Increasing Life Expectancy with Plant Polyphenols: Lessons from the Mediterranean and Japanese Diets
Abstract
1. Introduction
2. Materials and Methods
3. Plant Polyphenols: Chemistry and Mechanisms of Action
3.1. Classification and Chemical Description
3.2. Antioxidant and Anti-Inflammatory Mechanisms
- Fine Regulation of Cellular Signaling: Many plant polyphenols modulate key intracellular pathways, involving the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2), which has a crucial role in the expression of antioxidant peptides [49,50,51,52]. Furthermore, they reduce the nuclear factor kappa-light-chain-enhancer of the activated B cell (NF-κB) path, thus decreasing the activity of pro-inflammatory cytokines [11,53,54,55,56].
- Hormetic Actions: Hormesis is a two-step dose–response association with an environmental agent, whereby low-dose amounts may have a positive effect and high-dose quantities could be functionally inhibitory or toxic [57,58]. Indeed, at low doses, polyphenols may elicit a minor stress response aimed at enhancing cellular resilience, which may modulate stress resistance and regulate endogenous repair mechanisms [59,60,61,62].
3.3. Polyphenols’ Role in Cellular Metabolism and Longevity
4. The Mediterranean Diet: A Polyphenol-Rich Nutritional Paradigm
4.1. Dietary Pattern and Polyphenol Sources
- -
- -
- Resveratrol: Found in the skin of grapes, blueberries, raspberries, mulberries, and peanuts [95,96,97], resveratrol has been associated with the activation of longevity-related pathways such as the Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/Akt), Sirtuin 1 (SIRT1), and AMP-Activated Protein Kinase (AMPK) pathways [98,99,100,101] while inhibiting MTOR (mammalian target of rapamycin), a protein kinase that plays a crucial role in cell growth, proliferation, and metabolism [102].
- -
4.2. Health Outcomes Correlated with the Mediterranean Diet
4.3. Further Elements from Mediterranean Diet Studies
5. Japanese Dietary Patterns and the Role of Polyphenols in Longevity
5.1. Traditional Japanese Diet and Its Polyphenol Profile
5.2. Epidemiological Proof of Longevity
5.3. Biomolecular Mechanisms of the Japanese Dietary Schedule
6. Relationship Between Mediterranean and Japanese Polyphenol Contents
6.1. Major Polyphenolic Presence Characterizing the Japanese/Mediterranean Diet Profiles
| Polyphenolic Class | Mediterranean Diet | Japanese Diet |
|---|---|---|
| Stilbenes | Resveratrol (from red wine, grapes, and berries) [169] | – |
| Phenolic Alcohols | Hydroxytyrosol, tyrosol, and oleuropein (from extra-virgin olive oil and olive leaves) [81] | – |
| Flavonoids | Quercetin and catechins (from fruits/vegetables) [170] | Catechins (EGCG from green tea) [171] |
| Isoflavones | – | Genistein and daidzein (from soy products) [172] |
| Other Phenolic Chemicals | Various minor polyphenols (nuts and legumes) [173] | Unique compounds from seaweeds and mushrooms [174] |
6.2. Cooperative Effects and Nutrient Connections
6.3. The Impact on Aging Critical Biomarkers
7. Preclinical and Clinical Findings
7.1. Preclinical Studies
7.2. Clinical Studies and Epidemiological Findings
8. The Impact of Sugary Carbonated Drinks, Processed Foods and Cooking Practices on Hydroxynonenal Formation on Aging Acceleration
9. The Dual Janus-Faced Role of Alcohol Consumption Within These Dietary Patterns
9.1. Toxic Effects of Alcohol Abuse
9.2. Alcohol Drinking During Pregnancy: Fetal Alcohol Spectrum Disorders
9.3. Effects of Moderate Alcohol Consumption
9.4. Balancing the Dual Effects: A Context-Dependent Paradigm
10. The Close Relationship Between the Mediterranean/Japanese Diets and the Vegan/Vegetarian Diets
11. Future Assessments and Proposals
11.1. Improving Polyphenol Administration
11.2. Investigating Metabolic Contents and Bioavailability
11.3. Integration with Lifestyle and Cultural Practices
11.4. Limitations and Emerging Directions
12. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Joubert, C.; Chainay, H. Aging brain: The effect of combined cognitive and physical training on cognition as compared to cognitive and physical training alone—A systematic review. Clin. Interv. Aging 2018, 13, 1267–1301. [Google Scholar] [CrossRef] [PubMed]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini1, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.N.; Agrawal, N.; Moglad, E.; Priya, G.P.; Srivastava, M.; Chennakesavulu, K.; Mohanty, B.; Arya, R.; Kazmi, I.; Alzarea, S.I.; et al. CHIP and aging: A key regulator of proteostasis and cellular senescence. Biogerontology 2025, 26, 104. [Google Scholar] [CrossRef]
- Ungvari, A.; Nyúl-Tóth, Á.; Patai, R.; Csik, B.; Gulej, R.; Nagy, D.; Shanmugarama, S.; Benyó, Z.; Kiss, T.; Ungvari, Z.; et al. Cerebromicrovascular senescence in vascular cognitive impairment: Does accelerated microvascular aging accompany atherosclerosis? GeroScience 2025. ahead of print. [Google Scholar] [CrossRef]
- Beaver, L.M.; Jamieson, P.E.; Wong, C.P.; Hosseinikia, M.; Stevens, J.F.; Ho, E. Promotion of Healthy Aging Through the Nexus of Gut Microbiota and Dietary Phytochemicals. Adv. Nutr. 2025, 16, 100376. [Google Scholar] [CrossRef]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. GeroScience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Coradduzza, D.; Congiargiu, A.; Chen, Z.; Zinellu, A.; Carru, C.; Medici, S. Ferroptosis and Senescence: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3658. [Google Scholar] [CrossRef]
- Wang, K.; Dong, Y.; Liu, J.; Qian, L.; Wang, T.; Gao, X.; Wang, K.; Zhou, L. Effects of REDOX in Regulating and Treatment of Metabolic and Inflammatory Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 5860356. [Google Scholar] [CrossRef]
- Barnes, P.J. Mechanisms of development of multimorbidity in the elderly. Eur. Respir. J. 2015, 45, 790–806. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.V.; Khan, S.; Misri, S.; Gaira, K.S.; Rawat, S.; Rawat, B.; Khan, M.A.H.; Shah, A.A.; Asgher, M.; Ahmad, S. High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders. Pharmaceuticals 2024, 17, 975. [Google Scholar] [CrossRef]
- Fiore, M.; Messina, M.P.; Petrella, C.; D’Angelo, A.; Greco, A.; Ralli, M.; Ferraguti, G.; Tarani, L.; Vitali, M.; Ceccanti, M. Antioxidant properties of plant polyphenols in the counteraction of alcohol-abuse induced damage: Impact on the Mediterranean diet. J. Funct. Foods 2020, 71, 104012. [Google Scholar] [CrossRef]
- Raederstorff, D. Antioxidant activity of olive polyphenols in humans: A review. Int. J. Vitam. Nutr. Res. 2009, 79, 152–165. [Google Scholar] [CrossRef]
- Trichopoulou, A. From research to education: The Greek experience. Nutrition 2000, 16, 528–531. [Google Scholar] [CrossRef]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv. Nutr. 2019, 10, 422–436. [Google Scholar] [CrossRef]
- Casamenti, F.; Stefani, M. Olive polyphenols: New promising agents to combat aging-associated neurodegeneration. Expert Rev. Neurother. 2017, 17, 345–358. [Google Scholar] [CrossRef]
- Giacosa, A.; Barale, R.; Bavaresco, L.; Faliva, M.A.; Gerbi, V.; La Vecchia, C.; Negri, E.; Opizzi, A.; Perna, S.; Pezzotti, M.; et al. Mediterranean Way of Drinking and Longevity. Crit. Rev. Food Sci. Nutr. 2016, 56, 635–640. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Goto, Y.; Ota, H.; Kito, K.; Mano, F.; Joo, E.; Ikeda, K.; Inagaki, N.; Nakayama, T. Characteristics of the japanese diet described in epidemiologic publications: A qualitative systematic review. J. Nutr. Sci. Vitaminol. 2018, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Shirota, M.; Watanabe, N.; Suzuki, M.; Kobori, M. Japanese-Style Diet and Cardiovascular Disease Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients 2022, 14, 2008. [Google Scholar] [CrossRef]
- Matsuyama, S.; Shimazu, T.; Tomata, Y.; Zhang, S.; Abe, S.; Lu, Y.; Tsuji, I. Japanese Diet and Mortality, Disability, and Dementia: Evidence from the Ohsaki Cohort Study. Nutrients 2022, 14, 2034. [Google Scholar] [CrossRef]
- Vitale, E. An Optimal Dietary Pattern for Healthy Longevity: Scoping Differencing Review between the Mediterranean and the Japanese Diet. Endocr. Metab. Immune Disord.—Drug Targets 2023, 24, 1711–1720. [Google Scholar] [CrossRef]
- Moriki, D.; Koumpagioti, D.; Francino, M.P.; Rufián-Henares, J.Á.; Kalogiannis, M.; Priftis, K.N.; Douros, K. How Different Are the Influences of Mediterranean and Japanese Diets on the Gut Microbiome? Endocr. Metab. Immune Disord.—Drug Targets 2024, 24, 1733–1745. [Google Scholar] [CrossRef]
- Damigou, E.; Kosti, R.I.; Downs, S.M.; Naumovski, N.; Panagiotakos, D. Comparing The Mediterranean and The Japanese Dietary Pattern in Relation to Longevity—A Narrative Review. Endocr. Metab. Immune Disord.—Drug Targets 2024, 24, 1746–1755. [Google Scholar] [CrossRef]
