Abstract
A novel, eco-friendly, and efficient method for constructing 2,3-disubstituted chromone skeletons from readily available water, o-hydroxyaryl enaminones (o-HPEs), and aryldiazonium salts has been developed under mild reaction conditions. This α,β-C(sp2)–H bond difunctionalization/chromone annulation reaction strategy is achieved by building two C(sp3)–O bonds and a C(sp2)-N bond, which provides a practical pathway for the preparation of 2-hydroxy-3-hydrazono-chromones in moderate to excellent yields, enabling broad substrate scope and good functional group tolerance, as well as gram-scale synthesis. This protocol offers a valuable tool for synthesizing diverse functionalized chromones with potential applications in drug discovery and industrial synthesis.
1. Introduction
2,3-Disubstituted chromones are an important class of O-heterocyclic compounds that are widely distributed in the skeleton of natural products and drug molecules [1,2,3,4,5]. In the field of medicinal chemistry, chromones exhibit a wide range of pharmacological properties [6,7,8,9,10], including anti-inflammatory, antioxidant, anticholinergic, and anticancer activities, making them valuable lead compounds for drug discovery (Figure 1). Consequently, numerous efforts for developing novel and efficient strategies to these 2,3-disubstituted chromone molecules have been developed. In recent decades, conventional synthetic approaches toward 2,3-disubstituted chromones synthesis primarily involve the following chromone annulation reactions: (1) starting from 3-substituted chromones through a four-component reaction or transition metal (e.g., Pd)-catalyzed functionalization reactions [11,12]; (2) cyclization reactions such as the Kostanecki–Robinson reaction and Baker–Venkataraman rearrangement [13,14]; (3) two-step electrophilic cyclization or Suzuki cross-coupling reaction using 1-(2-methoxyphenyl)but-2-yn-1-one as the starting material [15]; (4) transition metal-catalyzed or electro-catalyzed coupling reactions of salicylaldehydes with alkynes [16]; (5) Claisen condensation of o-hydroxyphenylacetone derivatives with esters[17]; and (6) Pd-catalyzed SNAr reactions of 2-fluorobromobenzenes with ketones [18]. Despite the development of numerous synthetic methods for 2,3-disubstituted chromones, several challenges remain to be addressed, including the requirement for specialized and pre-functionalized substrates, the need for transition metal catalysts, and harsh reaction conditions. Consequently, there is an urgent need to develop new, green, and efficient methods for constructing such compounds.
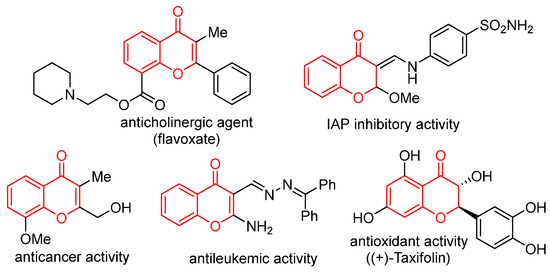
Figure 1.
Selected biologically active 2,3-disubstituted chromones.
o-Hydroxyaryl enaminones (o-HPEs), as a structurally unique class of enaminone compounds, are widely used in the synthesis of chromone derivatives due to their ease of preparation and high reactivity [19,20]. In this context, the distinctive conjugated structure of o-HPEs makes their vinyl α-position highly susceptible to attack by various electrophiles for the simple and efficient construction of 3-substituted chromones through cascade vinyl α-C(sp2)–H bond functionalization/intramolecular chromone annulation process (Scheme 1a). Various vinyl α-C(sp2)–H bond functionalizations include trifluoromethylation [21,22,23], alkylation [24,25,26,27], allylation [28,29], alkenylation [30], arylation [31,32,33], acylation [34,35], amination [36,37], halogenation [38,39], thiolation [40,41,42], selenylation [43,44], and phosphorylation [45], acyloxylation [46], sulfonylation [47,48], etc. In contrast, the vinyl β-position of o-HPEs is relatively inert but can be activated under specific conditions to construct 2-substituted chromone frameworks through the selective cascade vinyl β-C(sp2)–H bond functionalization/intramolecular chromone annulation process (Scheme 1b). In 2020, Wan’s group developed a copper-catalyzed tandem cyclization reaction of o-HPEs with secondary amines for the construction of 2-nitrogenated chromones [49]. In 2023, they further discovered that o-HPEs could undergo β-cyanation in the presence of potassium ferricyanide and iodine, enabling the introduction of cyanyl at the 2-position of chromones [50]. Nevertheless, these forementioned α- or β-C(sp2)–H bond functionalization/intramolecular chromone annulation reactions of o-HPEs only form a new bond at the 2- or 3-position of vinyl on the newly formed chromone skeleton to construct 2-substituted or 3-substituted chromones. On the other hand, regarding the α,β-C(sp2)–H bond difunctionalization/chromone annulation reaction of o-HPEs, it has recently been disclosed that two new bonds simultaneously form at the 2- and 3-position of vinyl on the newly formed chromone skeleton to construct 2,3-disubstituted chromones (Scheme 1c). At this stage, such difunctionalization/chromone annulation reactions are scarce and remain underdeveloped. Recently, Yang’s group reported the first example of a difunctionalization reaction at both the α- and β-positions of o-HPEs using tert-butyl nitrite as an electrophile and water as a nucleophile, leading to the synthesis of 2,3-disubstituted chromones [51]. Subsequently, in 2024, our group developed a similar difunctionalization strategy using aryldiazonium salts as electrophiles and alcohols as nucleophiles [52]. As a part of our ongoing interest in chromone annulation reactions [24,25,52] and enaminones chemistry [53,54,55,56], we report herein a novel α,β-C(sp2)–H bond difunctionalization/chromone annulation reaction of o-HPEs, aryldiazonium salts, and water (Scheme 1d). Notably, the synthesis approach is greener and milder to efficiently yield structurally diverse 2-hydroxy-3-hydrazono-chromones.
