p-Coumaroyl Amides from the Plant Kingdom: A Comprehensive Review of Natural Sources, Biosynthesis, and Biological Activities
Abstract
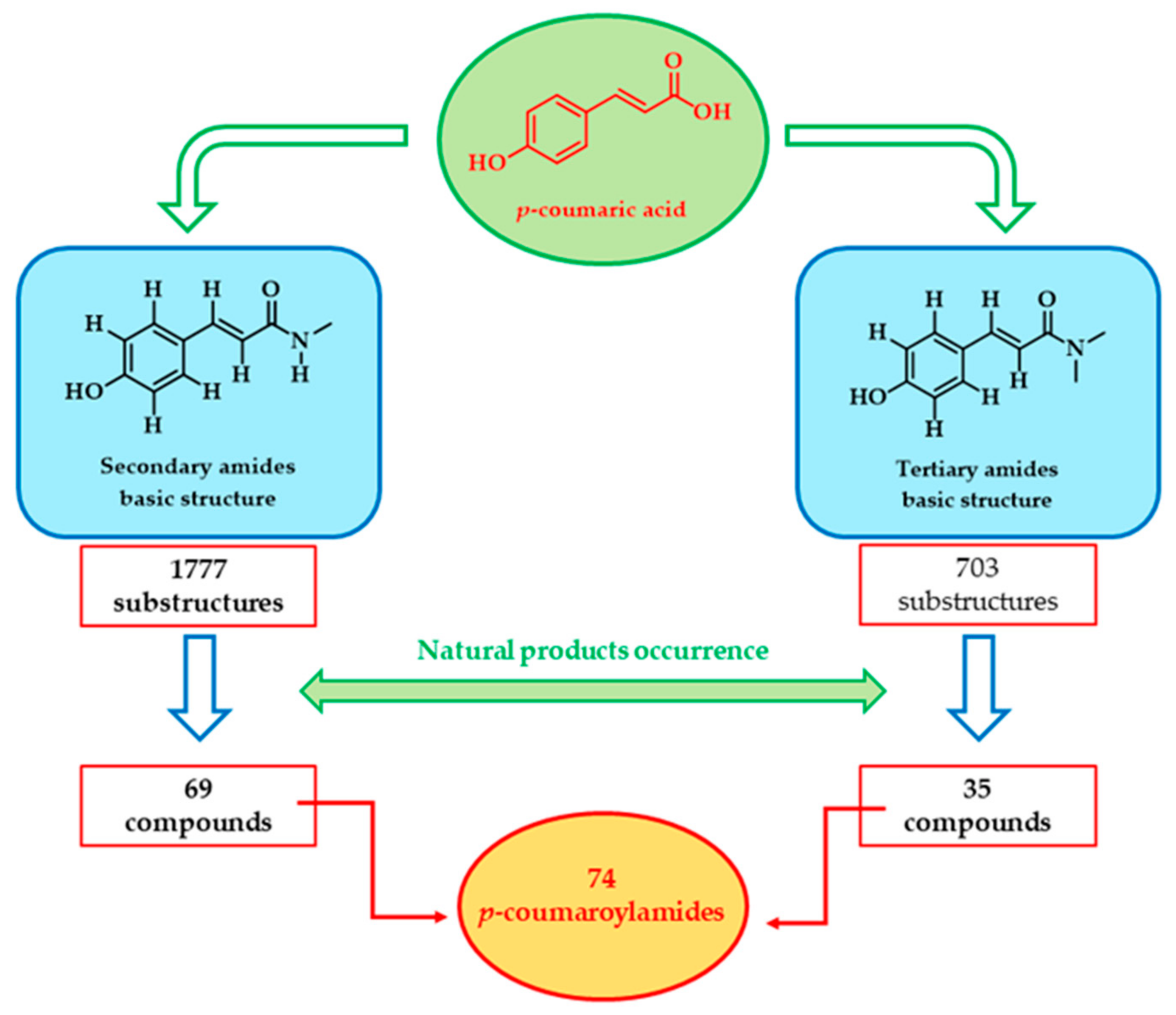
1. Introduction
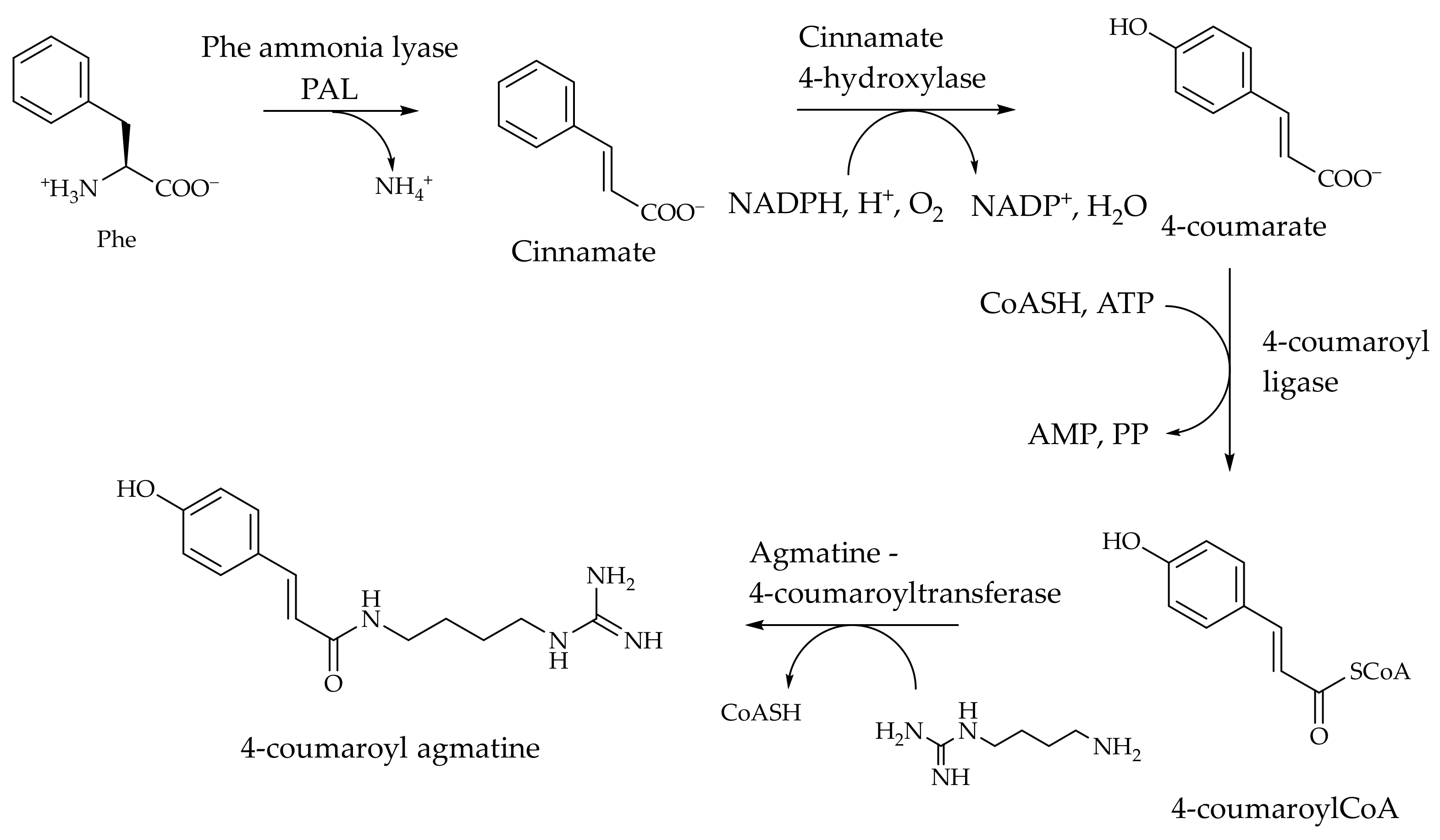
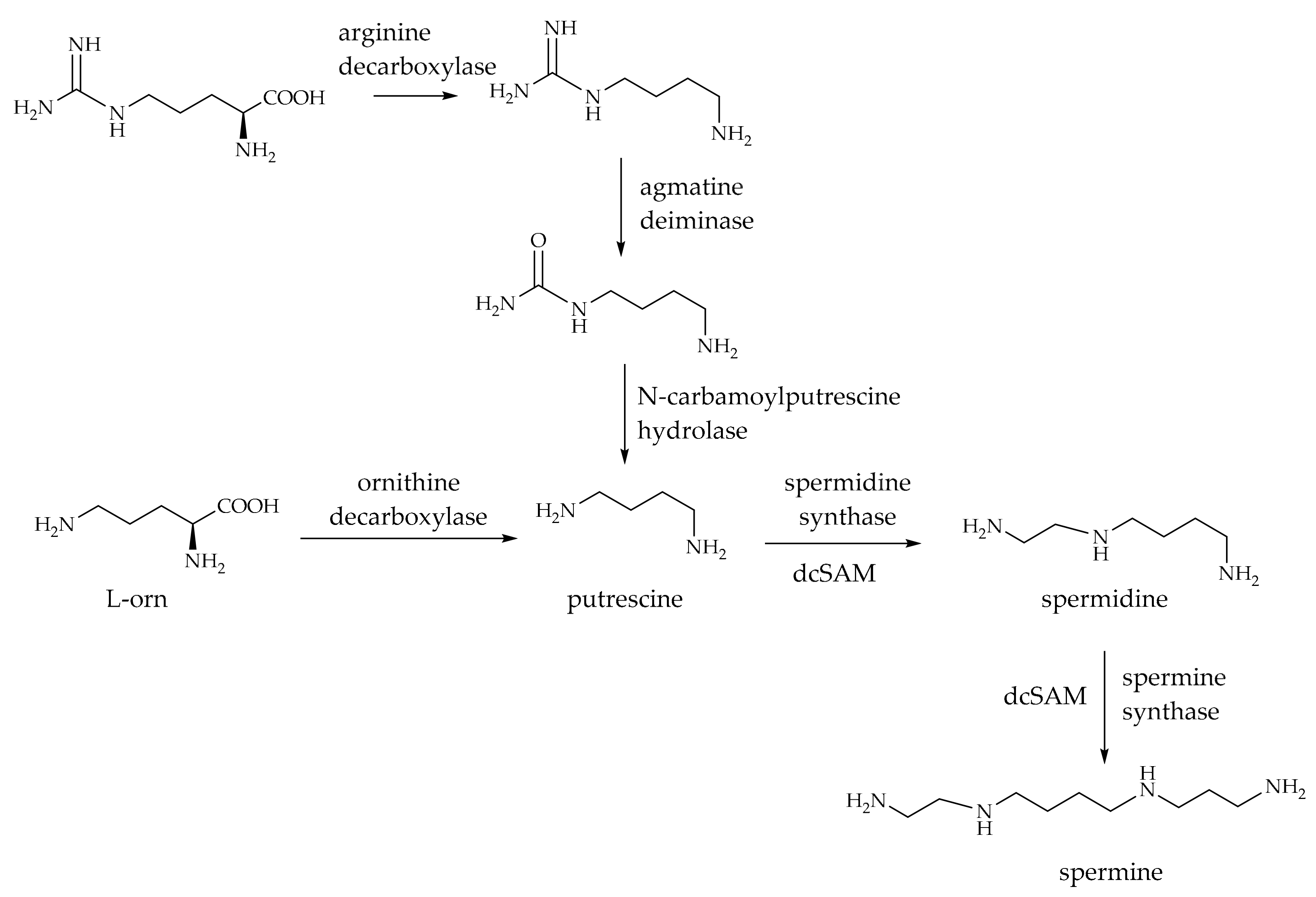
2. p-Coumaroyl Agmatine and Its Biosynthesis
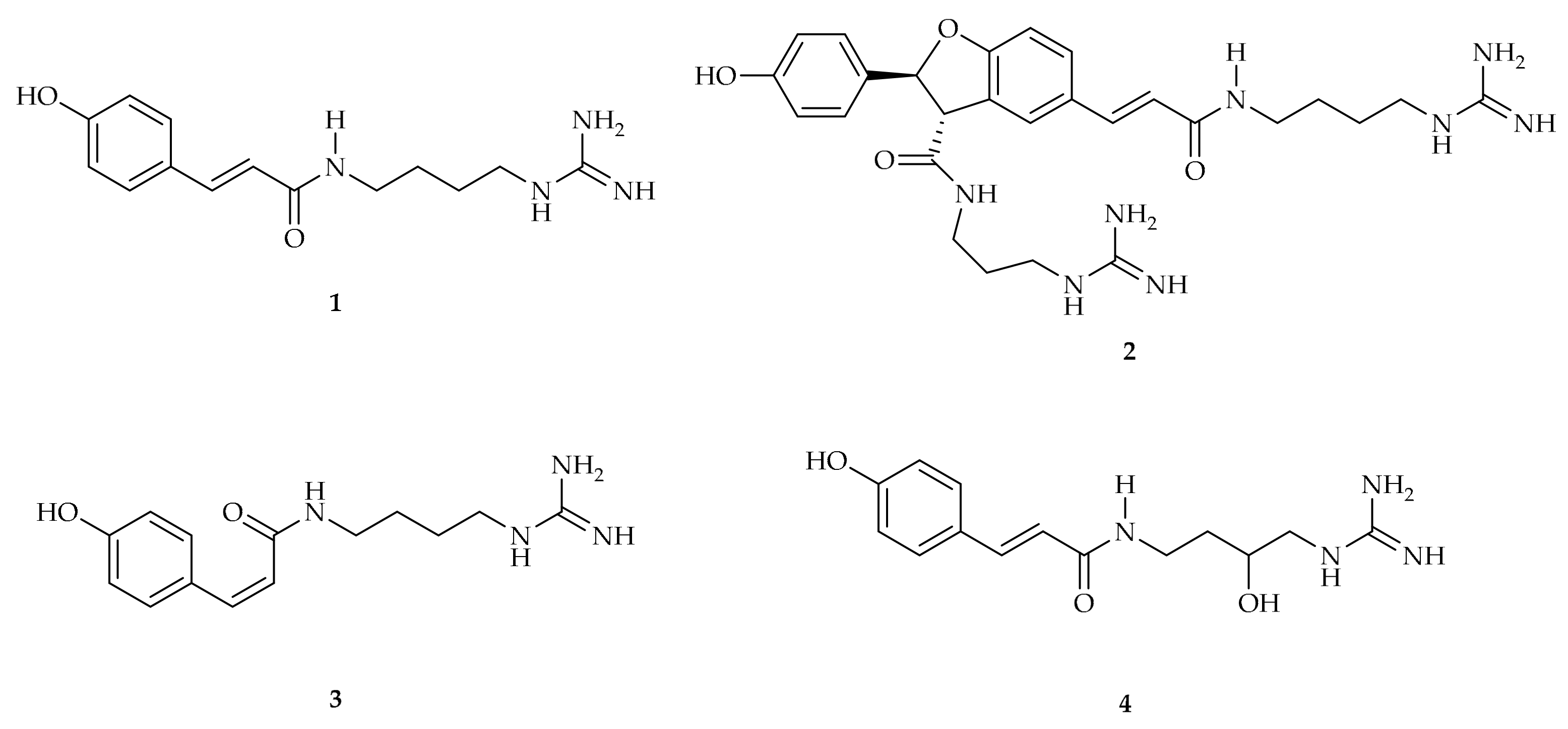
3. Secondary Amides
3.1. Isolation, Characterization, and Role in Plants
3.2. Biological Activity
3.2.1. Antioxidant, Anti-Inflammatory, and Cardiovascular Activities
3.2.2. Anticancer Activity
3.2.3. Antidiabetic Activity
3.2.4. Melanogenesis
3.2.5. Neurological Effects
3.2.6. Antiviral Activity
3.2.7. Antimicrobial Activity
3.2.8. Anthelmintic Activity
3.2.9. Other Activities
4. Tertiary Amides
4.1. Isolation, Characterization, and Role in Plants
4.2. Biological Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Liu, Y.; Han, H.; Lu, C.; Chen, H.; Chai, Y. Identification and Characterization of Phenolamides in Tea (Camellia sinensis) Flowers Using Ultra-High-Performance Liquid Chromatography/Q-Exactive Orbitrap Mass Spectrometry. Food Chem. 2023, 424, 136402. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Ma, Z.; Xiao, M.; Yang, L.; Tian, B.; Yu, Y.; Bi, C.; Fang, A.; Yang, Y. The Role of Hydroxycinnamic Acid Amide Pathway in Plant Immunity. Front. Plant Sci. 2022, 13, 922119. [Google Scholar] [CrossRef] [PubMed]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef]
- Berti, F.; Navarini, L.; Colomban, S.; Forzato, C. Hydroxycinnamoyl Amino Acids Conjugates: A Chiral Pool to Distinguish Commercially Exploited Coffea Spp. Molecules 2020, 25, 1704. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, X.; Wang, J.; Wang, F.; Mao, J. P-Coumaric Acid: Advances in Pharmacological Research Based on Oxidative Stress. Curr. Top. Med. Chem. 2024, 24, 416–436. [Google Scholar] [CrossRef]
- Stoessl, A. The Antifungal Factors in Barley-III.: Isolation of p-Coumaroylagmatine. Phytochemistry 1965, 4, 973–976. [Google Scholar] [CrossRef]
- Bird, C.R.; Smith, T.A. The Biosynthesis of Coumarylagmatine in Barley Seedlings. Phytochemistry 1981, 20, 2345–2346. [Google Scholar] [CrossRef]
- Burhenne, K.; Kristensen, B.K.; Rasmussen, S.K. A New Class of N-Hydroxycinnamoyltransferases: PURIFICATION, CLONING, AND EXPRESSION OF A BARLEY AGMATINE COUMAROYLTRANSFERASE (EC 2.3.1.64)*. J. Biol. Chem. 2003, 278, 13919–13927. [Google Scholar] [CrossRef]
- Ube, N.; Ishihara, A.; Yabuta, Y.; Taketa, S.; Kato, Y.; Nomura, T. Molecular Identification of a Laccase That Catalyzes the Oxidative Coupling of a Hydroxycinnamic Acid Amide for Hordatine Biosynthesis in Barley. Plant J. 2023, 115, 1037–1050. [Google Scholar] [CrossRef]
- Dong, X.; Gao, Y.; Chen, W.; Wang, W.; Gong, L.; Liu, X.; Luo, J. Spatiotemporal Distribution of Phenolamides and the Genetics of Natural Variation of Hydroxycinnamoyl Spermidine in Rice. Mol. Plant 2015, 8, 111–121. [Google Scholar] [CrossRef]
- Lagnika, L.; Weniger, B.; Attioua, B.; Jensen, O.; Anthaume, C.; Sanni, A.; Kaiser, M.; Lobstein, A.; Vonthron-Senecheau, C. Trypanocidal Activity of Diarylheptanoids from Schrankia Leptocarpa DC. South Afr. J. Bot. 2012, 83, 92–97. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Ullah, C.; Giddings Vassão, D.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Sclerotinia Sclerotiorum Infection Triggers Changes in Primary and Secondary Metabolism in Arabidopsis Thaliana. Phytopathol. 2021, 111, 559–569. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Sun, Q.-Y.; Yang, F.-M.; Long, C.-L.; Zhao, F.-W.; Tang, G.-H.; Niu, H.-M.; Wang, H.; Huang, Q.-Q.; Xu, J.-J.; et al. Neolignans and Caffeoyl Derivatives from Selaginella Moellendorffii. Helv. Chim. Acta 2010, 93, 2467–2477. [Google Scholar] [CrossRef]
- Ueda, M.; Tashiro, C.; Yamamura, S. Cis-p-Coumaroylagmatine, the Genuine Leaf-Opening Substance of a Nyctinastic Plant, Albizzia Julibrissin Durazz. Tetrahedron Lett. 1997, 38, 3253–3256. [Google Scholar] [CrossRef]
- Laupheimer, S.; Kurzweil, L.; Proels, R.; Unsicker, S.B.; Stark, T.D.; Dawid, C.; Hückelhoven, R. Volatile-Mediated Signalling in Barley Induces Metabolic Reprogramming and Resistance against the Biotrophic Fungus Blumeria Hordei. Plant Biol. 2023, 25, 72–84. [Google Scholar] [CrossRef]
- von Röpenack, E.; Parr, A.; Schulze-Lefert, P. Structural Analyses and Dynamics of Soluble and Cell Wall-Bound Phenolics in a Broad Spectrum Resistance to the Powdery Mildew Fungus in Barley. J. Biol. Chem. 1998, 273, 9013–9022. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in Plants: A Good Time to Be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef]
