Design and Application of Mesoporous Catalysts for Liquid-Phase Furfural Hydrogenation
Abstract
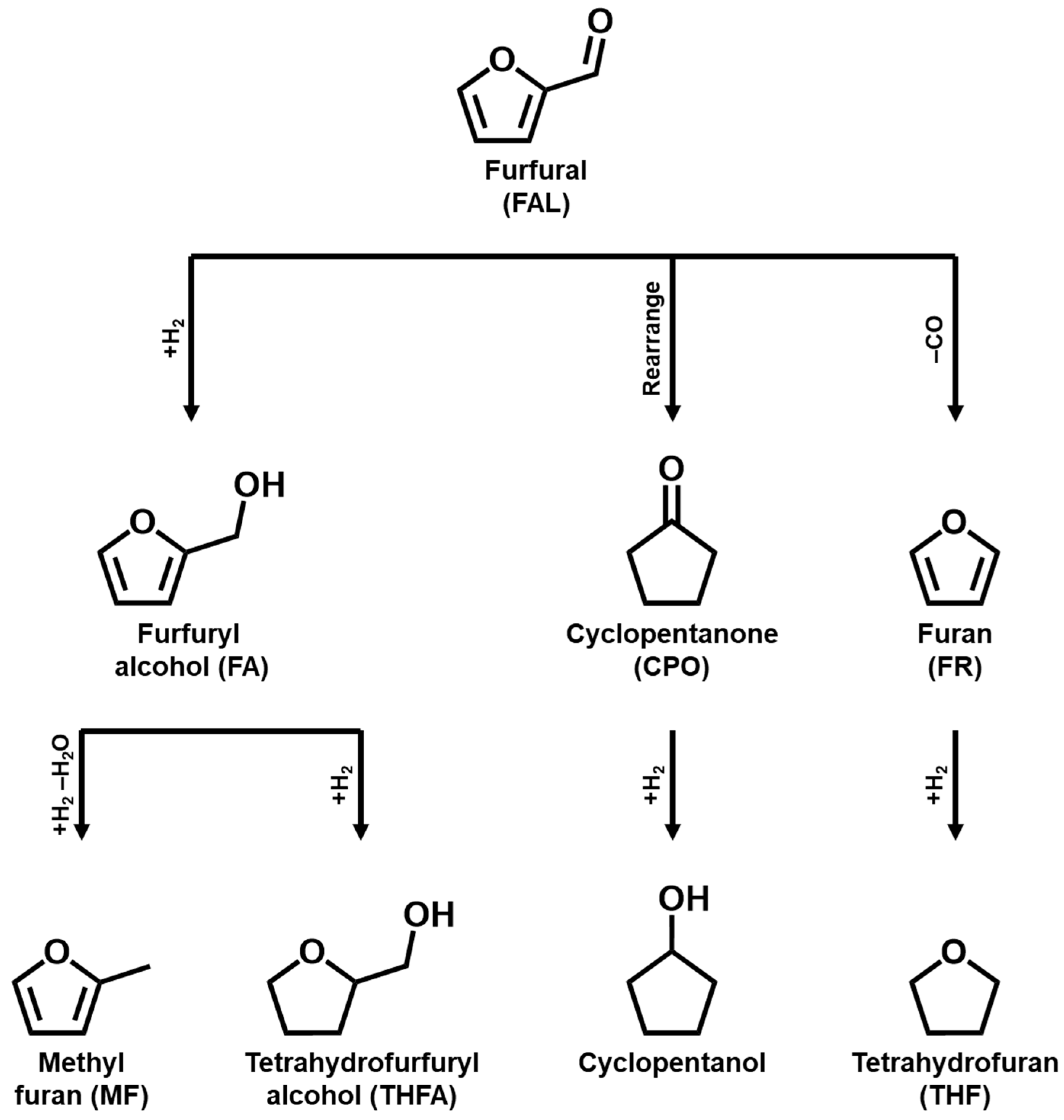
1. Introduction
2. Synthesis of Mesoporous Materials
2.1. Mesoporous Materials
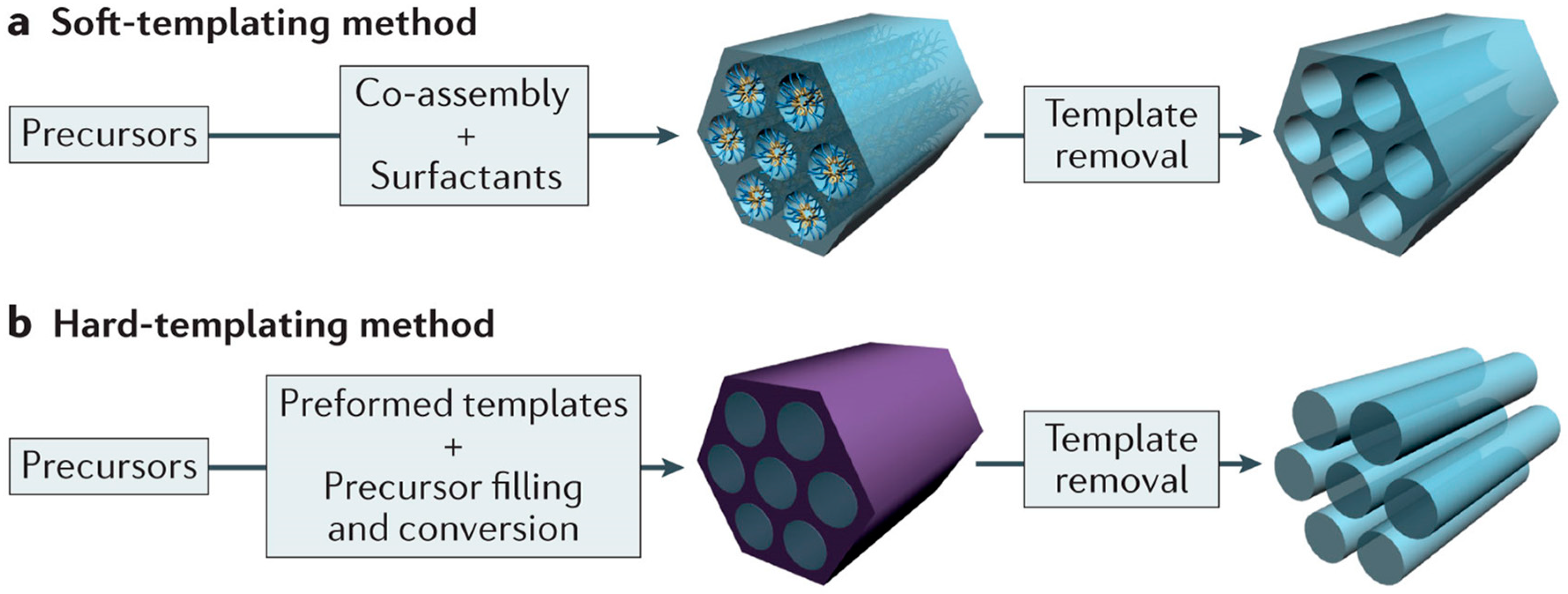
2.2. Templating Methods
2.3. Other Methods
3. Noble-Metal-Based Catalysts for FAL Hydrogenation
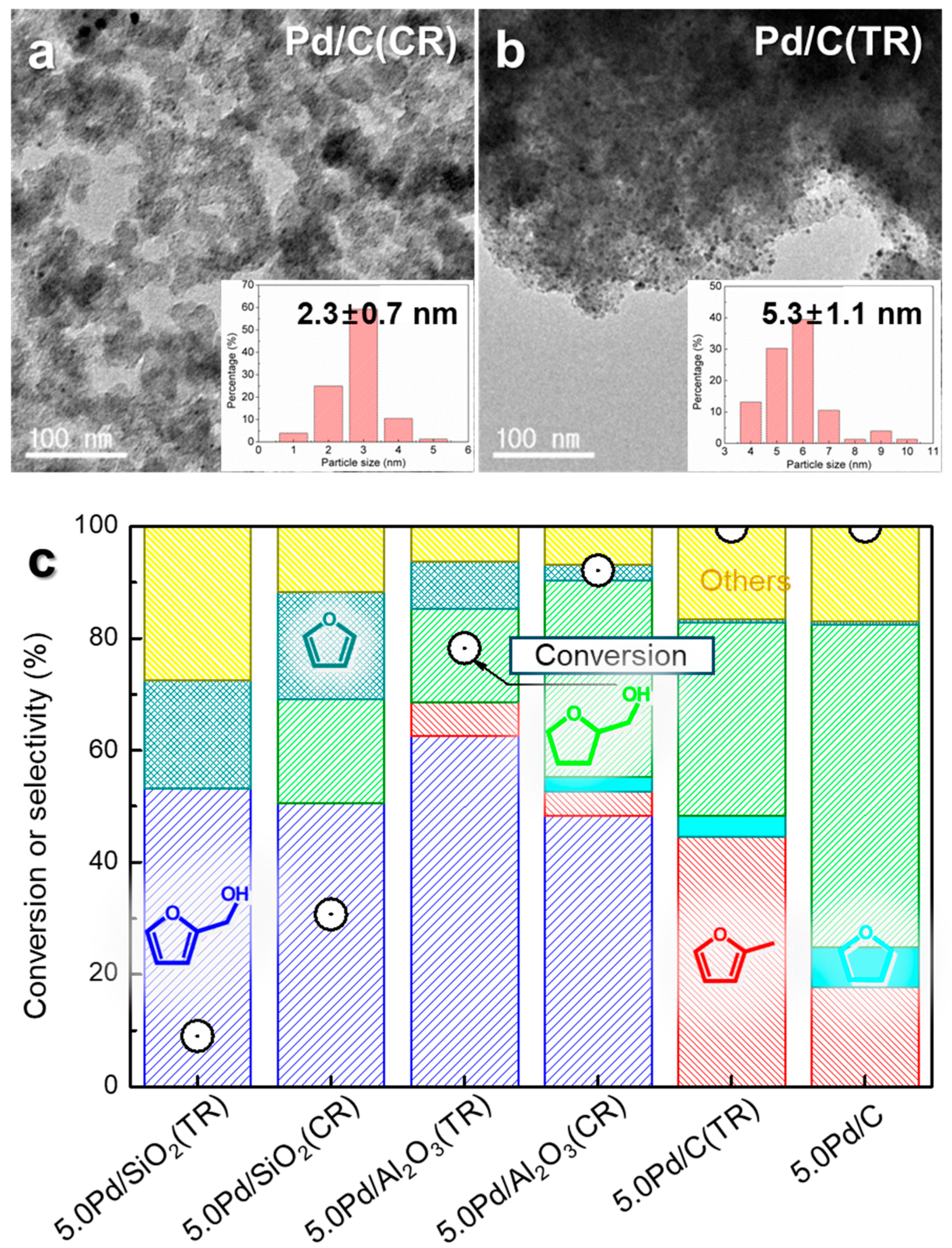
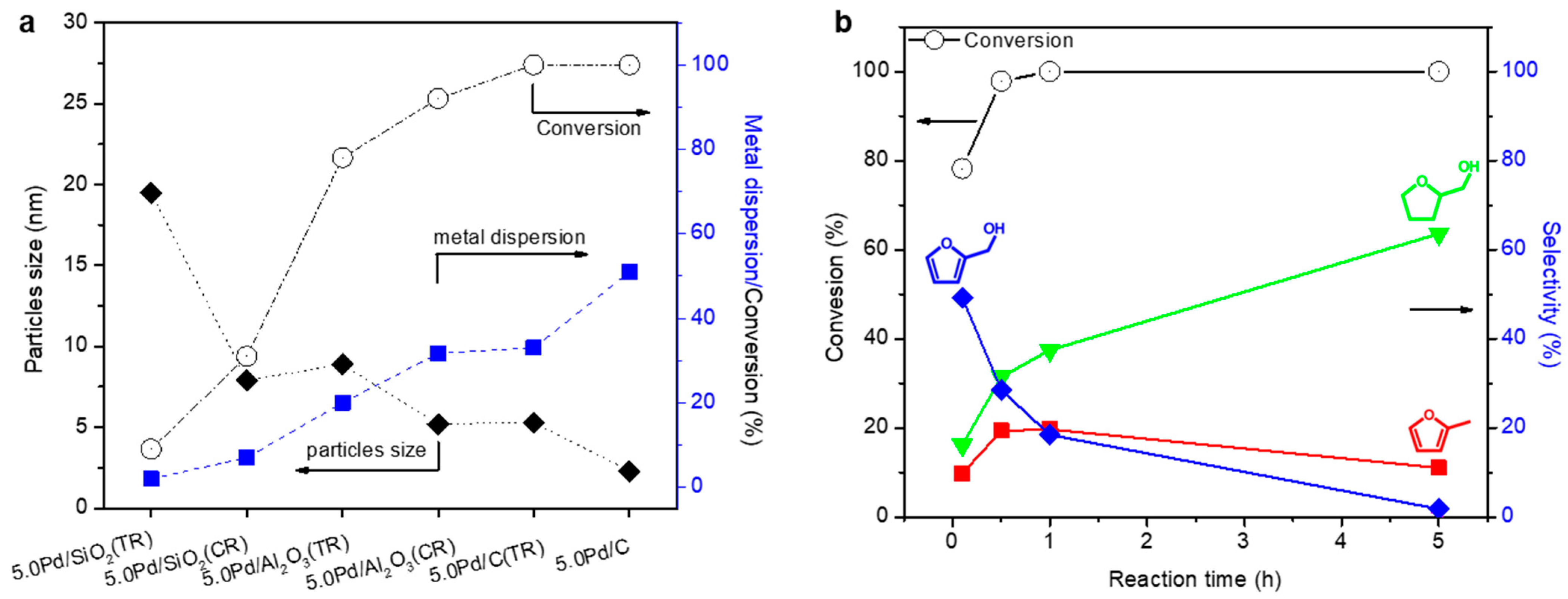
3.1. Supported Pd Nanoparticle Catalysts
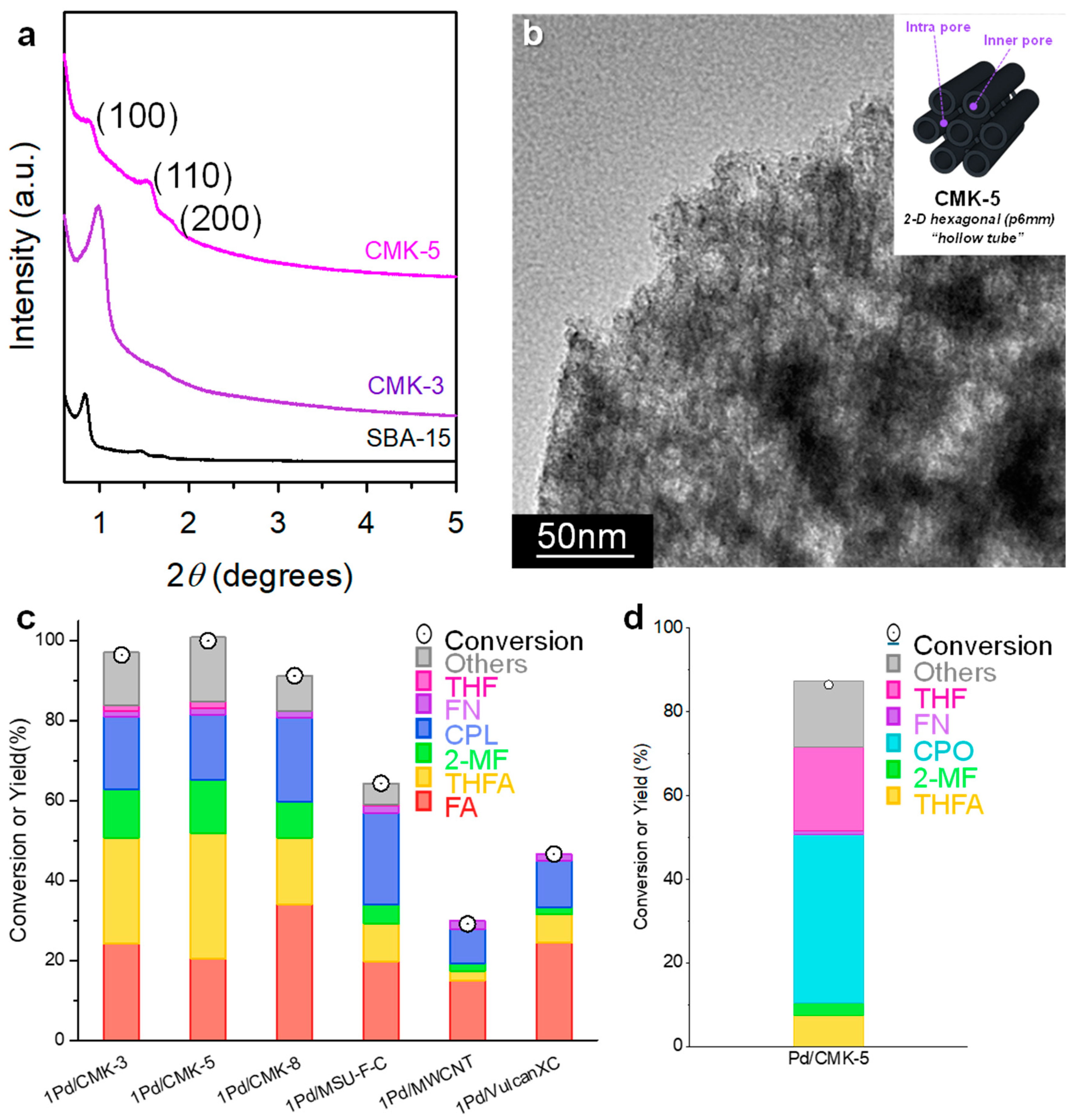
3.2. Highly Dispersed Pd Catalysts Supported on Various Carbons
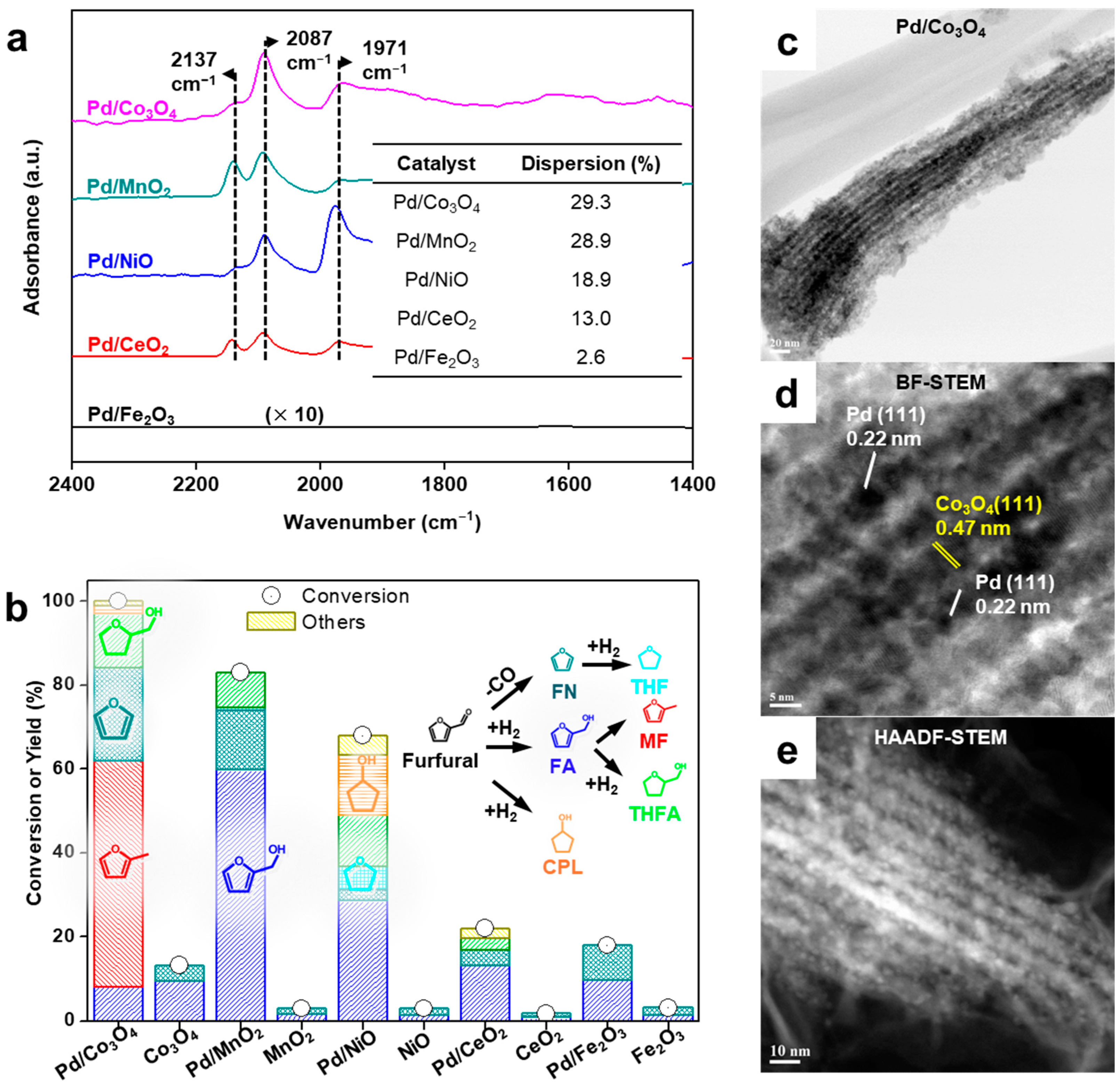
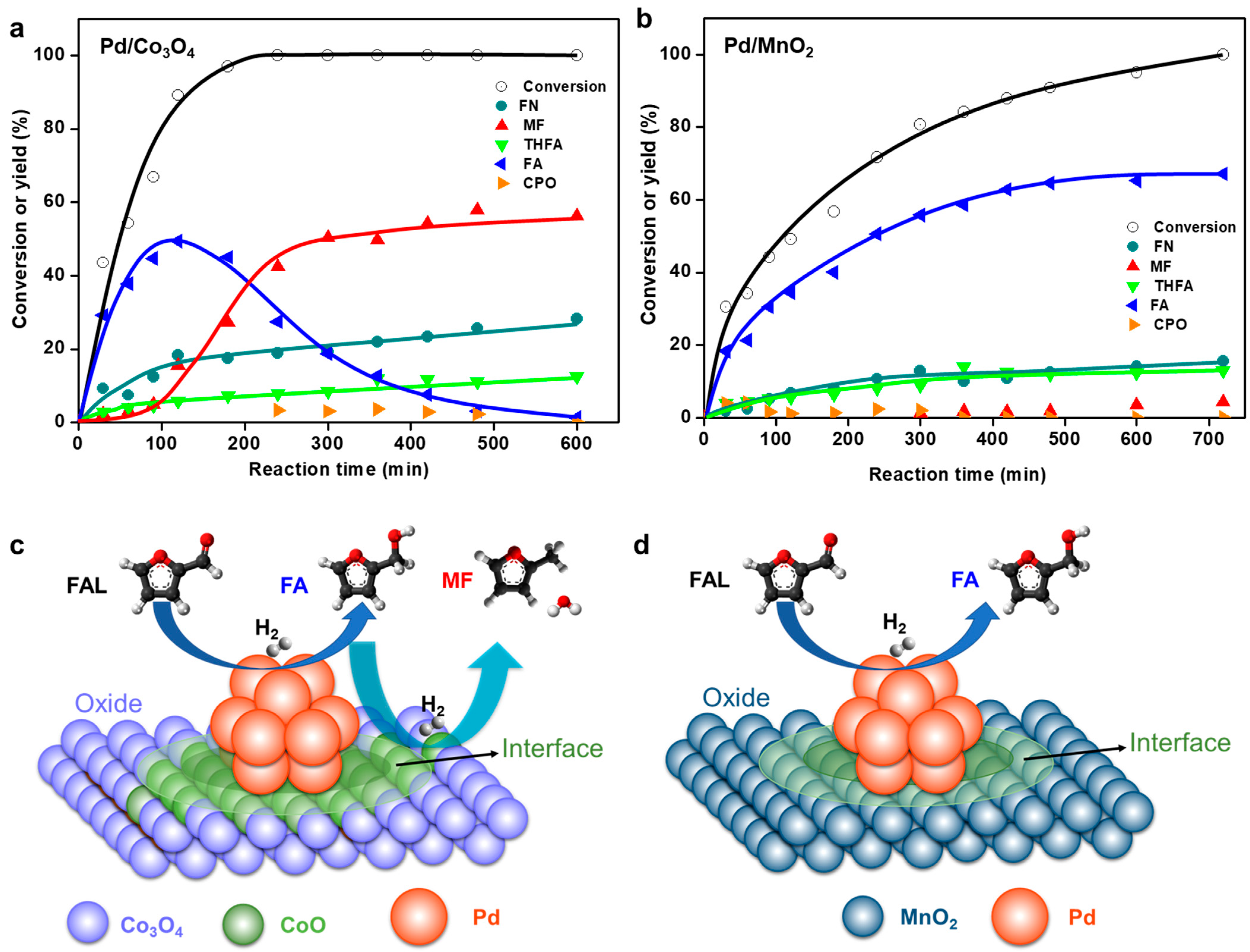
3.3. Interfacial Effect of Pd Supported on Mesoporous Oxides
3.4. Overview of Noble Metal Catalysis
4. Transition-Metal-Based Catalysts for FAL Hydrogenation
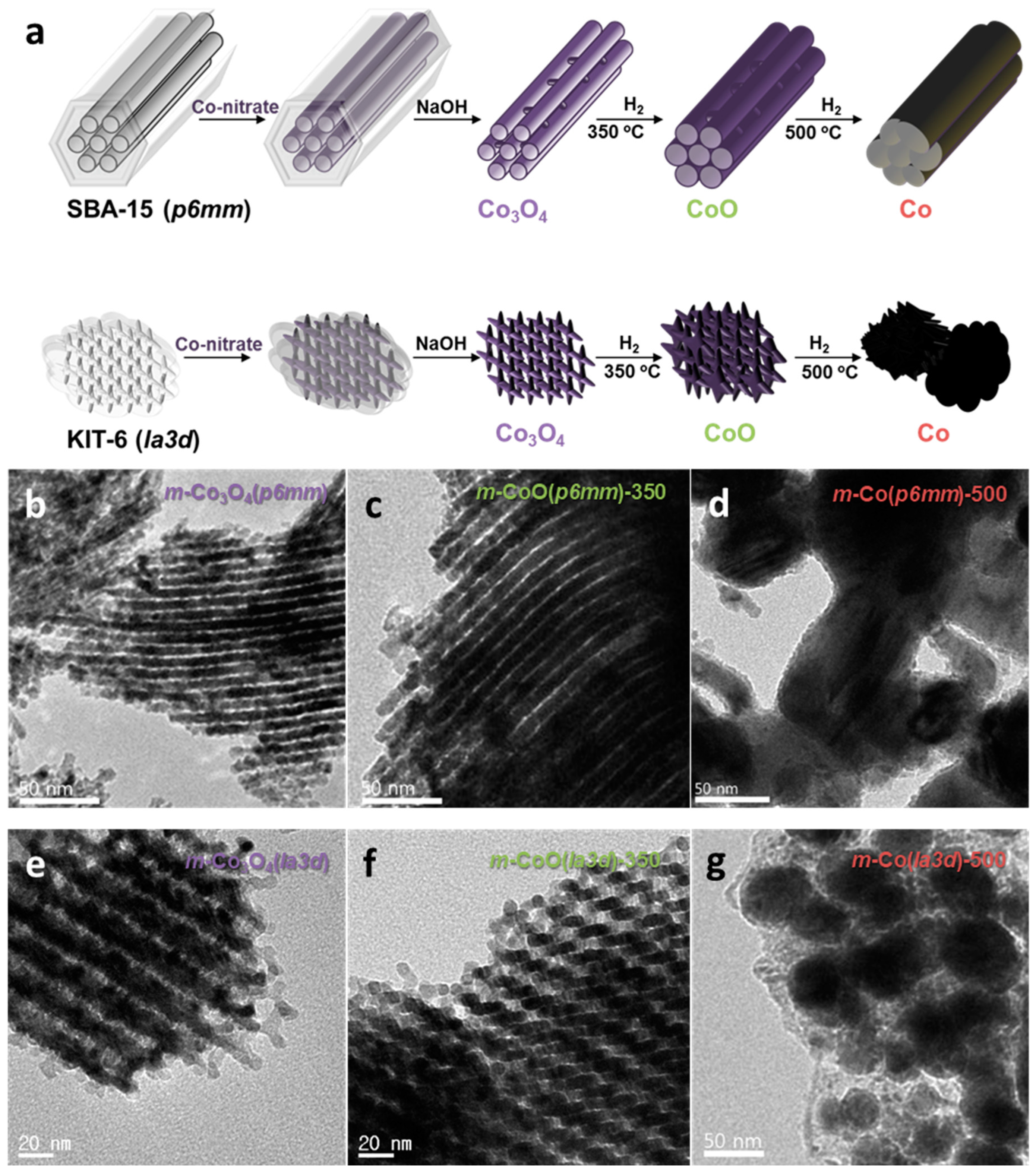
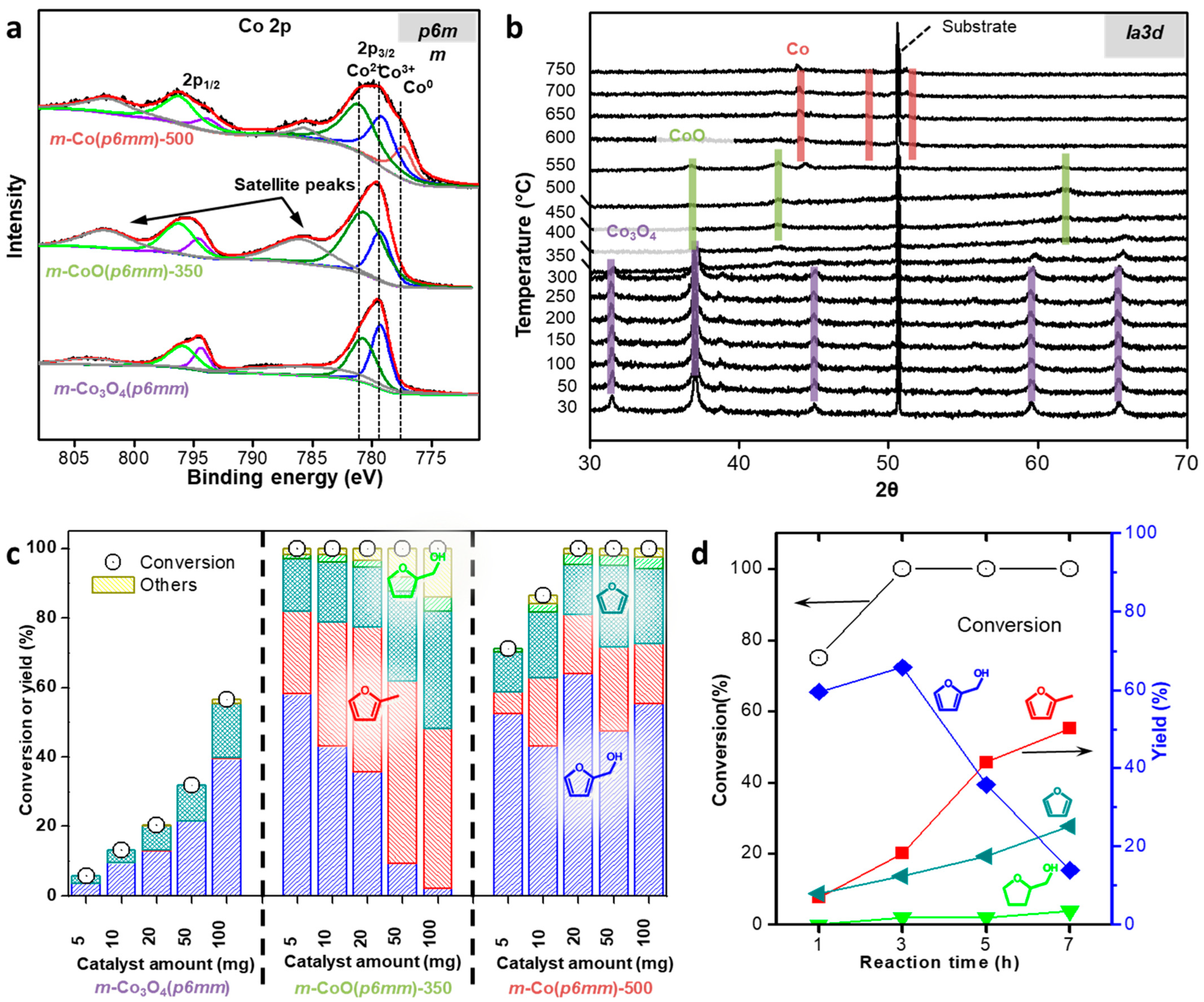
4.1. Structure-Dependent Catalytic Properties of Mesoporous Cobalt Oxides
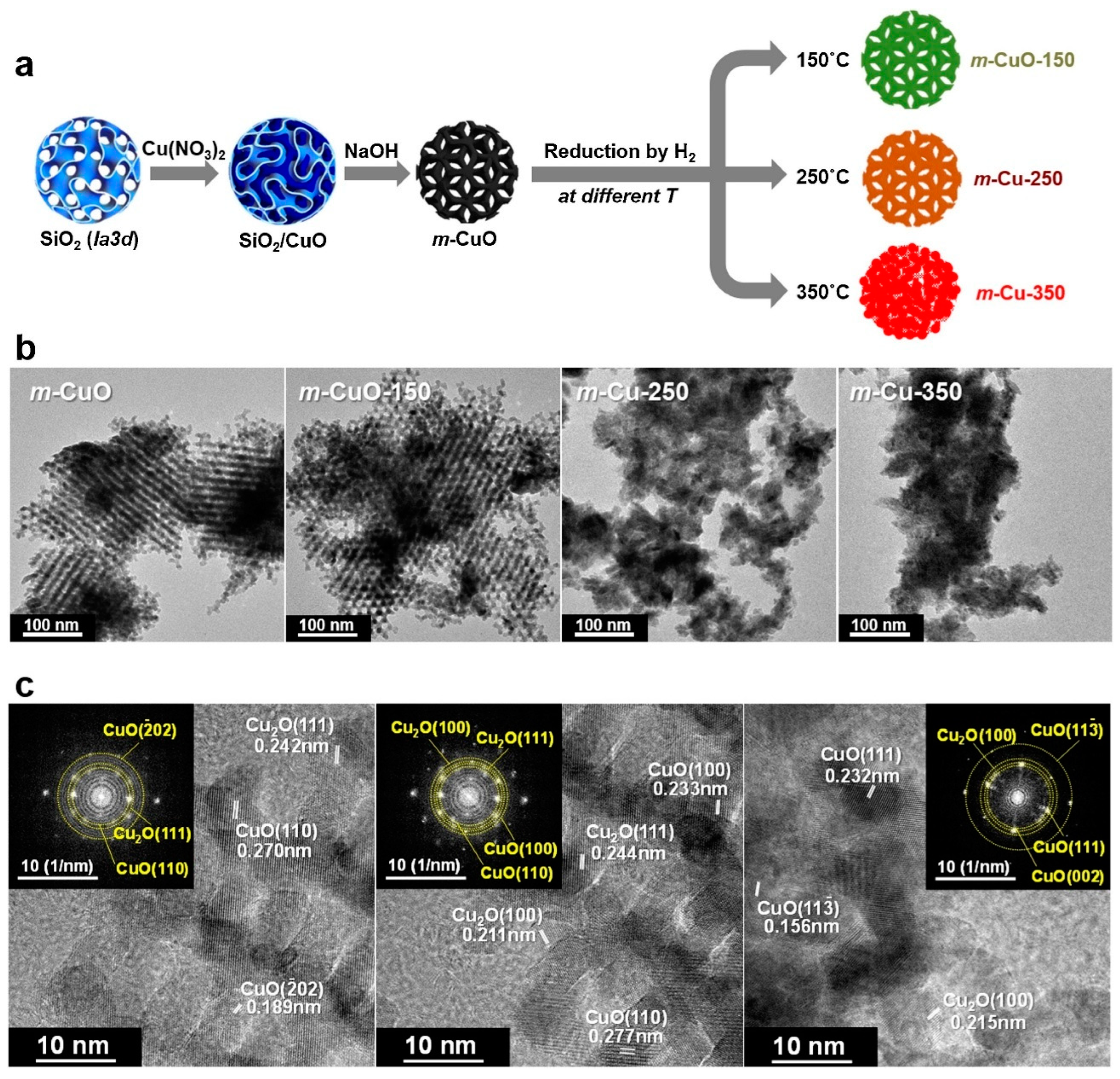
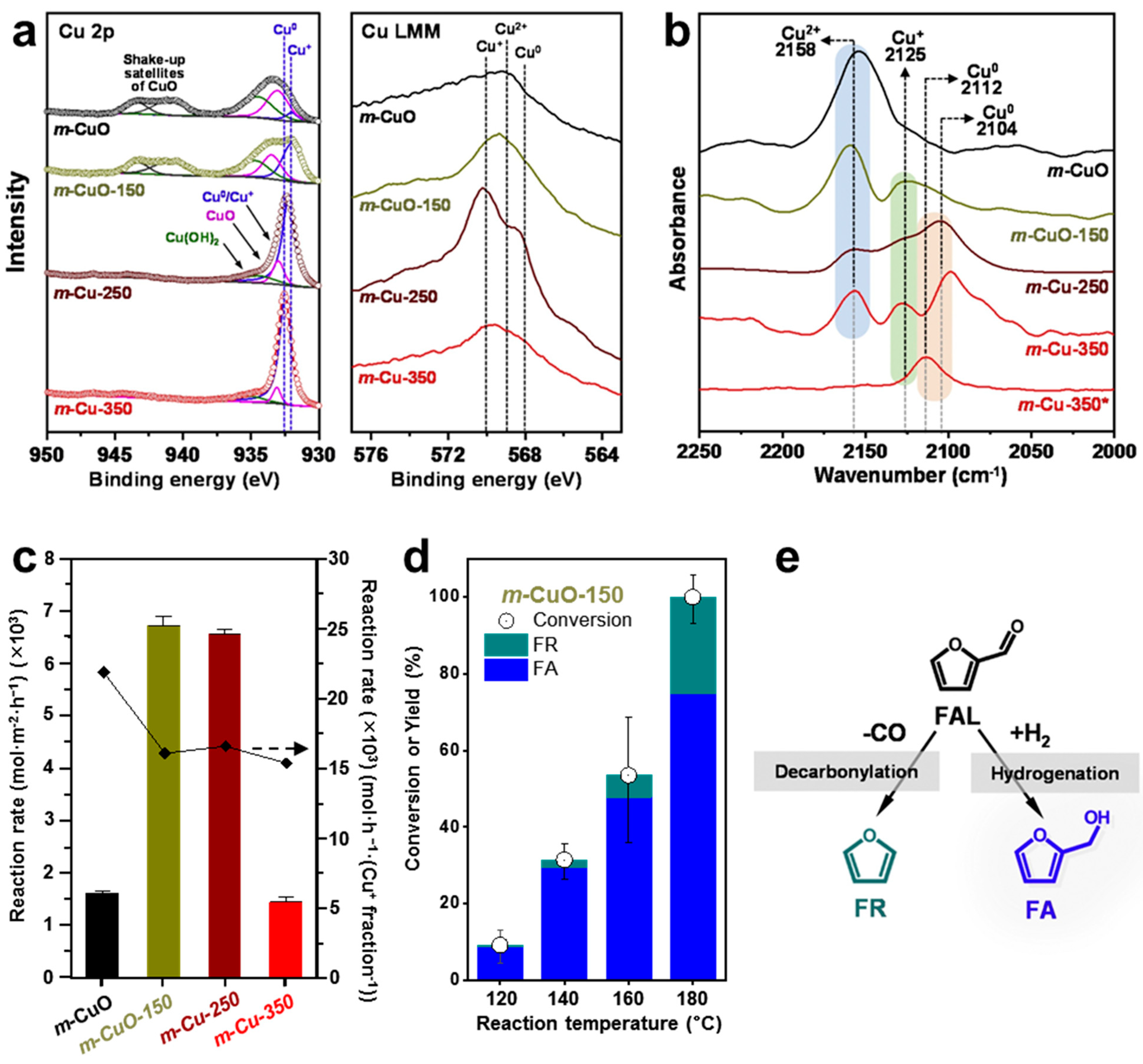
4.2. Cu2O (100) Surface as an Active Site
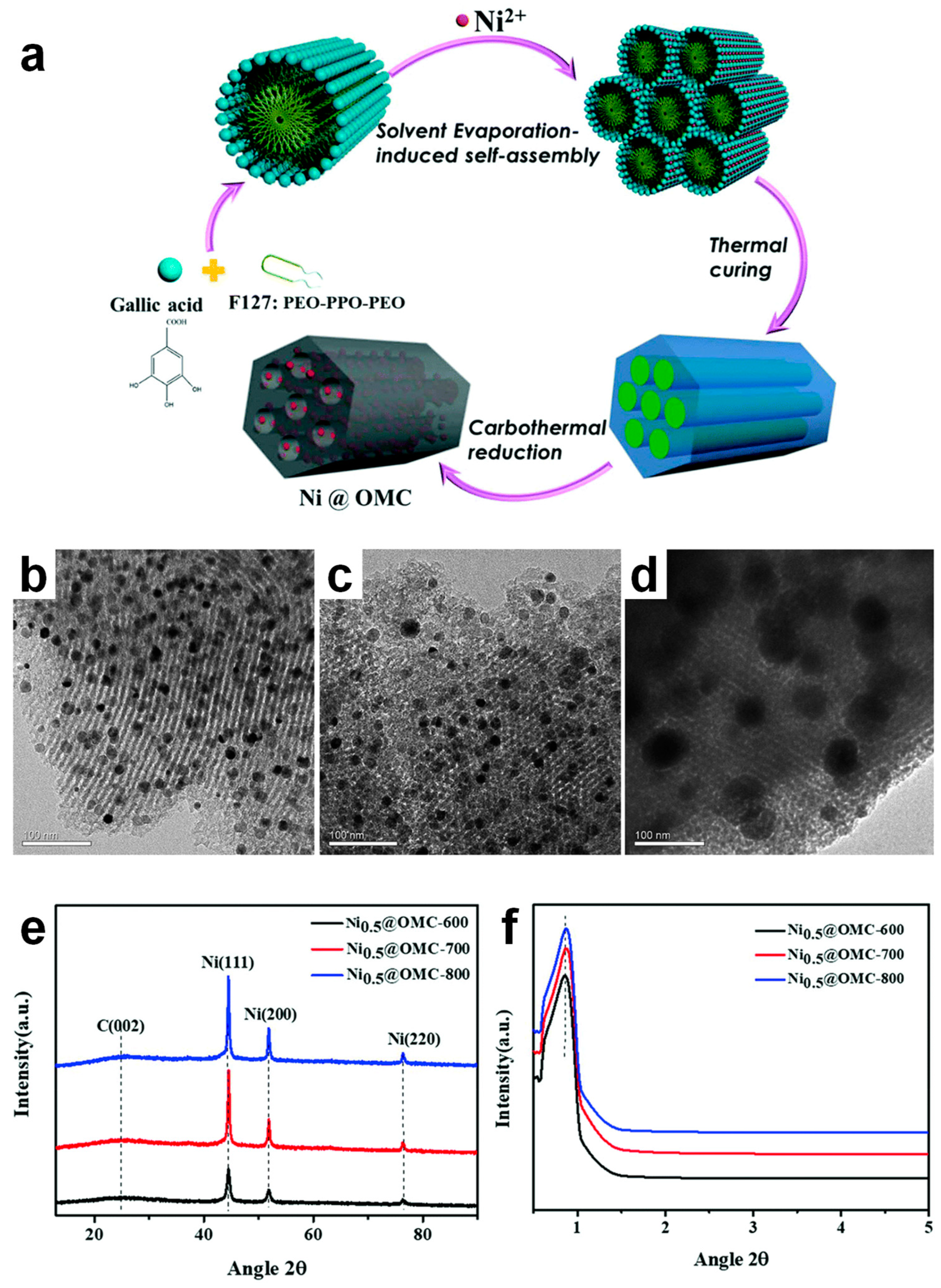
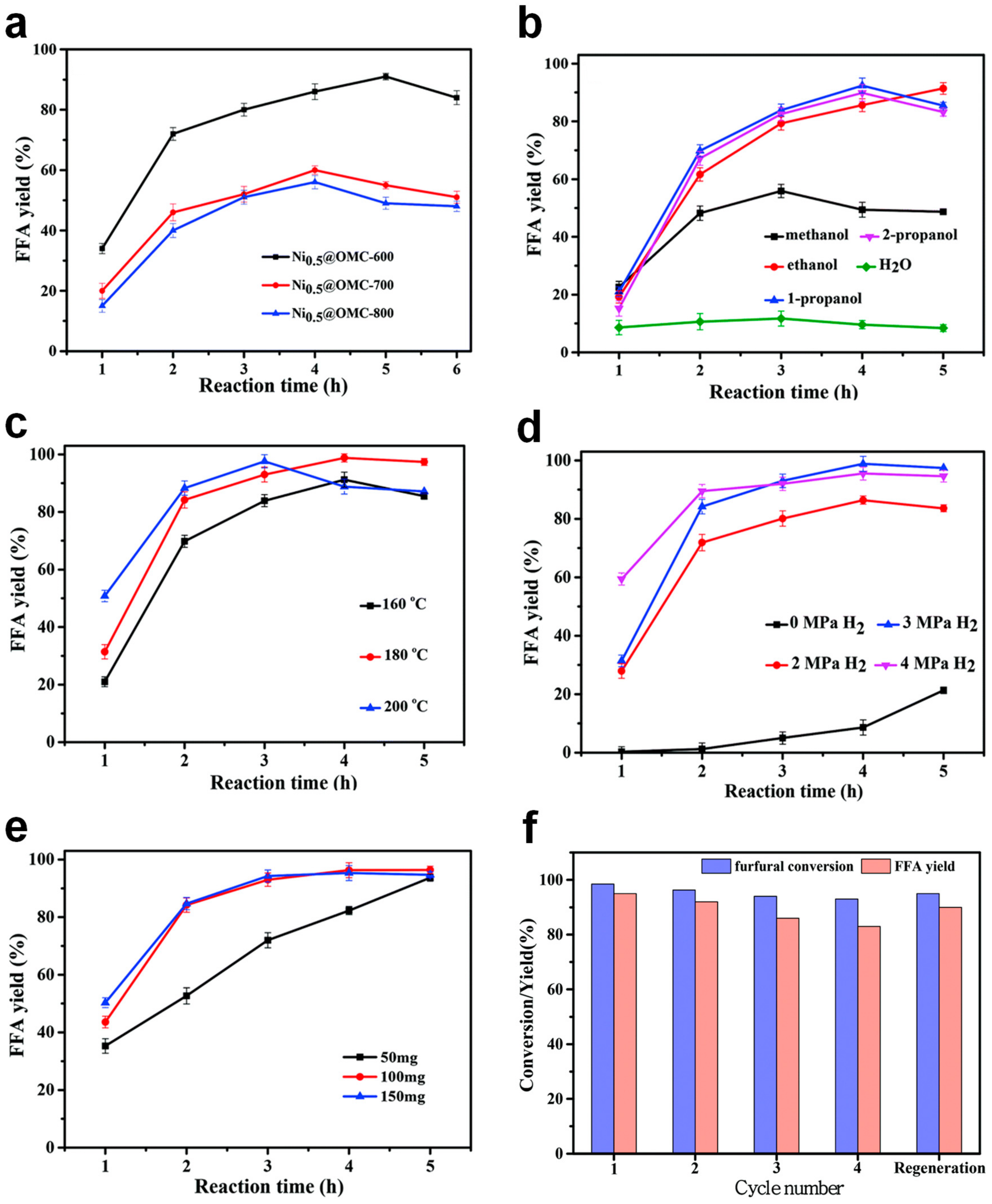
4.3. Ni-Doped Ordered Mesoporous Carbon Catalysts
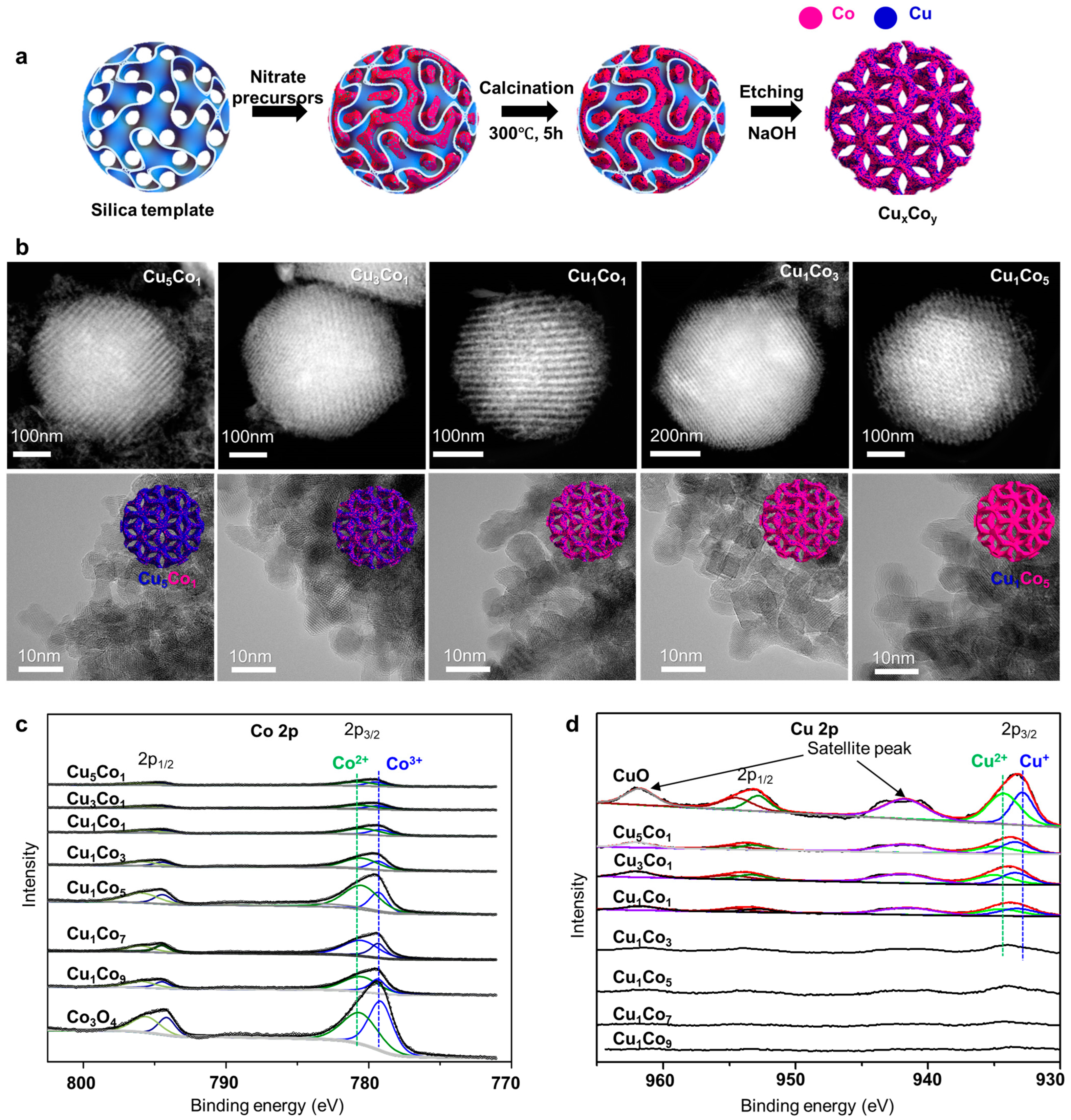
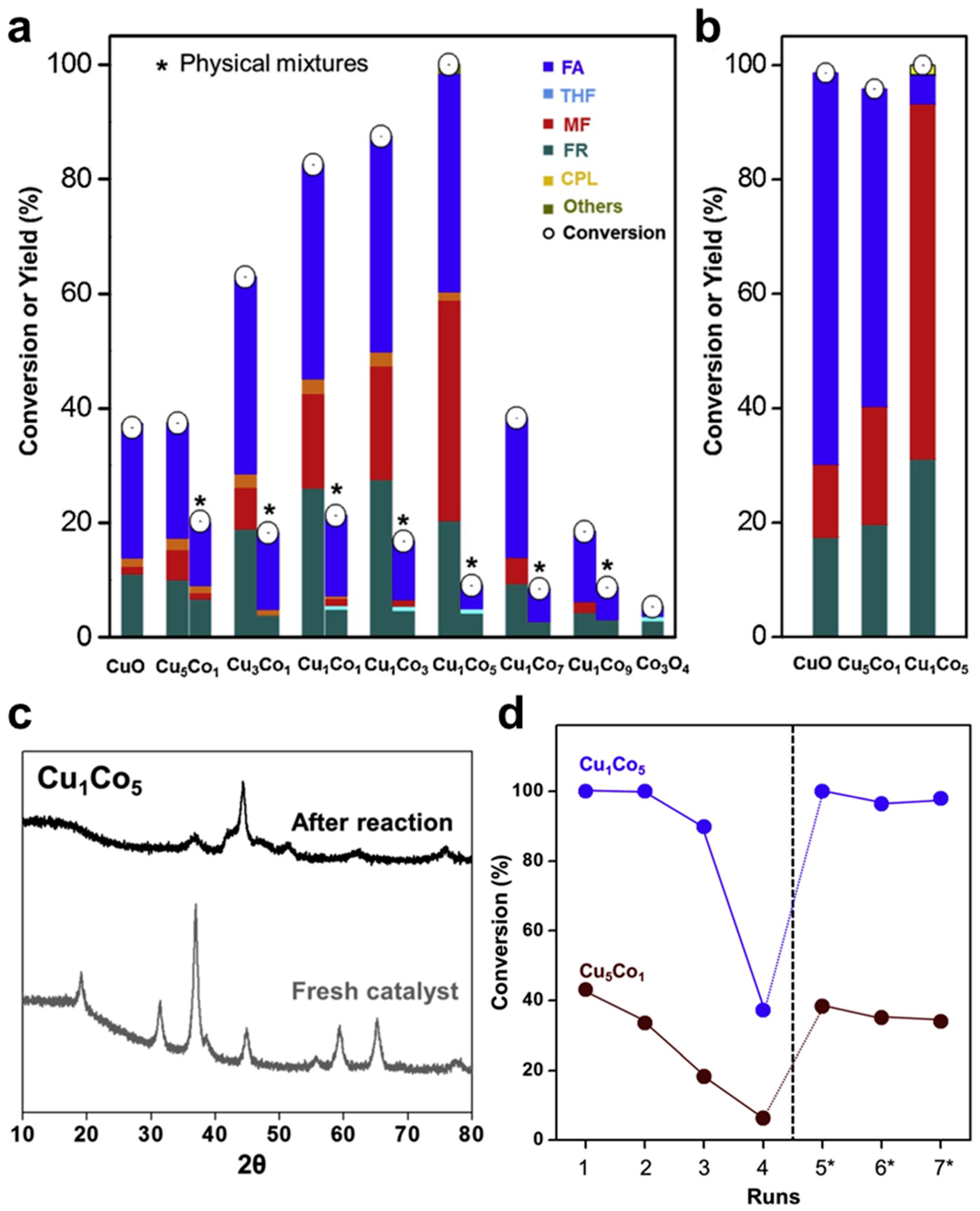
4.4. Mesoporous Mixed CuCo Oxides as Robust Catalysts
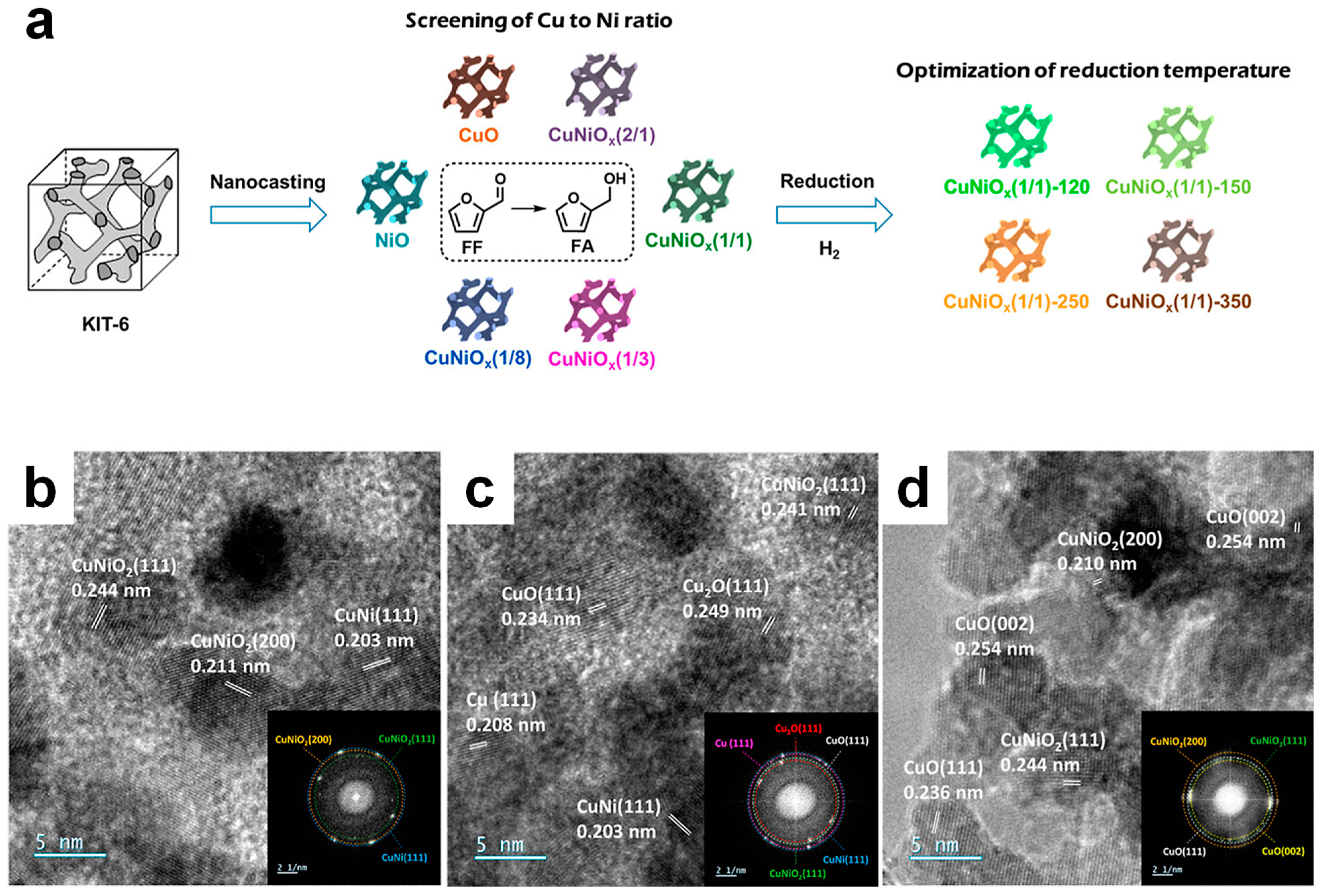
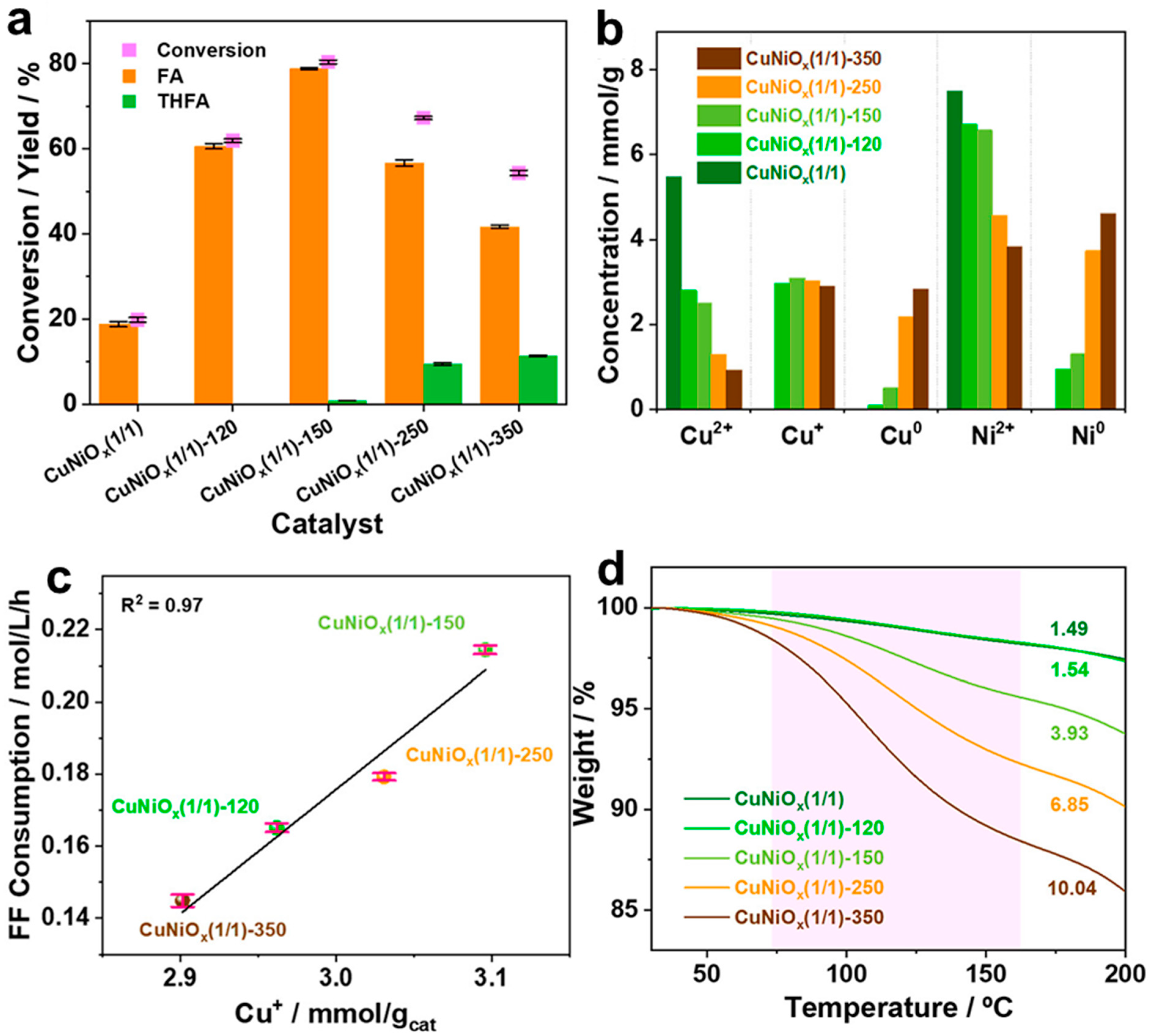
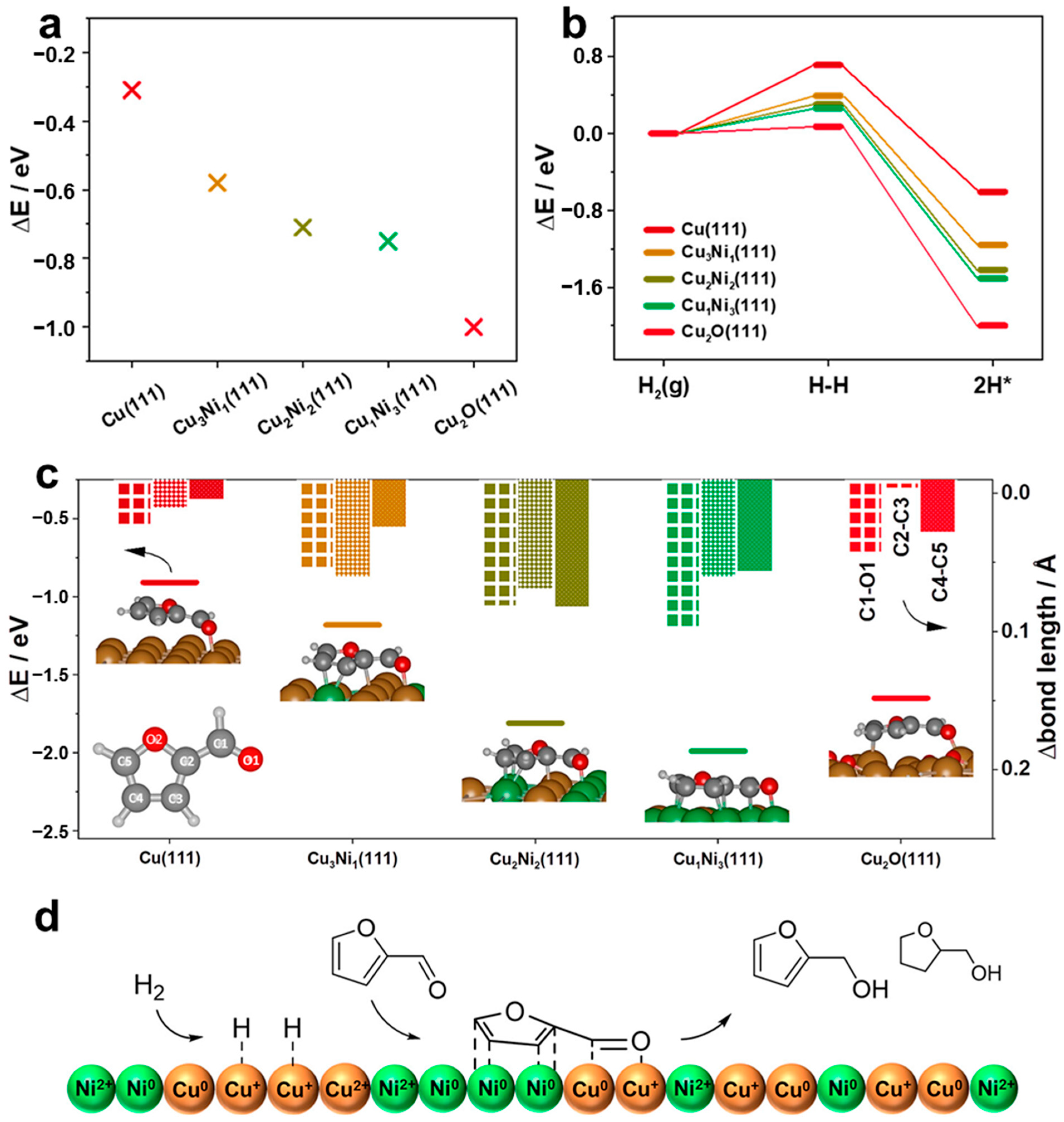
4.5. Role of Cu+ and CuNi Alloy in Mesoporous CuNi Catalysts
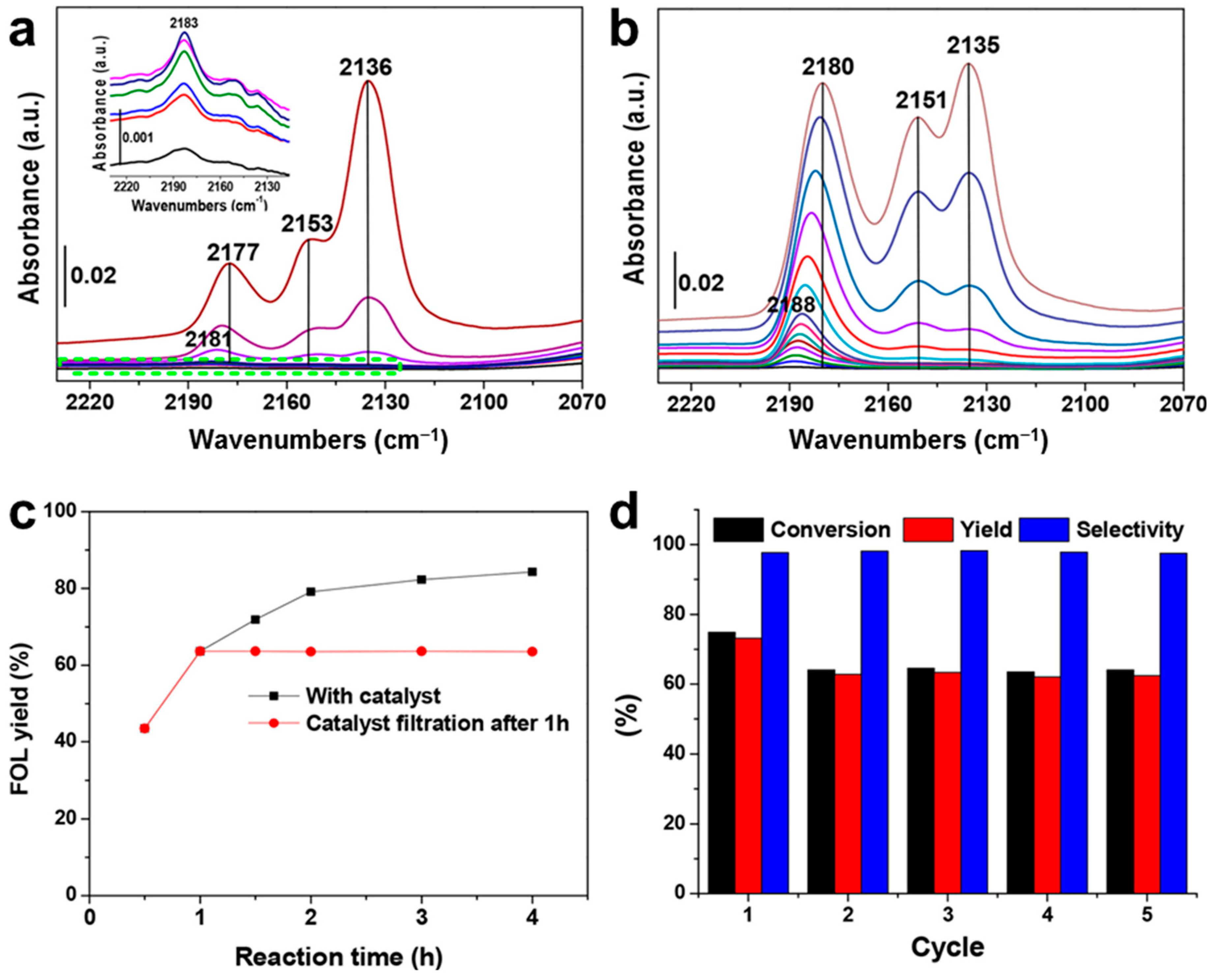
4.6. Role of Metal Coordination on MOF Catalysts
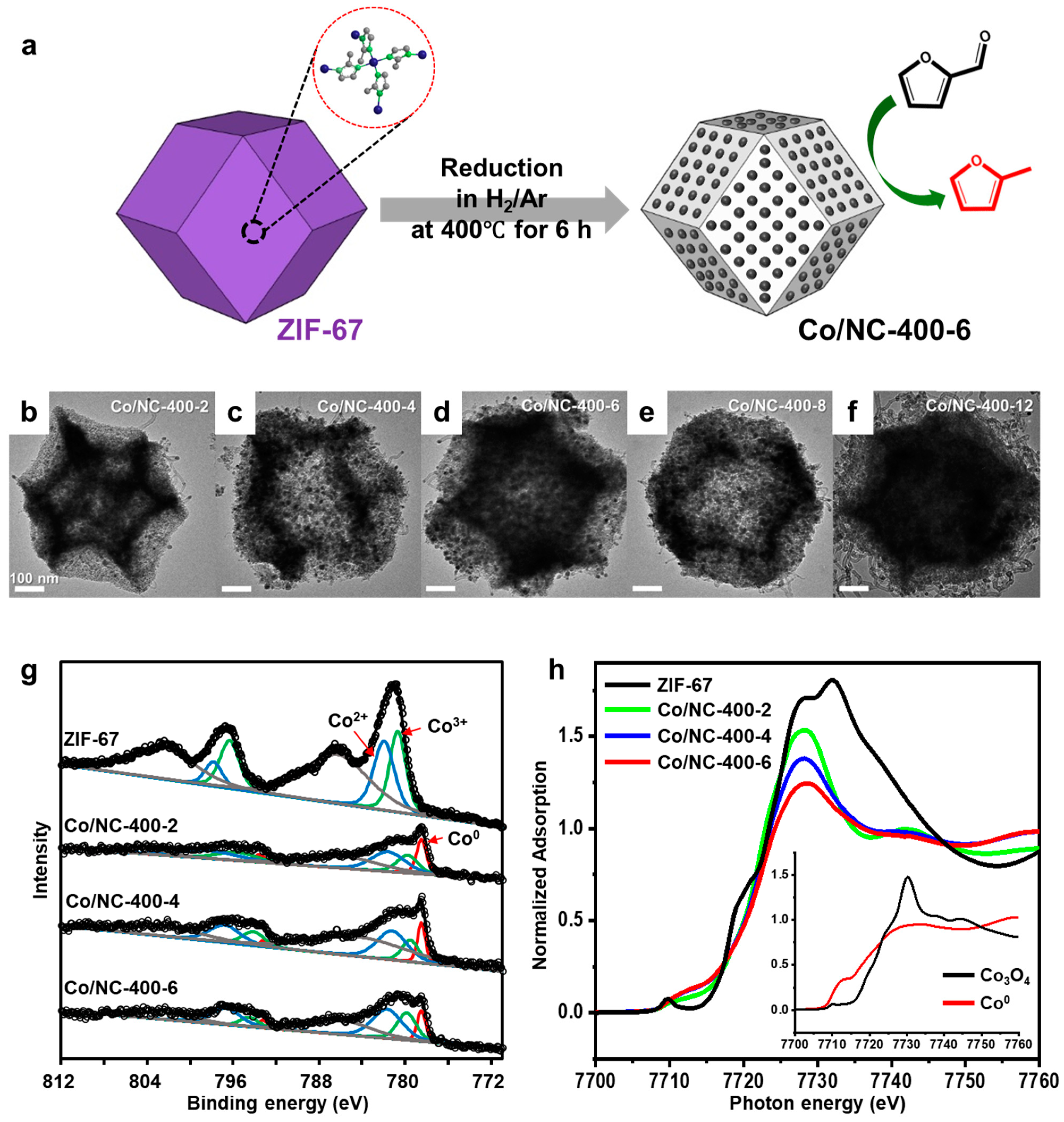
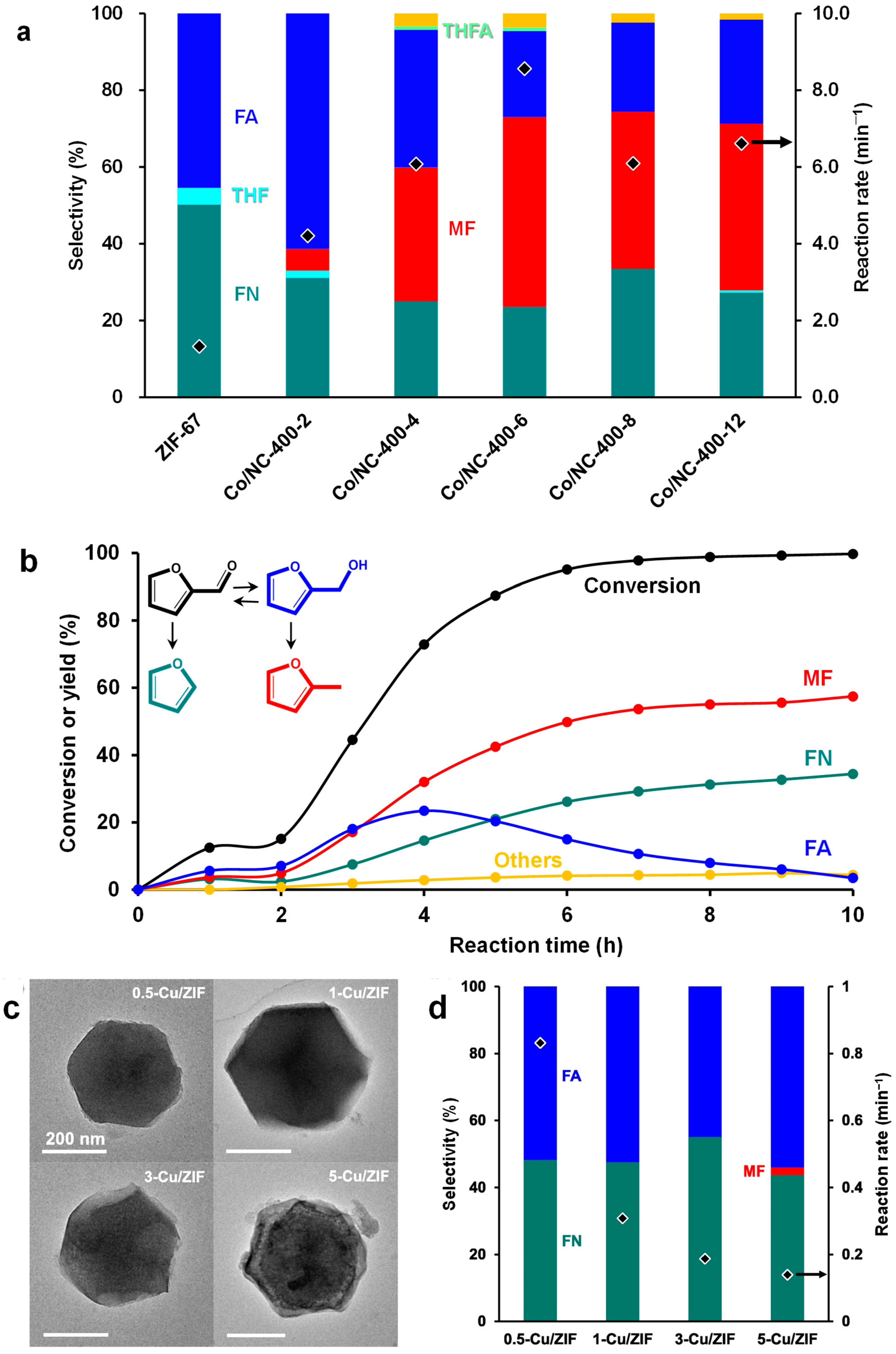
4.7. Structural Evolution of ZIF-67-Derived Catalysts
4.8. Overview on Transition Metal Catalysis
5. Conclusions and Perspectives
5.1. Summary and Significance
5.2. Spent Catalyst Analysis for Better Catalyst Design
5.3. Reducing Use of Gaseous Hydrogen
5.4. Effect of Solvent on Product Selectivity
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FAL | Furfural |
| FA | Furfuryl alcohol |
| FR | Furan |
| MF | Methylfuran |
| THF | Tetrahydrofuran |
| THFA | Tetrahydrofurfuryl alcohol |
| CPO | Cyclopentanone |
| MOF | Metal–organic framework |
| EISA | Evaporation-induced self-assembly |
| ZIF-67 | Zeolitic imidazolate framework-67 |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscopy |
| OMC | Ordered mesoporous carbon |
| BET | Brunauer–Emmett–Teller |
| XPS | X-ray photoelectron spectroscopy |
| ICP–OES | Inductively coupled plasma–optical emission spectroscopy |
| TPD | Temperature-programmed desorption |
| DRIFT | Diffuse reflectance infrared Fourier transform |
| HAADF | High-angle annular dark field |
| EDS | Energy dispersive spectroscopy |
| CTH | Catalytic transfer hydrogenation |
