Abstract
The rational design of ordered chromogenic supramolecular polymeric systems is critical for the advancement of next-generation stimuli-responsive, optical, and semiconducting materials. Previously, we reported the design of a stimuli-responsive, lamellar self-assembled platform composed of an imidazole-appended perylene diimide of varying methylene spacer length (n = 3, 4, and 6) and a commercially available diacid-functionalized diacetylene monomer, 10, 12 docosadiynedioic acid, in a 1:1 molar ratio. Herein, we expound on the importance of the composition of the imidazole-appended perylene diimide of varying methylene spacer length (n = 3, 4, and 6) and 10, 12 docosadiynedioic acid in the ratio of 2:1 to the supramolecular self-assembly, final morphology, and properties. Topochemical polymerization of the drop-cast films by UV radiation yielded blue-phase polydiacetylene formation, and subsequent thermal treatment of the films produced a thermoresponsive blue-to-red phase transformation. Differential scanning calorimetry (DSC) studies revealed a dual dependence of the methylene spacer length and stimuli treatment (UV and/or heat) on the thermal transitions of the films. Furthermore, small-angle X-ray scattering (SAXS) and wide-angle X-ray scattering (WAXS) showed well-defined hierarchical semiconducting nanostructures with interconnected “chessboard”-patterned lamellar stacking. Upon doping with an ionic liquid, the 2:1 platform showed higher ionic conductivity than the previous 1:1 one. The results presented here illustrate the importance of the composition and architecture to the ionic domain connectivity and ionic conductivity, which will have far-reaching implications for the rational design of semiconducting polymers for energy applications including fuel cells, batteries, ion-exchange membranes, and mixed ionic conductors.
1. Introduction
Polydiacetylenes (PDAs) have emerged as a useful class of stimuli-responsive polymeric materials [1,2,3,4]. They have garnered much interest for their potential in a wide range of colorimetric applications based on their known chromogenic transitions upon polymerization and stimuli-induced “blue→red phase” transition [5,6,7,8]. The pioneering work on PDAs began with Wegner in the 1960s with his investigations into the 1, 4 solid-state topochemical polymerization of hexadiyne-diol [9,10,11]. Since then, PDA research has grown substantially, establishing itself in such potential applications as biomedical devices [12], damage-sensing materials [13], optoelectronic devices [14], and chemosensors [15,16]. There are strict geometrical requirements for neighboring DA monomers to polymerize [17]. To meet DA monomer topochemical prerequisites, a variety of functionalities are attached to the monomer, including, but not limited to, hydrogen-bonding moieties [18,19,20], π-π stacking groups [21,22,23], and metal-coordinated motifs [24,25,26,27,28,29].
A variety of supramolecular PDAs with an array of architectures have been developed for sensing applications [30,31,32,33]. In many cases, the formation of supramolecular PDA platforms is facilitated by hydrogen-bonding groups, π-π stacking moieties, or a combination of the two [34,35]. Due to the strong affinity for perylene diimides (PDIs) to form into columnar objects by π-π stacking [36,37,38,39] and their known conductive properties [40,41], they have been utilized as ideal candidates for novel, conductive PDA systems. Both covalent and supramolecular PDI-PDA systems have been studied for their potential use in colorimetric and conductive applications [9,42,43,44,45,46]. Recently, our group developed a series of novel PDI-PDA templated polymeric structures capable of reversible multichromic and multiresponsive pathways [47]. The supramolecular templated formation relied on the imidazole–acid interactions of commercially available 10, 12 pentacosadiynoic acid (PCDA) and imidazole-appended PDI templates of various spacer length by hydrogen bonding. UV irradiation of solution-cast films yielded films capable of solvato- and thermochromic reversibility switching from the purple and red phases of PDA. The phase reversibility of these systems may be a result of the hydrogen bonding of the PDI–imidazole and carboxylic acid groups lowering the difference in conformational energy between the purple and red phases. X-ray studies including SAXS and WAXS studies revealed an arrangement of hydrogen-bonded PDI-PCDA cylinders amongst poly(PCDA)-rich domains composed of red and blue phases. These templated supramolecular polymeric PDI-PDA systems may have potential applications in sensors and conductive materials [48].
Due to the semicrystalline properties of PDAs and their derivatives, it is extremely challenging to synthesize architectures that can self-assemble into interconnected, gyroid-like or bicontinuous morphologies [18,49,50,51]. These morphologies would be immensely interesting to address if the transport and diffusion of ions and charged molecules are higher or lower than those of randomly oriented or aligned lamellar or cylindrical structures, which has been a major point of contention in the block copolymer field [51,52,53]. Furthermore, the transport of penetrants through nanoscale-oriented and disordered morphologies has been of interest for electrochemical devices [53], fuel cells [54], separation membranes [55], and batteries [56].
In this work, we designed a general strategy using compositions with different architectures as a tool to synthesize PDA-PDI systems that self-assembled into interconnected morphologies. Building on this framework of supramolecular PDI-PDA systems, this work seeks to investigate a supramolecular PDI-PDA polymer system composed of a central diacid DA monomer, commercially available 10, 12 docosadiynedioic acid (DCDDA), hydrogen-bonded to a series of two imidazole-appended PDI molecules, where the imidazole group is attached to PDI by a variable methylene spacer length, n (n = 3, 4, and 6) (PDI-mono(n-imz)). Upon complexation in solution, supramolecular formation occurs in a 2:1 molar ratio of PDI-mono(n-imz) and DCDDA (2:1 PDI-mono(n-imz)/DCDDA) through intermolecular π-π stacking of neighboring PDI molecules and imidazole–acid hydrogen bonding. This method allows for a modular approach to supramolecular PDI-PDCDDA systems where the properties of the materials can be easily tailored by substitution of the diacid-functionalized DA monomer or imidazole-appended PDI molecule prior to polymerization. Morphological studies before and after UV polymerization of the supramolecular systems displayed hierarchical ordering on multiple length scales and the effects of crystallinity on varying the methylene spacer length. Additionally, thermal studies revealed several trends in relation to varying the methylene spacer lengths of the PDI molecules in the supramolecular systems. Finally, the chromatic transitions were investigated and showed various pathways to achieve blue- and red-phase transitions in the PDA polymer backbone, where these 2:1 systems were compared and contrasted with the corresponding 1:1 architectures and used as model systems to investigate ionic conductivity in these films compared with lamellar-domain-forming PDA-PDI structures.
2. Results and Discussion
The PDI molecules used in this study (PDI-mono(n-imz)) were synthesized according to the previous literature [47,57,58] and were composed of unsymmetrical PDI functionalized with a variable methylene spacer length, n (n = # of methylene units: 3, 4, or 6), attached to a basic imidazole headgroup on one end and a dialkyl-solubilizing moiety on the other end (Figure 1). The diacid DA monomer used in this work, 10, 12 docosadiynedioic acid (DCDDA), is commercially available, and it was purified to remove any polymerized byproducts. The PDI-mono(n-imz) molecule and purified DCDDA monomer were dissolved in a 2:1 molar ratio (2:1 PDI-mono(n-imz)/DCDDA) at 2 wt % in anhydrous THF and stirred overnight in a sealed, dark container at room temperature. The resulting solutions were drop-cast onto dry quartz substrates at room temperature with no ambient light and allowed to dry completely to prepare supramolecular hydrogen-bonded structures. Samples that were examined prior to photopolymerization were analyzed upon drying, and a 254 nm UV source (7.6 mW cm−2, 10 min) was used to polymerize the drop-cast films prior to further analysis. Films that underwent stimuli treatment are denoted by “UV mx Heat mx”, where “UV” = 254 nm exposure for 10 min at room temperature and “Heat” = 150 °C for 1 h in air. “UV mx Heat mx” refers to “m” # of treatments by “UV” and/or “Heat”.
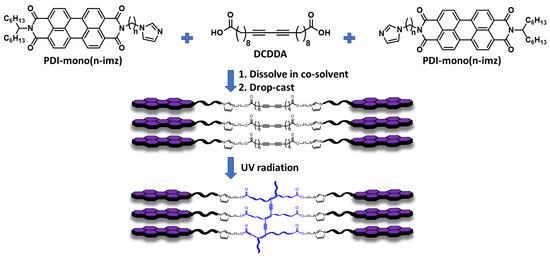
Figure 1.