- Mensah, E.O.; Danyo, E.K.; Asase, R.V. Exploring the effect of different diet types on ageing and age-related diseases. Nutrition 2025, 129, 112596. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem.—Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Nonaka, G. Isolation and structure elucidation of tannins. Pure Appl. Chem. 1989, 61, 357–360. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- De Souza Farias, S.A.; Da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Saleem, M.; Hyoung, J.K.; Ali, M.S.; Yong, S.L. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 1468475118. [Google Scholar]
- Scalbert, A. Quantitative Methods for the Estimation of Tannins in Plant Tissues. In Plant Polyphenols; Springer: Berlin/Heidelberg, Germany, 1992; pp. 259–280. [Google Scholar]
- Rivas, F.; Poblete-Aro, C.; Pando, M.E.; Allel, M.J.; Fernandez, V.; Soto, A.; Nova, P.; Garcia-Diaz, D. Effects of Polyphenols in Aging and Neurodegeneration Associated with Oxidative Stress. Curr. Med. Chem. 2021, 29, 1045–1060. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Ahmadinejad, F.; Møller, S.G.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Romano, G.L.; Laudani, S.; Gozzo, L.; Guerrera, I.; Dominguez Azpíroz, I.; Martínez Diaz, R.; Quiles, J.L.; Battino, M.; Drago, F.; et al. Flavan-3-ols and Vascular Health: Clinical Evidence and Mechanisms of Action. Nutrients 2024, 16, 2471. [Google Scholar] [CrossRef] [PubMed]
- Boonyong, C.; Vardhanabhuti, N.; Jianmongkol, S. Natural polyphenols prevent indomethacin-induced and diclofenac-induced Caco-2 cell death by reducing endoplasmic reticulum stress regardless of their direct reactive oxygen species scavenging capacity. J. Pharm. Pharmacol. 2020, 72, 583–591. [Google Scholar] [CrossRef]
- Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Curr. Issues Mol. Biol. 2022, 44, 5209–5220. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J. Vitagenes, cellular stress response, and acetylcarnitine: Relevance to hormesis. BioFactors 2009, 35, 146–160. [Google Scholar] [CrossRef]
- Divyajanani, S.; Harithpriya, K.; Ganesan, K.; Ramkumar, K.M. Dietary Polyphenols Remodel DNA Methylation Patterns of NRF2 in Chronic Disease. Nutrients 2023, 15, 3347. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Kamiński, P.; Bilski, R.; Kołodziejska, R.; Woźniak, A.; Tkaczenko, H. Role of Antioxidants in Modulating the Microbiota–Gut–Brain Axis and Their Impact on Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 3658. [Google Scholar] [CrossRef]
- Young, A.K.; Kim, G.Y.; Park, K.Y.; Yung, H.C. Resveratrol inhibits nitric oxide and prostaglandin E2 production by lipopolysaccharide-activated C6 microglia. J. Med. Food 2007, 10, 218–224. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Bhakta-Guha, D.; Efferth, T. Hormesis: Decoding two sides of the same coin. Pharmaceuticals 2015, 8, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis: A fundamental concept in biology. Microb. Cell 2014, 1, 145–149. [Google Scholar] [CrossRef]
- Murakami, A. Impact of hormesis to deepen our understanding of the mechanisms underlying the bioactivities of polyphenols. Curr. Opin. Biotechnol. 2024, 86, 103074. [Google Scholar] [CrossRef]
- Calabrese, V.; Wenzel, U.; Piccoli, T.; Jacob, U.M.; Nicolosi, L.; Fazzolari, G.; Failla, G.; Fritsch, T.; Osakabe, N.; Calabrese, E.J. Investigating hormesis, aging, and neurodegeneration: From bench to clinics. Open Med. 2024, 19, 20240986. [Google Scholar] [CrossRef]
- Calabrese, V.; Osakabe, N.; Siracusa, R.; Modafferi, S.; Di Paola, R.; Cuzzocrea, S.; Jacob, U.M.; Fritsch, T.; Abdelhameed, A.S.; Rashan, L.; et al. Transgenerational hormesis in healthy aging and antiaging medicine from bench to clinics: Role of food components. Mech. Ageing Dev. 2024, 220, 111960. [Google Scholar] [CrossRef]
- Chirumbolo, S. Hormesis, resveratrol and plant-derived polyphenols: Some comments. Hum. Exp. Toxicol. 2011, 30, 2027–2030. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M.; Nabavi, S.F. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol. Adv. 2016, 34, 813–826. [Google Scholar] [CrossRef]
- Meira Martins, L.A.; Vieira, M.Q.; Ilha, M.; de Vasconcelos, M.; Biehl, H.B.; Lima, D.B.; Schein, V.; Barbé-Tuana, F.; Borojevic, R.; Guma, F.C.R. The Interplay Between Apoptosis, Mitophagy and Mitochondrial Biogenesis Induced by Resveratrol Can Determine Activated Hepatic Stellate Cells Death or Survival. Cell Biochem. Biophys. 2015, 71, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chhabra, V.; Shenoy, S.; Daksh, R.; Ravichandiran, V.; Swamy, R.S.; Kumar, N. Role of Flavonoids in Modulation of Mitochondria Dynamics during Oxidative Stress. Mini-Rev. Med. Chem. 2023, 24, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondria in Neuroprotection by Phytochemicals: Bioactive Polyphenols Modulate Mitochondrial Apoptosis System, Function and Structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Sharma, R.; Bhate, L.; Agrawal, Y.; Aspatwar, A. Advanced nutraceutical approaches to Parkinson’s disease: Bridging nutrition and neuroprotection. Nutr. Neurosci. 2025, 1–17. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Q.; Chen, J.Y.; OuYang, D.; Lu, J.H. The pharmacological activity of epigallocatechin-3-gallate (EGCG) on Alzheimer’s disease animal model: A systematic review. Phytomedicine 2020, 79, 153316. [Google Scholar] [CrossRef]
- Sinclair, D.A. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 2005, 126, 987–1002. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Du, Y.; Li, G.; Wang, L.; Zhou, F. Resveratrol Ameliorated Vascular Calcification by Regulating Sirt-1 and Nrf2. Transplant. Proc. 2016, 48, 3378–3386. [Google Scholar] [CrossRef]
- Opie, L.H.; Lecour, S. The red wine hypothesis: From concepts to protective signalling molecules. Eur. Heart J. 2007, 28, 1683–1693. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; MacK, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Daimiel, L.; Micó, V.; Valls, R.M.; Pedret, A.; Motilva, M.J.; Rubió, L.; Fitó, M.; Farrás, M.; Covas, M.I.; Solá, R.; et al. Impact of Phenol-Enriched Virgin Olive Oils on the Postprandial Levels of Circulating microRNAs Related to Cardiovascular Disease. Mol. Nutr. Food Res. 2020, 64, e2000049. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Salazar, L.A.; Abdalla, D.S.P. Modulation of immune function by polyphenols: Possible contribution of epigenetic factors. Nutrients 2013, 5, 2314–2332. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Wu, J.-C.; Ho, C.-T. Epigenetic and Disease Targets by Polyphenols. Curr. Pharm. Des. 2013, 19, 6156–6185. [Google Scholar] [CrossRef]
- Izzotti, A.; Cartiglia, C.; Steele, V.E.; De Flora, S. MicroRNAs as targets for dietary and pharmacological inhibitors of mutagenesis and carcinogenesis. Mutat. Res. 2012, 751, 287–303. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive polyphenols: Antioxidant and anti-inflammatory properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Carito, V.; Ceccanti, M.; Tarani, L.; Ferraguti, G.; Chaldakov, G.N.; Fiore, M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016, 23, 3189–3197. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Santamato, A.; Ranieri, M.; Fiore, P.; Capurso, A.; Panza, F. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev. Neurother. 2008, 8, 133–158. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Châlons, P.; Aires, V.; Delmas, D. Polyphenol Extracts from Red Wine and Grapevine: Potential Effects on Cancers. Diseases 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, I.; Corcione, G.; Digiorgio, C.; Fioschi, G.; Paradiso, V.M. Ageing of Red Wine (cv. Negroamaro) in Mediterranean Areas: Impact of Different Barrels and Apulian Traditional Amphorae on Phenolic Indices, Volatile Composition and Sensory Analysis. Foods 2025, 14, 650. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; Cianci, R.; Raffaelli, G.; Peluso, D.; Bigioni, G.; De Lorenzo, A. Sex-Specific Adherence to the Mediterranean Diet in Obese Individuals. Nutrients 2024, 16, 3076. [Google Scholar] [CrossRef]
- Reytor-González, C.; Zambrano, A.K.; Montalvan, M.; Frias-Toral, E.; Simancas-Racines, A.; Simancas-Racines, D. Adherence to the Mediterranean Diet and its association with gastric cancer: Health benefits from a Planeterranean perspective. J. Transl. Med. 2024, 22, 483. [Google Scholar] [CrossRef]
- Godos, J.; Scazzina, F.; Paternò Castello, C.; Giampieri, F.; Quiles, J.L.; Briones Urbano, M.; Battino, M.; Galvano, F.; Iacoviello, L.; de Gaetano, G.; et al. Underrated aspects of a true Mediterranean diet: Understanding traditional features for worldwide application of a “Planeterranean” diet. J. Transl. Med. 2024, 22, 294. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Frumuzachi, O.; Kieserling, H.; Rohn, S.; Mocan, A. The impact of oleuropein, hydroxytyrosol, and tyrosol on cardiometabolic risk factors: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2025, 1–21. [Google Scholar] [CrossRef]
