Ruthenium Decorated Tris-Silylated Germanium Zintl Clusters Featuring an Unexpected Ligand Arrangement
Abstract
:1. Introduction
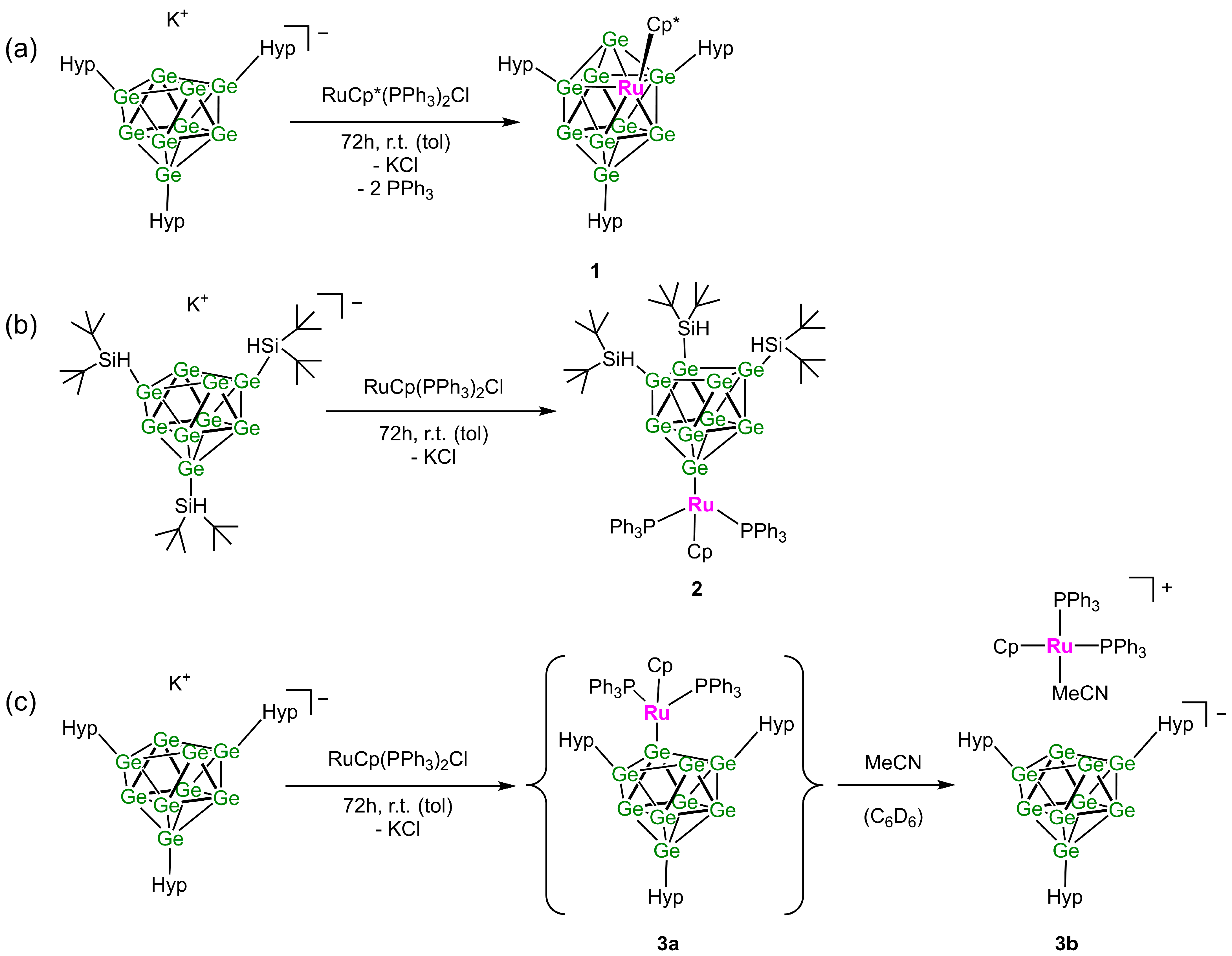
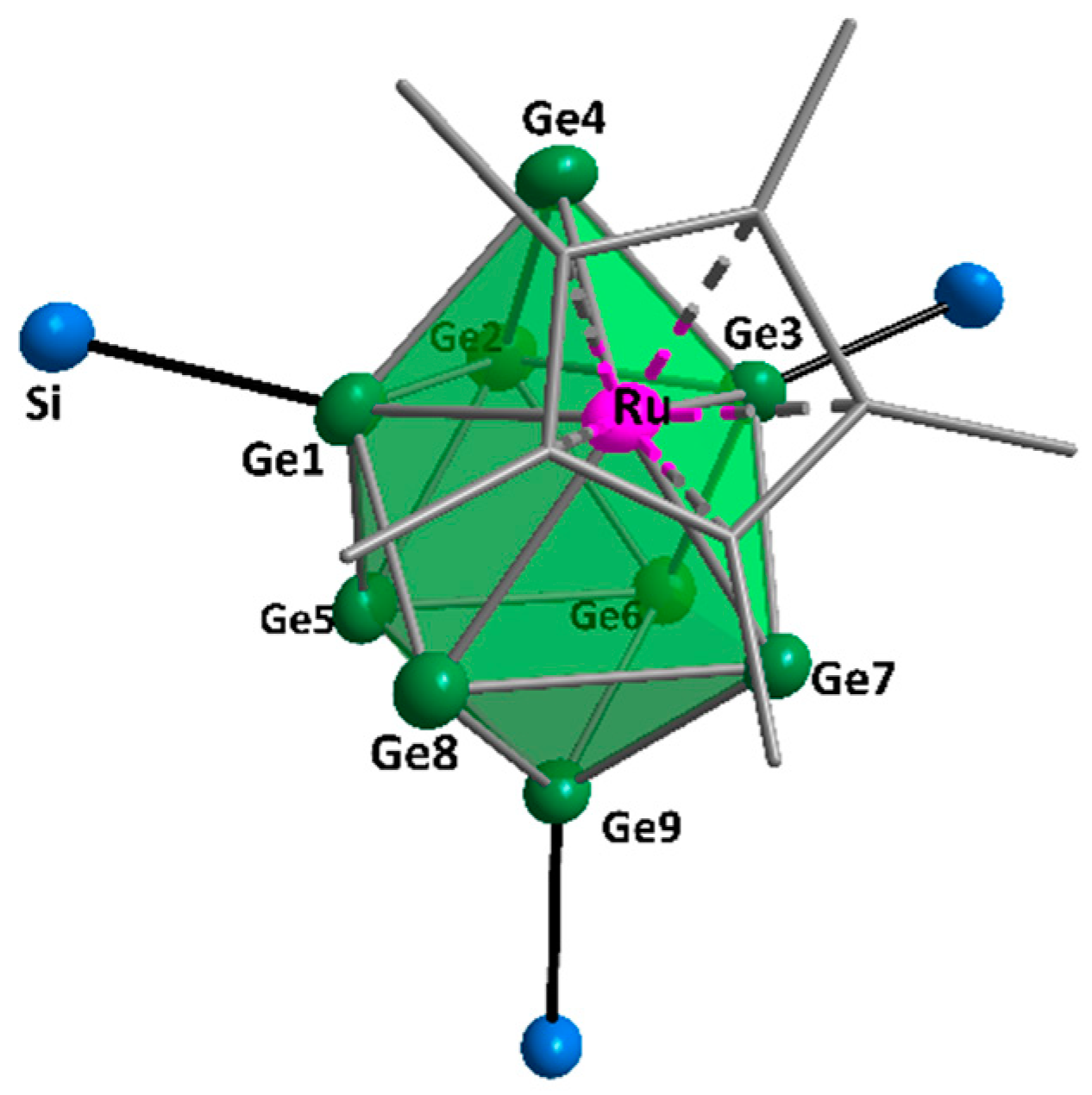
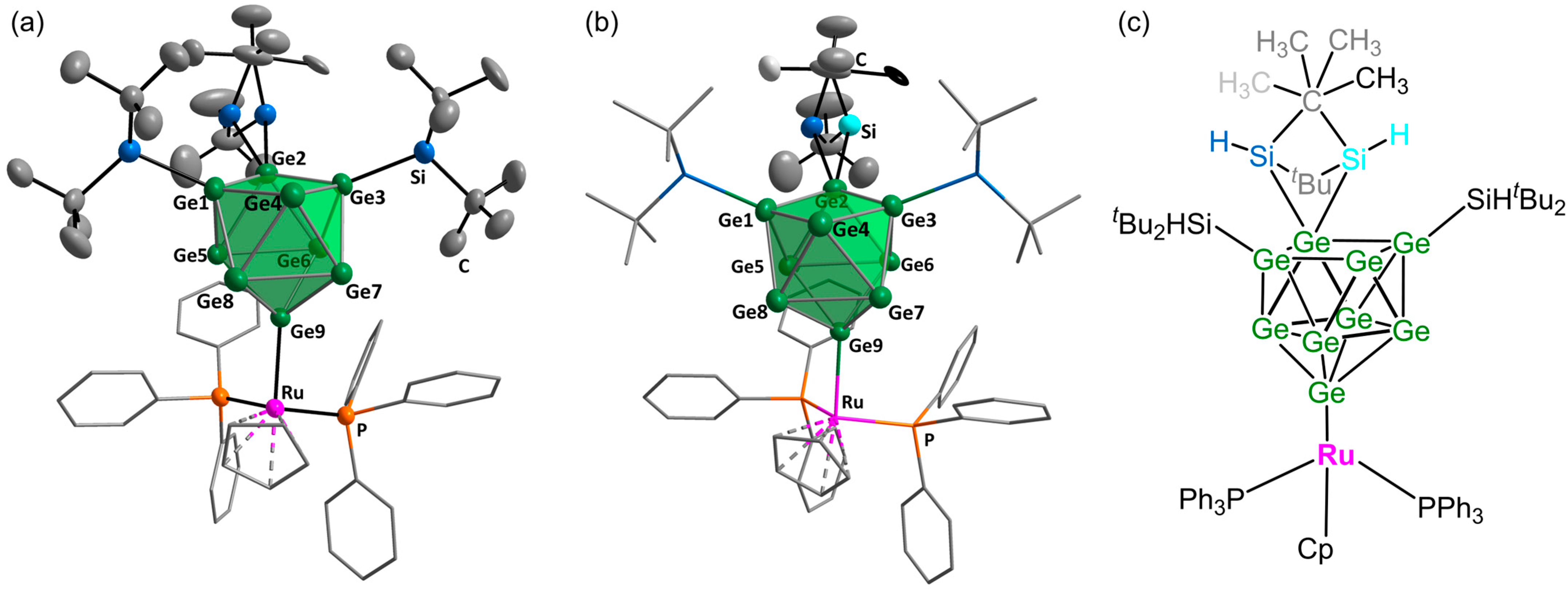
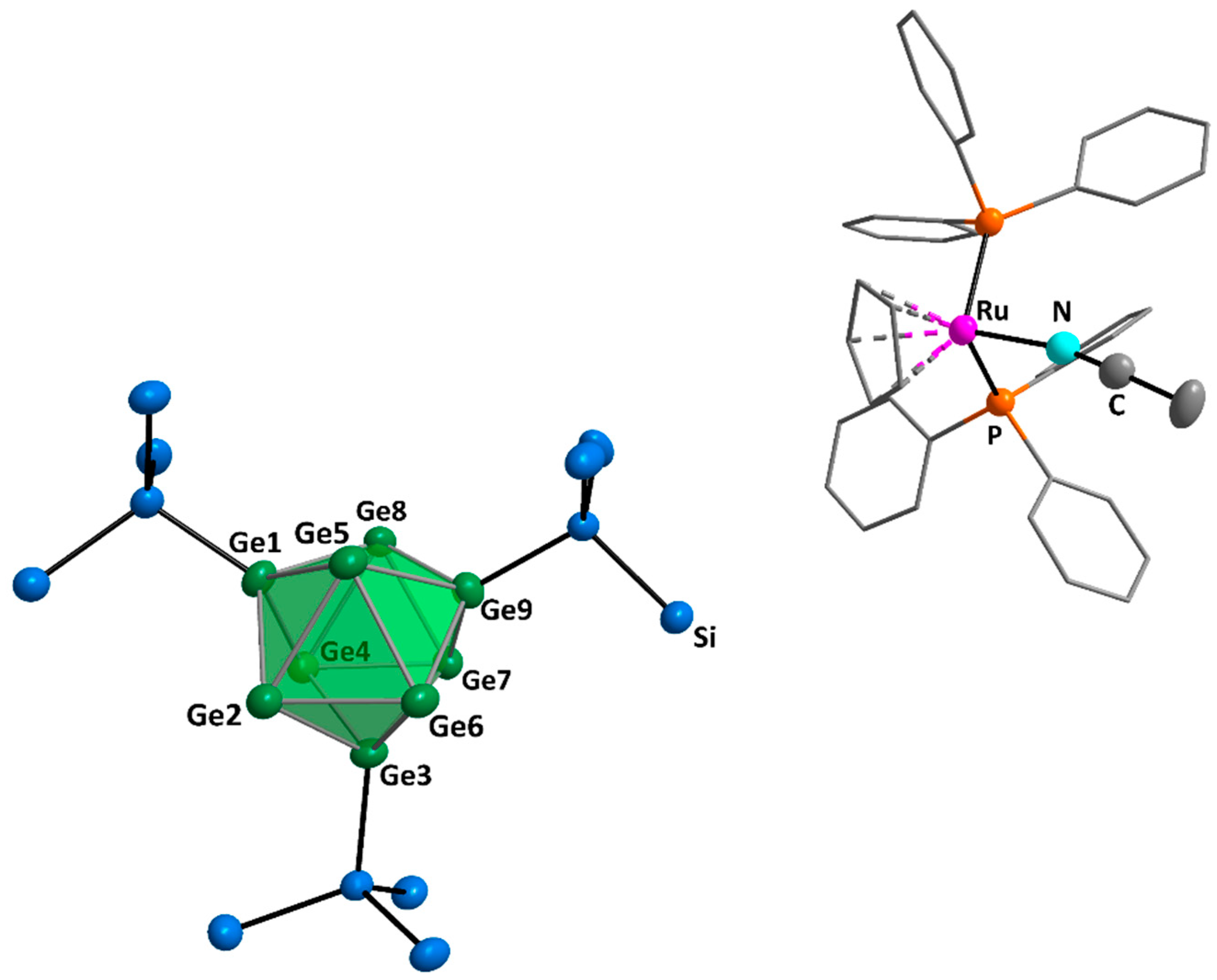
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. NMR Spectroscopy
3.3. Single-Crystal Structure Determination
3.4. LIFDI/MS
3.5. Elemental Analysis
3.6. Syntheses
3.6.1. Synthesis of K[Hyp3Ge9]
3.6.2. Synthesis of K[(tBu2HSi)3Ge9]
3.6.3. Synthesis of [5-Ge9Hyp3]RuCp* (1)
3.6.4. Synthesis of [η1-Ge9(SitBu2H)3]RuCp(PPh3)2 (2)
3.6.5. Synthesis of [Hyp3Ge9][RuCp(PPh3)2] or [Hyp3Ge9][RuCp(PPh3)2(MeCN)] (3a and 3b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoch, C.; Wendorff, M.; Röhr, C. Tetrapotassium nonastannide, K4Sn9. Acta Crystallogr. Sect. C 2002, 58, i45–i46. [Google Scholar] [CrossRef] [PubMed]
- Ponou, S.; Fässler, T.F. Crystal Growth and Structure Refinement of K4Ge9. Z. Anorg. Allg. Chem. 2007, 633, 393–397. [Google Scholar] [CrossRef]
- Queneau, V.; Sevov, S.C. Synthesis and Structure of the Zintl-Phase K4Pb9 Containing Isolated Pb94− Clusters of Two Different Geometries. Inorg. Chem. 1998, 37, 1358–1360. [Google Scholar] [CrossRef]
- Queneau, V.; Sevov, S.C. Ge: A Deltahedral Zintl Ion Now Made in the Solid-State. Angew. Chem. Int. Ed. 1997, 36, 1754–1756. [Google Scholar] [CrossRef]