Schematic illustration of the supramolecular formation of DCDDA and PDI-mono(n-imz) to form 2:1 PDI-mono(n-imz)/DCDDA and their subsequent polymerization to the blue phase by 254 nm UV radiation.
2.1. Supramolecular Complexation
The supramolecular PDI-mono(6-imz)/DCDDA structures formed in solution were analyzed by 1H NMR spectroscopy in tetrahydrofuran-d8 (THF-d8) at room temperature at increasing concentrations of DCDDA (Figure 2). The aromatic proton in the imidazole ring (labeled with *) successively shifts downfield from 7.49 ppm to 7.57 ppm upon increasing equivalents of DCDDA. This is a result of the hydrogen bonding between the carboxylic acid groups of DCDDA and the imidazole head groups of PDI-mono(6-imz). Additionally, the proton shifts associated with the PDI aromatic core broaden, indicating supramolecular formation, in agreement with the previous literature [58]. The titration profile obtained with increasing amounts of DCDDA added shows a critical transition when approximately 2 equiv of PDI-mono(6-imz) is used for 1.25 equiv of the diacid, which deviates from the expected 2:1 supramolecular structure. This deviation may be a result of the limited solubility of PDI-mono(6-imz) in THF-d8 in comparison to DCDDA at 2 wt%. This will lead to a lower concentration of PDI-mono(6-imz):DCDDA in solution, which may be the reason for the greater-than-expected experimental equivalence point. This may result in the drop-cast films not adopting the expected 2:1 supramolecular structure, as not all of the DCDDA carboxylic acid units complex with the PDI-mono(n-imz) molecules. This phenomenon is consistently observed for shorter methylene spacer lengths of n = 3 and 4 (Figures S1 and S2, respectively) as well.
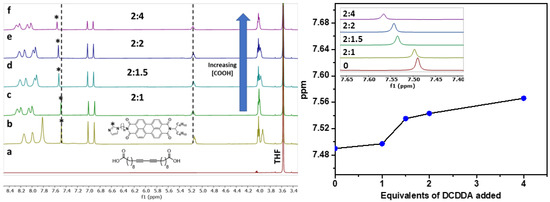
Figure 2.
(Left) 1H NMR spectrum overlay of (a) DCDDA, (b) PDI-mono(6-imz), (c) 2:1 PDI-mono(6-imz)/DCDDA, (d) 2:1.5 PDI-mono(6-imz)/DCDDA, (e) 2:2 PDI-mono(6-imz)/DCDDA, and (f) 2:4 PDI-mono(6-imz)/DCDDA in THF-d8 at 25 °C. The imidazole proton that is denoted by an asterisk (*) shifts from 7.48 ppm to 7.56 ppm, and the tertiary proton chemical shift at 5.18 ppm does not shift upon hydrogen bonding. (Right) Chemical shift change of PDI aromatic imidazole proton (labeled with * in the 1H NMR spectrum overlay) upon the addition of increasing amounts of DCDDA. Points represent experimental data. The inset shows the 1H NMR spectrum overlay of the aromatic imidazole proton peak from 7.64 ppm to 7.40 ppm of PDI-mono(6-imz):DCDDA with increasing amounts of DCDDA.
Upon drop-cast film formation, the supramolecular structures of PDI-mono(6-imz):DCDDA were shown to maintain imidazole–acid complexation in the solid state according to Fourier transform infrared (FTIR) spectroscopy analysis (Figure 3). Similar acid-appended DA monomers organize in the solid state by carboxylic acid dimerization [59]; therefore, the addition of a molecule with a basic binding site, such as imidazole, should disrupt the dimerization phenomenon [60,61]. This is seen when comparing the absorbance from 1725 cm−1 to 1700 cm−1, which represents carboxylic acid dimerization stretching, of pure DCDDA (red line) and 2:1 PDI-mono(6-imz):DCDDA (blue line), where there is clearly a large decrease in the signal intensity. Furthermore, there is a gradual increase in the intensity of this band as the concentration of DCDDA is increased. Additionally, the solid blue vertical line shifting at 1690 cm−1 to 1693 cm−1 (dashed blue vertical line) represents the change in C=O stretching when carboxylic acid dimerization is disturbed. This phenomenon is also consistent with complexes composed of shorter methylene spacer lengths of n = 3 and 4 (Figures S3 and S4, respectively).
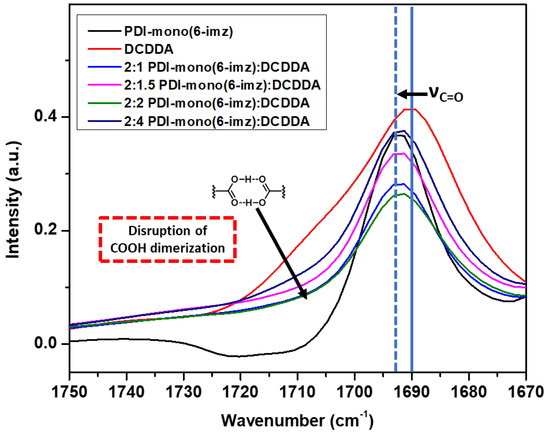
Figure 3.
FTIR–ATR spectrum overlay of PDI-mono(6-imz) (black line), DCDDA (red line), 2:1 PDI-mono(6-imz)/DCDDA (blue line), 2:1.5 PDI-mono(6-imz)/DCDDA (pink line), 2:2 PDI-mono(6-imz)/DCDDA (green line), and 2:4 PDI-mono(6-imz)/DCDDA (purple line) powder at 25 °C. The downward arrow at ~1710 cm−1 shows the decrease in free COOH absorbance after imidazole–acid complexation. The solid–to–dashed blue line shift from 1690 cm−1 to 1693 cm−1 represents the disruption of dimerized COOH moieties after imidazole–acid complexation.
2.2. Chromatic Transitions
The chromatic/stimuli-responsive phase behavior of the 2:1 PDI-mono(3-imz)/DCDDA drop-cast film on soda-lime (microscope) glass is shown in Figure 4. Prior to UV treatment, the UV–Vis spectrum overlay in Figure 4a shows only a contribution of PDI-mono(3-imz). However, after UV exposure, an absorption band at ~625 nm appears, which can be attributed to polymerized DCDDA (labeled PDCDDA) (Figure S5). Interestingly, thermal treatment of the 2:1 PDI-mono(3-imz)/DCDDA drop-cast film at 150 °C for one hour prior to UV exposure did not show a new absorption band indicating red- or blue-phase formation (Figure 4b). Thermal treatment at 150 °C was chosen because it is above the Tm of DCDDA, as determined by differential scanning calorimetry (DSC) (Figure S9). This contrasts with the thermal treatment of the DCDDA powder (red line, Figure S5), where there is an increase in the absorption intensity at ~575 nm, which is typically where the red-phase PDA absorption band appears [62,63,64,65]. This discrepancy may be a result of the PDI-mono(3-imz) molecule absorption band overlapping with this in the UV–Vis spectrum, or a result of the PDI molecule inhibiting or decreasing the formation of a red phase in the PDCDDA backbone. In Figure 4c, it is shown that red-phase formation may occur by heating the blue-phase 2:1 PDI-mono(3-imz)/DCDDA drop-cast film to 150 °C for one hour, indicated by the disappearance of the blue-phase absorption band (blue line) and the appearance of a red-phase absorption band (red line). From this, a phase diagram was constructed to determine routes to achieve various PDCDDA phases in the 2:1 PDI-mono(3-imz)/DCDDA drop-cast films (Figure 4d) based on the UV–Vis absorption data. It can be concluded that PDCDDA blue-phase formation occurs due to UV irradiation and not heat treatment. It can also be inferred that red-phase formation occurs through forming blue-phase PDA first by UV radiation and then by heat treatment. Interestingly, this occurs at longer methylene spacer lengths of n = 4 and 6 (Figures S6 and S7, respectively), which suggests that the effect of the methylene spacer length is minimal when transitioning between the blue and red PDCDDA phases.
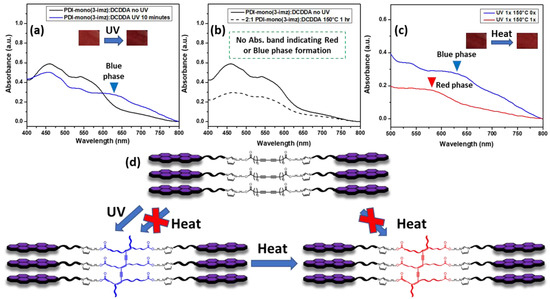
Figure 4.
