[3+2] Cycloaddition to a Chiral 5-Methylene-1,3-dioxolan-4-one and Pyrolysis of the Spiro Adducts
Abstract
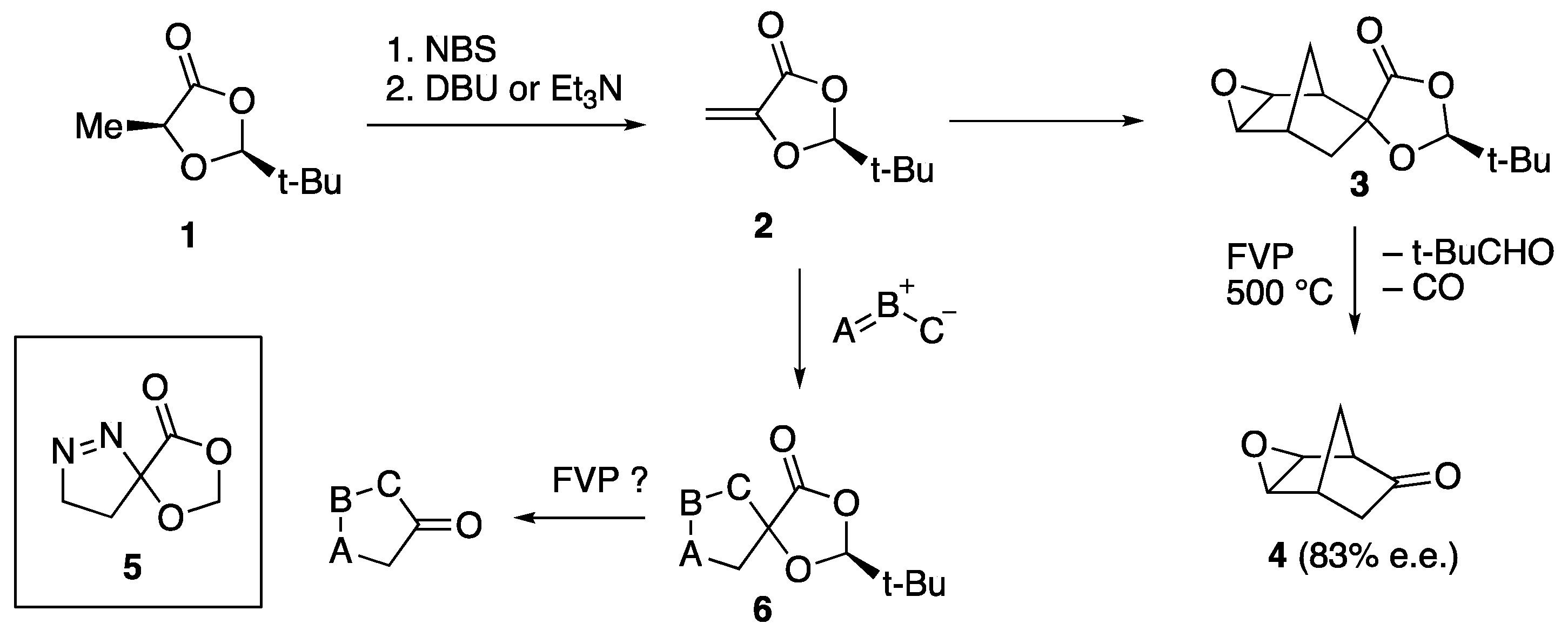
1. Introduction
2. Results and Discussion
2.1. [3+2] Cycloaddition to Methylenedioxolanone 2
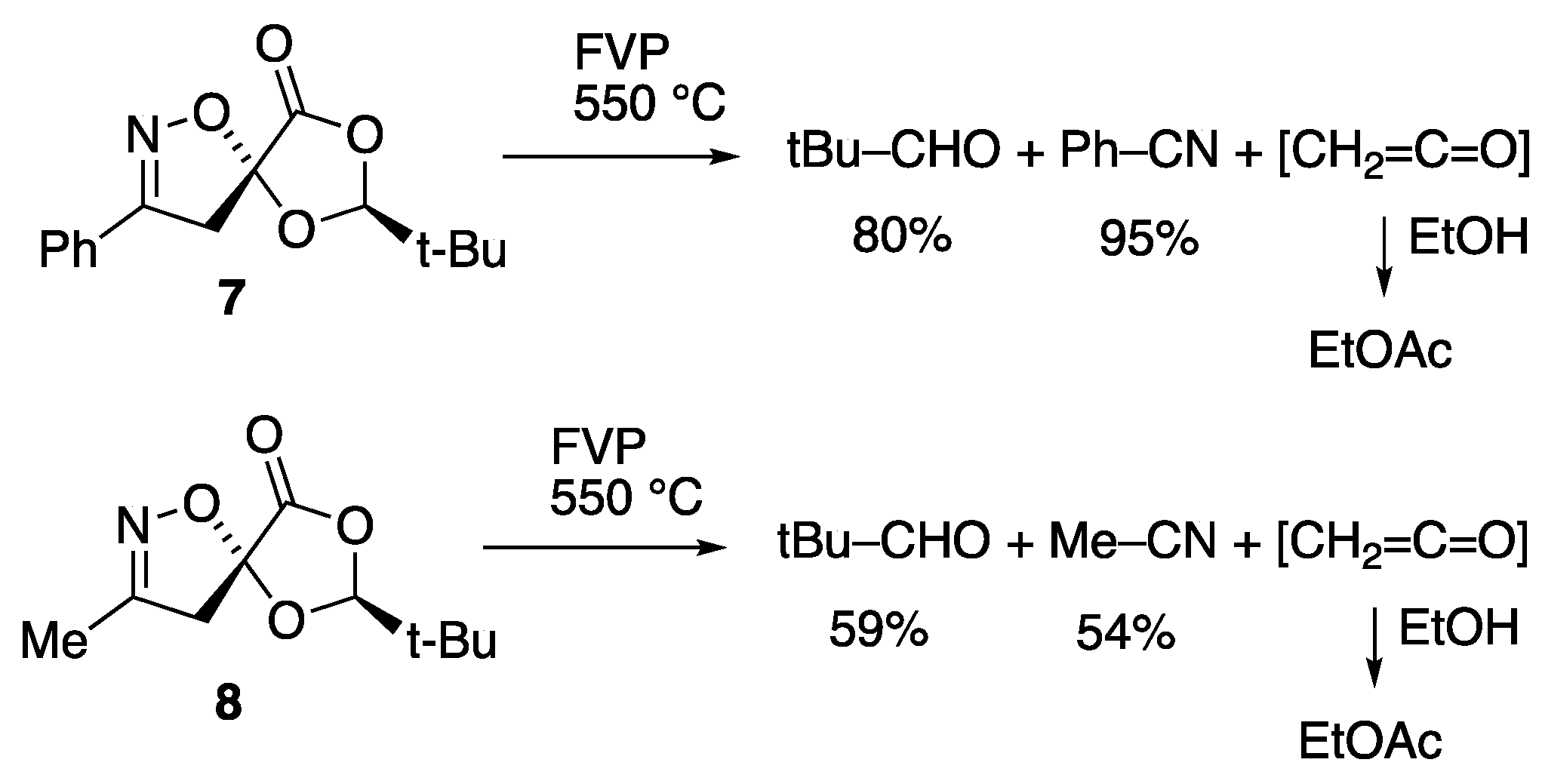
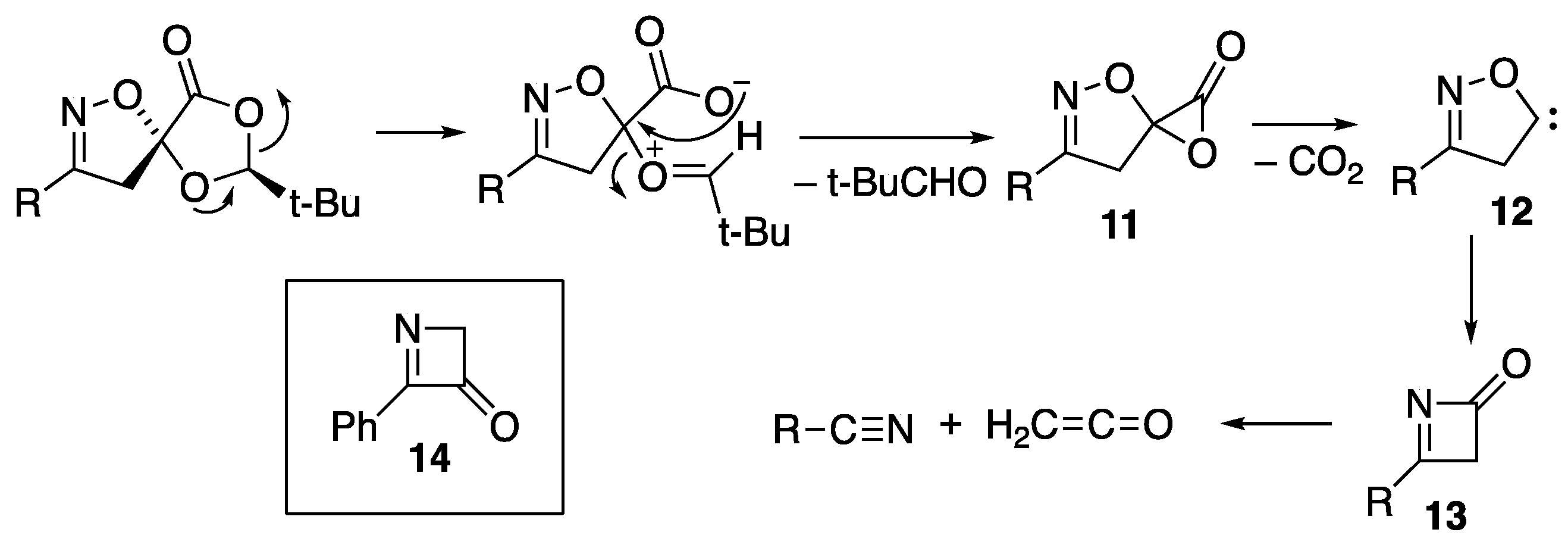
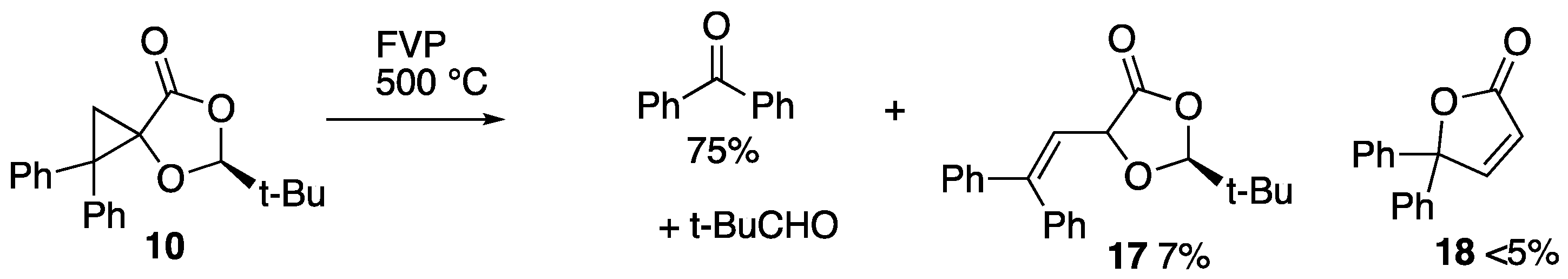
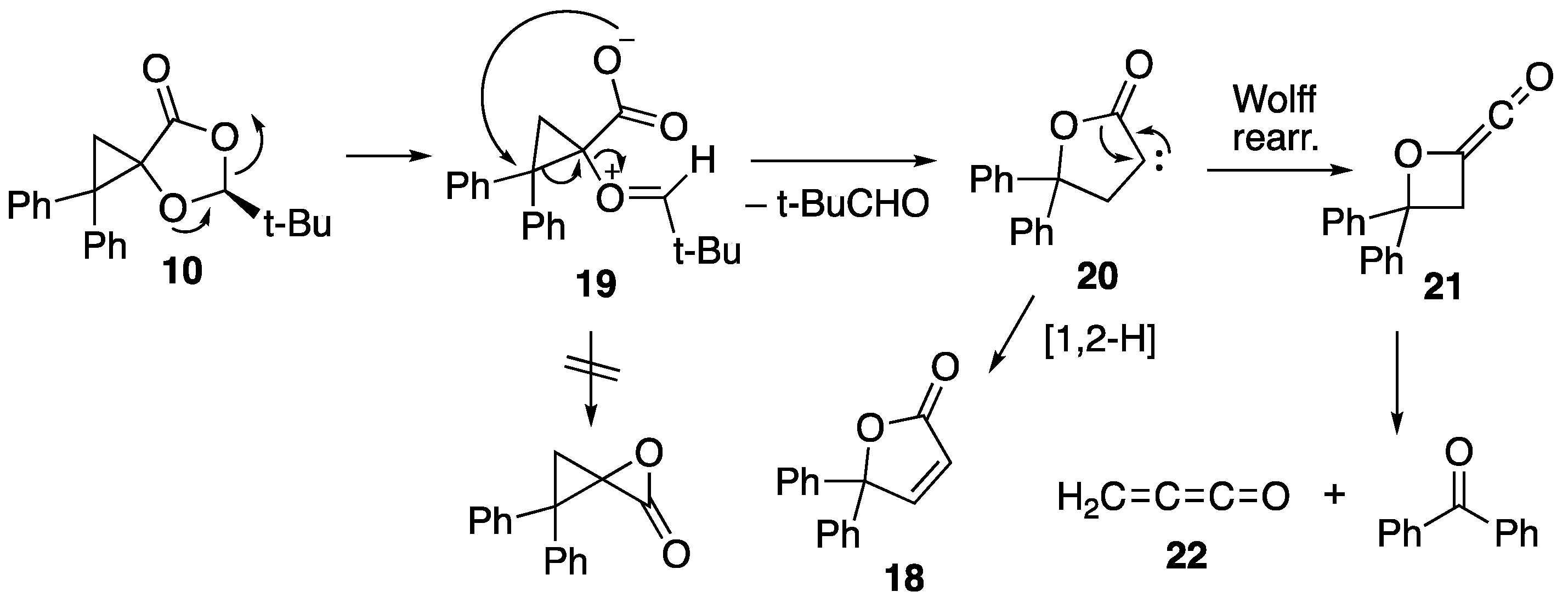
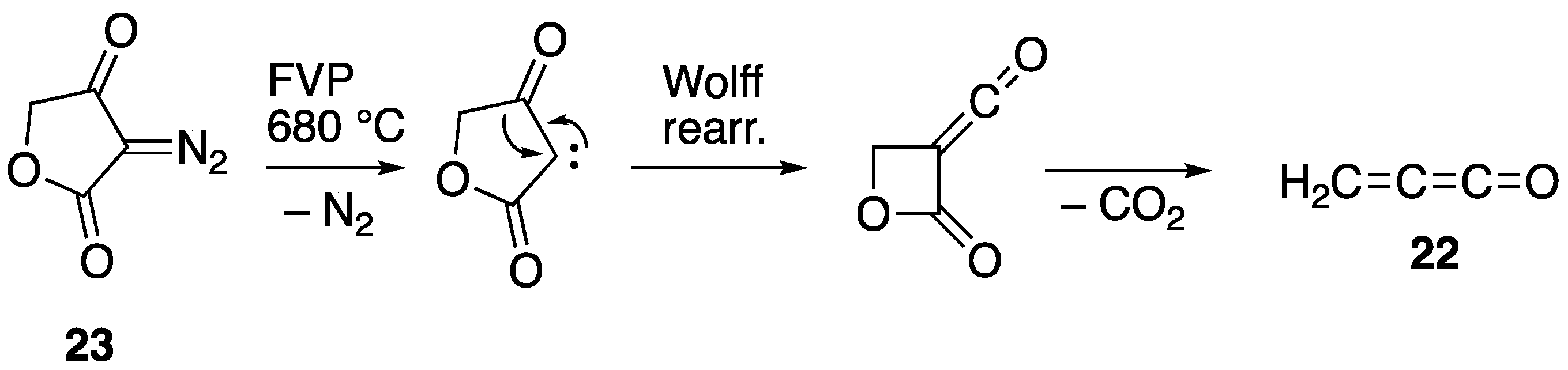
2.2. Flash Vacuum Pyrolysis of the Spiro Cycloadducts
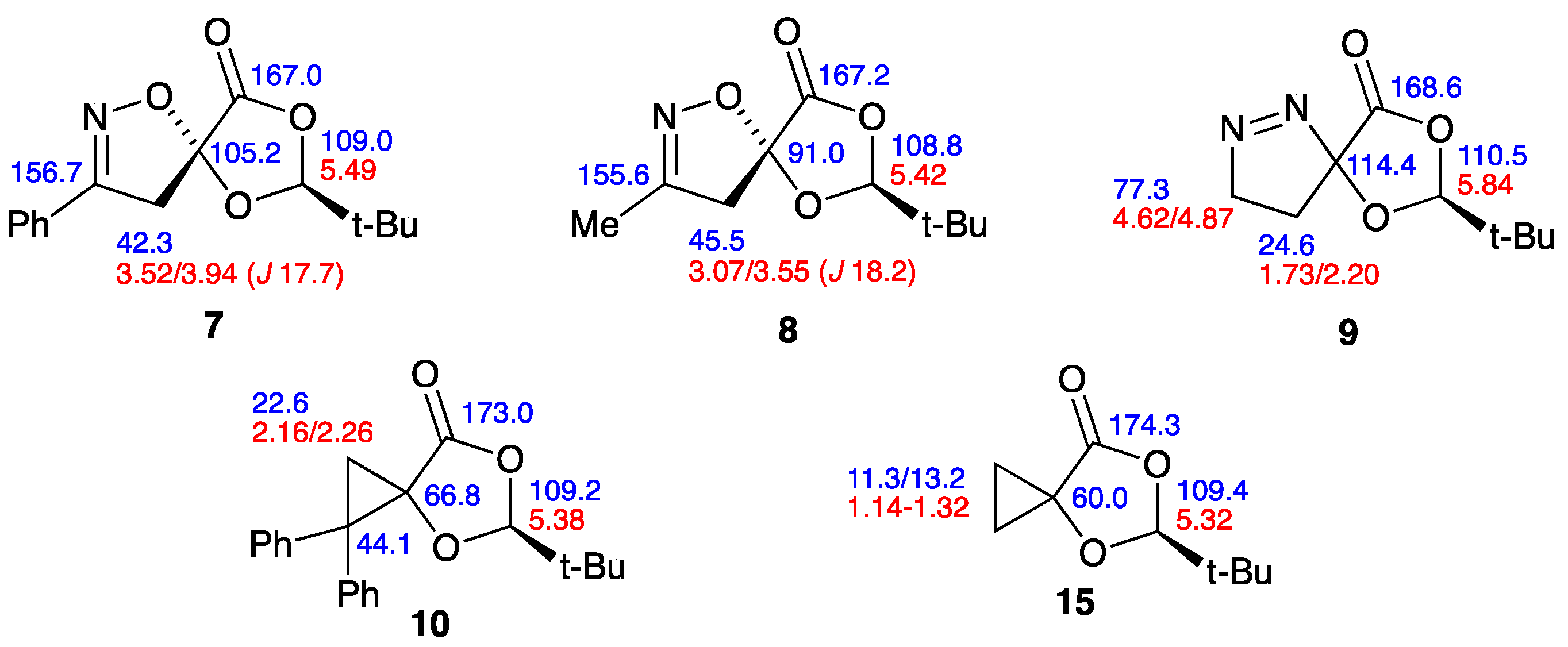
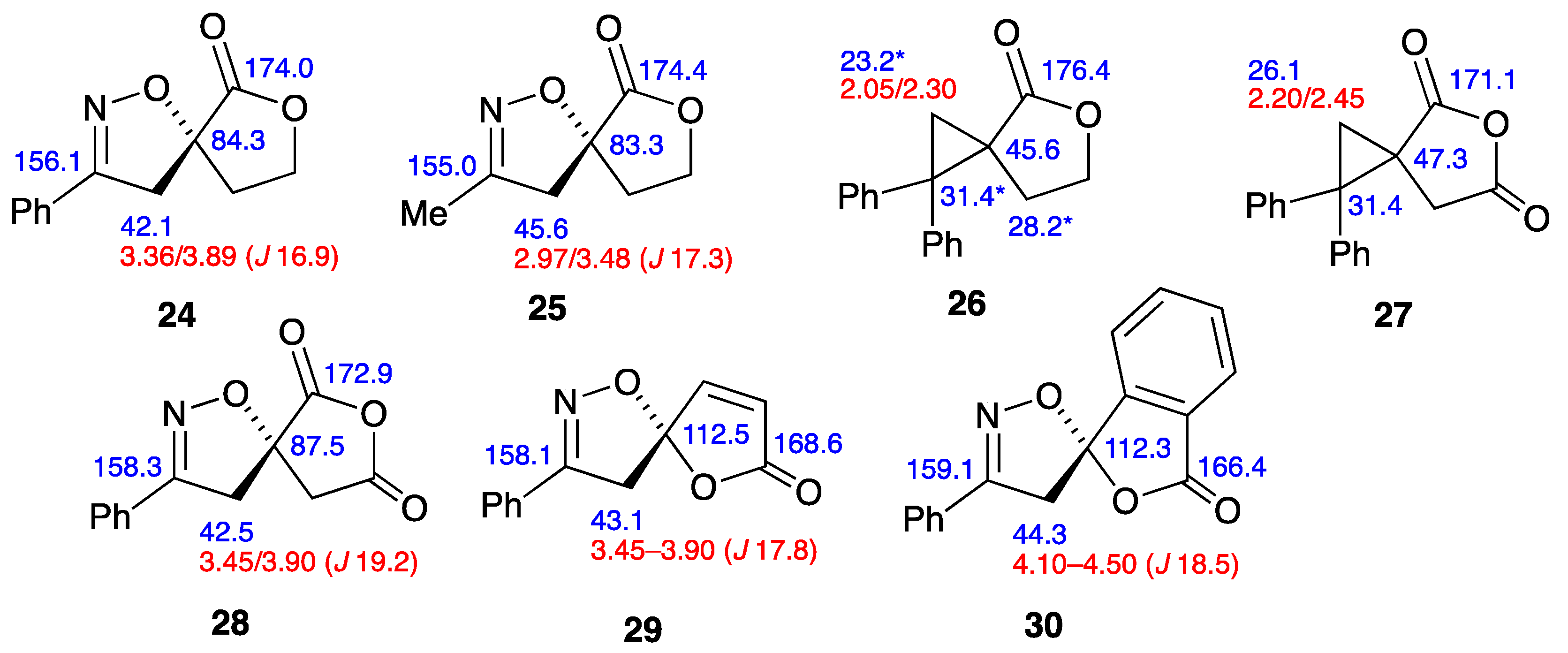
2.3. NMR Data for the Spiro Compounds
3. Experimental Procedure
3.1. General Experimental Details
3.2. Preparation of [3+2] Cycloadducts of (2R)-2-t-Butyl-5-methylene-1,3-dioxolan-4-one 2
3.2.1. Preparation of (2S,5R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,5′-4′,5′-dihydro-3′-phenylisoxazole] 7
3.2.2. Preparation of (2S,5R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,5′-4′,5′-dihydro-3′-methylisoxazole] 8
3.2.3. Preparation of (2S)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,3′-3′,4′-dihydro-5H-pyrazole] 9
3.2.4. Preparation of (2S)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,1′-2′,2′-diphenylcyclopropane] 10