- Castillo-Luna, A.; Priego-Capote, F. Phenolic enrichment of foods curated in olive oil: Kinetics and chemical evaluation. Food Chem. X 2024, 22, 101398. [Google Scholar] [CrossRef]
- Uğuz, A.C.; Rocha-Pimienta, J.; Martillanes, S.; Garrido, M.; Espino, J.; Delgado-Adámez, J. Chlorophyll Pigments of Olive Leaves and Green Tea Extracts Differentially Affect Their Antioxidant and Anticancer Properties. Molecules 2023, 28, 2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Medical Benefits and Polymer Applications of Grapes. Polymers 2025, 17, 750. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Justyńska, W.; Czpakowska, J.; Smolińska, E.; Bielenin, A.; Glabinski, A.; Szpakowski, P. Role of Plant Phytochemicals: Resveratrol, Curcumin, Luteolin and Quercetin in Demyelination, Neurodegeneration, and Epilepsy. Antioxidants 2024, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Zebeaman, M.; Tadesse, M.G.; Bachheti, R.K.; Bachheti, A.; Gebeyhu, R.; Chaubey, K.K. Plants and Plant-Derived Molecules as Natural Immunomodulators. Biomed. Res. Int. 2023, 2023, 7711297. [Google Scholar] [CrossRef]
- Shuid, A.N.; Abdul Nasir, N.A.; Ab Azis, N.; Shuid, A.N.; Razali, N.; Ahmad Hairi, H.; Mohd Miswan, M.F.; Naina Mohamed, I. A Systematic Review on the Molecular Mechanisms of Resveratrol in Protecting Against Osteoporosis. Int. J. Mol. Sci. 2025, 26, 2893. [Google Scholar] [CrossRef]
- Doghish, A.S.; El-Dakroury, W.A.; Abulsoud, A.I.; Abdelmaksoud, N.M.; Aly, S.H.; Elbadry, A.M.M.; Mohammed, O.A.; Abdel-Reheim, M.A.; Zaki, M.B.; Rizk, N.I.; et al. Natural compounds as regulators of miRNAs: Exploring a new avenue for treating brain cancer. Naunyn Schmiedebergs Arch. Pharmacol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Ruggiero, M.; Motti, M.L.; Meccariello, R.; Mazzeo, F. Resveratrol and Physical Activity: A Successful Combination for the Maintenance of Health and Wellbeing? Nutrients 2025, 17, 837. [Google Scholar] [CrossRef]
- Carollo, C.; Sorce, A.; Cirafici, E.; Mulè, G.; Caimi, G. Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential. Nutrients 2025, 17, 1212. [Google Scholar] [CrossRef]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef]
- Christimann, G.; Rocha, G.; Sattler, J.A.G. Bioactive compounds and dietary patterns in Alzheimer’s disease. J. Alzheimers Dis. 2025, 104, 597–610. [Google Scholar] [CrossRef]
- Sbai, O.; Torrisi, F.; Fabrizio, F.P.; Rabbeni, G.; Perrone, L. Effect of the Mediterranean Diet (MeDi) on the Progression of Retinal Disease: A Narrative Review. Nutrients 2024, 16, 3169. [Google Scholar] [CrossRef]
- Ross, F.C.; Mayer, D.E.; Horn, J.; Cryan, J.F.; Del Rio, D.; Randolph, E.; Gill, C.I.R.; Gupta, A.; Ross, R.P.; Stanton, C.; et al. Potential of dietary polyphenols for protection from age-related decline and neurodegeneration: A role for gut microbiota? Nutr. Neurosci. 2024, 27, 1058–1076. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Zuk, J.B.; Khavaran, H. Plant bioactive compounds from Mediterranean diet improve risk factors for metabolic syndrome. Int. J. Food Sci. Nutr. 2023, 74, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Salvo, A.; Tuttolomondo, A. The Role of Olive Oil in Cardiometabolic Risk. Metabolites 2025, 15, 190. [Google Scholar] [CrossRef]
- Aznar de la Riera, M.d.C.; Ortolá, R.; Kales, S.N.; Graciani, A.; Diaz-Gutierrez, J.; Banegas, J.R.; Rodríguez-Artalejo, F.; Sotos-Prieto, M. Health and environmental dietary impact: Planetary health diet vs. Mediterranean diet. A nationwide cohort in Spain. Sci. Total Environ. 2025, 968, 178924. [Google Scholar] [CrossRef]
- Zalaquett, N.; Lidoriki, I.; Lampou, M.; Saab, J.; Hadkhale, K.; Christophi, C.; Kales, S.N. Adherence to the Mediterranean Diet and the Risk of Head and Neck Cancer: A Systematic Review and Meta-Analysis of Case–Control Studies. Nutrients 2025, 17, 287. [Google Scholar] [CrossRef]
- Veronese, N.; Ragusa, F.S.; Maggi, S.; Witard, O.C.; Smith, L.; Dominguez, L.J.; Barbagallo, M.; Isanejad, M.; Prokopidis, K. Effect of the Mediterranean diet on incidence of heart failure in European countries: A systematic review and meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2024, 79, 195–199. [Google Scholar] [CrossRef]
- Bakis, H.; Chauveau, P.; Combe, C.; Pfirmann, P. Mediterranean Diet for Cardiovascular Risk Reduction in Chronic Kidney Disease. Adv. Kidney Dis. Health 2023, 30, 496–501. [Google Scholar] [CrossRef]
- Hernandez, A.V.; Marti, K.M.; Marti, K.E.; Weisman, N.; Cardona, M.; Biello, D.M.; Pasupuleti, V.; Benites-Zapata, V.A.; Roman, Y.M.; Piscoya, A. Effect of Mediterranean Diets on Cardiovascular Risk Factors and Disease in Overweight and Obese Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Nutr. Assoc. 2025, 44, 387–404. [Google Scholar] [CrossRef]
- Fernández-Lázaro, C.I.; Ruiz-Canela, M.; Martínez-González, M.Á. Deep dive to the secrets of the PREDIMED trial. Curr. Opin. Lipidol. 2021, 32, 62–69. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Petrella, C.; Carito, V.; Carere, C.; Ferraguti, G.; Ciafrè, S.; Natella, F.; Bello, C.; Greco, A.; Ralli, M.; Mancinelli, R.; et al. Oxidative stress inhibition by resveratrol in alcohol-dependent mice. Nutrition 2020, 79–80, 110783. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Venditti, A.; Bianco, A.; Ceccanti, M.; Serrilli, A.M.; Chaldakov, G.; Tarani, L.; De Nicolò, S.; Fiore, M. Effects of olive leaf polyphenols on male mouse brain NGF, BDNF and their receptors TrkA, TrkB and p75. Nat. Prod. Res. 2014, 28, 1970–1984. [Google Scholar] [CrossRef]
- De Nicoló, S.; Tarani, L.; Ceccanti, M.; Maldini, M.; Natella, F.; Vania, A.; Chaldakov, G.N.; Fiore, M. Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition 2013, 29, 681–687. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Calabrese, G.; Tornese, R.; Montefusco, A.; Placì, R.; Semeraro, T.; Durante, M.; De Caroli, M.; Lenucci, M.S. Pomegranate Extracts as Dual Regulators of Angiogenesis: A Systematic Review of Preclinical Evidence in Cancer and Chronic Wound Healing. Mol. Nutr. Food Res. 2025, 69, e70060. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, H.; He, Z. Recent advances in polyphenols improving vascular endothelial dysfunction induced by endogenous toxicity. J. Appl. Toxicol. 2021, 41, 701–712. [Google Scholar] [CrossRef]
- Otręba, M.; Kośmider, L.; Stojko, J.; Rzepecka-Stojko, A. Cardioprotective activity of selected polyphenols based on epithelial and aortic cell lines. A review. Molecules 2020, 25, 5343. [Google Scholar] [CrossRef]
- Akbari, M.; Tamtaji, O.R.; Lankarani, K.B.; Tabrizi, R.; Dadgostar, E.; Kolahdooz, F.; Jamilian, M.; Mirzaei, H.; Asemi, Z. The Effects of Resveratrol Supplementation on Endothelial Function and Blood Pressures Among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. High Blood Press. Cardiovasc. Prev. 2019, 26, 305–319. [Google Scholar] [CrossRef]
- Chong, M.F.F.; MacDonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, S28–S39. [Google Scholar] [CrossRef]
- Milena, E.; Maurizio, M. Exploring the Cardiovascular Benefits of Extra Virgin Olive Oil: Insights into Mechanisms and Therapeutic Potential. Biomolecules 2025, 15, 284. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Liu, Z.; Zhao, Z. Protective effect and mechanism of resveratrol on vascular endothelial cells. Chin. Crit. Care Med. 2024, 36, 664–668. [Google Scholar] [CrossRef]
- Silva, H.; Bárbara, R. Exploring the Anti-Hypertensive Potential of Lemongrass—A Comprehensive Review. Biology 2022, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological basis for nutraceutical supplementation in heart failure: A comprehensive review. Nutrients 2021, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Hu, T.; Jiang, J.G.; Zhao, J.W.; Zhu, W. Antioxidant and anti-inflammatory effects of polyphenols extracted from: Ilex latifolia Thunb. RSC Adv. 2018, 8, 7134–7141. [Google Scholar] [CrossRef]
- Wisnuwardani, R.W.; De Henauw, S.; Ferrari, M.; Forsner, M.; Gottrand, F.; Huybrechts, I.; Kafatos, A.G.; Kersting, M.; Knaze, V.; Manios, Y.; et al. Total Polyphenol Intake Is Inversely Associated with a Pro/Anti-Inflammatory Biomarker Ratio in European Adolescents of the HELENA Study. J. Nutr. 2020, 150, 1610–1618. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- De Bruyne, T.; Steenput, B.; Roth, L.; De Meyer, G.R.Y.; Dos Santos, C.N.; Valentová, K.; Dambrova, M.; Hermans, N. Dietary polyphenols targeting arterial stiffness: Interplay of contributing mechanisms and gut microbiome-related Metabolism. Nutrients 2019, 11, 578. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Life Expectancy and Healthy Life Expectancy Data by Country 2022. Available online: https://apps.who.int/gho/data/node.main.688 (accessed on 29 June 2025).