- Moghe, G.; Kruse, L.H.; Petersen, M.; Scossa, F.; Fernie, A.R.; Gaquerel, E.; D’Auria, J.C. BAHD Company: The Ever-Expanding Roles of the BAHD Acyltransferase Gene Family in Plants. Annu. Rev. Plant Biol. 2023, 74, 165–194. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Jeske, L.; Ulbrich, S.; Hofmann, J.; Koblitz, J.; Schomburg, I.; Neumann-Schaal, M.; Jahn, D.; Schomburg, D. BRENDA, the ELIXIR Core Data Resource in 2021: New Developments and Updates. Nucleic Acids Res. 2021, 49, D498–D508. [Google Scholar] [CrossRef]
- Jang, S.-M.; Ishihara, A.; Back, K. Production of Coumaroylserotonin and Feruloylserotonin in Transgenic Rice Expressing Pepper Hydroxycinnamoyl-Coenzyme A:Serotonin N-(Hydroxycinnamoyl)Transferase. Plant Physiol. 2004, 135, 346–356. [Google Scholar] [CrossRef][Green Version]
- Schmidt, A.; Grimm, R.; Schmidt, J.; Scheel, D.; Strack, D.; Rosahl, S. Cloning and Expression of a Potato cDNA Encoding Hydroxycinnamoyl-CoA:TyramineN-(Hydroxycinnamoyl)Transferase. J. Biol. Chem. 1999, 274, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2024, 53, D609–D617. [CrossRef]
- Yamane, M.; Takenoya, M.; Yajima, S.; Sue, M. Crystal Structure of Barley Agmatine Coumaroyltransferase, an It N-Acyltransferase from the BAHD Superfamily. Acta Crystallogr. Sect. F 2020, 76, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Yamane, M.; Takenoya, M.; Yajima, S.; Sue, M. Molecular and Structural Characterization of Agmatine Coumaroyltransferase in Triticeae, the Key Regulator of Hydroxycinnamic Acid Amide Accumulation. Phytochemistry 2021, 189, 112825. [Google Scholar] [CrossRef]
- Stark, T.; Justus, H.; Hofmann, T. Quantitative Analysis of N-Phenylpropenoyl-l-Amino Acids in Roasted Coffee and Cocoa Powder by Means of a Stable Isotope Dilution Assay. J. Agric. Food Chem. 2006, 54, 2859–2867. [Google Scholar] [CrossRef]
- Men, L.; Liu, Y.; Qiu, Y.; Yuan, X. An Effective UPLC Method for the Quantification and Fingerprint Analysis of Amides in a South China Native Medicinal Herb, Abri Herba. J. Food Compos. Anal. 2021, 96, 103723. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Y.; Zhao, H.; Men, L.; He, C.; Qiu, Y.; Yu, Q.; Li, K.; Qi, L.; Chen, D. The Isolation, Structure and Fragmentation Characteristics of Natural Truxillic and Truxinic Acid Derivatives in Abrus mollis Leaves. Phytochemistry 2021, 181, 112572. [Google Scholar] [CrossRef]
- Watanabe, M. Antioxidative Phenolic Compounds from Japanese Barnyard Millet (Echinochloa utilis) Grains. J. Agric. Food Chem. 1999, 47, 4500–4505. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, J.; Zheng, B.; Sun, J.; Luo, D.; Li, P.; Fan, J. Diversity of Phenolics Including Hydroxycinnamic Acid Amide Derivatives, Phenolic Acids Contribute to Antioxidant Properties of Proso Millet. LWT 2022, 154, 112611. [Google Scholar] [CrossRef]
- Roh, J.S.; Han, J.Y.; Kim, J.H.; Hwang, J.K. Inhibitory Effects of Active Compounds Isolated from Safflower (Carthamus tinctorius L.) Seeds for Melanogenesis. Biol. Pharm. Bull. 2004, 27, 1976–1978. [Google Scholar] [CrossRef]
- Middleton, M.; Cox, P.J.; Jaspars, M.; Kumarasamy, Y.; Nahar, L.; Reid, R.; Sarker, S.D. Dibenzylbutyrolactone Lignans and Indole Alkaloids from the Seeds of Centaurea nigra (Asteraceae). Biochem. Syst. Ecol. 2003, 31, 653–656. [Google Scholar] [CrossRef]
- Novello, C.R.; Marques, L.C.; Pires, M.E.; Kutschenco, A.P.; Nakamura, C.V.; Nocchi, S.; Sarragiotto, M.H.; Mello, J.C.P. Bioactive Indole Alkaloids from Croton echioides. J. Braz. Chem. Soc. 2016, 27, 2203–2209. [Google Scholar]
- Aderogba, M.A.; Ndhlala, A.R.; Rengasamy, K.R.R.; Van Staden, J. Antimicrobial and Selected In Vitro Enzyme Inhibitory Effects of Leaf Extracts, Flavonols and Indole Alkaloids Isolated from Croton menyharthii. Molecules 2013, 18, 12633–12644. [Google Scholar] [CrossRef]
- Sim, J.Y.; Kim, M.A.; Kim, M.J.; Chun, W.J.; Kwon, Y.S. Acetylcholinesterase Inhibitors from the Stem of Zea mays. Nat. Prod. Sci. 2014, 20, 13–16. [Google Scholar]
- Chen, Y.; Li, L.; Jiang, L.-R.; Tan, J.-Y.; Guo, L.-N.; Wang, X.-L.; Dong, W.; Wang, W.-B.; Sun, J.-K.; Song, B. Alkaloids Constituents from the Roots of Phragmites australis (Cav.) Trin. Ex Steud. with Their Cytotoxic Activities. Nat. Prod. Res. 2022, 36, 1454–1459. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Li, J.; Zhao, X.-F.; Xu, B.; Xiao, X.-H.; Ren, J.; Li, S.-X. Alkaloids and Phenylpropanoid from Rhizomes of Arundo donax L. Nat. Prod. Res. 2021, 35, 465–470. [Google Scholar] [CrossRef]
- Tian, X.; Guo, S.; He, K.; Roller, M.; Yang, M.; Liu, Q.; Zhang, L.; Ho, C.-T.; Bai, N. Qualitative and Quantitative Analysis of Chemical Constituents of Ptychopetalum olacoides Benth. Nat. Prod. Res. 2018, 32, 354–357. [Google Scholar] [CrossRef]
- Shoeb, M.; MacManus, S.M.; Jaspars, M.; Trevidu, J.; Nahar, L.; Kong-Thoo-Lin, P.; Sarker, S.D. Montamine, a Unique Dimeric Indole Alkaloid, from the Seeds of Centaurea montana (Asteraceae), and Its in Vitro Cytotoxic Activity against the CaCo2 Colon Cancer Cells. Tetrahedron 2006, 62, 11172–11177. [Google Scholar] [CrossRef]