- Todorov, E.; Sevov, S.C. Deltahedral Clusters in Neat Solids: Synthesis and Structure of the Zintl Phase Cs4Pb9 with Discrete Pb94− Clusters. Inorg. Chem. 1998, 37, 3889–3891. [Google Scholar] [CrossRef]
- Hoch, C.; Wendorff, M.; Röhr, C. Synthesis and crystal structure of the tetrelides A12M17 (A=Na, K, Rb, Cs; M=Si, Ge, Sn) and A4Pb9 (A=K, Rb). J. Alloys Compd. 2003, 361, 206–221. [Google Scholar] [CrossRef]
- Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T.F. Zintl ions, cage compounds, and intermetalloid clusters of Group 14 and Group 15 elements. Angew. Chem. Int. Ed. 2011, 50, 3630–3670. [Google Scholar] [CrossRef]
- Li, F.; Sevov, S.C. Rational synthesis of [Ge9{Si(SiMe3)3}3]− from its parent Zintl ion Ge9(4-). Inorg. Chem. 2012, 51, 2706–2708. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Z.-M. Recent advances in structural chemistry of Group 14 Zintl ions. Coord. Chem. Rev. 2019, 382, 32–56. [Google Scholar] [CrossRef]
- Wilson, R.J.; Lichtenberger, N.; Weinert, B.; Dehnen, S. Intermetalloid and Heterometallic Clusters Combining p-Block (Semi)Metals with d- or f-Block Metals. Chem. Rev. 2019, 119, 8506–8554. [Google Scholar] [CrossRef]
- Schiegerl, L.J.; Geitner, F.S.; Fischer, C.; Klein, W.; Fässler, T.F. Functionalization of [Ge9] with Small Silanes:[Ge9(SiR3)3]– (R = iBu, iPr, Et) and the Structures of (CuNHCDipp)[Ge9{Si(iBu)3}3], (K-18c6)Au[Ge9{Si(iBu)3}3]2, and (K-18c6)2[Ge9{Si(iBu)3}2]. Z. Anorg. Allg. Chem. 2016, 642, 1419–1426. [Google Scholar] [CrossRef]