- Kwack, S.J.; Kim, K.B.; Kim, H.S.; Yoon, K.S.; Lee, B.M. Risk assessment of soybean-based phytoestrogens. J. Toxicol. Environ. Health—Part A Curr. Issues 2009, 72, 1254–1261. [Google Scholar] [CrossRef]
- Ohishi, T.; Miyoshi, N.; Mori, M.; Sagara, M.; Yamori, Y. Health Effects of Soy Isoflavones and Green Tea Catechins on Cancer and Cardiovascular Diseases Based on Urinary Biomarker Levels. Molecules 2022, 27, 8899. [Google Scholar] [CrossRef]
- Radeva-Ilieva, M.; Stoeva, S.; Hvarchanova, N.; Georgiev, K.D. Green Tea: Current Knowledge and Issues. Foods 2025, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.; Inoue, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; Noda, M.; Iso, H.; Tsugane, S.; et al. Association of green tea consumption with mortality due to all causes and major causes of death in a Japanese population: The Japan Public Health Center-based Prospective Study (JPHC Study). Ann. Epidemiol. 2015, 25, 512–518.e3. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.K.; Saito, E.; Sawada, N.; Tsugane, S.; Ito, H.; Lin, Y.; Tamakoshi, A.; Sado, J.; Kitamura, Y.; Sugawara, Y.; et al. Green tea consumption and mortality in Japanese men and women: A pooled analysis of eight population-based cohort studies in Japan. Eur. J. Epidemiol. 2019, 34, 917–926. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.O.; Li, H.; Yang, G.; Li, Q.; Gao, Y.T.; Zheng, W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch. Intern. Med. 2005, 165, 1890–1895. [Google Scholar] [CrossRef]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials2. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar] [CrossRef]
- Baglia, M.L.; Gu, K.; Zhang, X.; Zheng, Y.; Peng, P.; Cai, H.; Bao, P.P.; Zheng, W.; Lu, W.; Shu, X.O. Soy isoflavone intake and bone mineral density in breast cancer survivors. Cancer Causes Control 2015, 26, 571–580. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Portillo, M.P. Scientific evidence supporting the beneficial effects of isoflavones on human health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef]
- Clarke, E.D.; Collins, C.E.; Rollo, M.E.; Kroon, P.A.; Philo, M.; Haslam, R.L. The relationship between urinary polyphenol metabolites and dietary polyphenol intakes in young adults. Br. J. Nutr. 2022, 127, 589–598. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Achaintre, D.; Rothwell, J.A.; Rinaldi, S.; Assi, N.; Ferrari, P.; Leitzmann, M.; Boutron-Ruault, M.C.; Fagherazzi, G.; Auffret, A.; et al. Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study. Sci. Rep. 2016, 6, 26905. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, R.; Sharma, A.; Goel, A.; Padwad, Y. Long-term consumption of green tea EGCG enhances murine health span by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis, and immunosenescence. J. Nutr. Biochem. 2022, 107, 109068. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y. Green Tea Mitigates the Hallmarks of Aging and Age-Related Multisystem Deterioration. Aging Dis. 2025. ahead of print. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, Z.; Chen, J.; Zhao, M.; Guo, C.; Wang, T.; Li, R.; Zhang, H.; Ma, X.; et al. Preclinical evidence construction for epigallocatechin-3-gallate against non-alcoholic fatty liver disease: A meta-analysis and machine learning study. Phytomedicine 2025, 142, 156651. [Google Scholar] [CrossRef] [PubMed]
- Al-Regaiey, K. Crosstalk between adipogenesis and aging: Role of polyphenols in combating adipogenic-associated aging. Immun. Ageing 2024, 21, 76. [Google Scholar] [CrossRef]
- Messina, M.; Duncan, A.; Messina, V.; Lynch, H.; Kiel, J.; Erdman, J.W. The health effects of soy: A reference guide for health professionals. Front. Nutr. 2022, 9, 970364. [Google Scholar] [CrossRef]
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Kenny, A.M.; Mangano, K.M.; Abourizk, R.H.; Bruno, R.S.; Anamani, D.E.; Kleppinger, A.; Walsh, S.J.; Prestwood, K.M.; Kerstetter, J.E. Soy proteins and isoflavones affect bone mineral density in older women: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 234–242. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Covas, M.I. Olive oil and the cardiovascular system. Pharmacol. Res. 2007, 55, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Della Ragione, F.; Cucciolla, V.; Borriello, A.; D’Angelo, S.; Galletti, P.; Zappia, V. Biological effects of hydroxytyrosol, a polyphenol from olive oil endowed with antioxidant activity. Adv. Nutr. Cancer 1999, 472, 115–130. [Google Scholar]
- Vivancos, M.; Moreno, J.J. Effect of resveratrol, tyrosol and beta-sitosterol on oxidised low-density lipoprotein-stimulated oxidative stress, arachidonic acid release and prostaglandin E2 synthesis by RAW 264.7 macrophages. Br. J. Nutr. 2008, 99, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, S.; Zhang, P.; Zhu, J.; Meng, G.; Xie, L.; Yu, Y.; Ji, Y.; Han, Y. Soy Isoflavone Protects Myocardial Ischemia/Reperfusion Injury through Increasing Endothelial Nitric Oxide Synthase and Decreasing Oxidative Stress in Ovariectomized Rats. Oxid. Med. Cell. Longev. 2016, 2016, 5057405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yu, S.; Zhang, W.; Peng, Y.; Pu, M.; Kang, T.; Zeng, J.; Yu, Y.; Li, G. Genistein attenuates monocrotaline-induced pulmonary arterial hypertension in rats by activating PI3K/Akt/eNOS signaling. Histol. Histopathol. 2017, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Zachariah, B.; Vickneshwaran, V.; Jacob, S.E.; Sridhar, M.G. Amelioration of oxidative stress and insulin resistance by soy isoflavones (from Glycine max) in ovariectomized Wistar rats fed with high fat diet: The molecular mechanisms. Exp. Gerontol. 2015, 63, 67–75. [Google Scholar] [CrossRef]
- Sharma, M.; Tollefsbol, T.O. Combinatorial epigenetic mechanisms of sulforaphane, genistein and sodium butyrate in breast cancer inhibition. Exp. Cell Res. 2022, 416, 113160. [Google Scholar] [CrossRef]
- De La Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef]
- Phillip, C.J.; Giardina, C.K.; Bilir, B.; Cutler, D.J.; Lai, Y.H.; Kucuk, O.; Moreno, C.S. Genistein cooperates with the histone deacetylase inhibitor vorinostat to induce cell death in prostate cancer cells. BMC Cancer 2012, 12, 145. [Google Scholar] [CrossRef]
- Xie, X.; Cong, L.; Liu, S.; Xiang, L.; Fu, X. Genistein alleviates chronic vascular inflammatory response via the miR-21/NF-κB p65 axis in lipopolysaccharide-treated mice. Mol. Med. Rep. 2021, 23, 192. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y. Japanese food culture and human health–what we can learn from Japan. J. Dis. Prev. Health Promot. 2024, 8, 9–12. [Google Scholar]
- Gabriel, A.S.; Ninomiya, K.; Uneyama, H. The role of the Japanese traditional diet in healthy and sustainable dietary patterns around the world. Nutrients 2018, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Phenol-Explorer. Show All Foods Which Polyphen Resveratrol is Found n.d. Available online: http://phenol-explorer.eu/contents/polyphenol/592 (accessed on 24 June 2025).
- Micronutrient Information Center; Linus Pauling Institute; Oregon State University; Corvallis OU. Flavonoids. Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/flavonoids (accessed on 24 June 2025).
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M.; U.S. Department of Agriculture; Agricultural Research Service. USDA Database for the Flavonoid Content of Selected Foods, Release 3.0. 2011; pp. 2+98–103. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03.pdf (accessed on 24 June 2025).
- Linus Pauling Institute; Micronutrients Information Center. Soy Isoflavones. Oregon State University: Corvallis, OR, USA, 2024; Available online: https://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/soy-isoflavones (accessed on 24 June 2025).
- Phenol-Explorer 3.6. Database on Polyphenol Content in Foods. Available online: http://phenol-explorer.eu/ (accessed on 24 June 2025).