- Niwa, T.; Etoh, H.; Shimizu, A.; Shimizu, Y. Cis-N-(p-Coumaroyl)Serotonin from Konnyaku, Amorphophallus Konjac K. Koch. Biosci. Biotechnol. Biochem. 2000, 64, 2269–2271. [Google Scholar] [CrossRef][Green Version]
- Elbandy, M.; Lerche, H.; Wagner, H.; Lacaille-Dubois, M.-A. Constituents of the Rhizome of Homalomena Occulta. Biochem. Syst. Ecol. 2004, 32, 1209–1213. [Google Scholar] [CrossRef]
- Chakradhari, S.; Perkons, I.; Mišina, I.; Sipeniece, E.; Radziejewska-Kubzdela, E.; Grygier, A.; Rudzińska, M.; Patel, K.S.; Radzimirska-Graczyk, M.; Górnaś, P. Profiling of the Bioactive Components of Safflower Seeds and Seed Oil: Cultivated (Carthamus tinctorius L.) vs. Wild (Carthamus oxyacantha M. Bieb.). Eur. Food Res. Technol. 2020, 246, 449–459. [Google Scholar] [CrossRef]
- Jenett-Siems, K.; Weigl, R.; Kaloga, M.; Schulz, J.; Eich, E. Ipobscurines C and D: Macrolactam-Type Indole Alkaloids from the Seeds of Ipomoea Obscura. Phytochemistry 2003, 62, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.J.; Park, S.; Kim, H.K.; Jeong, W.Y.; Choi, J.Y.; Sung, N.-J.; Lee, W.S.; Lim, C.-S.; Kim, G.-S.; et al. Characterisation of Flavonoids in Orostachys japonicus A. Berger Using HPLC–MS/MS: Contribution to the Overall Antioxidant Effect. Food Chem. 2011, 124, 1627–1633. [Google Scholar] [CrossRef]
- Ma, C.-M.; Nakamura, N.; Hattori, M. Inhibitory Effects on HIV-1 Protease of Tri-p-Coumaroylspermidine from Artemisia Caruifolia and Related Amides. Chem. Pharm. Bull. 2001, 49, 915–917. [Google Scholar] [CrossRef]
- Jiang, J.-S.; Lv, L.; Yang, Y.-J.; Zhang, J.-L.; Zhang, P.-C. New Spermidines from the Florets of Carthamus tinctorius. J. Asian Nat. Prod. Res. 2008, 10, 447–451. [Google Scholar] [CrossRef]
- Du, N.; Zhou, W.; Jin, H.; Liu, Y.; Zhou, H.; Liang, X. Characterization of Tropane and Cinnamamide Alkaloids from Scopolia tangutica by High-Performance Liquid Chromatography with Quadrupole Time-of-Flight Tandem Mass Spectrometry. J. Sep. Sci. 2019, 42, 1163–1173. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, Y.; Chen, B.; Ding, L.; Wu, F.E. A New Amide from Thyrocarpus glochidiatus. Nat. Prod. Res. 2006, 20, 1063–1066. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Wang, I.; Stark, R.E. Potato Wound-Healing Tissues: A Rich Source of Natural Antioxidant Molecules with Potential for Food Preservation. Food Chem. 2016, 210, 473–480. [Google Scholar] [CrossRef]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic Analysis of Rice (Oryza sativa) Metabolic Responses to Herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, X.; Llorca, L.C.; Lu, J.; Lou, Y.; Li, R. Role of Jasmonate Signaling in Rice Resistance to the Leaf Folder Cnaphalocrocis medinalis. Plant Mol. Biol. 2022, 109, 627–637. [Google Scholar] [CrossRef]
- Grossi, L.; Casadei, R. The Crucial Role of Non-Enzymatic NO-Production in Plants. An EPR Study. Phytochemistry 2021, 188, 112794. [Google Scholar] [CrossRef]
- Han, J.; Li, L.; Han, L.; Huang, X.; Yuan, T. Phenylpropanoid amides and phenylethanols from Nanophyton erinaceum, Biochemical Systematics and Ecology, 2015, 61, 399-401. 61. [CrossRef]
- Kim, K.H.; Choi, S.U.; Son, M.W.; Lee, K.R. Two New Phenolic Amides from the Seeds of Pharbitis nil. Chem. Pharm. Bull. 2010, 58, 1532–1535. [Google Scholar] [CrossRef][Green Version]
- Panagabko, C.; Chenier, D.; Fixon-Owoo, S.; Atkinson, J.K. Ion-Pair HPLC Determination of Hydroxycinnamic Acid Monoconjugates of Putrescine, Spermidine and Spermine. Phytochem. Anal. 2000, 11, 11–17. [Google Scholar] [CrossRef]
- Sagner, S.; Shen, Z.-W.; Deus-Neumann, B.; Zenk, M.H. The Biosynthesis of Lunarine in Seeds of Lunaria Annua. Phytochemistry 1998, 47, 375–387. [Google Scholar] [CrossRef]
- Zhang, L.; Peterson, D.G. Identification of Bitter Compounds in Extruded Corn Puffed Products. Food Chem. 2018, 254, 185–192. [Google Scholar] [CrossRef]
- Meurer, B.; Wray, V.; Wiermann, R.; Strack, D. Hydroxycinnamic Acid-Spermidine Amides from Pollen of Alnus glutinosa, Betula verrucosa and Pterocarya fraxinifolia. Phytochemistry 1988, 27, 839–843. [Google Scholar] [CrossRef]
- Meurer, B.; Wiermann, R.; Strack, D. Phenylpropanoid Patterns in Fagales Pollen and Their Phylogenetic Relevance. Phytochemistry 1988, 27, 823–828. [Google Scholar] [CrossRef]
- Werner, C.; Hu, W.; Lorenzi-Riatsch, A.; Hesse, M. Di-Coumaroylspermidines and Tri-Coumaroylspermidines in Anthers of Different Species of the Genus Aphelandra. Phytochemistry 1995, 40, 461–465. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green Extraction of Phenolic Compounds from Foxtail Millet Bran by Ultrasonic-Assisted Deep Eutectic Solvent Extraction: Optimization, Comparison and Bioactivities. LWT 2022, 154, 112740. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Zhao, X.; Xia, Y.; Sun, X.; Xie, W.; Ye, Y.; Lu, X.; Xu, G. Deep Annotation of Hydroxycinnamic Acid Amides in Plants Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry and Its In Silico Database. Anal. Chem. 2018, 90, 14321–14330. [Google Scholar] [CrossRef]