References
- Vogt, E.T.C.; Weckhuysen, B.M. The refinery of the future. Nature 2024, 629, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T. Taking on all of the biomass for conversion. Science 2020, 367, 1305–1306. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Liu, Z.; Wang, Y.; Xie, Z.; He, M. The future of green energy and chemicals: Rational design of catalysis routes. Joule 2022, 6, 1148–1159. [Google Scholar] [CrossRef]
- Seddon, N. Harnessing the potential of nature-based solutions for mitigating and adapting to climate change. Science 2022, 376, 1410–1416. [Google Scholar] [CrossRef]
- Gernaat, D.E.H.J.; de Boer, H.S.; Daioglou, V.; Yalew, S.G.; Müller, M.; van Vuuren, D.P. Climate change impacts on renewable energy supply. Nat. Clim. Change 2021, 11, 119–125. [Google Scholar] [CrossRef]
- Bogdanov, D.; Farfan, J.; Sadovskaia, K.; Aghahosseini, A.; Child, M.; Gulagi, A.; Oyewo, A.S.; Barbosa, L.S.N.S.; Breyer, C. Radical transformation pathway towards sustainable electricity via evolutionary steps. Nat. Commun. 2019, 10, 1077. [Google Scholar] [CrossRef]
- Li, G.; Wang, R.; Pang, J.; Wang, A.; Li, N.; Zhang, T. Production of Renewable Hydrocarbon Biofuels with Lignocellulose and Its Derivatives over Heterogeneous Catalysts. Chem. Rev. 2024, 124, 2889–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yin, G. Catalytic Transformation of the Furfural Platform into Bifunctionalized Monomers for Polymer Synthesis. ACS Catal. 2021, 11, 10058–10083. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, S.; Shen, X.; Chen, B.; Liu, H.; Han, B. Selective hydrogenation of aromatic furfurals into aliphatic tetrahydrofurfural derivatives. Green Chem. 2020, 22, 4937–4942. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass. Chem 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Rubin, E.M. Genomics of cellulosic biofuels. Nature 2008, 454, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Huber, G.H. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ. Sci. 2009, 2, 68–80. [Google Scholar] [CrossRef]
- Yang, T.; Gao, Y.; He, Q.; Chai, Y.; Qin, P.; Wu, Z.; Liu, C.; Gong, X.; Liang, Y. Preparation and application of UiO-66 (Zr) and its derivatives as catalysts in lignocellulosic biomass conversion. Chem. Eng. J. 2024, 486, 149971. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Xie, W.; Yang, L.; Yan, L.; Zhao, X. Recent advances in electrocatalytic upgrading of biomass-derived furfural. Chem. Eng. J. 2024, 485, 150083. [Google Scholar] [CrossRef]
- Date, N.S.; Hengne, A.M.; Huang, K.-W.; Chikate, R.C.; Rode, C.V. Single pot selective hydrogenation of fto 2-methylfuran over carbon supported iridium catalysts. Green Chem. 2018, 20, 2027–2037. [Google Scholar] [CrossRef]