- Van Doan, H.; Hoseinifar, S.H.; Esteban, M.Á.; Dadar, M.; Thu, T.T.N. Mushrooms, Seaweed, and Their Derivatives as Functional Feed Additives for Aquaculture: An Updated View. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 41–90. ISBN 1572-5995. [Google Scholar] [CrossRef]
- Terracina, S.; Petrella, C.; Francati, S.; Lucarelli, M.; Barbato, C.; Minni, A.; Ralli, M.; Greco, A.; Tarani, L.; Fiore, M.; et al. Antioxidant Intervention to Improve Cognition in the Aging Brain: The Example of Hydroxytyrosol and Resveratrol. Int. J. Mol. Sci. 2022, 23, 15674. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Barbouti, A.; Goulas, V. Dietary antioxidants in the mediterranean diet. Antioxidants 2021, 10, 1213. [Google Scholar] [CrossRef]
- De Santis, S.; Clodoveo, M.L.; Corbo, F. Correlation between Chemical Characterization and Biological Activity: An Urgent Need for Human Studies Using Extra Virgin Olive Oil. Antioxidants 2022, 11, 258. [Google Scholar] [CrossRef]
- Hsu, A.; Bruno, R.S.; Löhr, C.V.; Taylor, A.W.; Dashwood, R.H.; Bray, T.M.; Ho, E. Dietary soy and tea mitigate chronic inflammation and prostate cancer via NFκB pathway in the Noble rat model. J. Nutr. Biochem. 2011, 22, 502–510. [Google Scholar] [CrossRef]
- Tsuboi, H.; Takahashi, M.; Minamida, Y.; Yoshida, N. Psychological well-being and green tea consumption are associated with lower pentosidine serum levels among elderly female residents in Japan. J. Psychosom. Res. 2019, 126, 109825. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological potential and mechanisms of Tea’s bioactive compounds: An Updated review. J. Adv. Res. 2023, 65, 345–363. [Google Scholar] [CrossRef]
- Hernández-Silva, D.; López-Abellán, M.D.; Martínez-Navarro, F.J.; García-Castillo, J.; Cayuela, M.L.; Alcaraz-Pérez, F. Development of a Short Telomere Zebrafish Model for Accelerated Aging Research and Antiaging Drug Screening. Aging Cell 2025, 24, e70007. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Mukherjee, M.; Kumar, A.; Sharma, G.; Tabassum, F.; Akhtar, M.S.; Imam, M.T.; Almalki, Z.S. Preliminary investigation on impact of intergenerational treatment of resveratrol endorses the development of ‘super-pups’. Life Sci. 2023, 314, 121322. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Xicota, L.; Rodriguez-Morato, J.; Dierssen, M.; de la Torre, R. Potential Role of (-)-Epigallocatechin-3-Gallate (EGCG) in the Secondary Prevention of Alzheimer Disease. Curr. Drug Targets 2015, 18, 174–195. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef]
- Hector, K.L.; Lagisz, M.; Nakagawa, S. The effect of resveratrol on longevity across species: A meta-analysis. Biol. Lett. 2012, 8, 790–793. [Google Scholar] [CrossRef]
- Fumarola, S.; Cianfruglia, L.; Cecati, M.; Giammarchi, C.; Vaiasicca, S.; Gasparrini, M. Polyphenol Intake in Elderly Patients: A Novel Approach to Counteract Colorectal Cancer Risk? Int. J. Mol. Sci. 2025, 26, 2497. [Google Scholar] [CrossRef]
- Shinoda-Ito, Y.; Omori, K.; Ito, T.; Nakayama, M.; Ikeda, A.; Ito, M.; Ohara, T.; Takashiba, S. Novel Iron Chelators, Super-Polyphenols, Show Antimicrobial Effects against Cariogenic Streptococcus mutans. Antibiotics 2023, 12, 1562. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Soledade, F.; Martins, A.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Marine-Derived Components: Can They Be a Potential Therapeutic Approach to Parkinson’s Disease? Mar. Drugs 2023, 21, 451. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular targets of natural compounds with anti-cancer properties. Int. J. Mol. Sci. 2021, 22, 13659. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2016, 15, 562–594. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, W.; Hu, R.; Yang, X.; Gong, J.; Ma, S.; Xiang, H.; Yuan, X.; Zhang, H.; He, X.; et al. Dietary resveratrol improves semen quality by promoting mitochondrial function related genes expression in aging boars. J. Anim. Sci. 2025. ahead of print. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Sun, H.; Lei, D.; Liu, J.; Fei, Y.; Wang, C.; Han, C. Resveratrol ameliorates postoperative cognitive dysfunction in aged mice by regulating microglial polarization through CX3CL1/CX3CR1 signaling axis. Neurosci. Lett. 2025, 847, 138089. [Google Scholar] [CrossRef] [PubMed]
- Boondam, Y.; Saefoong, C.; Niltup, N.; Monteil, A.; Kitphati, W. The Cognitive Restoration Effects of Resveratrol: Insight Molecular through Behavioral Studies in Various Cognitive Impairment Models. ACS Pharmacol. Transl. Sci. 2024, 7, 3334–3357. [Google Scholar] [CrossRef]
- Zheng, X.M.; Zhang, X.D.; Tan, L.L.; Zhang, J.; Wang, T.T.; Ling, Q.; Wang, H.; Ouyang, K.W.; Wang, K.W.; Chang, W.; et al. Sirt1 m6A modification-evoked Leydig cell senescence promotes Cd-induced testosterone decline. Ecotoxicol. Environ. Saf. 2024, 284, 116884. [Google Scholar] [CrossRef]
- Napoli, C.; Coscioni, E.; Trama, U.; Strozziero, M.G.; Benincasa, G. An evidence-based debate on epigenetics and immunosenescence in COVID-19. Curr. Res. Immunol. 2023, 4, 100069. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef]
- Pan, A.L.; Hasalliu, E.; Hasalliu, M.; Angulo, J.A. Epigallocatechin Gallate Mitigates the Methamphetamine-Induced Striatal Dopamine Terminal Toxicity by Preventing Oxidative Stress in the Mouse Brain. Neurotox. Res. 2020, 37, 883–892. [Google Scholar] [CrossRef]
- Rasheed, N.O.A.; Ahmed, L.A.; Abdallah, D.M.; El-Sayeh, B.M. Nephro-toxic effects of intraperitoneally injected EGCG in diabetic mice: Involvement of oxidative stress, inflammation and apoptosis. Sci. Rep. 2017, 7, 40617. [Google Scholar] [CrossRef]
- Xu, T.; Liu, R.; Zhu, H.; Zhou, Y.; Pei, T.; Yang, Z. The Inhibition of LPS-Induced Oxidative Stress and Inflammatory Responses Is Associated with the Protective Effect of (-)-Epigallocatechin-3-Gallate on Bovine Hepatocytes and Murine Liver. Antioxidants 2022, 11, 914. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Márquez-Valadez, B.; Gómez-Sánchez, A.; Silva-Lucero, M.D.C.; Torres-Pérez, M.; Téllez-Ballesteros, R.I.; Ichwan, M.; Meraz-Ríos, M.A.; Kempermann, G.; Ramírez-Rodríguez, G.B. Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience 2016, 322, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, M.; Ge, Y.; Chen, J.; Wang, C.; Yao, C.; Lin, Y. EGCG protects the mouse brain against cerebral ischemia/reperfusion injury by suppressing autophagy via the AKT/AMPK/mTOR phosphorylation pathway. Front. Pharmacol. 2022, 13, 921394. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kuang, X.; Peng, Z.; Li, C.; Guo, C.; Fu, X.; Wu, J.; Luo, Y.; Rao, X.; Zhou, X.; et al. EGCG treats ICH via up-regulating miR-137-3p and inhibiting Parthanatos. Transl. Neurosci. 2020, 11, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Lee, S.M.; Kim, H.Y.; Lee, K.Y.; Lee, Y.J.; Kim, H.T.; Kim, J.; Kim, M.H.; Hwang, M.S.; Song, C.; et al. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci. Lett. 2006, 395, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.; Chen, J.H.; Lin, H.Y.; Liu, Y.S.; Tsai, C.F.; Chang, P.C.; Lu, D.Y.; Lin, C. Regulatory Effects of Neuroinflammatory Responses Through Brain-Derived Neurotrophic Factor Signaling in Microglial Cells. Mol. Neurobiol. 2018, 55, 7487–7499. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Lisco, G.; Corbo, F.; Crupi, P.; Sardone, R.; Catino, F.; Perna, S.; Gesualdo, L.; Lozupone, M.; et al. The Effect of a Mediterranean Diet on Arterial Stiffness: A Systematic Review. Nutrients 2025, 17, 1192. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef]