- Li, S.; Li, T.; Jin, Y.; Qin, X.; Tian, J.; Zhang, L. Antidepressant-Like Effects of Coumaroylspermidine Extract From Safflower Injection Residues. Front. Pharmacol. 2020, 11, 713. [Google Scholar] [CrossRef]
- Zhao, G.; Gai, Y.; Chu, W.-J.; Qin, G.-W.; Guo, L.-H. A Novel Compound N1,N5-(Z)-N10-(E)-Tri-p-Coumaroylspermidine Isolated from Carthamus tinctorius L. and Acting by Serotonin Transporter Inhibition. Eur. Neuropsychopharmacol. 2009, 19, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Zamble, A.; Sahpaz, S.; Hennebelle, T.; Carato, P.; Bailleul, F. N1,N5,N10-Tris(4-Hydroxycinnamoyl)Spermidines from Microdesmis keayana Roots. Chem. Biodivers. 2006, 3, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Meurer-Grimes, B.; Rovira, I. Antifungal Activity of Three Spermidine Conjugates. FEMS Microbiol. Lett. 2001, 201, 255–258. [Google Scholar] [CrossRef]
- Bokern, M.; Witte, L.; Wray, V.; Nimtz, M.; Meurer-Grimes, B. Trisubstituted Hydroxycinnamic Acid Spermidines from Quercus Dentata Pollen. Phytochemistry 1995, 39, 1371–1375. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nakamura, K.; Furukawa, K.; Konishi, Y.; Ogino, T.; Higashiura, K.; Yago, H.; Okamoto, K.; Otsuka, M. A New Nonpeptide Tachykinin NK1 Receptor Antagonist Isolated from the Plants of Compositae. Chem. Pharm. Bull. 2002, 50, 47–52. [Google Scholar] [CrossRef]
- Lam, S.-C.; Liu, X.; Chen, X.-Q.; Hu, D.-J.; Zhao, J.; Long, Z.-R.; Fan, B.; Li, S.-P. Chemical Characteristics of Different Parts of Coreopsis Tinctoria in China Using Microwave-Assisted Extraction and High-Performance Liquid Chromatography Followed by Chemometric Analysis. J. Sep. Sci. 2016, 39, 2919–2927. [Google Scholar] [CrossRef]
- Cheng, X.-R.; Ma, J.-H.; Amadou, I.; Zhao, W.; Chen, Y.-Y.; Zhang, C.-X.; Guan, B. Electrophilic Components from Xiaoheiyao (Rhizomes of Inula Nervosa Wall.) Alleviate the Production of Heterocyclic Aromatic Amines via Creatinine Inhibition. Food Chem. 2023, 404, 134561. [Google Scholar] [CrossRef]
- Xin, X.-L.; Yu, Z.-L.; Tian, X.-G.; Wei, J.-C.; Wang, C.; Huo, X.-K.; Ning, J.; Feng, L.; Sun, C.-P.; Deng, S.; et al. Phenylpropanoid Amides from Alisma orientalis and Their Protective Effects against H2O2-Induced Damage in SH-SY5Y Cells. Phytochem. Lett. 2017, 21, 46–50. [Google Scholar] [CrossRef]
- OuYang, J.; Zhou, W.-N.; Li, G.; Wang, X.-Y.; Ding, C.-X.; Suo, Y.-R.; Wang, H.-L. Three New Alkaloids from Hippophae rhamnoides Linn. Subsp. Sinensis Rousi. Helv. Chim. Acta 2015, 98, 1287–1291. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Lou, H.; Fan, P. Chemical Constituents of Hemp (Cannabis sativa L.) Seed with Potential Anti-Neuroinflammatory Activity. Phytochem. Lett. 2018, 23, 57–61. [Google Scholar] [CrossRef]
- Bakrim, S.; Elouafy, Y.; Touhtouh, J.; Aanniz, T.; El Kadri, K.; Khalid, A.; Fawzy, S.; Mesaik, M.A.; Lee, L.-H.; Chamkhi, I.; et al. Exploring the Chemistry, Biological Effects, and Mechanism Insights of Natural Coumaroyltyramine: First Report. Fitoterapia 2024, 178, 106182. [Google Scholar] [CrossRef]
- Yoshihara, T.; Takamatsu, S.; Sakamura, S. Three New Phenolic Amides from the Roots of Eggplant (Solanum melongena L.). Agric. Biol. Chem. 1978, 42, 623–627. [Google Scholar] [CrossRef]
- Ichikawa, M.; Ryu, K.; Yoshida, J.; Ide, N.; Kodera, Y.; Sasaoka, T.; Rosen, R.T. Identification of Six Phenylpropanoids from Garlic Skin as Major Antioxidants. J. Agric. Food Chem. 2003, 51, 7313–7317. [Google Scholar] [CrossRef]
- Liu, C.; Tian, J.; An, T.; Lyu, F.; Jia, P.; Zhou, M.; Liu, Z.; Feng, Y. Secondary Metabolites from Solanum rostratum and Their Antifeedant Defense Mechanisms against Helicoverpa armigera. J. Agric. Food Chem. 2020, 68, 88–96. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.W.; Jang, H.; Le, T.P.L.; Kim, J.G.; Lee, M.S.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Phenolic Amides from Tribulus terrestris and Their Inhibitory Effects on Nitric Oxide Production in RAW 264.7 Cells. Arch. Pharmacal Res. 2018, 41, 192–195. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Wang, Y.; Gao, S.-Y.; Zhu, L.-H.; Wang, F.; Li, H.; Chen, L.-X. Phenolic Amides with Anti-Parkinson’s Disease (PD) Effects from Nicandra physaloides. J. Funct. Foods 2017, 31, 229–236. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.-D.; Chen, J.-G.; Mao, X.; Yu, L.; Zhu, L. Three New Amides from Microlepia pilosissima. J. Asian Nat. Prod. Res. 2012, 14, 1027–1031. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yeh, Y.-T.; Yang, W.-L. Amides from the Stem of Capsicum Annuum. Nat. Prod. Commun. 2011, 6, 1934578X1100600217. [Google Scholar] [CrossRef]