- Mayer, K.; Schiegerl, L.J.; Kratky, T.; Gunther, S.; Fässler, T.F. Targeted attachment of functional groups at Ge9 clusters via silylation reactions. Chem. Commun. 2017, 53, 11798–11801. [Google Scholar] [CrossRef] [PubMed]
- Kysliak, O.; Kunz, T.; Schnepf, A. Metalloid Ge9R3−Clusters with Various Silyl Substituents: From Shielded to Open Cluster Cores. Eur. J. Inorg. Chem. 2017, 2017, 805–810. [Google Scholar] [CrossRef]
- Frischhut, S.; Fässler, T.F. Synthesis of low-oxidation-state germanium clusters comprising a functional anchor group—Synthesis and characterization of [(Ge(0))5(Ge-R)3(Ge-(CH2)n-CH=CH2)] with R = Si(SiMe3)3. Dalton Trans. 2018, 47, 3223–3226. [Google Scholar] [CrossRef]
- Frischhut, S.; Klein, W.; Drees, M.; Fässler, T.F. Acylation of Homoatomic Ge9 Cages and Subsequent Decarbonylation. Chem. Eur. J. 2018, 24, 9009–9014. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Munoz-Castro, A.; Sevov, S.C. [Ge9{Si(SiMe3)3}3{SnPh3}]: A tetrasubstituted and neutral deltahedral nine-atom cluster. Angew. Chem. Int. Ed. 2012, 51, 8581–8584. [Google Scholar] [CrossRef]
- Li, F.; Sevov, S.C. Synthesis, structures, and solution dynamics of tetrasubstituted nine-atom germanium deltahedral clusters. J. Am. Chem. Soc. 2014, 136, 12056–12063. [Google Scholar] [CrossRef]
- Schenk, C.; Schnepf, A. {Ge9R3Cr(CO)5}− and {Ge9R3Cr(CO)3}−: A metalloid cluster (Ge9R3−) as a flexible ligand in coordination chemistry [R=Si(SiMe3)3]. Chem. Commun. 2009, 22, 3208–3210. [Google Scholar] [CrossRef]