- Suzuki, E.; Yorifuji, T.; Takao, S.; Komatsu, H.; Sugiyama, M.; Ohta, T.; Ishikawa-Takata, K.; Doi, H. Green Tea Consumption and Mortality among Japanese Elderly People: The Prospective Shizuoka Elderly Cohort. Ann. Epidemiol. 2009, 19, 732–739. [Google Scholar] [CrossRef]
- Vázquez-Ruiz, Z.; Alonso, A.; Alonso-Gómez, Á.; Romaguera, D.; Martínez-González, M.Á.; Li, L.; Berrade, I.; Tojal-Sierra, L.; Noris, M.; Lamuela-Raventós, R.M.; et al. Associations between Dietary Phenolic Compounds and Biomarkers of Atrial Fibrillation Risk in Adults with Metabolic Syndrome: A Longitudinal Analysis. J. Nutr. 2025, in press. [Google Scholar] [CrossRef]
- Di Daniele, N.D.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A.D. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Mateos, D.; Ugarriza, L.; Gómez, C.; Sureda, A.; Tur, J.A. Long-Term Impact of Nutritional Intervention with Increased Polyphenol Intake and Physical Activity Promotion on Oxidative and Inflammatory Profiles in Patients with Metabolic Syndrome. Nutrients 2024, 16, 2121. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Fritsche, L.; Bombrich, M.; Machann, J.; Schick, F.; Staiger, H.; Kunz, I.; Schoop, R.; Lehn-Stefan, A.; Heni, M.; et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes. Metab. 2018, 20, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Izzo, C.; Annunziata, M.; Melara, G.; Sciorio, R.; Dallio, M.; Masarone, M.; Federico, A.; Persico, M. The role of resveratrol in liver disease: A comprehensive review from in vitro to clinical trials. Nutrients 2021, 13, 933. [Google Scholar] [CrossRef]
- Hosseini, H.; Koushki, M.; Khodabandehloo, H.; Fathi, M.; Panahi, G.; Teimouri, M.; Majidi, Z.; Meshkani, R. The effect of resveratrol supplementation on C-reactive protein (CRP) in type 2 diabetic patients: Results from a systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 49, 102251. [Google Scholar] [CrossRef]
- Forcano, L.; Fauria, K.; Soldevila-Domenech, N.; Minguillón, C.; Lorenzo, T.; Cuenca-Royo, A.; Menezes-Cabral, S.; Pizarro, N.; Boronat, A.; Molinuevo, J.L.; et al. Prevention of cognitive decline in subjective cognitive decline APOE ε4 carriers after EGCG and a multimodal intervention (PENSA): Study design. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12155. [Google Scholar] [CrossRef]
- Bazyar, H.; Hosseini, S.A.; Saradar, S.; Mombaini, D.; Allivand, M.; Labibzadeh, M.; Alipour, M. Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: A clinical trial. J. Complement. Integr. Med. 2021, 18, 405–411. [Google Scholar] [CrossRef]
- Settakorn, K.; Hantrakool, S.; Petiwathayakorn, T.; Hutachok, N.; Tantiworawit, A.; Charoenkwan, P.; Chalortham, N.; Chompupoung, A.; Paradee, N.; Koonyosying, P.; et al. A randomized placebo−controlled clinical trial of oral green tea epigallocatechin 3−gallate on erythropoiesis and oxidative stress in transfusion−dependent β−thalassemia patients. Front. Mol. Biosci. 2023, 10, 1248742. [Google Scholar] [CrossRef]
- Loftis, J.M.; Wilhelm, C.J.; Huckans, M. Effect of epigallocatechin gallate supplementation in schizophrenia and bipolar disorder: An 8-week, randomized, double-blind, placebo-controlled study. Ther. Adv. Psychopharmacol. 2013, 3, 21–27. [Google Scholar] [CrossRef]
- Cornali, K.; Di Lauro, M.; Marrone, G.; Masci, C.; Montalto, G.; Giovannelli, A.; Schievano, C.; Tesauro, M.; Pieri, M.; Bernardini, S.; et al. The Effects of a Food Supplement, Based on Co-Micronized Palmitoylethanolamide (PEA)–Rutin and Hydroxytyrosol, in Metabolic Syndrome Patients: Preliminary Results. Nutrients 2025, 17, 413. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) Leaf Polyphenols Improve Insulin Sensitivity in Middle-Aged Overweight Men: A Randomized, Placebo-Controlled, Crossover Trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H.; Lucas, E.A.; Khalil, D.A.; Devareddy, L.; Smith, B.J.; McDonald, J.; Arquitt, A.B.; Payton, M.E.; Mason, C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr. J. 2005, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J.B.; Chen, X.; Boass, A.; Symons, M.; Kohlmeier, M.; Renner, J.B.; Garner, S.C. Soy isoflavones: No effects on bone mineral content and bone mineral density in healthy, menstruating young adult women after one year. J. Am. Coll. Nutr. 2002, 21, 388–393. [Google Scholar] [CrossRef]
- Yamashima, T. 4-Hydroxynonenal from Mitochondrial and Dietary Sources Causes Lysosomal Cell Death for Lifestyle-Related Diseases. Nutrients 2024, 16, 4171. [Google Scholar] [CrossRef]
- Yamashima, T.; Seike, T.; Mochly-Rosen, D.; Chen, C.H.; Kikuchi, M.; Mizukoshi, E. Implication of the cooking oil-peroxidation product “hydroxynonenal” for Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1211141. [Google Scholar] [CrossRef]
- Yamashima, T.; Seike, T.; Oikawa, S.; Kobayashi, H.; Kido, H.; Yanagi, M.; Yamamiya, D.; Li, S.; Boontem, P.; Mizukoshi, E. Hsp70.1 carbonylation induces lysosomal cell death for lifestyle-related diseases. Front. Mol. Biosci. 2023, 9, 1063632. [Google Scholar] [CrossRef]
- Seike, T.; Boontem, P.; Yanagi, M.; Li, S.; Kido, H.; Yamamiya, D.; Nakagawa, H.; Okada, H.; Yamashita, T.; Harada, K.; et al. Hydroxynonenal Causes Hepatocyte Death by Disrupting Lysosomal Integrity in Nonalcoholic Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 925–944. [Google Scholar] [CrossRef]
- Boontem, P.; Yamashima, T. Hydroxynonenal causes Langerhans cell degeneration in the pancreas of Japanese macaque monkeys. PLoS ONE 2021, 16, e0245702. [Google Scholar] [CrossRef]
- Zhuang, Y.; Dong, J.; He, X.; Wang, J.; Li, C.; Dong, L.; Zhang, Y.; Zhou, X.; Wang, H.; Yi, Y.; et al. Impact of Heating Temperature and Fatty Acid Type on the Formation of Lipid Oxidation Products During Thermal Processing. Front. Nutr. 2022, 9, 913297. [Google Scholar] [CrossRef]
- Tranchida, N.; Molinari, F.; Franco, G.A.; Cordaro, M.; Di Paola, R. Potential Role of Dietary Antioxidants During Skin Aging. Food Sci. Nutr. 2025, 13, e70231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Tu, C.; Chen, X.; He, R. Advanced Glycation End Products in Disease Development and Potential Interventions. Antioxidants 2025, 14, 492. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Lin, S.; King, L.; Liu, L. The Potential Role of Advanced Glycation End Products in the Development of Kidney Disease. Nutrients 2025, 17, 758. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- Popkin, B.M.; Hawkes, C. Sweetening of the global diet, particularly beverages: Patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016, 4, 174–186. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Garrido-Miguel, M.; Martínez-García, I.; Lucerón-Lucas-Torres, M.; Rodríguez-Gutiérrez, E.; Berlanga-Macías, C.; Fernández-Bravo-Rodrigo, J.; Patiño-Cardona, S. Association of Dietary Advanced Glycation End Products with Overall and Site-Specific Cancer Risk and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1638. [Google Scholar] [CrossRef]
- Giraldo-Gonzalez, G.C.; Roman-Gonzalez, A.; Cañas, F.; Garcia, A. Molecular Mechanisms of Type 2 Diabetes-Related Heart Disease and Therapeutic Insights. Int. J. Mol. Sci. 2025, 26, 4548. [Google Scholar] [CrossRef]