- Zacarés, L.; López-Gresa, M.P.; Fayos, J.; Primo, J.; Bellés, J.M.; Conejero, V. Induction of p-Coumaroyldopamine and Feruloyldopamine, Two Novel Metabolites, in Tomato by the Bacterial Pathogen Pseudomonas syringae. MPMI 2007, 20, 1439–1448. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Tao, X.; Ying, X.; Stien, D. Two Amide Glycosides from Portulaca oleracea L. and Its Bioactivities. Nat. Prod. Res. 2021, 35, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-M.; Lan, Y.-H.; Hwang, T.-L.; Leu, Y.-L. Anti-Inflammatory and Antioxidant Components from Hygroryza aristata. Molecules 2011, 16, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.-L.; Zhang, N.; Sun, M.-G.; Huang, Y.-F.; Sun, Y.; Ma, H.-Y.; Hua, H.-M.; Pei, Y.-H. One New Cinnamic Imide Dervative from the Fruits of Tribulus terrestris. Nat. Prod. Res. 2008, 22, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapoom, T.; Kasai, R.; Chumsri, P.; Kraisintu, K.; Yamasaki, K. Lotthanongine, an Unprecedented Flavonoidal Indole Alkaloid from the Roots of Thai Medicinal Plant, Trigonostemon reidioides. Tetrahedron Lett. 2002, 43, 2941–2943. [Google Scholar] [CrossRef]
- Jágr, M.; Dvořáček, V.; Čepková, P.H.; Doležalová, J. Comprehensive Analysis of Oat Avenanthramides Using Hybrid Quadrupole-Orbitrap Mass Spectrometry: Possible Detection of New Compounds. Rapid Commun. Mass Spectrom. 2020, 34, e8718. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Borges, G.; Nagai, C.; Jackson, M.C.; Yokota, T.; Crozier, A.; Ashihara, H. Profiles of Phenolic Compounds and Purine Alkaloids during the Development of Seeds of Theobroma cacao Cv. Trinitario. J. Agric. Food Chem. 2013, 61, 427–434. [Google Scholar] [CrossRef]
- Stark, T.; Hofmann, T. Isolation, Structure Determination, Synthesis, and Sensory Activity of N-Phenylpropenoyl-l-Amino Acids from Cocoa (Theobroma cacao). J. Agric. Food Chem. 2005, 53, 5419–5428. [Google Scholar] [CrossRef]
- Lechtenberg, M.; Henschel, K.; Liefländer-Wulf, U.; Quandt, B.; Hensel, A. Fast Determination of N-Phenylpropenoyl-l-Amino Acids (NPA) in Cocoa Samples from Different Origins by Ultra-Performance Liquid Chromatography and Capillary Electrophoresis. Food Chem. 2012, 135, 1676–1684. [Google Scholar] [CrossRef]
- Alemanno, L.; Ramos, T.; Gargadenec, A.; Andary, C.; Ferriere, N. Localization and Identification of Phenolic Compounds in Theobroma cacao L. Somatic Embryogenesis. Ann. Bot. 2003, 92, 613–623. [Google Scholar] [CrossRef]
- Wei, Q.-Y.; Jiang, H.; Zhang, J.-X.; Guo, P.-F.; Wang, H. Synthesis of N-Hydroxycinnamoyl Amino Acid Ester Analogues and Their Free Radical Scavenging and Antioxidative Activities. Med. Chem. Res. 2012, 21, 1905–1911. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Liu, B.; Xiao, J.; Cheng, K.-W.; Chen, F.; Wang, M. Tricoumaroylspermidine from Rose Exhibits Inhibitory Activity against Ethanol-Induced Apoptosis in HepG2 Cells. Food Funct. 2021, 12, 5892–5902. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, A.Y.; Kim, J.H.; Seong, S.H.; Cho, E.J.; Choi, J.S.; Kim, M.J.; Yang, S.; Yokozawa, T.; Shin, Y.S. Protective Effects of Serotonin and Its Derivatives, N-Feruloylserotonin and N-(p-Coumaroyl) Serotonin, Against Cisplatin-Induced Renal Damage in Mice. Am. J. Chin. Med. 2019, 47, 369–383. [Google Scholar] [CrossRef]
- Kim, J.H.; He, M.T.; Kim, M.J.; Yang, C.Y.; Shin, Y.S.; Yokozawa, T.; Park, C.H.; Cho, E.J. Safflower (Carthamus tinctorius L.) Seed Attenuates Memory Impairment Induced by Scopolamine in Mice via Regulation of Cholinergic Dysfunction and Oxidative Stress. Food Funct. 2019, 10, 3650–3659. [Google Scholar] [CrossRef]
- Cho, S.-H.; Lee, H.-R.; Kim, T.-B.; Choi, S.-W.; Lee, W.-J.; Choi, Y. Effects of Defatted Safflower Seed Extract and Phenolic Compounds in Diet on Plasma and Liver Lipid in Ovariectomized Rats Fed High-Cholesterol Diets. J. Nutr. Sci. Vitaminol. 2004, 50, 32–37. [Google Scholar] [CrossRef]
- Božičević, A.; De Mieri, M.; Nassenstein, C.; Wiegand, S.; Hamburger, M. Secondary Metabolites in Allergic Plant Pollen Samples Modulate Afferent Neurons and Murine Tracheal Rings. J. Nat. Prod. 2017, 80, 2953–2961. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid Content of U.S. Fruits, Vegetables, and Nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Lee, Y.-J.; Kim, J.-D.; Kang, S.-N.; Lee, S.-K.; Jang, J.-Y.; Lee, H.-K.; Lim, J.-H.; Lee, O.-H. Phenolic Composition, Antioxidant Activity and Anti-Adipogenic Effect of Hot Water Extract from Safflower (Carthamus tinctorius L.) Seed. Nutrients 2013, 5, 4894–4907. [Google Scholar] [CrossRef]
- Katsuda, S.; Suzuki, K.; Koyama, N.; Takahashi, M.; Miyake, M.; Hazama, A.; Takazawa, K. Safflower Seed Polyphenols (N-(p-Coumaroyl)Serotonin and N-Feruloylserotonin) Ameliorate Atherosclerosis and Distensibility of the Aortic Wall in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) Rabbits. Hypertens. Res. 2009, 32, 944–949. [Google Scholar] [CrossRef]