- Geitner, F.S.; Fässler, T.F. Introducing Tetrel Zintl Ions to N-Heterocyclic Carbenes—Synthesis of Coinage Metal NHC Complexes of [Ge9{Si(SiMe3)3}3]. Eur. J. Inorg. Chem. 2016, 2016, 2688–2691. [Google Scholar] [CrossRef]
- Mayer, K.; Schiegerl, L.J.; Fässler, T.F. On the Reactivity of Silylated Ge9 Clusters: Synthesis and Characterization of [ZnCp*(Ge9{Si(SiMe3)3}3)], [CuPiPr3 (Ge9{Si(SiMe3)3}3)], and [(CuPiPr3)4{Ge9 (SiPh3)2}2]. Chem. Eur. J. 2016, 22, 18794–18800. [Google Scholar] [CrossRef]
- Schiegerl, L.J.; Melaimi, M.; Tolentino, D.R.; Klein, W.; Bertrand, G.; Fässler, T.F. Silylated Ge9 Clusters as New Ligands for Cyclic (Alkyl)amino and Mesoionic Carbene Copper Complexes. Inorg. Chem. 2019, 58, 3256–3264. [Google Scholar] [CrossRef]
- Townrow, O.P.E.; Duckett, S.B.; Weller, A.S.; Goicoechea, J.M. Zintl cluster supported low coordinate Rh(I) centers for catalytic H/D exchange between H2 and D2. Chem. Sci. 2022, 13, 7626–7633. [Google Scholar] [CrossRef] [PubMed]
- Willeit, N.S.; Hlukhyy, V.; Fässler, T.F. Synthesis, Structure and Catalytic Properties of Hyp3[Ge9Rh]PPh3. Z. Anorg. Allg. Chem. 2024, 650, e202400171. [Google Scholar] [CrossRef]
- Townrow, O.P.E.; Chung, C.; Macgregor, S.A.; Weller, A.S.; Goicoechea, J.M. A Neutral Heteroatomic Zintl Cluster for the Catalytic Hydrogenation of Cyclic Alkenes. J. Am. Chem. Soc. 2020, 142, 18330–18335. [Google Scholar] [CrossRef] [PubMed]
- Henke, F.; Schenk, C.; Schnepf, A. [Si(SiMe3)3]3Ge9M(CO)3(-) (M=Cr, Mo, W): Coordination chemistry with metalloid clusters. Dalton Trans. 2011, 40, 6704–6710. [Google Scholar] [CrossRef]