UV–Vis spectra of 2:1 PDI-mono(3-imz)/DCDDA drop-cast films indicating (a) blue-phase PDA formation after UV irradiation, (b) no PDA formation after thermal treatment at 150 °C for 1 h, (c) blue-to-red-phase PDA transformation upon after thermal treatment at 150 °C for 1 h, and (d) stimuli-specific chromatic transition diagram of 2:1 PDI-mono(3-imz)/DCDDA supramolecular system. The insets in (a,d) show photographs of 2:1 PDI-mono(3-imz)/DCDDA drop-cast films before/after UV and after UV/after UV and heating, respectively.
Thermal analysis of the DCDDA and 2:1 PDI-mono(n-imz)/DCDDA drop-cast films before and after UV irradiation was performed using thermogravimetric analysis (TGA) (Supporting Information, Figure S8, Table S1). The thermal stability of DCDDA was observed to exhibit a distinctive degradation behavior after UV irradiation, possibly resulting in an intermolecular 1,4-addition reaction to yield highly crosslinked networks [66,67]. Comparatively, the 2:1 PDI-mono(4-imz)/PCDA drop-cast films were more thermally stable than the 2:1 PDI-mono(3-imz)/PCDA films (Figure S8). This observation is attributed to the flexible alkyl spacers, which facilitate the PDI π-π interactions, thus forming ordered and stable assemblies.
The thermal transitions of these films before and after exposure to various stimuli were investigated by differential scanning calorimetry (DSC) after being subjected to a series of heating and cooling cycles at a ramp rate of 5 °C min−1 (Supporting Information, Figures S9–S12, Table S2). Similar to our initial report [47], all the drop-cast films exhibited a characteristic sharp melting transition of DCDDA but at higher temperatures in the second heating cycle. Upon cooling, the 2:1 PDI-mono(n-imz)/DCDDA drop-cast sharp crystalline transition temperature values of DCDDA (Tc,DCDDA) were observed. This suggests that at lower temperature, the disordered 2:1 PDI-mono(n-imz)/DCDDA supramolecular molecules lower the decoupling of the DCDDA domains from the arranged PDI templates, which undergo rearrangement to form well-packed crystalline structures [47].
2.3. Morphological Properties
Wide-angle X-ray scattering (WAXS) was used to study the effects of the methylene spacer length, n (where n = 3, 4, or 6), on crystallinity in the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films prior to DCDDA polymerization, after blue-phase formation by UV exposure and subsequent red-phase formation by heat treatment. The WAXS spectrum overlays of the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films after various stimuli treatments (Figures S13–S16) display peaks indicative of DCDDA-rich regions (d1, d4) and intermolecular π-π stacking of DCDDA (d6) and PDI-mono(n-imz) (d7). This, in conjunction with the FTIR data, shows that a combination of hydrogen bonding and π-π stacking aids in DA alignment in the supramolecular structures. The % crystallinity of the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films after various stimuli treatments was measured by comparing the crystalline to amorphous regions (2θ = 4–30°) by WAXS and plotted as a function of the methylene spacer length, n, in the supramolecular structures (Figure 5). The % crystallinity of the sample increases with increasing methylene spacer length, potentially as a result of the enhanced packing of PDI/PDCDDA cylinder domains with greater methylene spacer flexibility (peak d2, Figures S14–S16). Additionally, when n = 4 and 6, the crystallinity of the supramolecular structures is enhanced upon UV treatment, followed by a reduction upon heating. This may be a result of the longer, more flexible methylene spacer lengths attached to PDI-mono(n-imz) allowing for a higher degree of blue-phase formation of the PDCDDA domains as compared to when n = 3. Upon heating and red-phase formation, there is a reduction in the sample crystallinity, which may result from the PDCDDA backbone relaxing by undergoing a rotation about the C-C single bond [56]. This acts to disrupt the extent of crystallinity of the PDCDDA domains. Interestingly, the crystallinity slightly increases after heating when n = 3, which highlights how the increased methylene spacer length in PDI-mono(n-imz) may allow for a greater extent of blue-phase formation due to a higher degree of decoupling of the bulky, aromatic substituent from the PDCDDA backbone.
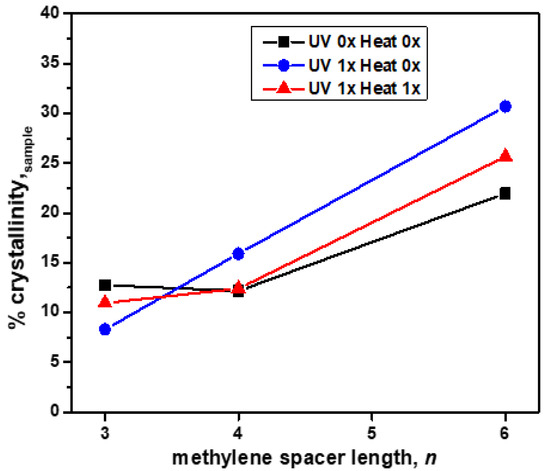
Figure 5.
The % crystallinity of samples of 2:1 PDI-mono(n-imz)/DCDDA drop-cast films calculated from WAXS (% crystallinity,sample) as a function of the methylene spacer length, n (where n = 3, 4, or 6), where black squares, blue circles, and red triangles represent the stimuli treatment provided to the drop-cast films (UV mx Heat mx).
Small-angle X-ray scattering (SAXS) was used to study the various structures within the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films. Figure 6 shows a representative 1D SAXS pattern of 2:1 PDI-mono(4-imz)/DCDDA after UV exposure for 10 min. The curve is best fit by several Gaussian peaks to resolve individual peak positions, qn*, and their values are listed in Table 1. Note that the peak positions cannot be interpreted by a known crystal structure. Herein, we propose stacking “chessboard”-patterned lamellae [as shown in the scheme of Figure 7a] to describe the SAXS pattern presented in Figure 6. The rationale of this proposed model originates from the combined effects of polymerization and π-π stacking. The first peak, q1* = 0.149 Å−1, corresponds to a d-spacing of (=42.1 Å), which is approximately the molecular length of nearly stretched PDI-mono(3-imz)/DCDDA. It should be noted that the π-π stacking at the end of the side chains promotes two potential PDI-mono(3-imz)/DCDDA aggregating configurations before the polymerization takes place, i.e., head-with-head (PDI-mono(3-imz)/DCDDA stacking with each other) and head-with-tail (PDI-mono(3-imz)/DCDDA connected by ends) (Figure 7b). Polymerization could chemically connect the head-with-head aggregates into a near-rectangular lamella (hereafter referred to as a “patch”), with its four corners remaining “active” for the head-with-tail п-п stacking as the “glue” points for other patches. As a result, all the corner-connected patches will form chessboard-like lamellae [Figure 7c]. We define the long axis of PDI-mono(3-imz)/DCDDA and the polymerization direction as the “a” and “b” axes, respectively, which form a 2D lattice crystal on the lamella. It should be noted that the “a” and “b” axes are not necessarily perpendicular to each other. The repeat spacing along the a axis is dictated by the molecular length of PDI-mono(3-imz)/DCDDA; hence, q1* is assigned to the first-order diffraction peak along the a axis, i.e., (1,0).
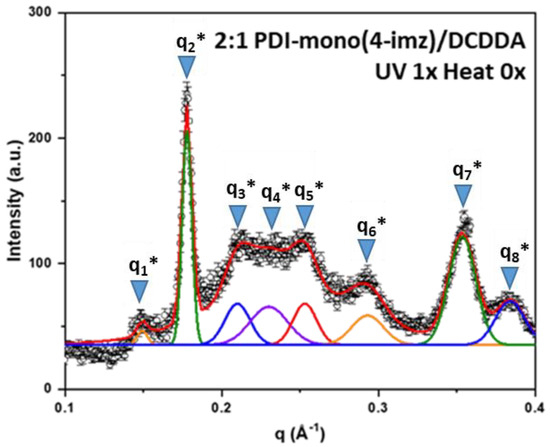
Figure 6.
The 1−D SAXS pattern of 2:1 PDI-mono(4-imz)/DCDDA after UV exposure (“UV” = 254 nm exposure for 10 min at room temperature, and “Heat” = 150 °C for 1 h). SAXS peak assignments (qn*) can be found in Table 1. Peak positions were determined using the fitting function in Origin.