- Takii, T.; Hayashi, M.; Hiroma, H.; Chiba, T.; Kawashima, S.; Zhang, H.L.; Nagatsu, A.; Sakakibara, J.; Onozaki, K. Serotonin Derivative, N-(p-Coumaroyl)Serotonin, Isolated from Safflower (Carthamus tinctorius L.) Oil Cake Augments the Proliferation of Normal Human and Mouse Fibroblasts in Synergy with Basic Fibroblast Growth Factor (bFGF) or Epidermal Growth Factor (EGF)1. J. Biochem. 1999, 125, 910–915. [Google Scholar] [CrossRef]
- Piga, R.; Naito, Y.; Kokura, S.; Handa, O.; Yoshikawa, T. Inhibitory Effect of Serotonin Derivatives on High Glucose-Induced Adhesion and Migration of Monocytes on Human Aortic Endothelial Cells. Br. J. Nutr. 2009, 102, 264–272. [Google Scholar] [CrossRef]
- Takii, T.; Kawashima, S.; Chiba, T.; Hayashi, H.; Hayashi, M.; Hiroma, H.; Kimura, H.; Inukai, Y.; Shibata, Y.; Nagatsu, A.; et al. Multiple Mechanisms Involved in the Inhibition of Proinflammatory Cytokine Production from Human Monocytes by N-(p-Coumaroyl)Serotonin and Its Derivatives. Int. Immunopharmacol. 2003, 3, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, A.; Zhang, H.; Mizukami, H.; Okuyama, H.; Sakakibara, J.; Tokuda, H.; Nishino, H. Tyrosinase Inhibitory and Anti-Tumor Promoting Activities of Compounds Isolated from Safflower (Carthamus tinctorius L.) and Cotton (Gossypium hirsutum L.) Oil Cakes. Nat. Prod. Lett. 2000, 14, 153–158. [Google Scholar] [CrossRef]
- Han, S.J.; Lim, M.J.; Lee, K.M.; Oh, E.; Shin, Y.S.; Kim, S.; Kim, J.S.; Yun, S.P.; Kang, L.-J. Safflower Seed Extract Attenuates the Development of Osteoarthritis by Blocking NF-κB Signaling. Pharmaceuticals 2021, 14, 258. [Google Scholar] [CrossRef]
- Choi, S.W.; Lee, S.K.; Kim, E.O.; Oh, J.H.; Yoon, K.S.; Parris, N.; Hicks, K.B.; Moreau, R.A. Antioxidant and Antimelanogenic Activities of Polyamine Conjugates from Corn Bran and Related Hydroxycinnamic Acids. J. Agric. Food Chem. 2007, 55, 3920–3925. [Google Scholar] [CrossRef]
- Lazari, D.; Alexiou, G.A.; Markopoulos, G.S.; Vartholomatos, E.; Hodaj, E.; Chousidis, I.; Leonardos, I.; Galani, V.; Kyritsis, A.P. N-(p-Coumaroyl) Serotonin Inhibits Glioblastoma Cells Growth through Triggering S-Phase Arrest and Apoptosis. J. Neuro-Oncol. 2017, 132, 373–381. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Gao, Y.; Xu, Q.; Ju, X.; Wang, L. Identification and Anti-Tumour Activities of Phenolic Compounds Isolated from Defatted Adlay (Coix lachryma-jobi L. Var. Ma-Yuen Stapf) Seed Meal. J. Funct. Foods 2016, 26, 394–405. [Google Scholar] [CrossRef]
- Xiong, W.; Li, Y.; Yao, Y.; Xu, Q.; Wang, L. Antioxidant Mechanism of a Newly Found Phenolic Compound from Adlay (NDPS) in HepG2 Cells via Nrf2 Signalling. Food Chem. 2022, 378, 132034. [Google Scholar] [CrossRef]
- Fu, R.; Dou, Z.; Li, N.; Fan, X.; Amin, S.; Zhang, J.; Wang, Y.; Li, Z.; Li, Z.; Yang, P. Avenanthramide A Potentiates Bim-Mediated Antineoplastic Properties of 5-Fluorouracil via Targeting KDM4C/MIR17HG/GSK-3β Negative Feedback Loop in Colorectal Cancer. Acta Pharm. Sin. B 2024, 14, 5321–5340. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical Profile of Launaea Nudicaulis Ethanolic Extract and Its Antidiabetic Effect in Streptozotocin-Induced Rats. Molecules 2021, 26, 1000. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Gouda, H.M.; Morsy, A.A.; Youssef, A.K.; Tolba, I.A.E.-M.; Selim, A.A.M.A. The Ethyl Acetate Extract from Abutilon Fruticosum Guill and Perr. as a Potential Diabetes–Cancer Prophylactic: A Cytotoxic, α-Glucosidase, and in-Silico Study. South Afr. J. Bot. 2023, 156, 110–114. [Google Scholar] [CrossRef]
- Ha, M.T.; Lee, T.H.; Kim, C.S.; Prajapati, R.; Kim, J.A.; Choi, J.S.; Min, B.S. PTP1B and α-Glucosidase Inhibitory Activities of the Chemical Constituents from Hedera rhombea Fruits: Kinetic Analysis and Molecular Docking Simulation. Phytochemistry 2022, 197, 113100. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, D.H.; Zhao, B.T.; Le, D.D.; Choi, D.H.; Kim, Y.H.; Nguyen, T.H.; Woo, M.H. A New Lignan and a New Alkaloid, and α-Glucosidase Inhibitory Compounds from the Grains of Echinochloa utilis Ohwi & Yabuno. Bioorganic Chem. 2017, 74, 221–227. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Potent α-Glucosidase Inhibitors from Safflower (Carthamus tinctorius L.) Seed. Phytother. Res. 2012, 26, 722–726. [Google Scholar] [CrossRef]
- Seo, K.-H.; Ra, J.-E.; Lee, S.-J.; Lee, J.H.; Kim, S.R.; Lee, J.H.; Seo, W.D. Anti-Hyperglycemic Activity of Polyphenols Isolated from Barnyard Millet (Echinochloa utilis L.) and Their Role Inhibiting α-Glucosidase. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 571–579. [Google Scholar] [CrossRef]
- Samad, A.H.; Ty Willing, T.S.; Alberti, K.G.M.; Taylor, R. Effects of BAYm 1099, New α-Glucosidase Inhibitor, on Acute Metabolic Responses and Metabolic Control in NIDDM Over 1 Mo. Diabetes Care 1988, 11, 337–344. [Google Scholar] [CrossRef]