3.3. Pyrolysis of [3+2] Cycloadducts
3.3.1. Pyrolysis of (2S,5R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,5′-4′,5′-dihydro-3′-phenylisoxazole] 7
3.3.2. Pyrolysis of (2S,5R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,5′-4′,5′-dihydro-3′-methylisoxazole] 8
3.3.3. Pyrolysis of (2S)-Spiro [2-t-butyl-1,3-dioxolan-4-one-5,3′-3′,4′-dihydro-5H-pyrazole] 9
3.3.4. Pyrolysis of (2S)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,1′-2′,2′-diphenylcyclopropane] 10
3.4. X-Ray Structure Determination of Adduct 7
(2S,5R)-Spiro[2-t-butyl-1,3-dioxolan-4-one-5,5′-4′,5′-dihydro-3′-phenylisoxazole] 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Torres, R.R. (Ed.) Spiro Compounds: Synthesis and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2022; 444p. [Google Scholar] [CrossRef]
- Ding, A.; Meazza, M.; Guo, H.; Yang, J.W.; Rios, R. New development in the enantioselective synthesis of spiro compounds. Chem. Soc. Rev. 2018, 47, 5946–5996. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef]
- Seebach, D.; Naef, R.; Calderari, G. α-Alkylation of α-heterosubstituted carboxylic acids without racemization: EPC-synthesis of tertiary alcohols and thiols. Tetrahedron 1984, 40, 1313–1324. [Google Scholar] [CrossRef]