Table 1.
SAXS peaks (Figure 6), qn*, and the corresponding d-spacing of the 2:1 PDI-mono(4-imz)/PDCDDA drop-cast films.
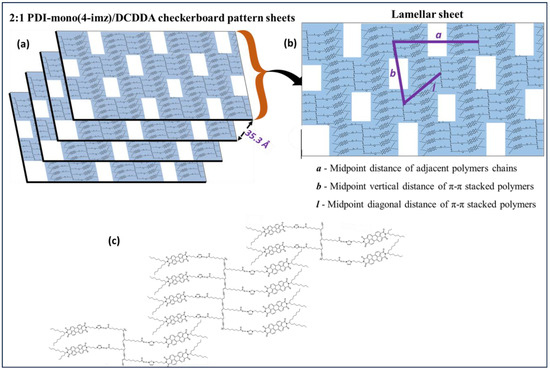
Figure 7.
Schematic illustration of (a) self-assembled 2:1 PDI-mono(4-imz)/PDCDDA into “chessboard” pattern sheets after UV exposure (“UV” = 254 nm exposure for 10 min at room temperature, and “Heat” = 150 °C for 1 h.). (b) Single lamellar sheet, and (c) head-with-head and head-with-tail configurations of the polymer.
The second peak, q2* (=0.178 Å−1), and the seventh peak, q7* (=0.353 Å−1), are much sharper than the others, suggestive of different origins. The fact that q7* is around 2q2* indicates that q7* is the second-order harmonic of q2*. The well-defined corresponding d-spacing of 35.3 Å (=) most likely originates from the interlamellar distance.
The third diffraction peak, q3*, at 0.210 Å−1 is assigned to the first-order Bragg peak along the b axis, i.e., (0,1), corresponding to a repeat spacing of (=29.9 Å) [Figure 7c]. The uniformity of the d-spacing along the b axis was not anticipated and is well understood since it is presumably controlled by entropy. Because of the “chessboard” configuration, we expect that there is a regular spacing along the diagonal direction [l in Figure 7c] between the a and b axes, which is (~25.8 Å) under the approximation of nearly orthogonal a and b axes, leading to a Bragg reflection at 0.243 Å−1, close to peak q4* (~0.240 Å−1), which is evidence of the “chessboard” configuration. The fifth peak, q5* (~0.253 Å−1) (if the a and b axes are nearly orthogonal), can be interpreted as the Bragg reflection from the (1,1) plane. The sixth peak, q6*~2q1*, is indicative of the second-order harmonic along the a axis, consistent with the WAXS pattern, where a higher-order (the fourth) Bragg reflection is found at q = 0.606 Å−1 for the DCDDA sample (Figure S13). Finally, the reflection of q8* is associated with PDI-mono(4-imz) [50], which is observed in PDI-rich regions within the supramolecular structures. The fact that the SAXS/WAXS data show a similar pattern to that from a reported intercalated layered structure [68] further validates the structural interpretation. Scanning electron micrographs of the samples provided inconclusive results and are not presented here.
Comparing the SAXS data (qn represents “UV 0x Heat 0x”, qn* represents “UV 1x Heat 0x”, and qn** represents “UV 1x Heat 1x” (Å−1)) and the corresponding d-values (dn, dn*, dn** (Å)) of the DCDDA powder before and after UV exposure (Figure S22, Table S10) to the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films (Figures S19–S21, Tables S7–S9) shows that the characteristic peak in DCDDA (before and after UV exposure) is present in the supramolecular structures that have spacer lengths of n = 4 and 6, both before and after UV exposure. This characteristic peak was only observed in the PDI-mono(n-imz)/DCDDA polymer with n = 3, after the film was exposed to UV, which enhanced the large-scale regularity of the supramolecular structure. This indicates that there are DCDDA-rich regions in the supramolecular structures. This may be a result of incomplete complexation of DCDDA acid end groups to PDI–imidazole moieties. This phenomenon is in agreement with the 1H NMR titration experiment (Figure 2), where the equivalent point was found to be 1.25 moles of DCDDA: 2 moles of PDI-mono(6-imz), in comparison to the theoretical 1:2 DCDDA:PDI-mono(6-imz) ratio. Since the 2:1 PDI-mono(n-imz):DCDDA drop-cast films were prepared at the same concentration as in the 1H NMR experiment (2 wt%), it is expected that the films will have a similar ratio, which may lead to DCDDA-rich regions because of incomplete acid complexation in solution.
Additionally, exposure of all spacer lengths to UV enhances the large-scale space regularity, where the length of the spacer is observed to play a very important role, resulting in the appearance of the q4* peak after UV exposure (Figures S19–S21). Subsequent thermal exposure of the films at 150 °C for one hour leads to peak broadening (q2** and q7** in all spacer lengths) and the disruption of the 4. q4* q4* distance between two adjacent stacked PDI/PDCDDA polymers (distance L), leading to the disappearance of q4* in all spacer lengths. This may be a result of thermal exposure disrupting the order and/or the thermochromic blue→red phase transition changing the domain sizes within the supramolecular structures.
2.4. Ionic Conductivity of the 2:1 PDI-Mono(4-imz)/PDCDDA and 1:1 Drop Cast Films
The ionic conductivity of the 2:1 PDI-mono(4-imz)/PDCDDA polymer was assessed using an Ossila Four-Point Probe System, and the values were compared with those from 1:1 PDI-mono(4-imz)/PDCDDA. Both polymers were doped with 0.04 mL of 1-Butyl-3-methylimidazolium acetate to enhance their ionic conductivity. The results show that the 2:1 PDI-mono(4-imz)/PDCDDA polymer chessboard pattern (Figure 7) exhibited a two-fold enhancement in ionic conductivity compared to the 1:1 PDI-mono(4-imz)/PDCDDA lamellar structures formed upon evaporation-induced self-assembly (Table 2). These well-defined chessboard patterns facilitate the close π-π bond stacking interactions, well suited for transferring electrons from molecule to molecule, and therefore play a key role in organic conductors [69]. Note that the PDI films were spiked with 1-butyl-3-methylimidazolium acetate, an ionic liquid with an ionic conductivity value of 1.44 mScm−1 [70], to enhance the conductivity of the films. These data values agree with the SAXS data, where the diffraction pattern attributed to the π-π stacking is sharp and well defined. When the samples were subjected to UV exposure, higher conductivity values were observed in both cases, thus indicating an improvement in the well-defined chessboard patterns (Tables S11 and S12).

Table 2.
Ionic conductivity values of 1:1 PDI-mono(4-imz)/PCDDA and 2:1 PDI-mono(4-imz)/PDCDDA before UV exposure in the presence of ionic liquid.
Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were used to analyze the thermal properties of the materials. After DCDDA complexation with PDI-mono(n-imz), the decomposition temperatures (Td) decreased as compared to pure DCDDA, as analyzed by TGA (Figure S8). DSC analysis revealed varying effects of the methylene spacer length, n, and stimuli treatment method on the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films and DCDDA powder in the second heating cycle (Figures S9–S12). The melting transition temperatures (Tm,DCDDA), enthalpies of melting transition (∆Hm,DCDDA), crystallization transition temperatures (Tc,DCDDA), and enthalpies of crystallization transition (∆Hc,DCDDA) of the DCDDA regions as a function of the methylene spacer length, n, and stimuli treatment can be found in Table S2. For 2:1 PDI-mono(3-imz)/DCDDA (Figure S10), all samples have a Tm,DCDDA on the heating cycle and all have a Tc,DCDDA on the cooling cycle, besides the sample with no UV or heat treatment (dashed black line). This potentially indicates that UV/thermal exposure allows for greater packing in the supramolecular structures at smaller methylene spacer lengths, whereas the sample treated with neither stimulus has a lower degree of packing. Interestingly, at a longer methylene spacer length of n = 6 (Figure S12), all samples have a Tm,DCDDA and Tc,DCDDA regardless of UV or heat exposure. This may be a result of the extended spacer length, which may decouple the bulky aromatic PDI core from the PDCDDA backbone and/or DA crystalline regions, allowing for greater packing. This is in agreement with the trend of increasing methylene spacer length and subsequent increased crystallinity in the supramolecular structures found in the WAXS analysis.