- Yu, Z.; Ji, N.; Xiong, J.; Han, Y.; Li, X.; Zhang, R.; Qiao, Y.; Zhang, M.; Lu, X. Ultrafine Ruthenium Clusters Shell-Embedded Hollow Carbon Spheres as Nanoreactors for Channel Microenvironment-Modulated Furfural Tandem Hydrogenation. Small 2022, 18, 2201361. [Google Scholar] [CrossRef]
- Wang, A.; Balsara, N.P.; Bell, A.T. Continuous pervaporation-assisted furfural production catalyzed by CrCl3. Green Chem. 2018, 20, 2903–2912. [Google Scholar] [CrossRef]
- Kolykhalov, D.A.; Golysheva, A.N.; Erokhin, K.S.; Karlinskii, B.Y.; Ananikov, V.P. The Stability Challenge of Furanic Platform Chemicals in Acidic and Basic Conditions. ChemSusChem 2025, 18, e202401849. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y.; Lee, C.B.T.L.; Lee, Z.J.; Liu, Q.; Jahim, J.M.; Zhou, Q.; Zhang, L. Furfural Production from Biomass Residues: Current Technologies, Challenges and Future Prospects. Biomass Bioenergy 2022, 161, 106458. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, Z.; Yu, L.; Li, X.; Yang, W. Trimetallic Cu-Ni-Re/Hβ catalyst for the direct conversion of furfural to 2-methyltetrahydrofuran. Chem. Eng. J. 2023, 454, 139746. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Huy, C.; Kim, J.S.; Yoon, S.; Yang, E.; Kwak, J.H.; Lee, M.S.; An, K. Supported Pd nanoparticle catalysts with high activities and selectivities in liquid-phase furfural hydrogenation. Fuel 2018, 226, 607–617. [Google Scholar] [CrossRef]
- Lee, J.; Woo, J.; Nguyen-Huy, C.; Lee, M.S.; Joo, S.H.; An, K. Highly dispersed Pd catalysts supported on various carbons for furfural hydrogenation. Catal. Today 2020, 350, 71–79. [Google Scholar] [CrossRef]
- Lee, H.; Nguyen-Huy, C.; Jang, E.J.; Lee, J.; Yang, E.; Lee, M.S.; Kwak, J.H.; An, L. Interfacial effect of Pd supported on mesoporous oxide for catalytic furfural hydrogenation. Catal. Today 2021, 365, 291–300. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Lee, J.; Seo, J.H.; Yang, E.; Lee, J.; Choi, K.; Lee, H.; Kim, J.H.; Lee, M.S.; Joo, S.H.; et al. Structure-dependent catalytic properties of mesoporous cobalt oxides in furfural hydrogenation. Appl. Catal. A Gen. 2019, 583, 117125. [Google Scholar] [CrossRef]
- Lee, J.; Seo, J.H.; Nguyen-Huy, C.; Yang, E.; Lee, J.G.; Lee, H.; Jang, E.J.; Kwak, J.H.; Lee, J.H.; Lee, H.; et al. Cu2O (100) surface as an active site for catalytic furfural hydrogenation. Appl. Catal. B Environ. 2021, 282, 119576. [Google Scholar] [CrossRef]
- Tang, Y.; Qiu, M.; Yang, J.; Shen, F.; Wang, X.; Qi, X. One-Pot Self-Assembly Synthesis of Ni-Doped Ordered Mesoporous Carbon for Quantitative Hydrogenation of Furfural to Furfuryl Alcohol. Green Chem. 2021, 23, 1861–1870. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Lee, H.; Lee, J.; Kwak, J.H.; An, K. Mesoporous mixed CuCo oxides as robust catalysts for liquid-phase furfural hydrogenation. Appl. Catal. A Gen. 2019, 571, 118–126. [Google Scholar] [CrossRef]