- Calderari, G.; Seebach, D. Asymmetrische Michael-Additionen. Stereoselektive Alkylierung chiraler, nicht racemischer Enolate durch Nitroolefine. Herstellung enantiomerenreiner γ-aminobuttersäure- und Bernsteinsäure-Derivate. Helv. Chim. Acta 1985, 68, 1592–1604. [Google Scholar] [CrossRef]
- Aitken, R.A.; Thomas, A.W. Behaviour of dioxolanones as chiral acyl anion equivalents. Synlett 1998, 1998, 102–104. [Google Scholar] [CrossRef]
- Aitken, R.A.; Thomas, A.W. Heterocyclic acyl and formyl anion equivalents. Adv. Heterocycl. Chem. 2001, 79, 89–114. [Google Scholar] [CrossRef]
- Zimmermann, J.; Seebach, D. Brominations of cyclic acetals from α-amino acids and α- or β-hydroxy acids with N-bromosuccinimide. Helv. Chim. Acta 1987, 70, 1104–1114. [Google Scholar] [CrossRef]
- Mattay, J.; Mertes, J.; Maas, G. Asymmetric induction in the Diels-Alder reaction of chiral (2S)-2-(tert-butyl)-5-methylene-1,3-dioxolan-4-one. Chem. Ber. 1989, 122, 327–330. [Google Scholar] [CrossRef]
- Mattay, J.; Kneer, G.; Mertes, J. Asymmetric induction in inverse Diels-Alder reactions of the chiral c,d-olefin (2S)-2-(tert-butyl)-5-methylene-1,3-dioxolan-4-one. Synlett 1990, 1990, 145–147. [Google Scholar] [CrossRef]