- Willeit, N.S.; Klein, W.; Coburger, P.; Fritz-Langhals, E.; Fässler, T.F. Functionalised NiGe9 Clusters as Homogeneous Single-Site Catalysts for Olefin Isomerisation Reactions. ChemCatChem 2024, 16, e202301200. [Google Scholar] [CrossRef]
- Henke, F.; Schenk, C.; Schnepf, A. [Si(SiMe3)3]6Ge18M (M = Zn, Cd, Hg): Neutral metalloid cluster compounds of germanium as highly soluble building blocks for supramolecular chemistry. Dalton Trans. 2009, 42, 9141–9145. [Google Scholar] [CrossRef]
- Kysliak, O.; Schrenk, C.; Schnepf, A. Reactivity of [Ge9{Si(SiMe3)3}3](-) Towards Transition-Metal M(2+) Cations: Coordination and Redox Chemistry. Chem. Eur. J. 2016, 22, 18787–18793. [Google Scholar] [CrossRef]
- Li, F.; Sevov, S.C. Coordination of Tri-Substituted Nona-Germanium Clusters to Cu(I) and Pd(0). Inorg. Chem. 2015, 54, 8121–8125. [Google Scholar] [CrossRef]
- Schenk, C.; Henke, F.; Santiso-Quiñones, G.; Krossing, I.; Schnepf, A. [Si(SiMe3)3]6Ge18M (M = Cu, Ag, Au): Metalloid cluster compounds as unusual building blocks for a supramolecular chemistry The coordination chemistry of group 15 element ligand complexes-a developing area. Dalton Trans. 2008, 33, 4436–4441. [Google Scholar] [CrossRef]