- Fang, W.; Liu, S.; Steffensen, A.K.; Schill, L.; Kastlunger, G.; Riisager, A. On the Role of Cu+ and CuNi Alloy Phases in Mesoporous CuNi Catalyst for Furfural Hydrogenation. ACS Catal. 2023, 13, 8437–8444. [Google Scholar] [CrossRef]
- Valekar, A.H.; Lee, M.; Yoon, J.W.; Kwak, J.; Hong, D.-Y.; Oh, K.-R.; Cha, G.-Y.; Kwon, Y.-U.; Jung, J.; Chang, J.S. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol under Mild Conditions over Zr-MOFs: Exploring the Role of Metal Node Coordination and Modification. ACS Catal. 2020, 10, 3720–3732. [Google Scholar] [CrossRef]
- Lee, J.G.; Yoon, S.; Yang, E.; Lee, J.H.; Song, K.; Moon, H.R.; An, K. Structural evolution of ZIF-67-derived catalysts for furfural hydrogenation. J. Catal. 2020, 392, 302–312. [Google Scholar] [CrossRef]
- Hu, L.; Sha, B.; Shi, Y.; Shen, N.; Yang, M.; Chen, K.; Wu, Z.; Tang, X.; He, A.; Lin, L. Switchable transformation of biomass-derived furfural to furfuryl alcohol and isopropyl furfuryl ether over a zirconium-based bifunctional catalyst. Chem. Eng. J. 2024, 498, 155725. [Google Scholar] [CrossRef]
- Sheng, Y.; Tian, F.; Wang, X.; Jiang, N.; Zhang, X.; Chen, X.; Liang, C.; Wang, A. Carbon-encapsulated Ni catalysts derived from citrate complexes for highly efficient hydrogenation of furfural to tetrahydrofurfuryl alcohol. Energy 2024, 292, 130360. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, T.; Zhao, J.; Zhou, W.; Libretto, N.J.; Miller, J.T. NiSn intermetallic nanoparticles with geometrically isolated Ni sites for selective C–O cleavage of furfural. Appl. Catal. B Environ. 2024, 340, 123176. [Google Scholar] [CrossRef]
- Ward, C.J.; Chen, M.; Lamkins, A.; Ordonez, C.; Sun, R.; Chatterjee, P.; Niu, M.; Cui, R.; Liu, D.-J.; Huang, W. Achieving Product Control in Furfural Hydrogenation Using Intermetallic Catalysts. ACS Catal. 2024, 14, 17113–17122. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Y.; Ma, Z.; Sun, Z.; Liu, X.; Xu, C.C.; Nie, R. Synergy between Ni3Sn2 alloy and lewis acidic ReOx enables selectivity control of furfural hydrogenation to cyclopentanone. Appl. Catal. B Environ. 2024, 340, 123191. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, G.; Lan, T.; Ma, Z.; Wang, H.; Liu, Y.; Xu, B.; Lu, Y. Gas-phase hydrogenation of furfural to furfuryl alcohol: A promising Cu/SiO2 catalyst derived from lamellar Cu-based hydroxy double salt. Fuel 2024, 372, 132095. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Bing, Z.; Gao, Y.; Yang, T.; Liu, Q.; Zhang, M.; Liu, Z. Refining Surface Copper Species on Cu/SiO2 Catalysts to Boost Furfural Hydrogenation to Furfuryl Alcohol. Molecules 2025, 30, 225. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.G.; Sung, K.; Lee, H.; Kim, J.; Nam, E.; Han, J.W.; An, K. Reversible Pd Catalysts Supported on Hierarchical Titanate Nanosheets for an N-Methylindole-Based Liquid Organic Hydrogen Carrier. ACS Catal. 2023, 13, 13691–13703. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, S.; Lee, H.; Kurisingal, J.F.; Han, S.H.; Kim, Y.H.; An, K. Complete utilization of waste lignin: Preparation of lignin-derived carbon supports and conversion of lignin-derived guaiacol to nylon precursors. Catal. Sci. Technol. 2022, 12, 5021–5031. [Google Scholar] [CrossRef]