Figure 7 displays a schematic illustration detailing the phase transitions observed in 2:1 PDI-mono(n-imz)/DCDDA drop-cast films based on SAXS measurements after various stimuli treatments (Figures S19–S21, Tables S7–S9). Prior to any stimuli treatments (“UV 0 × Heat 0×”), the supramolecular structures at all methylene spacer lengths n show some uniform ordering that is most likely a result of hydrogen bonding due to imidazole–acid interactions, π-π stacking of adjacent PDI molecules, and, to a lesser extent, van der Waals attractions of long alkyl chains. Upon UV exposure for 10 min (“UV 1x Heat 0x”), blue-phase formation by covalent linkages of neighboring DCDDA monomers increases the uniform ordering within the films. Thermal treatment of the blue-phase films (“UV 1x Heat 1x”) for 60 min at 150 °C results in red-phase PDCDDA formation, which in turn disrupts the uniform ordering within the supramolecular structures.
A comparison of morphologies to our previous work [47] on supramolecular templated 1:1 PDI-mono(n-imz)/PCDA drop-cast films shows the similarities of PDI and DCDDA π-π stacking and acid–imidazole hydrogen bonding from 1H NMR, FTIR, and WAXS analyses, and similarities in the supramolecular structure formed are seen in the SAXS data. The 1:1 structure formed PPCDA-rich regions that alternated with cylindrical PDI-PPCDA hydrogen-bonded columnar stacks. The SAXS analysis of the 1:1 mono(4-imz)/PCDA drop-cast films also shows evidence of PPCDA-rich regions that somewhat alternate to an extent with the PDI cylindrical structures. Interestingly, the comparison of the DSC data does not show the same thermal transition trends of crystallinity. The 1:1 templated structure only exhibits a Tc at shorter methylene spacer lengths, whereas the 2:1 structure displays a Tc at the three- and six-spacer lengths (Figures S10 and S12). This may be a result of the “di-complexation” of DCDDA by the PDI-mono(n-imz) molecules providing less mobility, which causes crystallization to occur at longer methylene spacer lengths. Furthermore, the drop-cast films did not exhibit purple-phase PDCDDA, which is understood as a coexistence of the red and blue phases. Instead, the blue phase and red phase could be selectively triggered through distinct stimuli pathways, which enables applications in sensing and stimuli-responsive functional materials.
3. Materials
N-(1-hexylheptyl)-N′-((1-imidazole)propyl)perylene-3,4,9,19-tetracarboxyl-3,4-anhydride-9,10-bisimide (PDI-mono(3-imz) [47], N-(1-hexylheptyl)-N′-((1-imidazole)butyl)perylene-3,4,9,19-tetracarboxyl-3,4-anhydride-9,10-bisimide (PDI-mono(4-imz) [47], and N-(1-hexylheptyl)-N′-((1-imidazole)hexyl)perylene-3,4,9,19-tetracarboxyl-3,4-anhydride-9,10-bisimide (PDI-mono(6-imz)) [58] were synthesized following previously reported procedures. 1-(4-aminobutyl)imidazole (2) was synthesized following modified procedures [71,72]. Anhydrous dichloromethane (CH2Cl2) (99.8%) was purchased from Acros Organics and used without further purification. 10,12 docosadiynedioic acid (DCDDA) (97%) was purchased from Alfa Aesar, dissolved in anhydrous THF, and passed through a 0.45 μm GPC filter to remove polymerized byproducts prior to use. Tetrahydrofuran-d8 (D, 99.5%) was purchased from Cambridge Isotope Laboratories Inc. (Tewksbury, MA 01876, USA) and used without further purification.
3.1. Materials Synthesis
3.1.1. Representative Drop-Cast Film Preparation
A clean, dry 15 mL plastic amber-colored bottle was tared with the cap on and a small magnetic stir bar inside. PDI-mono(3-imz) (2.0 equiv.) was added to the plastic bottle, followed by a pure DCDDA stock solution (20 mg/mL, 1.0 equiv.) and anhydrous CHCl3 in a sunlight/UV-free room. The resulting 2 wt% 2:1 PDI-mono(3-imz)/DCDDA solution was allowed to gently stir at room temperature in the sealed plastic bottle overnight. The solution was drop-cast onto a clean, dry quartz microscope slide and was allowed to dry overnight in a UV-proof room. The “Before UV” samples were analyzed upon drying, and the “After UV” samples were polymerized in a 254 nm UV crosslinker chamber (power = 7.6 mW cm−2) for ten minutes prior to analysis.
3.1.2. Stimuli Treatment of Films
Films that underwent stimuli treatment are denoted by “UV mx Heat mx”, where “UV” = 254 nm exposure for 10 min at room temperature, “Heat” = 150 °C for 1 h, and m = # of treatments by “UV” and/or “Heat”.
Thermal treatment: Films that underwent thermal treatment were heated to 150 °C for 1 h in air.
UV polymerization: Solid-state polymerization by UV irradiation of DCDDA and the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films was performed in a Fisher Scientific UV Crosslinker equipped with 254 nm bulbs at a power of 7.6 mW cm−2 for 10 min at room temperature.
3.2. Instrumentation
Nuclear magnetic resonance (NMR) spectroscopy: 1H NMR spectra were recorded on a Bruker DMX 500 MHz NMR spectrometer at 24° C. Deuterated chloroform (CDCl3) was used as a solvent with the reference peak at 7.24 ppm. Deuterated tetrahydrofuran (THF-d8) was used as a solvent with the reference peak at 3.58 ppm.
Infrared (IR) spectroscopy: Infrared spectra were acquired on a Nicolet Magna-IR 560 spectrometer with a resolution of 2 cm−1 and 32 scans in micro-ATR mode. The spectrometer was equipped with a Specac Quest single-reflection ATR accessory containing a diamond crystal sample plate.
Dynamic scanning calorimetry (DSC): The indium standard-calibrated TA-2920 DSC (Q-100 series) instrument was used to analyze the thermal properties of DCDDA, PDI-mono(n-imz) small molecules, and the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films before and after UV irradiation. The amount of sample was 5–10 mg, with a scanning rate of 5 °C/min. Phase transition temperatures were determined using TA Universal Analysis software v5.5.24. The first heat cycle was used for the PDI-mono(n-imz) small molecules, and the second heat cycle was used in determining the phase transition temperature of DCDDA after UV irradiation, as well as for the 2:1 PDI-mono(n-imz)/DCDDA drop-cast films before and after UV irradiation.
Small-angle X-ray scattering (SAXS): SAXS was performed on a pin-hole collimated Bruker Nano STAR instrument configured with Cu-Kα radiation (1.5418 Å). The scattered intensity was recorded on a Mikro Gap VÅNTEC-2000 detector (pixel size = 67 mm) with a sample-to-detector distance of ~67 cm to cover a scattering vector, , ranging from 0.007 to 0.015 to 0.462 Å−1. Silver behenate was used to calibrate the sample-to-detector distance. The samples were prepared by sandwiching them between two pieces of scotch tape. A scotch tape substrate blank was analyzed to subtract the background from the SAXS patterns.
Wide-angle X-ray scattering (WAXS): Oxford Diffraction XCalibur PX Ultra (transmission mode) was used for the WAXS measurements with an Onyx detector (CuKα radiation 1.5418 Å, double mirror focusing, 35 kV and 35 mA). The samples were prepared by sandwiching them between two pieces of scotch tape. A scotch tape substrate blank was analyzed to subtract the background from the WAXS patterns.
UV–Vis absorbance: UV–Vis analysis of the drop-cast films of DCDDA after UV irradiation, PDI-mono(n-imz) small molecules, and drop-cast films of 2:1 PDI-mono(n-imz)/DCDDA before and after UV irradiation was performed on a Shimadzu UV-Vis Spectrometer (UV-2450) in absorbance mode with a wavelength range of 300–800 nm.
Electrical conductivity measurement by four-point probe: The Ossila four-point probe system (UK, T2001A, software version 1.0) was used to measure the sheet resistance, resistivity, and conductivity of the films. To reduce the impact of humidity on the ionic conductivity, we performed our experiments in a lab with a dehumidifier that served to reduce the relative humidity present in the room. Additionally, the experiments were performed at room temperature with a marginal error of (±2 °C).
The ionic conductivity of the x:y PDI-mono(n-imz)/DCDDA lamellar structures was assessed with the probes vertically hooked onto a horizontally lying drop-cast film. Several measurements from different regions of the film were recorded, and the average values were reported.