- Vianello, E.; Beltrami, A.P.; Aleksova, A.; Janjusevic, M.; Fluca, A.L.; Corsi Romanelli, M.M.; La Sala, L.; Dozio, E. The Advanced Glycation End-Products (AGE)–Receptor for AGE System (RAGE): An Inflammatory Pathway Linking Obesity and Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 3707. [Google Scholar] [CrossRef]
- Habes, D.; Kestranek, J.; Stranik, J.; Kacerovsky, M.; Spacek, J. Is there an association between pelvic organ prolapse and oxidative stress? A systematic review. PLoS ONE 2022, 17, e0271467. [Google Scholar] [CrossRef]
- Plotegher, N.; Bubacco, L. Lysines, Achilles’ heel in alpha-synuclein conversion to a deadly neuronal endotoxin. Ageing Res. Rev. 2016, 26, 62–71. [Google Scholar] [CrossRef]
- Steinerová, A.; Racek, J.; Stožický, F.; Zima, T.; Fialová, L.; Lapin, A. Antibodies against oxidized LDL—Theory and clinical use. Physiol. Res. 2001, 50, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. How much ultra-processed food do you eat? Blood and urine record it. Nature 2025. ahead of print. [Google Scholar] [CrossRef]
- Hafner, E.; Hribar, M.; Pravst, I. Ultra-Processed Foods in the Food Supply: Prevalence, Nutritional Composition and Use of Voluntary Labelling Schemes. Nutrients 2025, 17, 1731. [Google Scholar] [CrossRef] [PubMed]
- Fontalba-Navas, A.; Echeverria, R.; Larrea-Killinger, C.; Gracia-Arnaiz, M.; Soar, C.; Arrebola, J.P. Association Between the Healthy Eating Index and the Body Mass Index of Older Adults: An Analysis of Food Frequency and Preferences. Nutrients 2025, 17, 1717. [Google Scholar] [CrossRef]
- Han, I.H.; Csallany, A.S. Temperature dependence of HNE formation in vegetable oils and butter oil. J. Am. Oil Chem. Soc. 2008, 85, 777–782. [Google Scholar] [CrossRef]
- Yuan, J.; Shoeman, D.W.; Csallany, A.S. Formation of 4-Hydroxy-2-Trans-Nonenal, a Toxic Aldehyde, in Thermally Treated Olive and Sunflower Oils. J. Am. Oil Chem. Soc. 2018, 95, 813–823. [Google Scholar] [CrossRef]
- Golan, R.; Shai, I.; Gepner, Y.; Harman-Boehm, I.; Schwarzfuchs, D.; Spence, J.D.; Parraga, G.; Buchanan, D.; Witkow, S.; Friger, M.; et al. Effect of wine on carotid atherosclerosis in type 2 diabetes: A 2-year randomized controlled trial. Eur. J. Clin. Nutr. 2018, 72, 871–878. [Google Scholar] [CrossRef]
- Serio, F.; Imbriani, G.; Acito, M.; Moretti, M.; Fanizzi, F.P.; De Donno, A.; Valacchi, G. Moderate red wine intake and cardiovascular health protection: A literature review. Food Funct. 2023, 14, 6346–6362. [Google Scholar] [CrossRef]
- McNeill, K.A. Epidemiology of Brain Tumors. Neurol. Clin. 2016, 34, 981–998. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Varghese, J.; Dakhode, S. Effects of Alcohol Consumption on Various Systems of the Human Body: A Systematic Review. Cureus 2022, 14, e30057. [Google Scholar] [CrossRef]
- MacKillop, J.; Agabio, R.; Feldstein Ewing, S.W.; Heilig, M.; Kelly, J.F.; Leggio, L.; Lingford-Hughes, A.; Palmer, A.A.; Parry, C.D.; Ray, L.; et al. Hazardous drinking and alcohol use disorders. Nat. Rev. Dis. Prim. 2022, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2020, 19, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.D.; Coveñas, R. Biochemical mechanisms associating alcohol use disorders with cancers. Cancers 2021, 13, 3548. [Google Scholar] [CrossRef] [PubMed]
- Zorumski, C.F.; Mennerick, S.; Izumi, Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol 2014, 48, 1–17. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol metabolizing enzymes, microsomal ethanol oxidizing system, cytochrome P450 2E1, catalase, and aldehyde dehydrogenase in alcohol-associated liver disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Millonig, G.; Wang, Y.; Homann, N.; Bernhardt, F.; Qin, H.; Mueller, S.; Bartsch, H.; Seitz, H.K. Ethanol-mediated carcinogenesis in the human esophagus implicates CYP2E1 induction and the generation of carcinogenic DNA-lesions. Int. J. Cancer 2011, 128, 533–540. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Alcohol and cardiovascular disease--modulation of vascular cell function. Nutrients 2012, 4, 297–318. [Google Scholar] [CrossRef]
- Ceccanti, M.; Coriale, G.; Fiorentino, D.; Iannitelli, A.; Tarani, L.; Fiore, M. Italian Guidelines for the diagnosis and treatment of Fetal Alcohol Spectrum Disorders. Riv. Psichiatr. 2024, 59, 191–193. [Google Scholar] [CrossRef]
- Ferraguti, G.; Fanfarillo, F.; Nicotera, S.; Terracina, S.; Moschella, C.; Mattia, A.; David, M.C.; Pichini, S.; Coriale, G.; Fiorentino, D.; et al. Italian Guidelines for the diagnosis and treatment of Fetal Alcohol Spectrum Disorders: Detecting alcohol drinking during pregnancy. Riv. Psichiatr. 2024, 59, 241–249. [Google Scholar] [CrossRef]
- Popova, S.; Dozet, D.; Shield, K.; Rehm, J.; Burd, L. Alcohol’s impact on the fetus. Nutrients 2021, 13, 3452. [Google Scholar] [CrossRef] [PubMed]
- Carpita, B.; Migli, L.; Chiarantini, I.; Battaglini, S.; Montalbano, C.; Carmassi, C.; Cremone, I.M.; Dell’osso, L. Autism Spectrum Disorder and Fetal Alcohol Spectrum Disorder: A Literature Review. Brain Sci. 2022, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Wilhoit, L.F.; Scott, D.A.; Simecka, B.A. Fetal Alcohol Spectrum Disorders: Characteristics, Complications, and Treatment. Community Ment. Health J. 2017, 53, 711–718. [Google Scholar] [CrossRef] [PubMed]
- del Campo, M.; Jones, K.L. A review of the physical features of the fetal alcohol spectrum disorders. Eur. J. Med. Genet. 2017, 60, 55–64. [Google Scholar] [CrossRef]
- Ceccanti, M.; Coriale, G.; Fiorentino, D.; Tarani, L.; Messina, M.P.; Vitali, M.; Fiore, M.; May, P.A. Italian Guidelines for the diagnosis and treatment of Fetal Alcohol Spectrum Disorders: Epidemiology. Riv. Psichiatr. 2024, 59, 259–268. [Google Scholar] [CrossRef]
- Menghi, M.; Micangeli, G.; Paparella, R.; Ceccanti, M.; Coriale, G.; Ferraguti, G.; Fiore, M.; Fiorentino, D.; Piccioni, M.G.; Tarani, L. Italian Guidelines for the diagnosis and treatment of Fetal Alcohol Spectrum Disorders: Clinical hallmarks. Riv. Psichiatr. 2024, 59, 203–211. [Google Scholar] [CrossRef]
- Micangeli, G.; Menghi, M.; Paparella, R.; Ceccanti, M.; Coriale, G.; Fiorentino, D.; Ferraguti, G.; Fiore, M.; Tarani, L. Italian Guidelines for the diagnosis and treatment of Fetal Alcohol Spectrum Disorders: Diagnostic criteria. Riv. Psichiatr. 2024, 59, 195–202. [Google Scholar] [CrossRef]
- Carito, V.; Ceccanti, M.; Ferraguti, G.; Coccurello, R.; Ciafrè, S.; Tirassa, P.; Fiore, M. NGF and BDNF Alterations by Prenatal Alcohol Exposure. Curr. Neuropharmacol. 2019, 17, 308–317. [Google Scholar] [CrossRef]
- Chabenne, A.; Moon, C.; Ojo, C.; Khogali, A.; Nepal, B.; Sharma, S. Biomarkers in fetal alcohol syndrome. Biomark. Genom. Med. 2014, 6, 12–22. [Google Scholar] [CrossRef]
- Burd, L.; Klug, M.G.; Li, Q.; Kerbeshian, J.; Martsolf, J.T. Diagnosis of fetal alcohol spectrum disorders: A validity study of the fetal alcohol syndrome checklist. Alcohol 2010, 44, 605–614. [Google Scholar] [CrossRef]