- Yang, E.; Lee, J.G.; Park, E.D.; An, K. Methane oxidation to formaldehyde over vanadium oxide supported on various mesoporous silicas. Korean J. Chem. Eng. 2021, 38, 1224–1230. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Zhao, Y.; Liu, J.; Liu, C.; Wang, Z.; Zheng, G.; Huang, G.; Mei, Y. Atomic Layer Deposition Inducing Integration of Co, N Codoped Carbon Sphere on 3d Foam with Hierarchically Porous Structures for Flexible Hydrogen Producing Device. Adv. Funct. Mater. 2019, 29, 1906365. [Google Scholar] [CrossRef]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-Derived Electrocatalysts for Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered Mesoporous Metal Oxides: Synthesis and Applications. Chem. Soc. Rev. 2012, 41, 4909–4927. [Google Scholar] [CrossRef]
- Gu, D.; Schüth, F. Synthesis of Non-Siliceous Mesoporous Oxides. Chem. Soc. Rev. 2014, 43, 313–344. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-Induced Self-Assembly: Nanostructures Made Easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.d.A.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Seoane, B.; Castellanos, S.; Dikhtiarenko, A.; Kapteijn, F.; Gascon, J. Multi-Scale Crystal Engineering of Metal Organic Frameworks. Coord. Chem. Rev. 2016, 307, 147–187. [Google Scholar] [CrossRef]
- Rajesh, R.U.; Mathew, T.; Kumar, H.; Singhal, A.; Thomas, L. Metal-Organic Frameworks: Recent Advances in Synthesis Strategies and Applications. Inorg. Chem. Commun. 2024, 162, 112223. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal Conversion of Core–Shell Metal–Organic Frameworks: A New Method for Selectively Functionalized Nanoporous Hybrid Carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-H.; Zhang, B.-W.; Hu, Z.; Qu, X.-M.; Jiang, Y.-X.; Wang, Y.-X.; Wang, J.-Z.; Liu, H.K.; Chou, S.-L. The Quasi-Pt-Allotrope Catalyst: Hollow PtCo@ Single-Atom Pt1 on Nitrogen-Doped Carbon toward Superior Oxygen Reduction. Adv. Funct. Mater. 2019, 29, 1807340. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef]
- Bernsmeier, D.; Chuenchom, L.; Paul, B.; Rummler, S.; Smarsly, B.; Kraehnert, R. Highly Active Binder-Free Catalytic Coatings for Heterogeneous Catalysis and Electrocatalysis: Pd on Mesoporous Carbon and Its Application in Butadiene Hydrogenation and Hydrogen Evolution. ACS Catal. 2016, 6, 8255–8263. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Park, J.Y.; Baker, L.R.; Somorjai, G.A. Role of Hot Electrons and Metal–Oxide Interfaces in Surface Chemistry and Catalytic Reactions. Chem. Rev. 2015, 115, 2781–2817. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Xu, E.; Feng, H.; Wang, L.; Zhang, Y.; Liu, K.; Cui, S.; Meng, H.; Wang, G.; Yang, Y. Pt Single Atoms and Nanosized Clusters as Catalytic Reaction Platforms for Selective Hydrogenation Applications. ACS Appl. Nano Mater. 2023, 6, 14991–15001. [Google Scholar] [CrossRef]