The 4-point probe has a space distance of 1.27 mm between each probe. Initially, the 4-point probe instrument was calibrated by using an ITO glass (2 × 1.5 cm, 100 nm thickness) substrate, which had a resistance of 18 Ω/sq, resistivity of 1.8 µΩ/sq, and conductivity of 554.6 kS/m. The I–V curve and data were directly obtained from the instrument, and then the sheet resistance (R), resistivity, and conductivity values were obtained from the Ossila software version 1.0 by applying the sample and probe parameters. The thickness of ionic gels was measured using a micrometer vernier caliper. The resistance, resistivity, and conductivity of the sheet were calculated using the following formulas:
where ΔV is the change in voltage measured between the inner probes, I is the current applied between the outer probes, Rs is the resistance of the sheet (Ω/sq), ρ is the resistivity (Ω m), σ is the conductivity (S/m), and t is the film thickness (µm) measured using a micrometer vernier caliper. The thickness of each gel varied from one sample to another. To obtain accurate data, the measurements were taken from the center of the sample or sheet in triplicates.
Rs = 4.5323 ∆V/I ρ = Rs × t σ = 1/ρ
4. Conclusions
A novel approach to supramolecular PDI-PDA polymeric systems has been developed from a DA diacid monomer hydrogen-bonded to imidazole-appended PDI molecules that are π-π-stacked. Here, we elucidated the importance of the composition of the imidazole-appended perylene diimide of varying methylene spacer length (n = 3, 4, and 6) and 10, 12 docosadiynedioic acid in the ratio of 2:1 to the supramolecular self-assembly, final morphology, and properties. The supramolecular formation was monitored in solution by 1H NMR spectroscopy and revealed a deviation from the theoretical 2:1 PDI-mono(n-imz)/DCDDA to an experimental 2:1.25 molar ratio. FTIR spectroscopy of the supramolecular structures in the solid state displayed retention of the supramolecular formation when comparing the characteristic C=O stretching in the pure DCDDA monomer to the supramolecular structures. UV–Vis spectroscopy was used to study various chromatic transitions, where topochemical polymerization of the drop-cast films proceeded upon UV radiation for 10 min, resulting in blue-phase PDCDDA formation. Subsequent thermal treatment displayed thermochromic behavior upon the blue→red phase formation in the films. Interestingly, thermal polymerization of the blue or red phase did not occur. Thermal studies by DSC showed a dual dependence of the methylene spacer length and stimuli treatment (UV or heat) on characteristic transitions in the films. Furthermore, small-angle X-ray scattering (SAXS) and wide-angle X-ray scattering (WAXS) showed well-defined hierarchical semiconducting nanostructures with interconnected “chessboard”-patterned lamellar stacking. This unusual morphology with domain connectivity directly enhanced the ionic conductivity of this ionic liquid-doped 2:1 system compared to our previously published 1:1 composition of PDI and PDCDDA. Thus, the rational design of this 2:1 system with a tailored composition, architecture, and self-assembly will be important for energy applications involving the transport of ions and charges.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30061207/s1, Figures S1 and S2: NMR spectra of various 2:1 PDI-mono(n-imz)/DCDDA, Figures S3 and S4: FTIR-ATR spectra of various 2:1 PDI-mono(n-imz)/DCDDA, Figures S5–S7: UV-visible spectra of 2:1 PDI-mono(n-imz)/DCDDA before and after UV or thermal treatment, Figure S6 and Table S1: TGA plot and numerical data for DCDDA, and 2:1 PDI-mono(n-imz)/DCDDA, Figures S7–S12 and Table S2: DSC data and corresponding numerical data table for DCDDA and 2:1 PDI-mono(n-imz)/DCDDA before and after UV and/or thermal treatment, Figures S13–S18 and Tables S3–S6: 1D and 2D WAXS data and corresponding peak assignments in the tables for DCDDA and 2:1 PDI-mono(n-imz)/DCDDA before and after UV and/or thermal treatment, Figures S19–S22 and Tables S7–S10: 1D SAXS data and corresponding peaks assignemnts in tables for DCDDA and 2:1 PDI-mono(n-imz)/DCDDA before and after UV and/or thermal treatment, Table S11 and Table S12: Ionic conductivity for 1:1 PDI-mono(4-imz)/PCDDA and 2:1 PDI-mono(4-imz)/PDCDDA in ionic liquids after UV exposure and various control samples.
Author Contributions
Conceptualization, I.J.M., M.-P.N. and R.M.K.; Methodology, I.J.M., K.-C.S., M.-P.N. and R.M.K.; Validation, F.K.M. and M.-P.N.; Formal analysis, F.K.M. and K.-C.S.; Investigation, R.M.K.; Resources, R.M.K.; Data curation, I.J.M., F.K.M. and K.-C.S.; Writing—original draft, I.J.M.; Writing—review & editing, F.K.M., K.-C.S., M.-P.N. and R.M.K.; Supervision, R.M.K.; Project administration, R.M.K.; Funding acquisition, R.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
I.J.M. acknowledges a fellowship from the Department of Education GAANN Program (P200A150330). This work was partly supported by the National Science Foundation under DMR-1507045. The SAXS data were collected at UCONN NanoStar SAXS instrument, acquired through the funding support from a NSF-Major Research Instrument (MRI) grant (1228817).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The central instrumentation facilities at the Institute of Materials Science and Chemistry Department at UConn are acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huo, J.; Deng, Q.; Fan, T.; He, G.; Hu, X.; Hong, X.; Chen, H.; Luo, S.; Wang, Z.; Chen, D. Advances in polydiacetylene development for the design of side chain groups in smart material applications—A mini review. Polym. Chem. 2017, 8, 7438–7445. [Google Scholar] [CrossRef]
- Okada, S.; Peng, S.; Spevak, W.; Charych, D. Color and chromism of polydiacetylene vesicles. Acc. Chem. Res. 1998, 31, 229–239. [Google Scholar] [CrossRef]
- Sun, X.; Chen, T.; Huang, S.; Li, L.; Peng, H. Chromatic polydiacetylene with novel sensitivity. Chem. Soc. Rev. 2010, 39, 4244–4257. [Google Scholar] [CrossRef]
- Reppy, M.A.; Pindzola, B.A. Biosensing with polydiacetylene materials: Structures, optical properties and applications. Chem. Commun. 2007, 4317–4338. [Google Scholar] [CrossRef]
- Chance, R.; Baughman, R.; Müller, H.; Eckhardt, C.J. Thermochromism in a polydiacetylene crystal. J. Chem. Phys. 1977, 67, 3616–3618. [Google Scholar] [CrossRef]
- Wenzel, M.; Atkinson, G.H. Chromatic properties of polydiacetylene films. J. Am. Chem. Soc. 1989, 111, 6123–6127. [Google Scholar] [CrossRef]
- Yoon, J.; Chae, S.K.; Kim, J.-M. Colorimetric sensors for volatile organic compounds (VOCs) based on conjugated polymer-embedded electrospun fibers. J. Am. Chem. Soc. 2007, 129, 3038–3039. [Google Scholar] [CrossRef]
- Tomioka, Y.; Tanaka, N.; Imazeki, S. Effects of side-group interactions on pressure-induced chromism of polydiacetylene monolayer at a gas-water interface. Thin Solid Film. 1989, 179, 27–31. [Google Scholar] [CrossRef]
- Qian, X.; Sädler, B. Recent developments in polydiacetylene-based sensors. Chem. Mater. 2019, 31, 1196–1222. [Google Scholar] [CrossRef]