- Ceccanti, M.; De Nicolò, S.; Mancinelli, R.; Chaldakov, G.; Carito, V.; Ceccanti, M.; Laviola, G.; Tirassa, P.; Fiore, M. NGF and BDNF long-term variations in the thyroid, testis and adrenal glands of a mouse model of fetal alcohol spectrum disorders. Ann. Ist. Super. Sanita 2013, 49, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Laviola, G.; Aloe, L.; di Fausto, V.; Mancinelli, R.; Ceccanti, M. Early exposure to ethanol but not red wine at the same alcohol concentration induces behavioral and brain neurotrophin alterations in young and adult mice. Neurotoxicology 2009, 30, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Mancinelli, R.; Aloe, L.; Laviola, G.; Sornelli, F.; Vitali, M.; Ceccanti, M. Hepatocyte growth factor, vascular endothelial growth factor, glial cell-derived neurotrophic factor and nerve growth factor are differentially affected by early chronic ethanol or red wine intake. Toxicol. Lett. 2009, 188, 208–213. [Google Scholar] [CrossRef]
- Ceccanti, M.; Mancinelli, R.; Tirassa, P.; Laviola, G.; Rossi, S.; Romeo, M.; Fiore, M. Early exposure to ethanol or red wine and long-lasting effects in aged mice. A study on nerve growth factor, brain-derived neurotrophic factor, hepatocyte growth factor, and vascular endothelial growth factor. Neurobiol. Aging 2012, 33, 359–367. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, C.K.; LaVoie, H.A.; DiPette, D.J.; Singh, U.S. Resveratrol restores Nrf2 Level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol. Pharmacol. 2011, 80, 446–457. [Google Scholar] [CrossRef]
- Małkowska, A.; Makarowa, K.; Zawada, K.; Grzelak, M.; Zmysłowska, A. Effect of curcumin on the embryotoxic effect of ethanol in a zebrafish model. Toxicol. Vitr. 2024, 101, 105951. [Google Scholar] [CrossRef]
- Ceccanti, M.; Coccurello, R.; Carito, V.; Ciafrè, S.; Ferraguti, G.; Giacovazzo, G.; Mancinelli, R.; Tirassa, P.; Chaldakov, G.N.; Pascale, E.; et al. Paternal alcohol exposure in mice alters brain NGF and BDNF and increases ethanol-elicited preference in male offspring. Addict. Biol. 2016, 21, 776–787. [Google Scholar] [CrossRef]
- Ciafrè, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Ceccanti, M.; Fiore, M. Alcohol as an early life stressor: Epigenetics, metabolic, neuroendocrine and neurobehavioral implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668. [Google Scholar] [CrossRef]
- Abel, E.L. Paternal contribution to fetal alcohol syndrome. Addict. Biol. 2004, 9, 127–133. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta—Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Critselis, E.; Vasilopoulou, E. Mediterranean diet and longevity. Eur. J. Cancer Prev. 2004, 13, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 2000, 131, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate Wine Consumption and Health: A Narrative Review. Nutrients 2023, 15, 175. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The fluid aspect of the mediterranean diet in the prevention and management of cardiovascular disease and diabetes: The role of polyphenol content in moderate consumption of wine and olive oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef]
- Lombardo, M.; Feraco, A.; Camajani, E.; Caprio, M.; Armani, A. Health Effects of Red Wine Consumption: A Narrative Review of an Issue That Still Deserves Debate. Nutrients 2023, 15, 1921. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Łuszczki, E.; Boakye, F.; Zielińska, M.; Dereń, K.; Bartosiewicz, A.; Oleksy, Ł.; Stolarczyk, A. Vegan diet: Nutritional components, implementation, and effects on adults’ health. Front. Nutr. 2023, 10, 1294497. [Google Scholar] [CrossRef]
- Bali, A.; Naik, R. The Impact of a Vegan Diet on Many Aspects of Health: The Overlooked Side of Veganism. Cureus 2023, 15, e35148. [Google Scholar] [CrossRef]
- Craig, W.J. Health effects of vegan diets. Am. J. Clin. Nutr. 2009, 89, 1627S–1633S. [Google Scholar] [CrossRef]
- Sebastiani, G.; Barbero, A.H.; Borrás-Novel, C.; Casanova, M.A.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Tutusaus, M.P.; Martínez, S.F.; Roig, M.D.G.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, S.M.; Raposo, A.; Saraiva, A.; Zandonadi, R.P. Vegetarian diet: An overview through the perspective of quality of life domains. Int. J. Environ. Res. Public Health 2021, 18, 4067. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, S.M.; Rosenfeld, D.L.; Moreira, A.V.B.; Zandonadi, R.P. Plant-based and vegetarian diets: An overview and definition of these dietary patterns. Eur. J. Nutr. 2023, 62, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef]
- Parker, H.W.; Vadiveloo, M.K. Diet quality of vegetarian diets compared with nonvegetarian diets: A systematic review. Nutr. Rev. 2019, 77, 144–160. [Google Scholar] [CrossRef]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef]
- Kamble, M.G.; Singh, A.; Singh, S.V.; Kamble, M.G.; Sagar, N.A.; Rani, N. Nanotechnology for encapsulation of bioactive components: A review. Discov. Food 2025, 5, 116. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Singh, R.; Westfall, S.; Herman, F.; Faith, J.; Ho, L. The Role of the Gut Microbiota in the Metabolism of Polyphenols as Characterized by Gnotobiotic Mice. J. Alzheimer’s Dis. 2018, 63, 409–421. [Google Scholar] [CrossRef]
- Pratelli, G.; Tamburini, B.; Carlisi, D.; De Blasio, A.; D’Anneo, A.; Emanuele, S.; Notaro, A.; Affranchi, F.; Giuliano, M.; Seidita, A.; et al. Foodomics-Based Approaches Shed Light on the Potential Protective Effects of Polyphenols in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 14619. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanova, S.; Chakarov, D.; Kraev, K.; Ivanov, K. Ecdysterone and Turkesterone-Compounds with Prominent Potential in Sport and Healthy Nutrition. Nutrients 2024, 16, 1382. [Google Scholar] [CrossRef]
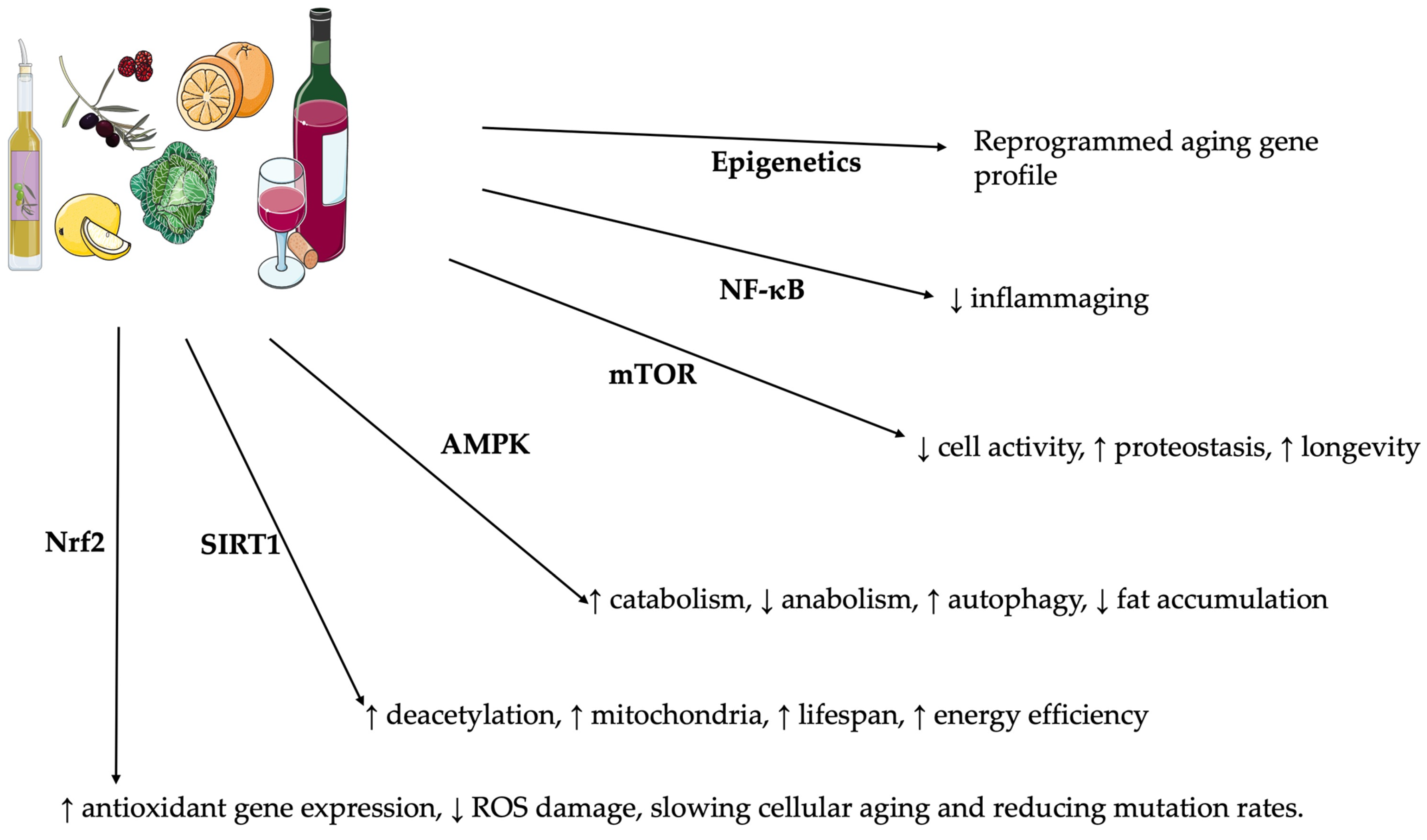
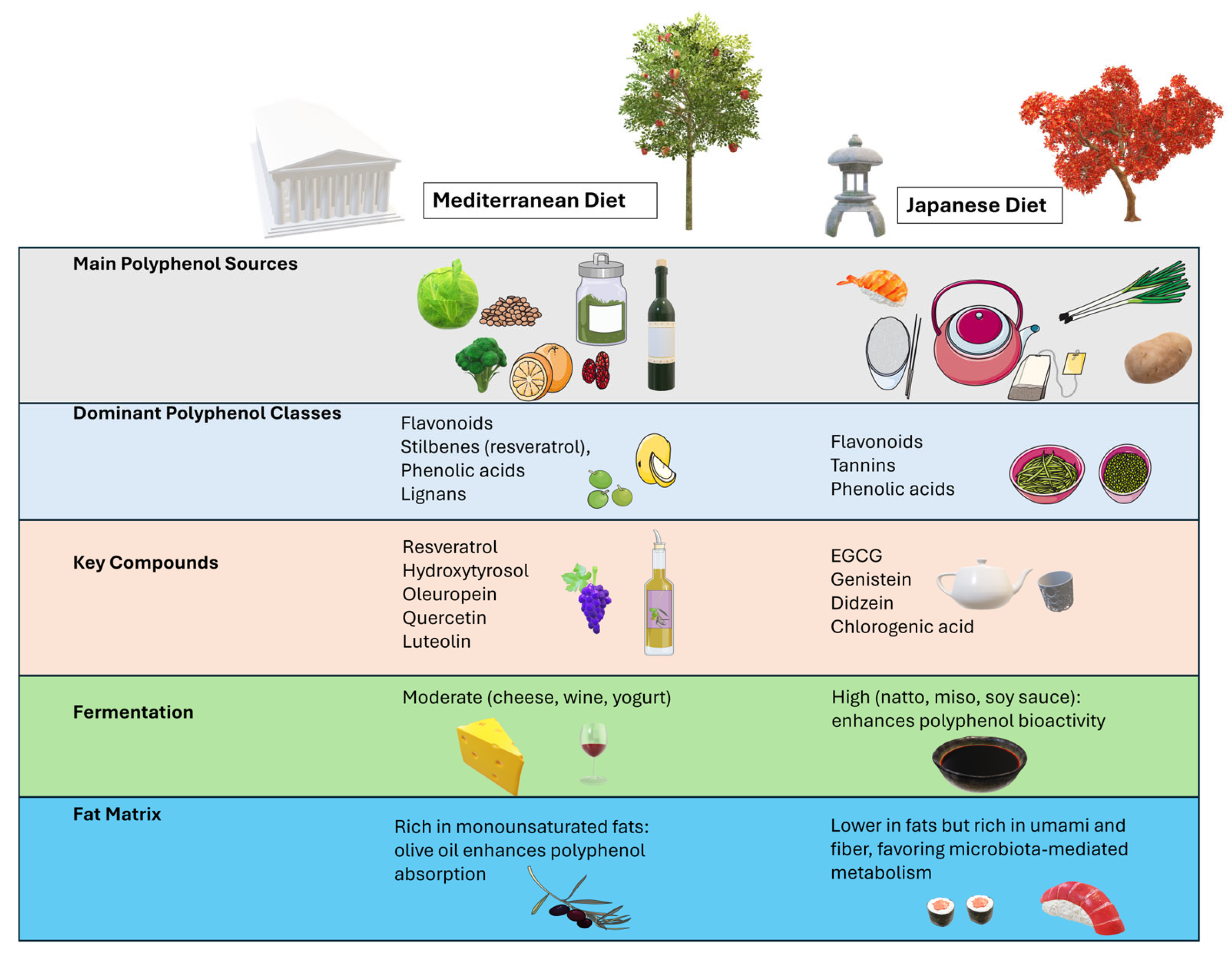
| Polyphenol Type | Formulation | Dose (Daily) | Population and Intervention | Primary Outcome | References |
|---|---|---|---|---|---|
| Resveratrol | Purified capsule | 150 mg | Overweight adults; several weeks | ↑ SIRT1 activity, ↓ CRP | [217,218,219,220] |
| EGCG | Green tea extract | 100/600 mg | Mild cognitive impairment; thalassemics; diabetics; 6/12 months | ↑ Cognitive scores, ↓ oxidative markers | [221,222,223,224] |
| Hydroxytyrosol | Olive oil phenolic extract | 10/15 mg | Metabolic syndrome; 8/12 weeks | ↓ ox-LDL, ↓ IL-6 | [225,226] |
| Soy Isoflavones | Soy protein isolate | 60/90 mg | Post-menopausal women; 1 year | ↑ Bone mineral density, ↓ LDL-cholesterol | [227,228] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.; Tonchev, A.B.; Pancheva, R.Z.; Yamashima, T.; Venditti, S.; Ferraguti, G.; Terracina, S. Increasing Life Expectancy with Plant Polyphenols: Lessons from the Mediterranean and Japanese Diets. Molecules 2025, 30, 2888. https://doi.org/10.3390/molecules30132888
Fiore M, Tonchev AB, Pancheva RZ, Yamashima T, Venditti S, Ferraguti G, Terracina S. Increasing Life Expectancy with Plant Polyphenols: Lessons from the Mediterranean and Japanese Diets. Molecules. 2025; 30(13):2888. https://doi.org/10.3390/molecules30132888
Chicago/Turabian StyleFiore, Marco, Anton B. Tonchev, Ruzha Z. Pancheva, Tetsumori Yamashima, Sabrina Venditti, Giampiero Ferraguti, and Sergio Terracina. 2025. "Increasing Life Expectancy with Plant Polyphenols: Lessons from the Mediterranean and Japanese Diets" Molecules 30, no. 13: 2888. https://doi.org/10.3390/molecules30132888
APA StyleFiore, M., Tonchev, A. B., Pancheva, R. Z., Yamashima, T., Venditti, S., Ferraguti, G., & Terracina, S. (2025). Increasing Life Expectancy with Plant Polyphenols: Lessons from the Mediterranean and Japanese Diets. Molecules, 30(13), 2888. https://doi.org/10.3390/molecules30132888