- Taylor, M.J.; Beaumont, S.K.; Islam, M.J.; Tsatsos, S.; Parlett, C.A.M.; Issacs, M.A.; Kyriakou, G. Atom Efficient PtCu Bimetallic Catalysts and Ultra Dilute Alloys for the Selective Hydrogenation of Furfural. Appl. Catal. B Environ. 2021, 284, 119737. [Google Scholar] [CrossRef]
- Yuan, E.; Wang, C.; Wu, C.; Shi, G.; Jian, P.; Hou, X. Constructing Hierarchical Structures of Pd Catalysts to Realize Reaction Pathway Regulation of Furfural Hydroconversion. J. Catal. 2023, 421, 30–44. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, J.; Zhang, M.; Li, H.; Yang, J.-H. Highly Dispersed PdCu Supported on MCM-41 for Efficiently Selective Transfer Hydrogenation of Furfural into Furfuryl Alcohol. Appl. Surf. Sci. 2023, 619, 156716. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Tu, R.; Liu, S.; Hu, J.; Li, Y.; Sun, Y. Quenching Method to Prepare Ultra-Low Loading High-Entropy Catalyst for Furfural Selectively Hydrogenation at Ambient Temperature. Appl. Catal. B Environ. 2024, 342, 123358. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Jiao, F. Ordered Mesoporous Cobalt Oxide as Highly Efficient Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2013, 135, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ciotonea, C.; Royer, S.; Dacquin, J.-P.; Vinod, C.P. Engineering Pore Morphology Using Silica Template Route over Mesoporous Cobalt Oxide and Its Implications in Atmospheric Pressure Carbon Dioxide Hydrogenation to Olefins. Appl. Mater. Today 2020, 19, 100586. [Google Scholar] [CrossRef]
- Jiao, F.; Jumas, J.-C.; Womes, M.; Chadwick, A.V.; Harrison, A.; Bruce, P.G. Synthesis of Ordered Mesoporous Fe3O4 and γ-Fe2O3 with Crystalline Walls Using Post-Template Reduction/Oxidation. J. Am. Chem. Soc. 2006, 128, 12905–12909. [Google Scholar] [CrossRef]
- Tusuz, H.; Liu, Y.; Weidenthaler, C.; Schuth, F. Pseudomorphic Transformation of Highly Ordered Mesoporous Co3O4 to CoO via Reduction with Glycerol. J. Am. Chem. Soc. 2008, 130, 14108–14110. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Jia, Z.; Han, Q.; Yu, S.; Liu, S.; Li, L.; Wu, Q.; Yu, H.; Liu, Y. Bi-Functional Active Sites Cu/MgO-La2O3 Catalysts the Selective Hydrogenation of Furfural to Furfuryl Alcohol. Chem. Eng. J. 2024, 500, 157400. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.; Yi, W.-J.; Yang, J.; Jiang, K.; Yang, T.; Li, Z.; Zhang, M.; Liu, Z.; Wu, B. Unveiling the Effects of Support Crystal Phase on Cu/Al2O3 Catalysts for Furfural Selective Hydrogenation to Furfuryl Alcohol. Chem. Eng. J. 2024, 498, 155620. [Google Scholar] [CrossRef]