- Roush, W.R.; Essenfeld, A.P.; Warmus, J.S.; Brown, B.B. Synthesis and Diels-Alder reactions of 2-alkyl-5-methylene-1,3-dioxolan-4-ones and 2-alkyl-3-acyl-5-methylene-1,3-oxazolidin-4-ones: Highly exo and diastereoface selective chiral ketene equivalents. Tetrahedron Lett. 1989, 30, 7305–7308. [Google Scholar] [CrossRef]
- Roush, W.R.; Brown, B.B. Enantioselective synthesis of 2-alkyl-5-methylene-1,3-dioxolan-4-ones and exo-selective Diels-Alder reactions with cyclopentadiene. J. Org. Chem. 1992, 57, 3380–3387. [Google Scholar] [CrossRef]
- Aitken, R.A.; Power, L.A.; Slawin, A.M.Z. New chemistry of chiral 1,3-dioxolan-4-ones. Molecules 2023, 28, 3845. [Google Scholar] [CrossRef]
- Likhterov, V.R.; Etlis, V.S. Dipolar addition of diazomethane to 5-methylene-1,3-dioxolan-4-one. Chem. Heterocycl. Compd. 1985, 21, 982–984. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.-U. The Huisgen reaction: Milestones of the 1,3-dipolar cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Bast, K.; Christl, M.; Huisgen, R.; Mack, W. 1,3-Dipolare Cycloadditionen, 68. Additionen der Nitriloxide an CN-Mehrfachbindungen. Chem. Ber. 1972, 105, 2825–2840. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Clapp, L.B. The mass spectra of 1,2,4-oxadiazolines. J. Heterocycl. Chem. 1968, 5, 61–68. [Google Scholar] [CrossRef]