- Wegner, G. Topochemische Reaktionen von Monomeren mit konjugierten Dreifachbindungen/Tochemical Reactions of Monomers with conjugated triple Bonds: I. Mitt.: Polymerisation von Derivaten des 2.4-Hexadiin-1.6-diols im kristallinen Zustand. Z. Naturforsch. B 1969, 24, 824–832. [Google Scholar] [CrossRef]
- Wegner, G. Topochemical reactions of monomers with conjugated triple-bonds. IV. Polymerization of bis-(p-toluene sulfonate) of 2.4-hexadiin-1.6-diol. Die Makromol. Chem. Macromol. Chem. Phys. 1971, 145, 85–94. [Google Scholar] [CrossRef]
- Fang, F.; Meng, F.; Luo, L. Recent advances on polydiacetylene-based smart materials for biomedical applications. Mater. Chem. Front. 2020, 4, 1089–1104. [Google Scholar] [CrossRef]
- Lee, J.P.; Hwang, H.; Chae, S.; Kim, J.-M. A reversibly mechanochromic conjugated polymer. Chem. Commun. 2019, 55, 9395–9398. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, M.; Song, M.; Jiang, L.; Li, J.; He, Y.; Zhou, L. Good conductivity of a single component polydiacetylene film. Org. Electron. 2017, 49, 174–178. [Google Scholar] [CrossRef]
- Rao, V.K.; Teradal, N.L.; Jelinek, R. Polydiacetylene capacitive artificial nose. ACS Appl. Mater. Interfaces 2019, 11, 4470–4479. [Google Scholar] [CrossRef]
- Dolai, S.; Bhunia, S.K.; Beglaryan, S.S.; Kolusheva, S.; Zeiri, L.; Jelinek, R. Colorimetric polydiacetylene–aerogel detector for volatile organic compounds (VOCs). ACS Appl. Mater. Interfaces 2017, 9, 2891–2898. [Google Scholar] [CrossRef]
- Enkelmann, V. Structural aspects of the topochemical polymerization of diacetylenes. In Polydiacetylenes; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1984; Volume 63, pp. 91–133. [Google Scholar]
- Krishnan, D.; RB, A.R.; Gowd, E.B. Topochemical polymerization of hierarchically ordered diacetylene monomers within the block copolymer domains. Polym. Chem. 2019, 10, 3154–3162. [Google Scholar] [CrossRef]
- Fan, J.; Xu, X.; Yu, W.; Wei, Z.; Zhang, D. Hydrogen-bond-driven supramolecular self-assembly of diacetylene derivatives for topochemical polymerization in solution. Polym. Chem. 2020, 11, 1947–1953. [Google Scholar] [CrossRef]
- Nyayachavadi, A.; Mason, G.T.; Nazir Tahir, M.; Ocheje, M.U.; Rondeau-Gagné, S. Covalent cross-linking of diketopyrrolopyrrole-based organogels with polydiacetylenes. Langmuir 2018, 34, 12126–12136. [Google Scholar] [CrossRef]
- Da Costa, V.; Le Moigne, J.; Oswald, L.; Pham, T.; Thierry, A. Thin film orientation by epitaxy of carbazolyl polydiacetylenes: Guest-host interaction on a crystal surface. Macromolecules 1998, 31, 1635–1643. [Google Scholar] [CrossRef]
- Ye, Q.; You, X.; Zou, G.; Yu, X.; Zhang, Q. Morphology, structure and chromatic properties of azobenzene-substituted polydiacetylene supramolelular assemblies. J. Mater. Chem. 2008, 18, 2775–2780. [Google Scholar] [CrossRef]
- Shirakawa, M.; Fujita, N.; Shinkai, S. A stable single piece of unimolecularly π-stacked porphyrin aggregate in a thixotropic low molecular weight gel: A one-dimensional molecular template for polydiacetylene wiring up to several tens of micrometers in length. J. Am. Chem. Soc. 2005, 127, 4164–4165. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Khazi, M.I.; Kundapur, U.; Kim, B.; Kim, Y.; Lee, C.W.; Kim, J.-M. Cation-directed self-assembly of macrocyclic diacetylene for developing chromogenic polydiacetylene. ACS Macro Lett. 2019, 8, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Phonchai, N.; Khanantong, C.; Kielar, F.; Traiphol, R.; Traiphol, N. Low-temperature reversible thermochromic polydiacetylene/zinc (II)/zinc oxide nanocomposites for colorimetric sensing. ACS Appl. Nano Mater. 2019, 2, 4489–4498. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.W.; Kim, J.-M. A magnetically responsive polydiacetylene precursor for latent fingerprint analysis. ACS Appl. Mater. Interfaces 2016, 8, 6245–6251. [Google Scholar] [CrossRef]
- Kim, B.; Khazi, M.I.; Kim, J.-M. Nickel-Ion-Coordinated Reversibly Solvatochromic Polydiacetylene Based on Tubular Assembly of Macrocyclic Diacetylene. Macromolecules 2021, 54, 8220–8228. [Google Scholar] [CrossRef]
- Shin, H.; Jannah, F.; Yoo, E.J.; Kim, J.-M. A colorimetric and fluorescence “turn-on” sensor for Fe (III) ion based on imidazole-functionalized polydiacetylene. Sens. Actuators B Chem. 2022, 350, 130885. [Google Scholar] [CrossRef]
- Finney, T.J.; Parikh, S.J.; Berman, A.; Sasaki, D.Y.; Kuhl, T.L. Characterizing and Tuning the Properties of Polydiacetylene Films for Sensing Applications. Langmuir 2021, 37, 12940–12951. [Google Scholar] [CrossRef]
- Yarimaga, O.; Jaworski, J.; Yoon, B.; Kim, J.-M. Polydiacetylenes: Supramolecular smart materials with a structural hierarchy for sensing, imaging and display applications. Chem. Commun. 2012, 48, 2469–2485. [Google Scholar] [CrossRef]
- Ahn, D.J.; Kim, J.-M. Fluorogenic polydiacetylene supramolecules: Immobilization, micropatterning, and application to label-free chemosensors. Acc. Chem. Res. 2008, 41, 805–816. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, S.; Kim, J.-M. Recent conceptual and technological advances in polydiacetylene-based supramolecular chemosensors. Chem. Soc. Rev. 2009, 38, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Cordova, D.L.M.; Robang, A.S.; Kuang, Y.; Ogura, K.S.; Paravastu, A.K.; Arguilla, M.Q.; Ardoña, H.A.M. Thermochromic behavior of polydiacetylene nanomaterials driven by charged peptide amphiphiles. Biomacromolecules 2023, 24, 4051–4063. [Google Scholar] [CrossRef] [PubMed]
- Kadamannil, N.N.; Shames, A.I.; Bisht, R.; Biswas, S.; Shauloff, N.; Lee, H.; Kim, J.-M.; Jelinek, R. Light-Induced Self-Assembled Polydiacetylene/Carbon Dot Functional “Honeycomb”. ACS Appl. Mater. Interfaces 2024, 16, 22593–22603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Q.; Tong, X.; Wang, Y.; Cai, K.; Ji, W. Rational Design of Bio-Inspired Peptide Electronic Materials toward Bionanotechnology: Strategies and Applications. Adv. Funct. Mater. 2024, 34, 2401466. [Google Scholar] [CrossRef]
- Würthner, F.; Thalacker, C.; Diele, S.; Tschierske, C. Fluorescent J-type aggregates and thermotropic columnar mesophases of perylene bisimide dyes. Chem.– Eur. J. 2001, 7, 2245–2253. [Google Scholar] [CrossRef]
- Marty, R.; Szilluweit, R.; Sánchez-Ferrer, A.; Bolisetty, S.; Adamcik, J.; Mezzenga, R.; Spitzner, E.-C.; Feifer, M.; Steinmann, S.N.; Corminboeuf, C. Hierarchically structured microfibers of “single stack” perylene bisimide and quaterthiophene nanowires. ACS Nano 2013, 7, 8498–8508. [Google Scholar] [CrossRef]
- Würthner, F.; Chen, Z.; Hoeben, F.J.; Osswald, P.; You, C.-C.; Jonkheijm, P.; Herrikhuyzen, J.v.; Schenning, A.P.; van der Schoot, P.P.; Meijer, E. Supramolecular p− n-heterojunctions by co-self-organization of oligo (p-phenylene vinylene) and perylene bisimide dyes. J. Am. Chem. Soc. 2004, 126, 10611–10618. [Google Scholar] [CrossRef]
- Yagai, S.; Seki, T.; Karatsu, T.; Kitamura, A.; Würthner, F. Transformation from H-to J-aggregated perylene bisimide dyes by complexation with cyanurates. Angew. Chem. Int. Ed. 2008, 47, 3367–3371. [Google Scholar] [CrossRef]
- Chen, Z.; Stepanenko, V.; Dehm, V.; Prins, P.; Siebbeles, L.D.; Seibt, J.; Marquetand, P.; Engel, V.; Würthner, F. Photoluminescence and conductivity of self-assembled π–π stacks of perylene bisimide dyes. Chem.–Eur. J. 2007, 13, 436–449. [Google Scholar] [CrossRef]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef]