- Zhai, Z.; Chu, J.; Sun, L.; Zhao, X.; Huang, D.; Yang, X.; Zhuang, C.; Min, C.; Wang, Y. Ultrahigh Metal Content Carbon-Based Catalyst for Efficient Hydrogenation of Furfural: The Regulatory Effect of Glycerol. ACS Appl. Mater. Interfaces 2022, 14, 44439–44449. [Google Scholar] [CrossRef]
- Ahn, C.-I.; Koo, H.M.; Jo, J.M.; Roh, H.-S.; Lee, J.-B.; Lee, Y.-J.; Jang, E.J.; Bae, J.W. Stabilized ordered-mesoporous Co3O4 structures using Al pillar for the superior Co hydrogenation activity to hydrocarbons. Appl. Catal. B Environ. 2016, 180, 139–149. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, X.; Zeng, F.; Li, Q.; Liang, X. Optimization and Reaction Kinetics of Catalytic Transfer Hydrogenation of 5-Hydroxymethylfurfural to 2, 5-Dimethylfuran over CuCoOx Catalysts. ChemCatChem 2025, 17, e202401412. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Jia, Z.; Liu, S.; Li, L.; Wu, Q.; Yu, H.; Liu, Y.; Jiang, X.; Liu, Y. Selective Hydrogenation of Furfural to Furfuryl Alcohol over Copper-Cobalt Bimetallic Catalyst. Chem. Eng. J. 2024, 490, 151677. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, H.; Liu, R.; Luo, H.; Lv, Y.; Liu, P. Highly Efficient and Selective Hydrogenation of Furfural to Furfuryl Alcohol and Cyclopentanone over Cu-Ni Bimetallic Catalysts: The Crucial Role of CuNi Alloys and Cu+ Species. J. Catal. 2024, 436, 115603. [Google Scholar] [CrossRef]
- Shoosri, T.; Chotiwilaiwan, P.; Rattanapornchaiwat, T.; Teerawatananond, T.; Miyake, T.; Panpranot, J.; Weerachawanasak, P. Bimetallic Copper-and Nickel-Rich Cu–Ni Phyllosilicate Catalysts for the Liquid Phase Selective Hydrogenation of Furfural to Furfuryl Alcohol. RSC Adv. 2024, 14, 38232–38244. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Huang, L.; Liu, P. Highly Efficient Selective Hydrogenation of Furfural to Tetrahydrofurfuryl Alcohol over MOF-Derived Co-Ni Bimetallic Catalysts: The Effects of Co-Ni Alloy and Adsorption Configuration. J. Catal. 2024, 440, 115824. [Google Scholar] [CrossRef]
- Kulkarni, B.B.; Maradur, S.P. Tandem Hydrogenation/Hydrogenolysis of Furfural to 2-Methylfuran over Multifunctional Metallic Cu Nanoparticles Supported ZIF-8 Catalyst. Bioresour. Technol. 2024, 402, 130805. [Google Scholar] [CrossRef]
- Han, Y.-W.; Ye, L.; Gong, T.-J.; Lu, X.-B.; Fu, Y. Porous Composite-Mediated Bimetallic Cluster POMs/Zr-MOF for Catalytic Transfer Hydrogenation of Biomass-Derived Aldehydes and Ketones. Adv. Funct. Mater. 2024, 34, 2315044. [Google Scholar] [CrossRef]