- Paton, R.M. The chemistry of nitrile sulphides. Chem. Soc. Rev. 1989, 18, 33–52. [Google Scholar] [CrossRef]
- Huisgen, R.; Grashey, R.; Knupfer, H.; Kunz, R.; Seidel, M. 1,3-Dipolare Cycloadditionen, VI. Anlagerung der Nitrilimine an Azomethine und Isocyanate. Chem. Ber. 1964, 97, 1085–1095. [Google Scholar] [CrossRef]
- Mattay, J. Selectivity and charge transfer in photoreactions of arenes with olefins, 1. substitution versus cycloaddition. Tetrahedron 1985, 41, 2393–2404. [Google Scholar] [CrossRef]
- Canonne, P.; Akssira, M.; Fytas, G. Etude de l’action des alkylmagnesiens primaires et arylmagnesiens sur les anhydrides tricycliques pontes. Tetrahedron 1984, 40, 1809–1815. [Google Scholar] [CrossRef]
- Brown, R.F.C.; Eastwood, F.W.; McMullen, G.L. Evidence for the pyrolytic generation of methylene ketene (propadienone). J. Am. Chem. Soc. 1976, 98, 7421–7422. [Google Scholar] [CrossRef]
- Chapman, O.L.; Miller, M.D.; Pitzenberger, S.M. Infrared spectroscopy of matrix-isolated propadienone. J. Am. Chem. Soc. 1987, 109, 6867–6868. [Google Scholar] [CrossRef]