- Kysliak, O.; Nguyen, D.D.; Clayborne, A.Z.; Schnepf, A. [PtZn2Ge18(Hyp)8] (Hyp = Si(SiMe3)3): A Neutral Polynuclear Chain Compound with Ge9(Hyp)3 Units. Inorg. Chem. 2018, 57, 12603–12609. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Klein, W.; Jantke, L.A.; Schiegerl, L.J.; Fässler, T.F. Reaction of SiCl2·dipp with K[Ge9{Si(SiMe3)3}3]– Synthesis and Characterization of [K(dipp)2][Ge9{Si(SiMe3)3}3]·tol and [dipp-H][Ge9{Si(SiMe3)3}3]·2acn [dipp = 1,3-Bis(2,6-Diisopropylphenyl)imidazol-2-ylidene]. Z. Anorg. Allge. Chem. 2016, 642, 1314–1319. [Google Scholar] [CrossRef]
- Frischhut, S.; Kaiser, F.; Klein, W.; Drees, M.; Kühn, F.E.; Fässler, T.F. Capping nido-Nonagermanide Clusters with M-PPh3 and Dynamics in Solution: Synthesis and Structure of closo-[(Me3Si)3Si]3Et[Ge9M](PPh3) (M = Ni, Pt). Organometallics 2018, 37, 4560–4567. [Google Scholar] [CrossRef]
- Li, F.; Muñoz-Castro, A.; Sevov, S.C. [(Me3Si)Si]3EtGe9Pd(PPh3), a Pentafunctionalized Deltahedral Zintl Cluster: Synthesis, Structure, and Solution Dynamics. Angew. Chem. Int. Ed. 2016, 55, 8630–8633. [Google Scholar] [CrossRef]
- Tatebe, C.J.; Tong, Z.; Kiernicki, J.J.; Coughlin, E.J.; Zeller, M.; Bart, S.C. Activation of Triphenylphosphine Oxide Mediated by Trivalent Organouranium Species. Organometallics 2018, 37, 934–940. [Google Scholar] [CrossRef]
- Braun, P.A.; Westermair, F.F.; Gschwind, R.M.; Korber, N. [(K,Rb)@([2.2.2]crypt)]2(K,Rb)4[Si9W(CO)4] ⋅ 13.4 NH3—The First Tungsten Functionalized Silicon Zintl Cluster. Z. Anorg. Allg. Chem. 2023, 649, e202300117. [Google Scholar] [CrossRef]