- Tahir, M.N.; Nyayachavadi, A.; Morin, J.-F.; Rondeau-Gagné, S. Recent progress in the stabilization of supramolecular assemblies with functional polydiacetylenes. Polym. Chem. 2018, 9, 3019–3028. [Google Scholar] [CrossRef]
- Seo, J.; Khazi, M.I.; Kim, J.-M. Highly responsive triethylamine vapor sensor based on a perylene diimide-polydiacetylene system via heat-induced tuning of the molecular packing approach. Sens. Actuators B Chem. 2021, 334, 129660. [Google Scholar] [CrossRef]
- De Adhikari, A.; Morag, A.; Seo, J.; Kim, J.M.; Jelinek, R. Polydiacetylene–perylenediimide supercapacitors. ChemSusChem 2020, 13, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Kantha, C.; Joung, J.F.; Park, S.; Jelinek, R.; Kim, J.M. Covalently linked perylene diimide–polydiacetylene nanofibers display enhanced stability and photocurrent with reversible FRET phenomenon. Small 2019, 15, 1901342. [Google Scholar] [CrossRef]
- Jiang, H.; Hershtig, G.; Richter, S.; Jelinek, R. Light-induced conductivity in a solution-processed film of polydiacetylene and perylene diimide. J. Phys. Chem. Lett. 2016, 7, 1628–1631. [Google Scholar] [CrossRef]
- Martin, I.J.; Shih, K.-C.; Nieh, M.-P.; Kasi, R.M. Templated supramolecular structures of multichromic, multiresponsive perylene diimide-polydiacetylene films. Macromolecules 2020, 53, 4501–4510. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, W.; Ai, K.; Liu, J. Highly sensitive polydiacetylene ensembles for biosensing and bioimaging. Front. Chem. 2020, 8, 565782. [Google Scholar] [CrossRef]
- Lindsell, W.E.; Preston, P.N.; Seddon, J.M.; Rosair, G.M.; Woodman, T.A. Macroscopic helical and cylindrical morphologies from achiral 1, 3-diynes. Chem. Mater. 2000, 12, 1572–1576. [Google Scholar] [CrossRef]
- Sunilkumar, A.; Chethan, B.; Prasad, V.; Matteppanavar, S. An Overview of Stability, Lifetime, and Reuse of Surfactant Sensors; Royal Society of Chemistry: London, UK, 2023. [Google Scholar]
- Yan, L.; Rank, C.; Mecking, S.; Winey, K.I. Gyroid and other ordered morphologies in single-ion conducting polymers and their impact on ion conductivity. J. Am. Chem. Soc. 2019, 142, 857–866. [Google Scholar] [CrossRef]
- Krishnan, B.P.; Yang, Y.-F.; Woeppel, A.B.; Flores-Hansen, C.; Yeo, H.; Boudouris, B.W. Topochemical Synthesis of Conducting Radical Polymers. Macromolecules 2024, 57, 4747–4756. [Google Scholar] [CrossRef]
- Zhang, Z.; Krajniak, J.; Ganesan, V. A multiscale simulation study of influence of morphology on ion transport in block copolymeric ionic liquids. Macromolecules 2021, 54, 4997–5010. [Google Scholar] [CrossRef]
- He, G.; Li, Z.; Zhao, J.; Wang, S.; Wu, H.; Guiver, M.D.; Jiang, Z. Nanostructured ion-exchange membranes for fuel cells: Recent advances and perspectives. Adv. Mater. 2015, 27, 5280–5295. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Hayward, R.C. Interconnected Nanoporous Polysulfone by the Self-Assembly of Randomly Linked Copolymer Networks and Linear Multiblocks. ACS Appl. Mater. Interfaces 2024, 16, 34079–34088. [Google Scholar] [CrossRef]
- Jeong, K.j.; Jeong, S.; Lee, S.; Son, C.Y. Predictive Molecular Models for Charged Materials Systems: From Energy Materials to Biomacromolecules. Adv. Mater. 2023, 35, 2204272. [Google Scholar] [CrossRef]
- Holman, M.W.; Liu, R.; Adams, D.M. Single-molecule spectroscopy of interfacial electron transfer. J. Am. Chem. Soc. 2003, 125, 12649–12654. [Google Scholar] [CrossRef]
- Tran, H.; Gopinadhan, M.; Majewski, P.W.; Shade, R.; Steffes, V.; Osuji, C.O.; Campos, L.M. Monoliths of semiconducting block copolymers by magnetic alignment. ACS Nano 2013, 7, 5514–5521. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, H.; Liu, F.; Russell, T.P.; Hu, W. Approaching intra-and interchain charge transport of conjugated polymers facilely by topochemical polymerized single crystals. Adv. Mater. 2017, 29, 1701251. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, J.-S.; Choi, H.; Sohn, D.; Ahn, D.J. Rational design and in-situ FTIR analyses of colorimetrically reversibe polydiacetylene supramolecules. Macromolecules 2005, 38, 9366–9376. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H.; Zhang, Q.; Wang, F.; Zou, G. Helical polydiacetylene prepared in the liquid crystal phase using circular polarized ultraviolet light. Chem. Commun. 2014, 50, 365–367. [Google Scholar] [CrossRef]
- Orchard, B.; Tripathy, S. Molecular structure and electronic property modification of poly (diacetylenes). Macromolecules 1986, 19, 1844–1850. [Google Scholar] [CrossRef]
- Kim, H.; Khazi, M.I.; Kim, J.-M. Preparation and colorimetric response of an aldehyde-functionalized macrocyclic diacetylene-derived polydiacetylene. Dyes Pigments 2021, 187, 109114. [Google Scholar] [CrossRef]
- Kim, B.; Heo, J.-M.; Khazi, M.I.; Kim, J.-M. Reversible solvatochromism of polydiacetylenes based on extensively hydrogen-bonded tubular arrays. Macromolecules 2021, 54, 2485–2493. [Google Scholar] [CrossRef]
- Khazi, M.I.; Balachandra, C.; Shin, G.; Jang, G.-H.; Govindaraju, T.; Kim, J.-M. Co-solvent polarity tuned thermochromic nanotubes of cyclic dipeptide–polydiacetylene supramolecular system. RSC Adv. 2020, 10, 35389–35396. [Google Scholar] [CrossRef] [PubMed]
- Hema, K.; Ravi, A.; Raju, C.; Sureshan, K.M. Polymers with advanced structural and supramolecular features synthesized through topochemical polymerization. Chem. Sci. 2021, 12, 5361–5380. [Google Scholar] [CrossRef]
- Badarau, C.; Wang, Z.Y. Synthesis and optical properties of thermally and photochemically cross-linkable diacetylene-containing polymers. Macromolecules 2004, 37, 147–153. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kang, S.-H.; Doe, C.; Yu, J.; Sim, J.-B.; Kim, J.; Kline, S.R.; Choi, S.-M. Highly Ordered Self-Assembly of 1D Nanoparticles in Phospholipids Driven by Curvature and Electrostatic Interaction. J. Am. Chem. Soc. 2009, 131, 7456–7460. [Google Scholar] [CrossRef]
- Scherlis, D.A.; Marzari, N. π-Stacking in thiophene oligomers as the driving force for electroactive materials and devices. J. Am. Chem. Soc. 2005, 127, 3207–3212. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Y.; Li, Z.; Wang, J. Viscosities and conductivities of 1-butyl-3-methylimidazolium carboxylates ionic liquids at different temperatures. J. Chem. Eng. Data 2012, 57, 3102–3108. [Google Scholar] [CrossRef]
- Press, J.B.; Wright Jr, W.B.; Chan, P.S.; Marsico, J.W.; Haug, M.F.; Tauber, J.; Tomcufcik, A.S. Thromboxane synthetase inhibitors and antihypertensive agents. 2. N-[(1H-Imidazol-1-yl) alkyl]-1H-isoindole-1, 3 (2H)-diones and N-[(1H-1, 2, 4-triazol-1-yl) alkyl]-1H-isoindole-1, 3 (2H)-diones as unique antihypertensive agents. J. Med. Chem. 1986, 29, 816–819. [Google Scholar] [CrossRef]
- Hay, M.P.; Wilson, W.R.; Moselen, J.W.; Palmer, B.D.; Denny, W.A. Hypoxia-selective antitumor agents. 8. Bis (nitroimidazolyl) alkanecarboxamides: A new class of hypoxia-selective cytotoxins and hypoxic cell radiosensitizers. J. Med. Chem. 1994, 37, 381–391. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).