- Agusta, K.D.; Miharja, M.F.; Sakti, A.W.; Arrozi, U.S.F.; Mukaromah, L.; Patah, A.; Hara, T.; Permana, Y. Zr-MOFs–Catalyzed Transfer Hydrogenation of Furfural to Furfuryl Alcohol: Unveiled Performance of DUT-52. Mol. Catal. 2022, 524, 112265. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Lu, S.; Li, P.; Xia, X.; Li, C.; Li, F. Phosphorus-Modified Zirconium Metal Organic Frameworks for Catalytic Transfer Hydrogenation of Furfural. New J. Chem. 2020, 44, 20308–20315. [Google Scholar] [CrossRef]
- Kang, R.; Wang, S.; Guo, D.; Feng, J.; Pan, H. Trimetallic NiZr/CoOx Catalyst for the Selective Hydrogenation of Furfural into Furfuryl Alcohol. Int. J. Hydrogen Energy 2024, 51, 1471–1482. [Google Scholar] [CrossRef]
- Jia, S.; Yang, W.; Liu, H.; Chen, X.; Luo, J.; Liang, C. Regulation of Sol-Gel Derived Cu-M/Al2O3 by Metal Additives for the Selective Catalytic Transfer Hydrogenation of Furfural. Catal. Today 2025, 445, 115078. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Q.; Nian, Y.; Wang, F.; Zhang, X.; Zhang, Z.; Bing, C.; Fan, X.; Ahishakiye, R. Promoting Effect of Oxygen Vacancies in Co/CoAl2O4 Catalyst Steered with a Straightforward Method on Hydrogenation of Furfural to 2-Methylfuran. Appl. Catal. B Environ. 2024, 343, 123529. [Google Scholar] [CrossRef]
- Zhang, J.; Jian, C.; Wang, F.-F.; Zhang, W.; Tian, Z.; Chen, D.-L. The Role of Frustrated Lewis Pair in Catalytic Transfer Hydrogenation of Furfural Using Nickel Single-Atom Catalysts: A Theoretical Study. ChemPhysChem 2025, 26, e202400628. [Google Scholar] [CrossRef]
| Entry | Catalyst | T (°C) | Time (h) | Mass Ratio (Cat. 1/FAL/IPA) | Conversion (%) | YFA 2 (%) | SFA 3 (%) | TOF 4 (h–1) |
|---|---|---|---|---|---|---|---|---|
| 1 | UiO-66 | 82 | 2 | 0.1/1/25 | 2.3 | 1.3 | 65.5 | 0.26 |
| 2 | DUT-52 | 82 | 2 | 0.1/1/25 | 2.1 | 0.0 | 0.0 | 0.0 |
| 3 | UiO-67 | 82 | 2 | 0.1/1/25 | 5.2 | 0.1 | 1.9 | 0.02 |
| 4 | DUT-67 | 82 | 2 | 0.1/1/25 | 16.4 | 13.5 | 82.3 | 2.0 |
| 5 | MOF-808 | 82 | 2 | 0.1/1/25 | 81.3 | 66.4 | 81.7 | 11.6 |
| 6 | MOF-808 | 40 | 24 | 0.1/0.5/12.5 | 27.5 | 25.4 | 92.3 | 0.2 |
| 7 | M-MOF-808 | 82 | 2 | 0.1/1/25 | 89.3 | 79.1 | 88.6 | 15.0 |
| 8 | M-MOF-808 | 40 | 24 | 0.1/0.5/12.5 | 96.5 | 85.5 | 88.6 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Lee, S.; An, K. Design and Application of Mesoporous Catalysts for Liquid-Phase Furfural Hydrogenation. Molecules 2025, 30, 1270. https://doi.org/10.3390/molecules30061270
Lee H, Lee S, An K. Design and Application of Mesoporous Catalysts for Liquid-Phase Furfural Hydrogenation. Molecules. 2025; 30(6):1270. https://doi.org/10.3390/molecules30061270
Chicago/Turabian StyleLee, Hyeongeon, Shinjae Lee, and Kwangjin An. 2025. "Design and Application of Mesoporous Catalysts for Liquid-Phase Furfural Hydrogenation" Molecules 30, no. 6: 1270. https://doi.org/10.3390/molecules30061270
APA StyleLee, H., Lee, S., & An, K. (2025). Design and Application of Mesoporous Catalysts for Liquid-Phase Furfural Hydrogenation. Molecules, 30(6), 1270. https://doi.org/10.3390/molecules30061270