- Boyd, P.D.W.; Hart, M.C.; Pritzwald-Stegmann, J.R.F.; Roper, W.R.; Wright, L.J. Selective Substitution of One of the Substituents on Germanium in Coordinatively Unsaturated Ruthenium Germyl Complexes. Organometallics 2012, 31, 2914–2921. [Google Scholar] [CrossRef]
- Khan, M.M.; Alam, M.; Ghosh, S.; Rahaman, A.; Tocher, D.A.; Richmond, M.G.; Kabir, S.E.; Roesky, H.W. Reactions of Ru3(CO)10(μ-dppm) with Ph3GeH: Ge–H and Ge–C bond cleavage in Ph3GeH at triruthenium clusters. J. Organomet. Chem. 2017, 843, 75–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Xu, S.; Zhou, X. Unexpected Formation of the Ruthenium Carbonyl Cluster with a Trigonal-Bipyramidal Ge2Ru3 Core Accompanied by Loss of Germanium Methyl Groups. Organometallics 2001, 20, 3829–3832. [Google Scholar] [CrossRef]
- Wade, K. The Structural Significane of the Number of Skeletal Bonding Elektron-Pairs in Carboranes, the Higher Boranes and Borane Anions, and Various Transition-Metal Carbonyl Cluster Compounds. J. Chem. Soc. 1971, 15, 792–793. [Google Scholar] [CrossRef]
- Michael, D.; Mingos, P. Polyhedral Skeletal Electron Pair Approach. Acc. Chem. Res. 1984, 17, 311–319. [Google Scholar]
- Gutkin, V.; Gun, J.; Prikhodchenko, P.V.; Lev, O.; Gonsalvi, L.; Peruzzini, M.; Romerosa, A.; Malpartida, T.C.; Lidrissi, C. Electro-oxidation of Ruthenium Cyclopentadienyl PTA Complexes in DMF: ESI-MS, Cyclic Voltammetry, and Online Electrochemistry/ESI-MS Studies. J. Electrochem. Soc. 2007, 154, F7–F15. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- MestreNova; Version 14.2.2; Mestrelab Research; Bruker: Ettlingen, Germany, 2021.