- Stverkova, S.; Zak, Z.; Jonas, J. 1,3-Dipolar cycloaddition of diphenylnitrilimine to substituted dihydro-3-methylene-2(3H)-furanones. Liebigs Ann. Chem. 1995, 1995, 477–480. [Google Scholar] [CrossRef]
- Tran, N.C.; Dhondt, H.; Flipo, M.; Deprez, B.; Willand, N. Synthesis of functionalized 2-isoxazolines as three-dimensional fragments for fragment-based drug discovery. Tetrahedron Lett. 2015, 56, 4119–4123. [Google Scholar] [CrossRef]
- Roussel, C.; Ciamala, K.; Vebrel, J.; Knorr, M.; Kubicki, M.M. Reaction of 3-methylenedihydro-(3H)-furan-2-one with diazoalkanes. Synthesis and crystal structures of spiranic cyclopropyl compounds. Heterocycles 2007, 71, 1517–1528. [Google Scholar] [CrossRef]
- Roussel, C.; Ciamala, K.; Melot, J.M.; Vebrel, J.; Riche, C. The [3+2] cycloaddition of diphenyldiazomethane and ethyl diazoacetate with itaconic anhydride. A new access to spiranic cyclopropane derivatives. J. Chem. Res. (S) 2002, 2002, 449–451. [Google Scholar] [CrossRef]
- Roussel, C.; Ciamala, K.; Vebrel, J.; Riche, C. Reactivity of arylnitrile oxides and C-aroyl-N-phenylnitrones with 3-methylenedihydro-(3H)-furan-2-one and itaconic anhydride. Heterocycles 2009, 78, 1977–1991. [Google Scholar] [CrossRef]