- Niwa, T.; Doi, U.; Osawa, T. Inhibitory Activity of Corn-Derived Bisamide Compounds against α-Glucosidase. J. Agric. Food Chem. 2003, 51, 90–94. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Li, Q.; Zheng, W.; Wu, P.; Wu, R.; Chen, W.-H.; Li, C. N-p-Coumaroyloctopamine Ameliorates Hepatic Glucose Metabolism and Oxidative Stress Involved in a PI3K/AKT/GSK3β Pathway. Front. Pharmacol. 2024, 15, 1396641. [Google Scholar] [CrossRef]
- Georgiev, L.; Chochkova, M.; Totseva, I.; Seizova, K.; Marinova, E.; Ivanova, G.; Ninova, M.; Najdenski, H.; Milkova, T. Anti-Tyrosinase, Antioxidant and Antimicrobial Activities of Hydroxycinnamoylamides. Med. Chem. Res. 2013, 22, 4173–4182. [Google Scholar] [CrossRef]
- Seo, W.D.; Kim, J.Y.; Jang, K.C.; Han, S.-I.; Ra, J.-E.; Oh, S.-H.; Lee, J.H.; Kim, Y.-G.; Kang, H.-J.; Kim, B.-J.; et al. Anti-Pigmentation Effect of Serotonin Alkaloid Isolated from Korean Barnyard Millet (Echinochola utilis). J. Korean Soc. Appl. Biol. Chem. 2012, 55, 579–586. [Google Scholar] [CrossRef]
- Mercier, A.J.; Farragher, S.; Schmor, B.; Kamau, M.; Atkinson, J. Effect of a Plant-Derived Spider Toxin Analogue on Crayfish Neuromuscular Junctions. Can. J. Zool. 1998, 76, 2103–2107. [Google Scholar] [CrossRef]
- Zolfaghari, B.; Yazdiniapour, Z.; Sadeghi, M.; Akbari, M.; Troiano, R.; Lanzotti, V. Cinnamic Acid Derivatives from Welsh Onion (Allium fistulosum) and Their Antibacterial and Cytotoxic Activities. Phytochem. Anal. 2021, 32, 84–90. [Google Scholar] [CrossRef]
- Abdel-Moez, G.; Sayed, H.; Khalifa, A.; Abd-Elrahman, S.; Osman, M.; Mohamed, S. Evaluating Anthelmintic, Anti-Platelet, and Anti-Coagulant Activities, and Identifying the Bioactive Phytochemicals of Amaranthus blitum L. BMC Complement. Med. Ther. 2024, 24, 183. [Google Scholar] [CrossRef]
- Aderibigbe, S.A.; Idowu, S.O.; Olaniyi, A.A.; Wright, C.W.; Fatokun, A.A. Bioactivity and Cytotoxicity Profiling of Vincosamide and Strictosamide, Anthelmintic Epimers from Sarcocephalus latifolius (Smith) Bruce Leaf. J. Ethnopharmacol. 2021, 265, 113142. [Google Scholar] [CrossRef]
- Rajareddy, A.; Murthy M, S. Synthesis, Characterization, and Anthelmintic Activity of Novel Benzothiazole Derivatives Containing Indole Moieties. Asian J Pharm Clin Res 2019, 12, 321–325. [Google Scholar] [CrossRef]
- Hao, B.; Liu, G.-L.; Hu, X.-G.; Wang, G.-X. Bioassay-Guided Isolation and Identification of Active Compounds from Semen Pharbitidis against Dactylogyrus intermedius (Monogenea) in Goldfish (Carassius auratus). Vet. Parasitol. 2012, 187, 452–458. [Google Scholar] [CrossRef]
- Omosa, L.K.; Nchiozem-Ngnitedem, V.-A.; Guefack, M.-G.F.; Mbaveng, A.T.; Kuete, V. Antibacterial Activities of Thirteen Naturally Occuring Compounds from Two Kenyan Medicinal Plants: Zanthoxylum paracanthum (Mildbr). Kokwaro (Rutaceae) and Dracaena usambarensis Engl. (Asparagaceae) against MDR Phenotypes. South Afr. J. Bot. 2022, 151, 756–762. [Google Scholar] [CrossRef]
- Stark, T.; Lang, R.; Keller, D.; Hensel, A.; Hofmann, T. Absorption of N-Phenylpropenoyl-L-Amino Acids in Healthy Humans by Oral Administration of Cocoa (Theobroma cacao). Mol. Nutr. Food Res. 2008, 52, 1201–1214. [Google Scholar] [CrossRef]
- Tanaka, E.; Tanaka, C.; Mori, N.; Kuwahara, Y.; Tsuda, M. Phenylpropanoid Amides of Serotonin Accumulate in Witches’ Broom Diseased Bamboo. Phytochemistry 2003, 64, 965–969. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Wu, D.-D.; Zhuang, C.-C.; Ma, C.-M. Anti-Osteoporosis Effects of Mammalian Lignans and Their Precursors from Flaxseed and Safflower Seed Using Zebrafish Model. J. Food Sci. 2023, 88, 5278–5290. [Google Scholar] [CrossRef]
- Mellon, J.E.; Moreau, R.A. Inhibition of Aflatoxin Biosynthesis in Aspergillus flavus by Diferuloylputrescine and P-Coumaroylferuloylputrescine. J. Agric. Food Chem. 2004, 52, 6660–6663. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-Y.; Xia, Y.-G.; Wang, Q.-H.; Dou, D.-Q.; Kuang, H.-X. Two New Amide Alkaloids from the Flower of Datura metel L. Fitoterapia 2010, 81, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Chung, C.-Y.; Hwang, T.-L.; Chen, J.-F. Amides and Benzenoids from Zanthoxylum ailanthoides with Inhibitory Activity on Superoxide Generation and Elastase Release by Neutrophils. J. Nat. Prod. 2009, 72, 107–111. [Google Scholar] [CrossRef]
- Luo, X.; Huang, Y.; Zhang, S.; Yin, H.; Li, C.; Li, Q. Five New Phenethyl Cinnamides from the Mangrove Associates Micromelum falcatum. Biochem. Syst. Ecol. 2014, 56, 191–195. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Wang, W.; Wang, R.; Ding, Y.; Du, L. Alkaloids from Portulaca oleracea L. Phytochemistry 2005, 66, 2595–2601. [Google Scholar] [CrossRef]
- Murata, T.; Miyase, T.; Yoshizaki, F. Cyclic Spermidine Alkaloids and Flavone Glycosides from Meehania fargesii. Chem. Pharm. Bull. 2010, 58, 696–702. [Google Scholar] [CrossRef]
- Choi, G.-Y.; Kim, K.J.; Park, H.-S.; Hwang, E.-S.; Cho, J.-M.; Kim, H.-B.; Kim, D.-O.; Park, J.-H. Phenolic Changes in a Combined Herbal Extract of Lithospermum Erythrorhizon, Houttuynia Cordata, and Spirodela polyrhiza and Alleviation of DNCB-Induced Atopic Dermatitis in BALB/c Mice. Food Sci. Biotechnol. 2024, 33, 129–144. [Google Scholar] [CrossRef]
