef]
- Stéen, E.J.L.; Jørgensen, J.T.; Denk, C.; Battisti, U.M.; Nørregaard, K.; Edem, P.E.; Bratteby, K.; Shalgunov, V.; Wilkovitsch, M.; Svatunek, D.; et al. Lipophilicity and Click Reactivity Determine the Performance of Bioorthogonal Tetrazine Tools in Pretargeted In Vivo Chemistry. ACS Pharmacol. Transl. Sci. 2021, 4, 824–833. [Google Scholar] [CrossRef]
- Timperanza, C.; Jensen, H.; Bäck, T.; Lindegren, S.; Aneheim, E. Pretargeted Alpha Therapy of Disseminated Cancer Combining Click Chemistry and Astatine-211. Pharmaceuticals 2023, 16, 595. [Google Scholar] [CrossRef]


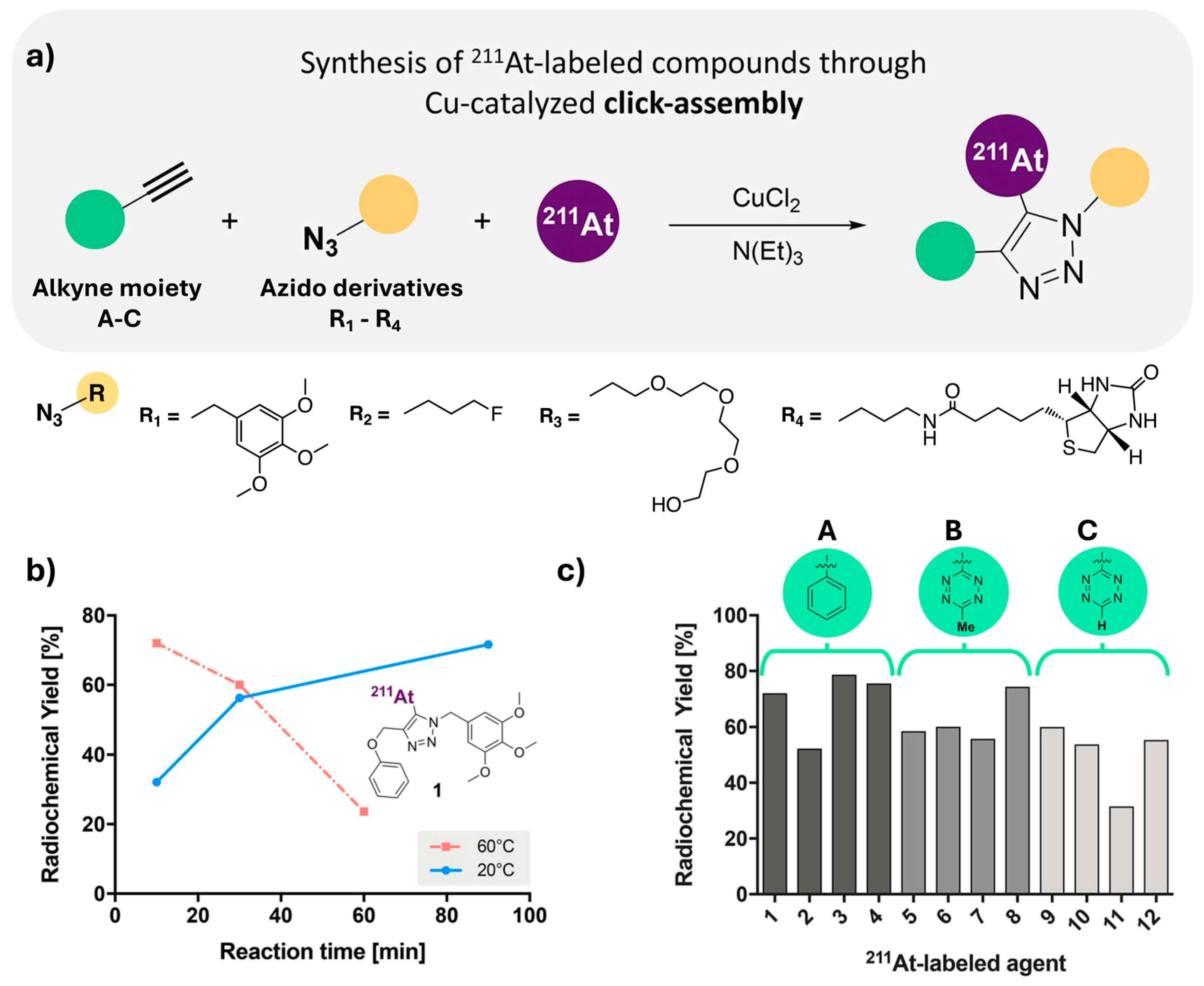
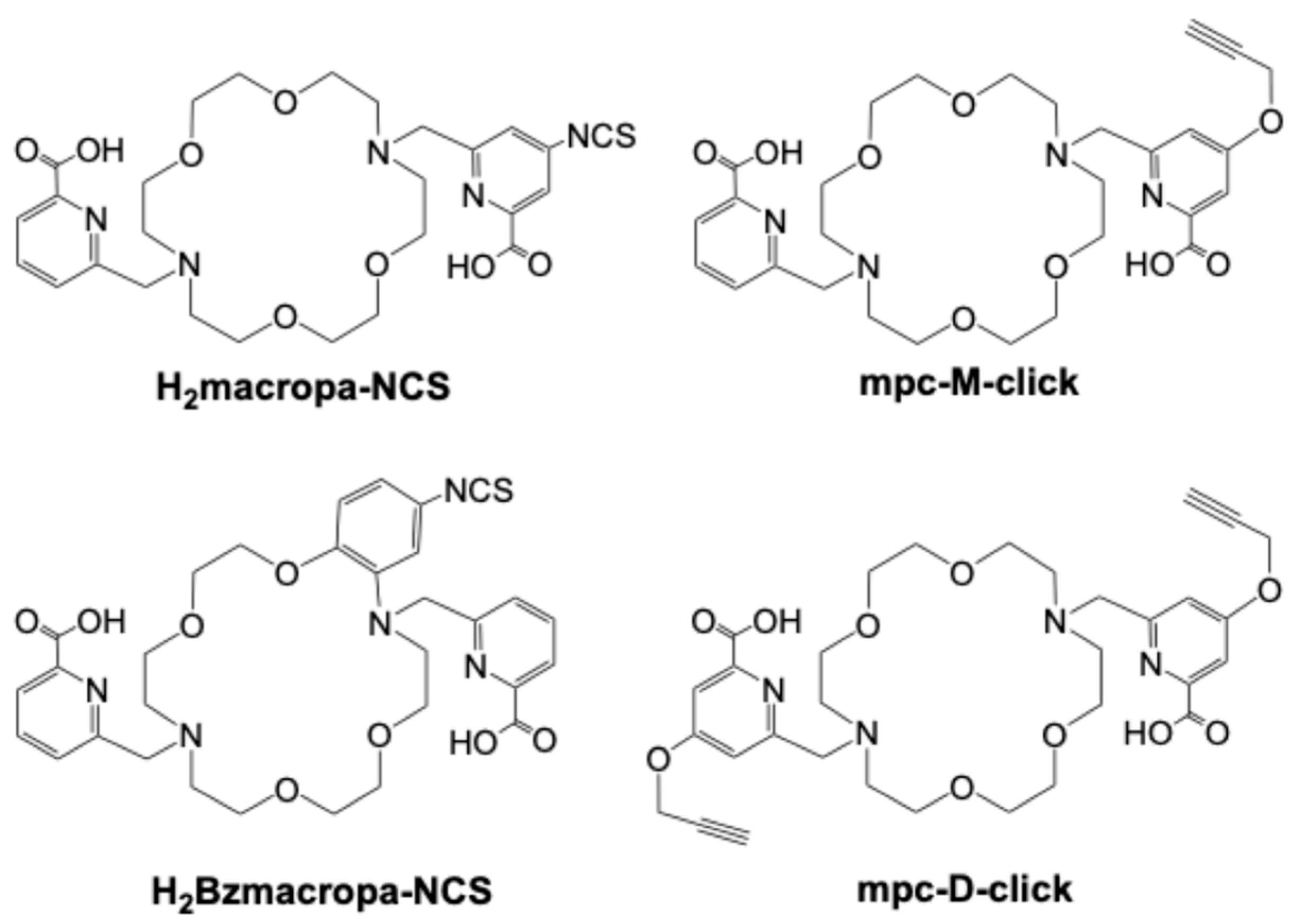
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerda, S.; de Kruijff, R.M.; Djanashvili, K. The Advancement of Targeted Alpha Therapy and the Role of Click Chemistry Therein. Molecules 2025, 30, 1296. https://doi.org/10.3390/molecules30061296
Lacerda S, de Kruijff RM, Djanashvili K. The Advancement of Targeted Alpha Therapy and the Role of Click Chemistry Therein. Molecules. 2025; 30(6):1296. https://doi.org/10.3390/molecules30061296
Chicago/Turabian StyleLacerda, Sara, Robin M. de Kruijff, and Kristina Djanashvili. 2025. "The Advancement of Targeted Alpha Therapy and the Role of Click Chemistry Therein" Molecules 30, no. 6: 1296. https://doi.org/10.3390/molecules30061296
APA StyleLacerda, S., de Kruijff, R. M., & Djanashvili, K. (2025). The Advancement of Targeted Alpha Therapy and the Role of Click Chemistry Therein. Molecules, 30(6), 1296. https://doi.org/10.3390/molecules30061296