- Roussel, C.; Fihi, R.; Ciamala, K.; Audebert, P.; Vebrel, J. Revisiting the addition of nitriloxides on protoanemonin. A new access to (2E)-3-(3′-arylisoxazol-5′-yl)propenoic acids. New J. Chem. 2000, 24, 471–476. [Google Scholar] [CrossRef]
- Roussel, C.; Ciamala, K.; Audebert, P.; Vebrel, J. Cycloaddition of some arylnitriloxides with 3-methylenephthalide: Electrocatalytic opening of the adducts. New J. Chem. 1999, 23, 989–992. [Google Scholar] [CrossRef]
- Werner, A.; Buss, H. Ueber Benzhyroximsäurechlorid. Ber. Dtsch. Chem. Ges. 1894, 27, 2193–2201. [Google Scholar] [CrossRef]
- Vogel, A.I. Textbook of Practical Organic Chemistry, 5th ed.; Longman: Harlow, UK, 1989; p. 432. [Google Scholar]
- Miller, J.B. Preparation of crystalline diphenyldiazomethane. J. Org. Chem. 1959, 24, 560–561. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]

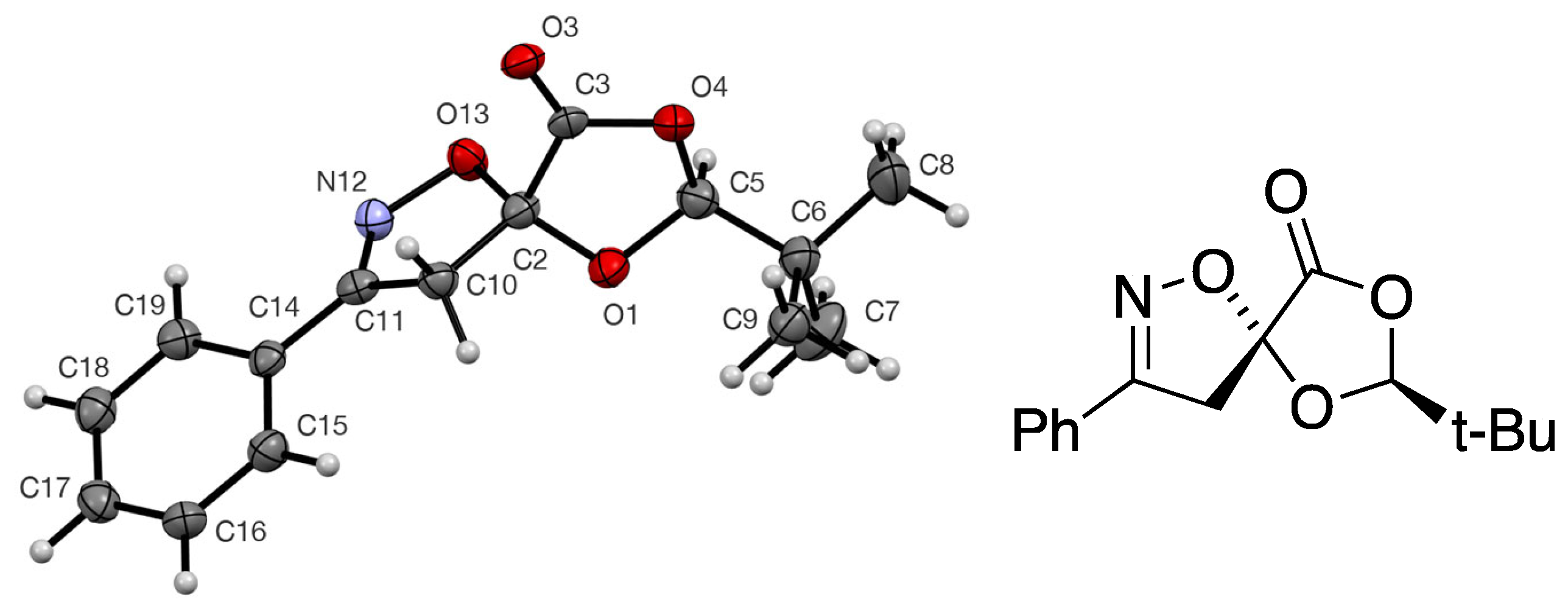

| Bond Lengths | Angles | ||||||
|---|---|---|---|---|---|---|---|
| C(2)–C(10) | 1.480(3) | C(2)–C(3) | 1.502(3) | O(13)–C(2)–C(10) | 104.98(16) | C(3)–C(2)–O(1) | 103.57(16) |
| C(10)–C(11) | 1.487(3) | C(3)–O(3) | 1.187(2) | C(2)–C(10)–C(11) | 101.08(17) | C(2)–O(1)–C(5) | 107.75(16) |
| C(11)–N(12) | 1.264(3) | C(3)–O(4) | 1.327(3) | C(10)–C(11)–N(12) | 113.4(2) | O(1)–C(5)–O(4) | 104.86(17) |