- Li, C.-Y.; Tsai, W.-J.; Damu, A.G.; Lee, E.-J.; Wu, T.-S.; Dung, N.X.; Thang, T.D.; Thanh, L. Isolation and Identification of Antiplatelet Aggregatory Principles from the Leaves of Piper Lolot. J. Agric. Food Chem. 2007, 55, 9436–9442. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; García-Mateos, R.; Becerra-Martínez, E.; Toledo-Aguilar, R.; Volke-Haller, V.H.; Jesús Magdaleno-Villar, J. Bioactive Compounds of Purslane (Portulaca oleracea L.) According to the Production System: A Review. Sci. Hortic. 2023, 308, 111584. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Xiang, L.; Zheng, Y. Phenolic Alkaloids as a New Class of Antioxidants in Portulaca Oleracea. Phytother. Res. 2009, 23, 1032–1035. [Google Scholar] [CrossRef]
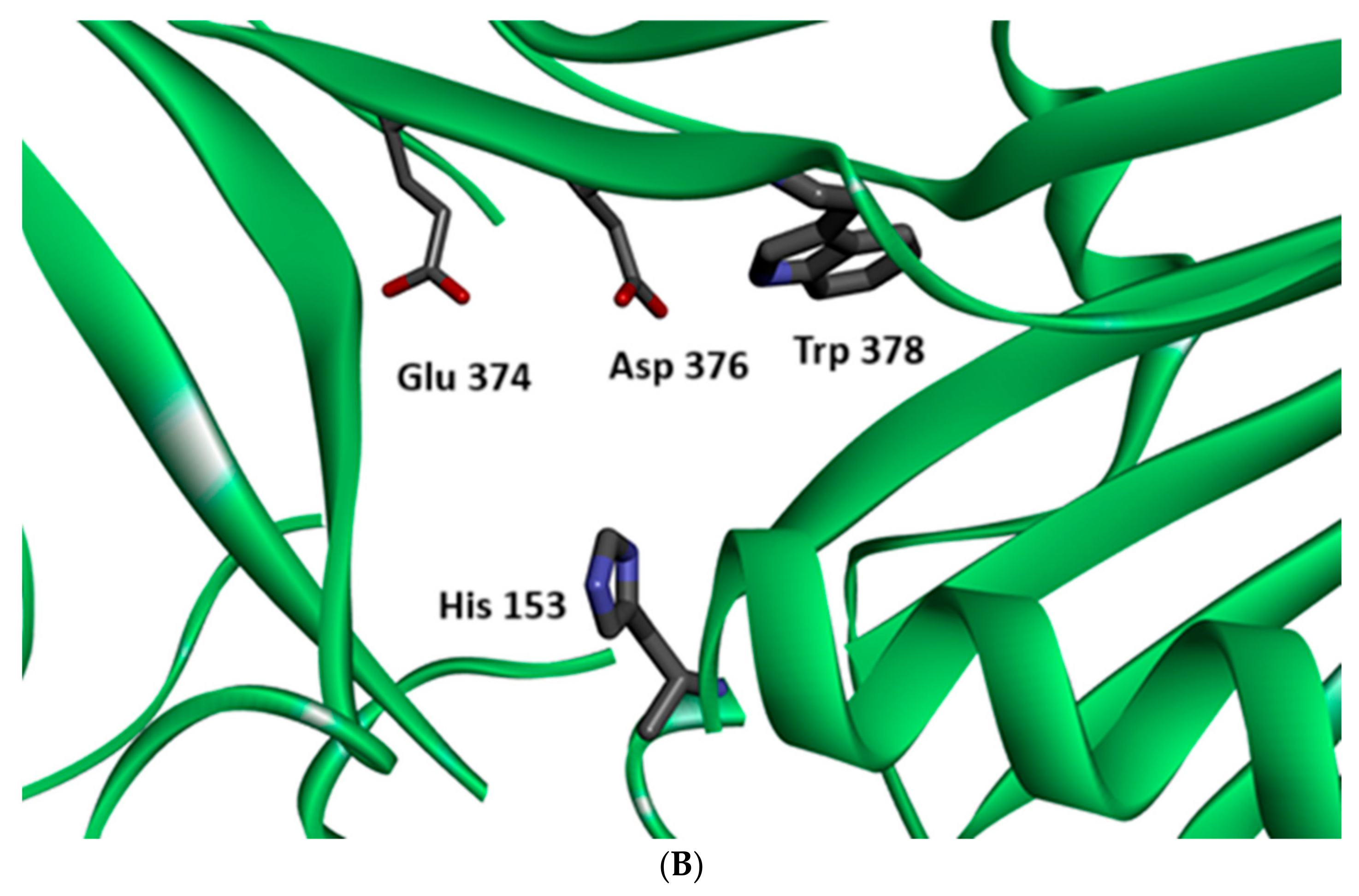
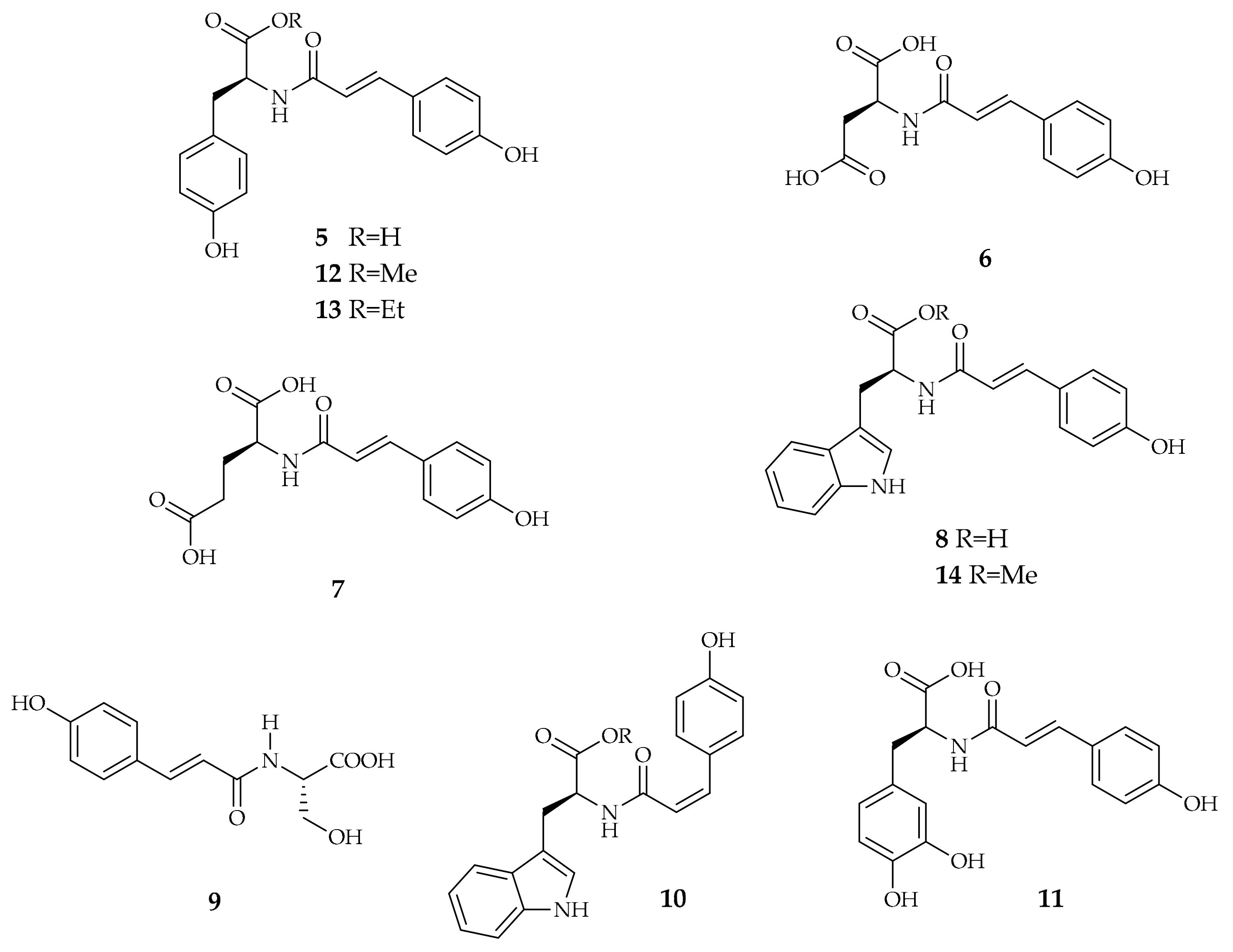


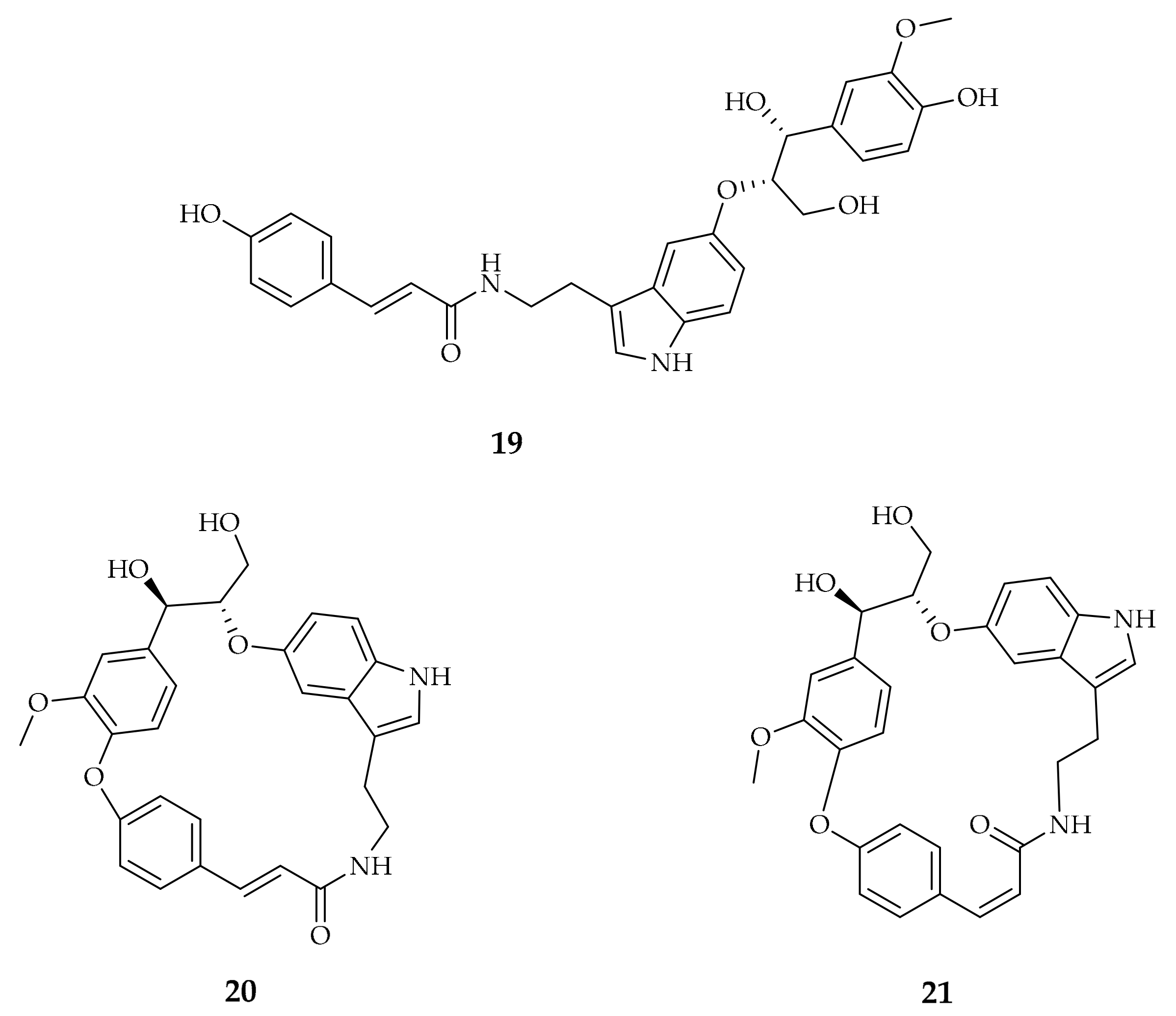
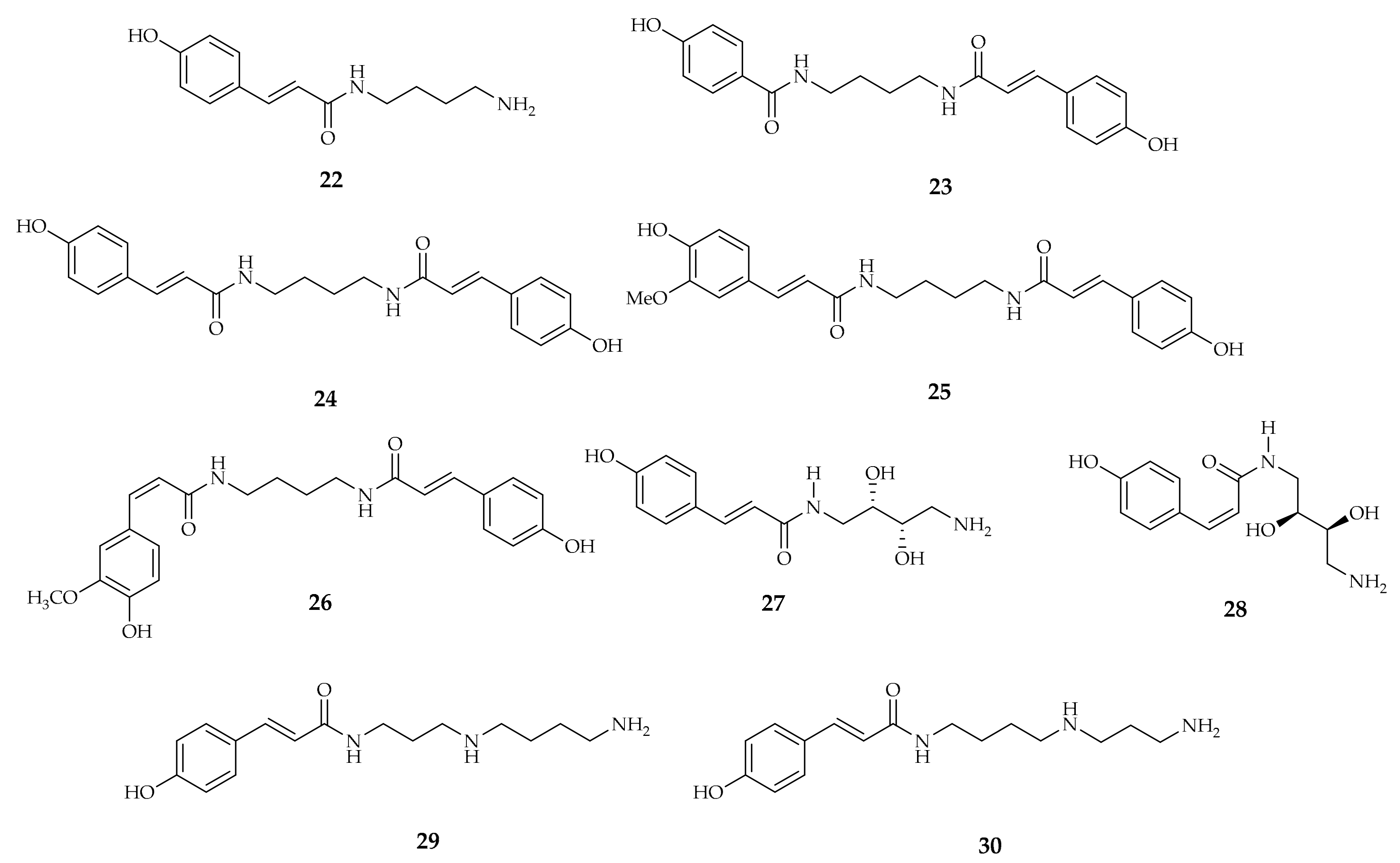
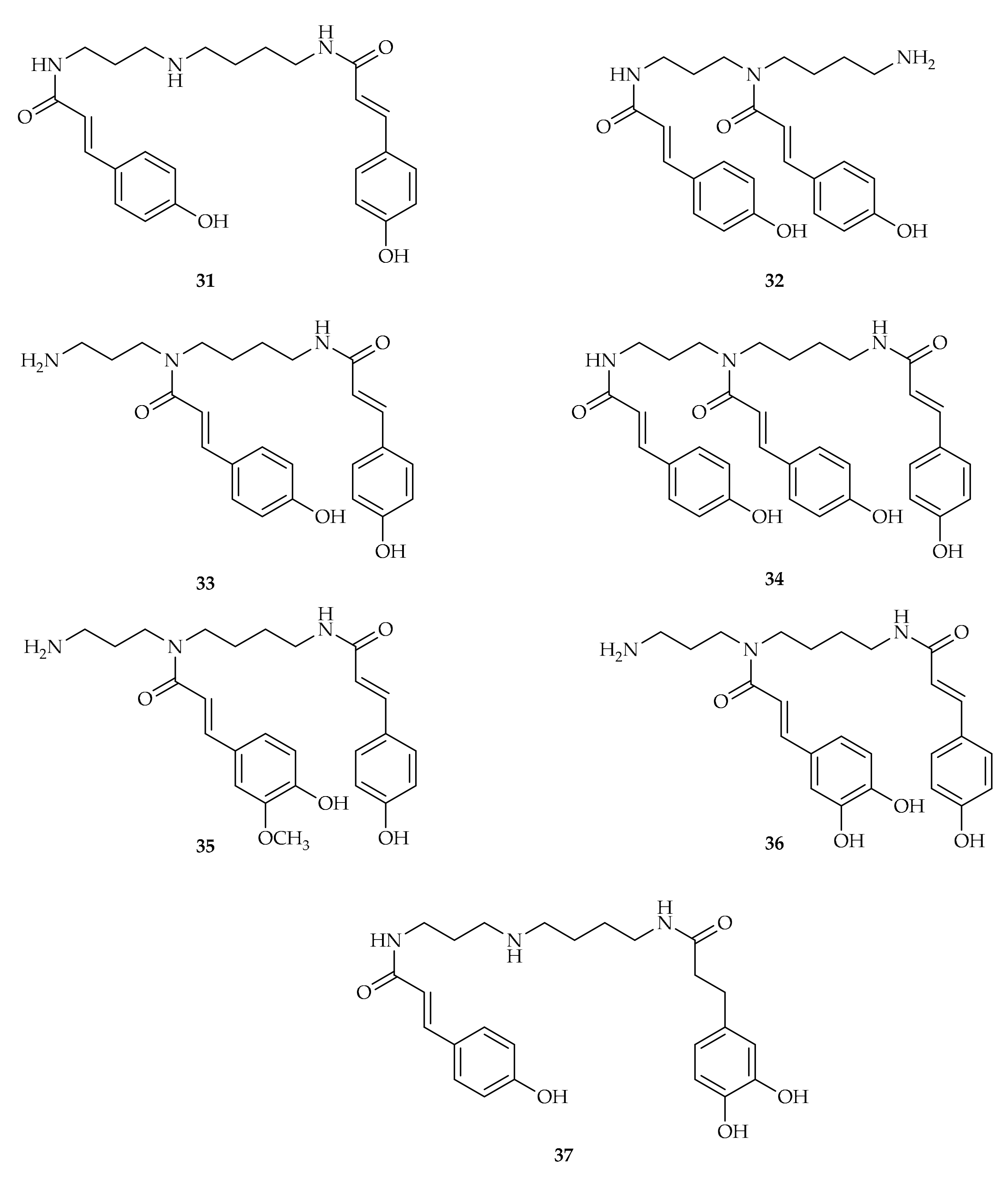
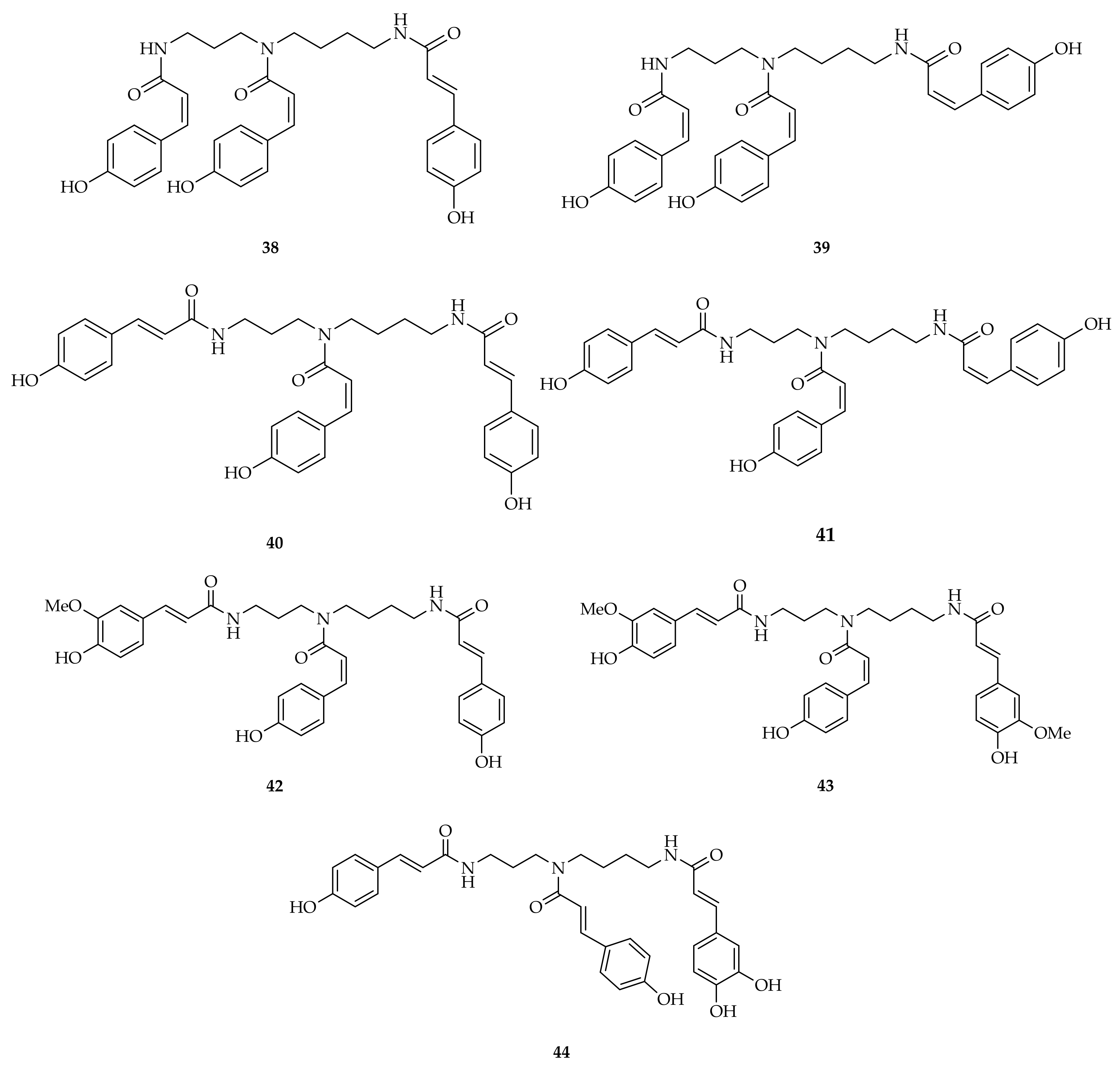
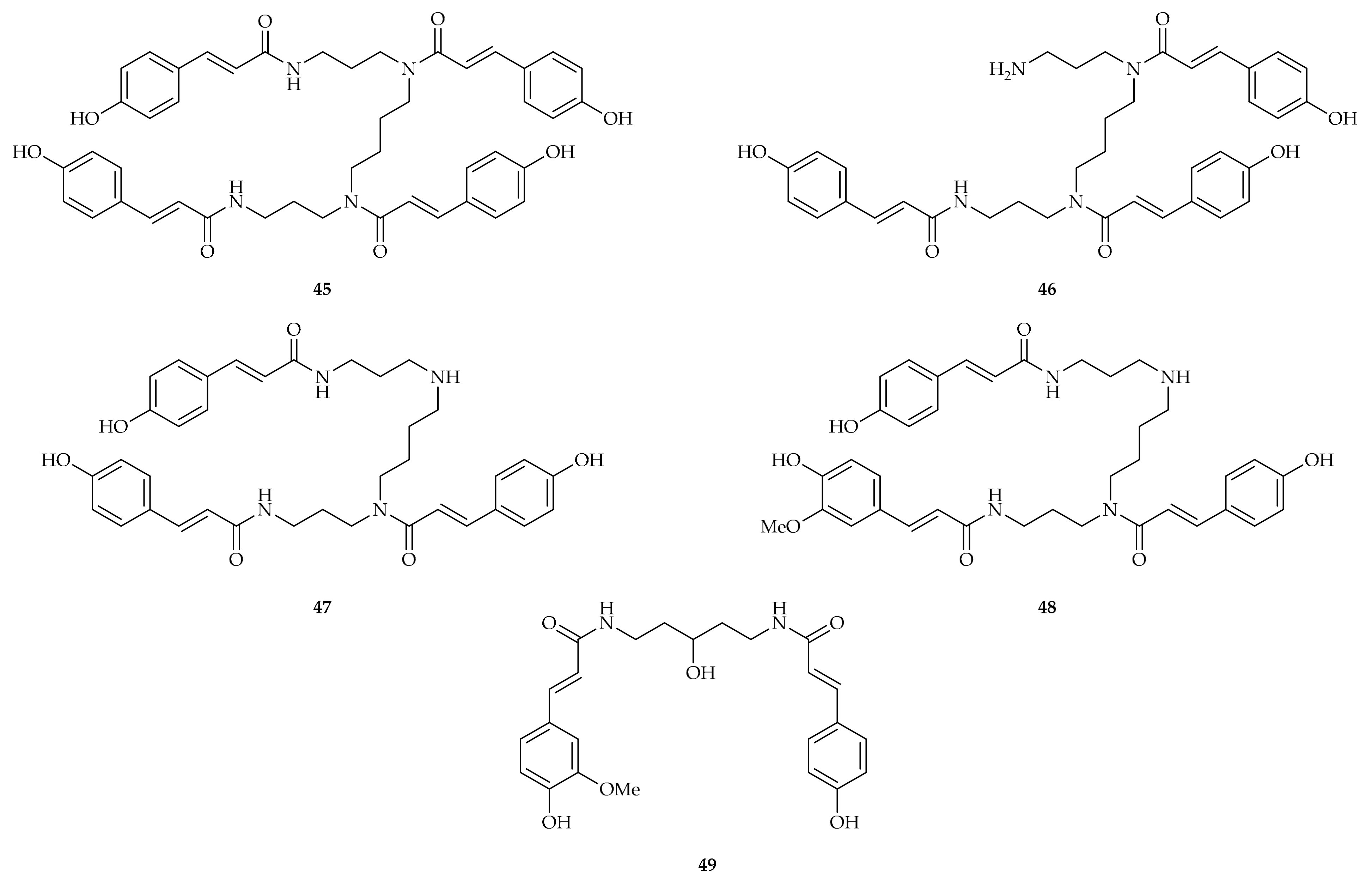
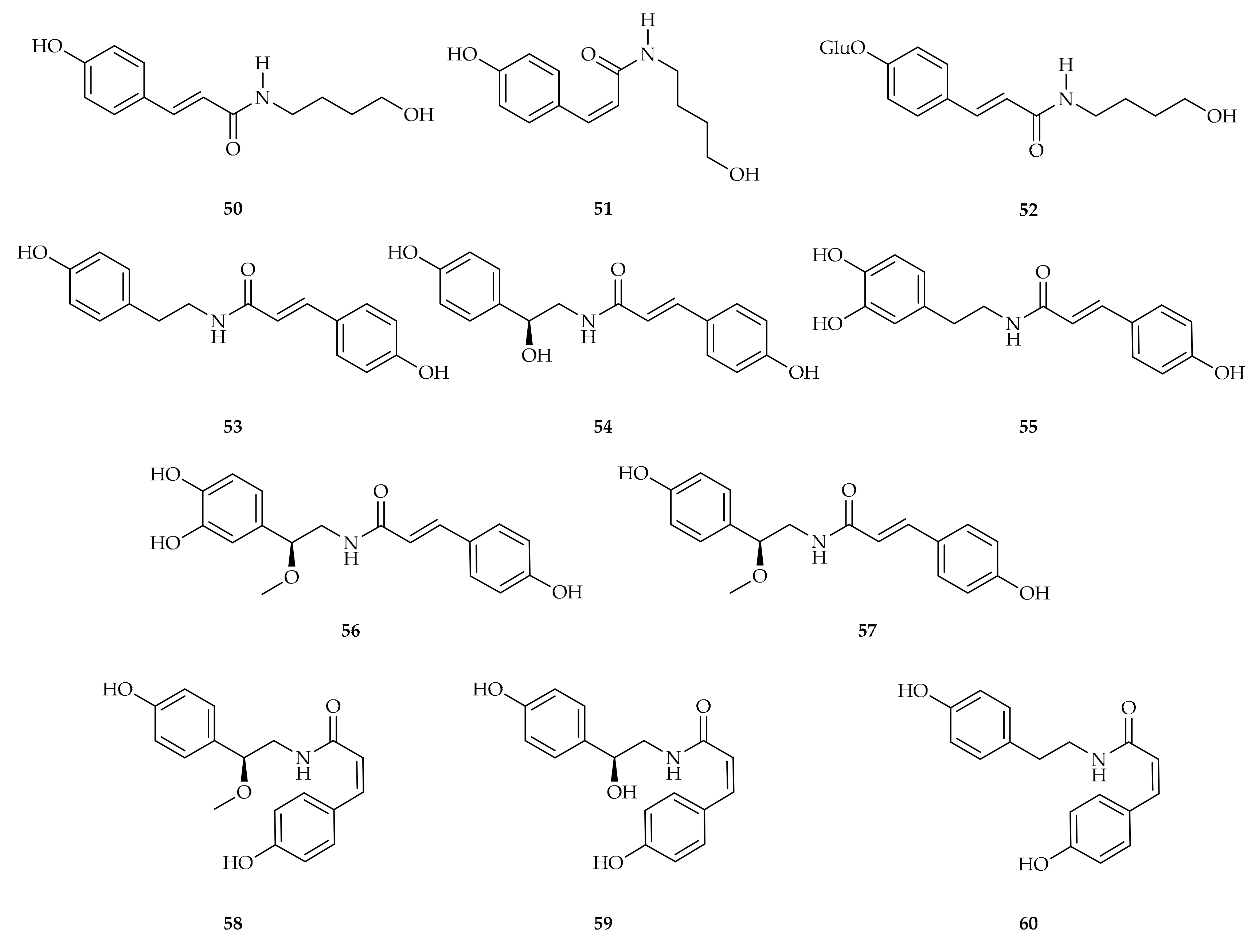
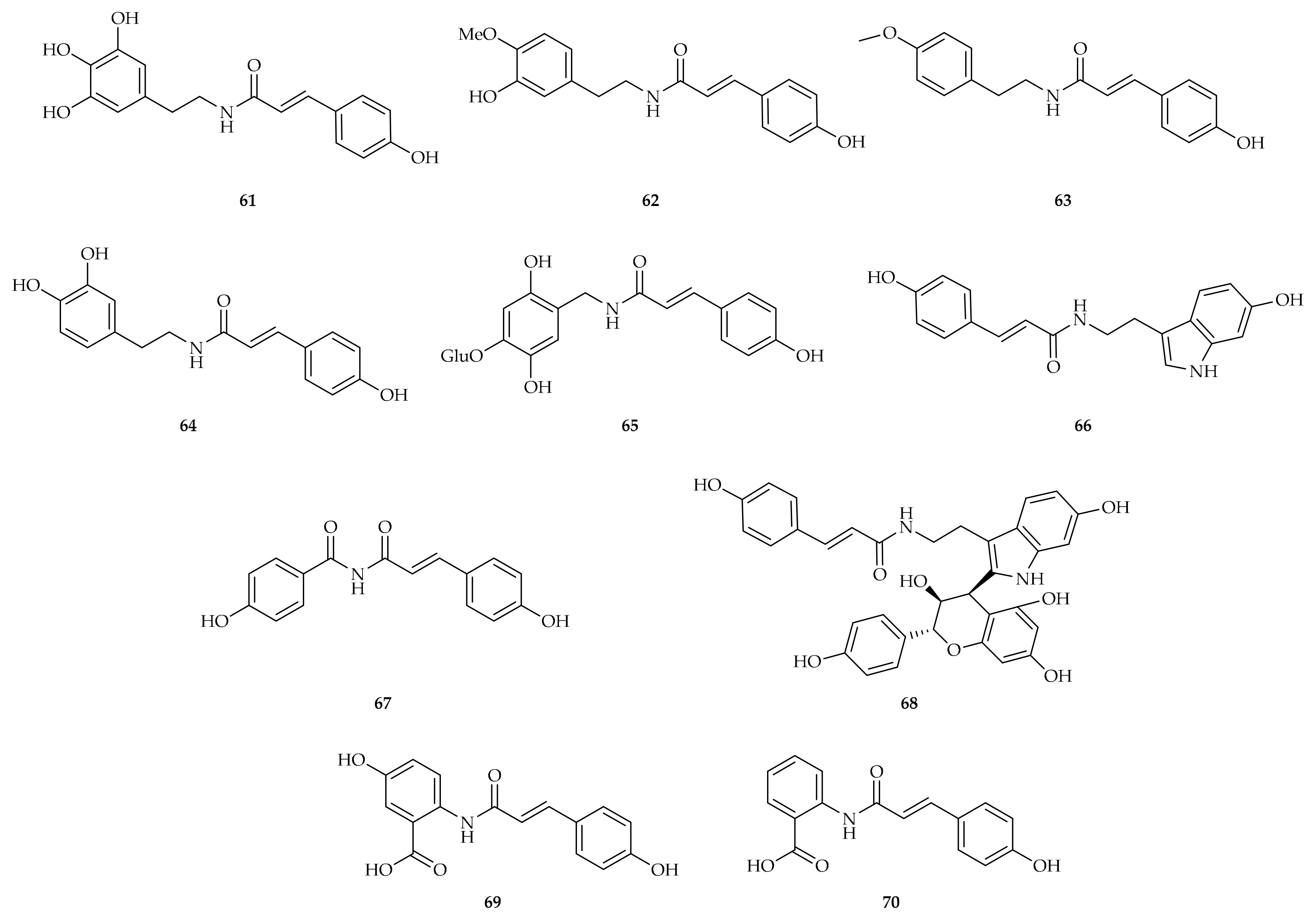
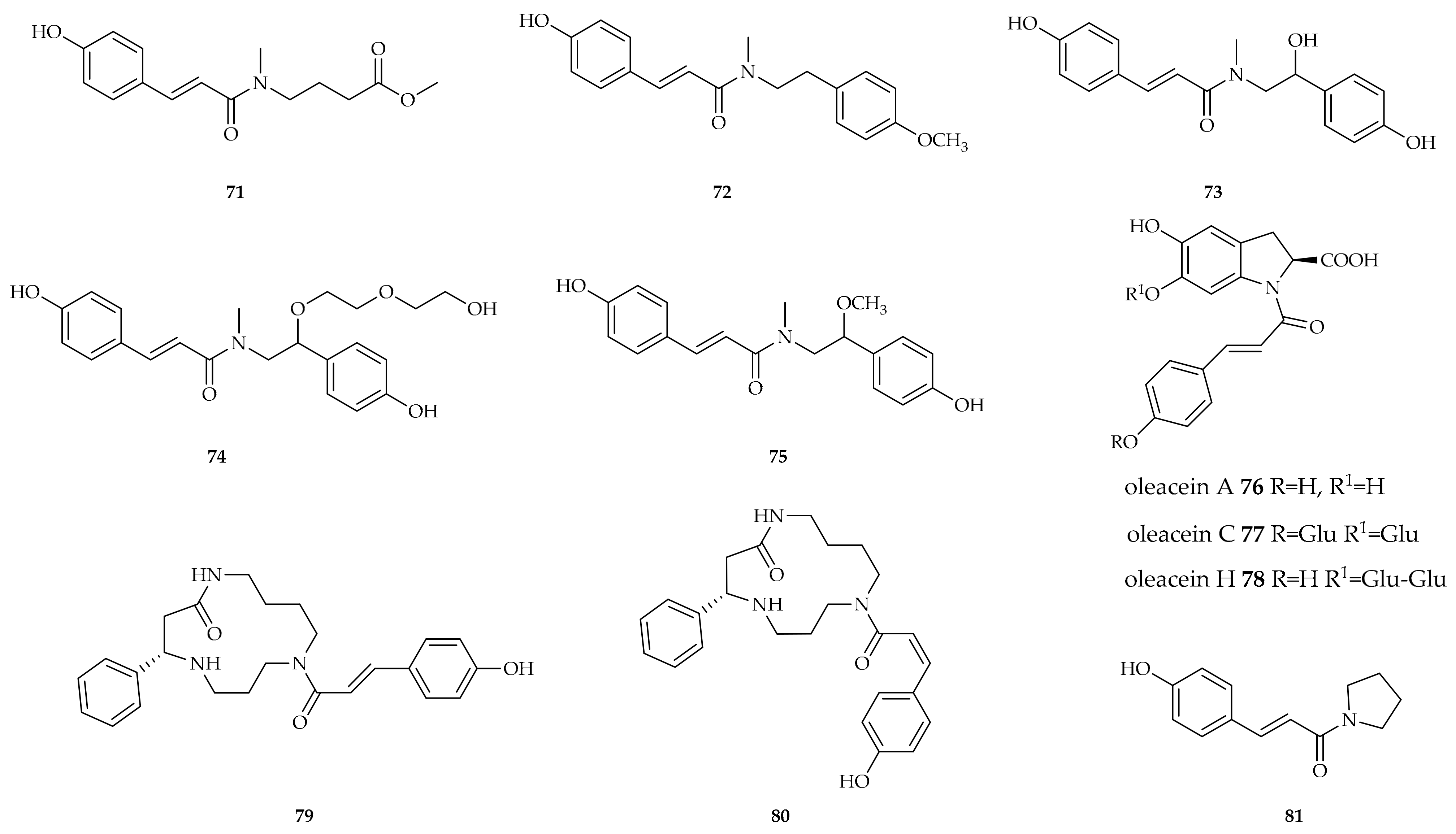
| Compound | Plant Source | Enzyme/System | Activity | Comments | Reference |
|---|---|---|---|---|---|
| p-coumaroyltyrosine methyl ester 12 | Hedera Rhombea | α-Glucosidase inhibition | 82.73 μM a | One order of magnitude better than the reference inhibitor acarbose. | [113] |
| Abrusamide A 15, Abrusamide D and H | NO synthase inhibition | 23.4, 25.2 and 28.3 μM a | In lipopolysaccharide (LPS)-induced RAW264.7 macrophages. | [27] | |
| Feruloylserotonin and p-coumaroylserotonin 16 | Tyrosinase inhibition | 23 and 74 μM a | Notably, the melanogenesis inhibition test has also been carried out on human enzymes in melanoma cells | [30] | |
| (E,E)-di-p-coumaroylputrescine 24 | Tyrosinase inhibition | 181.73 µM a | [45] | ||
| p-coumaroylserotonin 16 | Inhibition of and MAO-B AChE | MAO-B 1.2 μM AChE 15 μg/mL a | MAO-B inhibition is potentially involved in AD development. AChE in scopolamine-induced mice to ameliorate memory | [33] | |
| N1,N5-dicoumaroylspermidine 32 N5,N10-di-p-coumaroylspermidine 33 N1,N5,N10-tri-p-coumaroylspermidine 34 | AChE inhibition | 714.43 μg/mL a | Evaluation of an extract from foxtail millet bran | [60] | |
| oleraciamide E 65 | AChE inhibition | 52.43 ± 0.33 μM | [82] | ||
| N10-coumaroylspermidine 30 | Invertebrate neuromuscular junction receptors | 200 μM a | Antagonist action | [122] | |
| Compound 55 | Inhibition of AchE and CarE | 16.87 ± 0.83 and 23.14 ± 1.54 μM d | [76] | ||
| Compound 56 | Inhibition of AchE and CarE | 41.29 ± 1.54 and 46.87 ± 0.83 μM d | [76] | ||
| (Z)-N1,N5-(E)-N10-tri-p-coumaroylspermidine 38 | Inhibition of serotonin uptake in S6 cells and in synaptosomes | 0.74 ± 0.15 µM for S6 cells 1.07 ± 0.23 µM for synaptosomes a | [63] | ||
| N1,N5,N10-tri-p-coumaroylspermidine 34 | HIV protease inhibition | 83 μM a | [44] | ||
| ailanthamide 72 | MLP/CB-induced elastase release | 4.23 µg/mL a | [134] | ||
| N1,N5-dicoumaroylspermidine 32 | Growth of HepG-2, MCF-7, and CaCo-2 cancer cell lines | 46.34 ± 3.99 and 92.69 ± 5.63 μg/ml a | [107] | ||
| Piperlotine E 81 | Platelet aggregation induced by arachidonic acid and PAF | 11.5 µg/mL as anti-AA and 58.65 µg/mL as anti-PAF a | [139] | ||
| Compound 45 | inhibitory activity on contraction of smooth muscle in guinea pig ileum and antagonism towards NK1 receptor | 21.9 nM and 3.3 nM b | [67] | ||
| p-Coumaroyloctopamine 54 | Antimicrobial activity against S. aureus and K. pneumoniae | 64 mg/mL c | [128] | ||
| Compound 27 | anthelmintic activity against Dactylogyrus intermedius | 1.4 (mg/L) d | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berti, F.; Tamburello, E.M.; Forzato, C. p-Coumaroyl Amides from the Plant Kingdom: A Comprehensive Review of Natural Sources, Biosynthesis, and Biological Activities. Molecules 2025, 30, 1259. https://doi.org/10.3390/molecules30061259
Berti F, Tamburello EM, Forzato C. p-Coumaroyl Amides from the Plant Kingdom: A Comprehensive Review of Natural Sources, Biosynthesis, and Biological Activities. Molecules. 2025; 30(6):1259. https://doi.org/10.3390/molecules30061259
Chicago/Turabian StyleBerti, Federico, Elena Maria Tamburello, and Cristina Forzato. 2025. "p-Coumaroyl Amides from the Plant Kingdom: A Comprehensive Review of Natural Sources, Biosynthesis, and Biological Activities" Molecules 30, no. 6: 1259. https://doi.org/10.3390/molecules30061259
APA StyleBerti, F., Tamburello, E. M., & Forzato, C. (2025). p-Coumaroyl Amides from the Plant Kingdom: A Comprehensive Review of Natural Sources, Biosynthesis, and Biological Activities. Molecules, 30(6), 1259. https://doi.org/10.3390/molecules30061259