- X-Area; Version 1.76.8.1; Stoe & Cie GmbH: Darmstadt, Germany, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B.J. ShelXle: A Qt Graphical User Interface for SHELXL. Appl. Crystallogr. 2011, 44, 1281. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond—Crystal and Molecular Structure Visualization; Diamond Version 3.2k; Crystal Impact GbR: Bonn, Germany, 2014; Available online: https://www.crystalimpact.de/diamond (accessed on 6 March 2025).
- Muhr, M.; Heiß, P.; Schütz, M.; Bühler, R.; Gemel, C.; Linden, M.H.; Linden, H.B.; Fischer, R.A. Enabling LIFDI-MS measurements of highly air sensitive organometallic compounds: A combined MS/glovebox technique. Dalton Trans. 2021, 50, 9031–9036. [Google Scholar] [CrossRef]

| Compound | Prism Heights/Å | d1/d2 (1) | Torsion Angles/° (1) | Cluster Type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h1 | h2 | h3 | a | b | c | α1 | α2 | α3 | ||
| [η1-Ge9(SitBu2H)3]RuCp(PPh3)2, 2 | 3.700(2) | 2.985(2) | 3.068(1) | 1.08 | 1.35 | 1.30 | 3.93(5) | 29.48(5) | 27.31(5) | C4v |
| [Hyp3Ge9][RuCp(PPh3)2(MeCN)], 3b | 3.2746(4) | 3.2698(4) | 3.6934(4) | 1.13 | 1.13 | 1.08 | 26.78(2) | 26.48(2) | 14.80(2) | D3h |
| [(SitBu2H)3Ge9]− [13] | 3.4166(7) | 3.3979(7) | 3.3663(7) | 1.05 | 1.08 | 1.07 | 22.78(2) | 20.51(2) | 23.93(2) | D3h |
| (η3-Ge9Hyp3)ZnCp* [28] | 3.3509(6) | 3.2478(7) | 3.2994(6) | 1.08 | 1.14 | 1.11 | 23.61(2) | 26.09(2) | 25.01(2) | D3h |
| Ge9Hyp3Et [17] | 3.650(1) | 3.0694(9) | 3.0554(9) | 1.06 | 1.26 | 1.26 | 3.39(3) | 29.88(2) | 32.17(3) | C4v |
| [Ge9Hyp3(COPh)] [15] | 3.664(1) | 3.124(1) | 3.058(1) | 1.05 | 1.24 | 1.28 | 3.02(4) | 28.43(5) | 32,33(4) | C4v |
| [Ge9Hyp3(CH2)3CH=CH2] [14] | 3.6042(5) | 3.1176(5) | 3.1383(5) | 1.02 | 1.24 | 1.22 | 4.98(2) | 28.92(2) | 30.23(2) | C4v |
| [(η1-Ge9Hyp3)Cr(CO)5]− [18] | 3.812(1) | 3.090(1) | 3.014(1) | 1.14 | 1.23 | 1.29 | 5.21(2) | 29.32(2) | 32.08(2) | C4v |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willeit, N.S.; Hlukhyy, V.; Fässler, T.F. Ruthenium Decorated Tris-Silylated Germanium Zintl Clusters Featuring an Unexpected Ligand Arrangement. Molecules 2025, 30, 1247. https://doi.org/10.3390/molecules30061247
Willeit NS, Hlukhyy V, Fässler TF. Ruthenium Decorated Tris-Silylated Germanium Zintl Clusters Featuring an Unexpected Ligand Arrangement. Molecules. 2025; 30(6):1247. https://doi.org/10.3390/molecules30061247
Chicago/Turabian StyleWilleit, Nicole S., Viktor Hlukhyy, and Thomas F. Fässler. 2025. "Ruthenium Decorated Tris-Silylated Germanium Zintl Clusters Featuring an Unexpected Ligand Arrangement" Molecules 30, no. 6: 1247. https://doi.org/10.3390/molecules30061247
APA StyleWilleit, N. S., Hlukhyy, V., & Fässler, T. F. (2025). Ruthenium Decorated Tris-Silylated Germanium Zintl Clusters Featuring an Unexpected Ligand Arrangement. Molecules, 30(6), 1247. https://doi.org/10.3390/molecules30061247