| N(12)–O(13) | 1.421(2) | O(4)–C(5) | 1.435(3) | C(11)–N(12)–O(13) | 108.29(16) | C(5)–O(4)–C(3) | 108.44(17) |
| O(13)–C(2) | 1.420(3) | C(5)–O(1) | 1.405(3) | N(12)–O(13)–C(2) | 108.62(15) | O(4)–C(3)–C(2) | 108.02(19) |
| O(1)–C(2) | 1.383(3) | O(13)–C(2)–C(3) | 106.59(17) | O(1)–C(2)–C(10) | 112.03(18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Power, L.A.; Slawin, A.M.Z. [3+2] Cycloaddition to a Chiral 5-Methylene-1,3-dioxolan-4-one and Pyrolysis of the Spiro Adducts. Molecules 2025, 30, 1246. https://doi.org/10.3390/molecules30061246
Aitken RA, Power LA, Slawin AMZ. [3+2] Cycloaddition to a Chiral 5-Methylene-1,3-dioxolan-4-one and Pyrolysis of the Spiro Adducts. Molecules. 2025; 30(6):1246. https://doi.org/10.3390/molecules30061246
Chicago/Turabian StyleAitken, R. Alan, Lynn A. Power, and Alexandra M. Z. Slawin. 2025. "[3+2] Cycloaddition to a Chiral 5-Methylene-1,3-dioxolan-4-one and Pyrolysis of the Spiro Adducts" Molecules 30, no. 6: 1246. https://doi.org/10.3390/molecules30061246
APA StyleAitken, R. A., Power, L. A., & Slawin, A. M. Z. (2025). [3+2] Cycloaddition to a Chiral 5-Methylene-1,3-dioxolan-4-one and Pyrolysis of the Spiro Adducts. Molecules, 30(6), 1246. https://doi.org/10.3390/molecules30061246