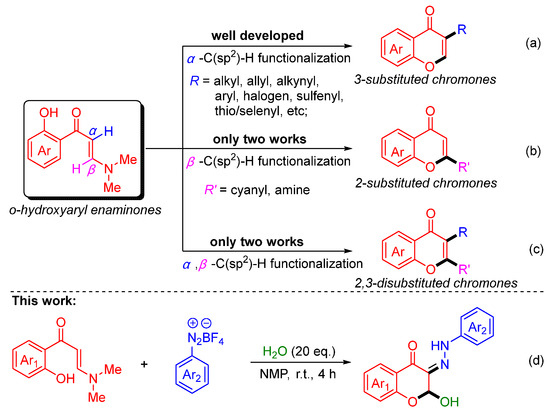
Scheme 1.
(a) The construction methods of 3-substituted chromones from o-HPEs; (b) The construction methods of 2-substituted chromones from o-HPEs; (c) The construction methods of 2,3-disubstituted chromones from o-HPEs; (d) This work: the construction methods of 2-hydroxy-3-hydrazono-chromones from o-HPEs.
2. Results and Discussion
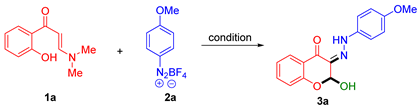
We commenced this study by exploring this cascade α,β-C(sp2)–H bond difunctionalization/chromone annulation reaction, and o-hydroxyarylenaminone 1a and p-methoxy diazonium salt 2a were chosen as model substrates to evaluate the reaction condition (Table 1). Initially, the reaction was carried out in THF with water (20 equiv.) as an additive, stirring at room temperature in air for 4 h, yielding the target 2-hydroxy-3-hydrazono-chromone 3a in 65% yield. To further improve the reaction yield, various solvents were screened, including 2-MeTHF, 1,4-dioxane, 1,3-dioxane, EA, CHCl3, DCE, toluene, chlorobenzene, DMF, DMA, and NMP (entries 2–12). The results showed that 3a was not formed in CHCl3, toluene, or chlorobenzene, while the highest yield was 88% in NMP. Subsequently, the amount of water was optimized. Without additional water, the yield was only 40% (entry 13), and by increasing or decreasing the amount of water, the yield was slightly lower (entries 14 and 15). The equivalent of diazonium salt 2a was also screened, with 1.5 equivalents found to be optimal (entries 16 and 17). Additionally, the reaction temperature, atmosphere, and time were also investigated (entries 18–23). The optimal reaction condition was determined as follows: 1a (0.1 mmol), 2a (0.15 mmol), and water (20 equiv.) in NMP at room temperature under air for 4 h.

Table 1.
Optimization of reaction conditions a.
Under the optimized reaction conditions, we initially investigated the substrate scope of o-HPEs with aryldiazonium salt 2a (Scheme 2). The results demonstrated excellent substrate compatibility of o-HPEs to afford the corresponding 2-hydroxy-3-hydrazono-chromones 3a–3p in 60–93% yields, which exhibited broad tolerance to both electron-donating groups (OMe, Me) and electron-withdrawing groups (Br, F, phenyl, biphenyl, naphthyl, amide, and thiophene) on the aromatic ring of o-HPEs. Generally, o-HPEs with substituents at the 6-position (3b), 5-position (3c–3i), or 4-position (3j–3l) all afforded the target products in moderate to excellent yields. Furthermore, o-HPEs with multiple substituents also performed well in this transformation (3m–3p). In addition, 1-naphthyl and 2-naphthyl replacement of the benzene ring of o-HPEs were further evaluated. The reaction also proceeded smoothly, providing the corresponding target compounds 3q–3r in 68–70% yields. The structure of 3a was unequivocally confirmed by X-ray crystallographic analysis (CCDC 2425569).
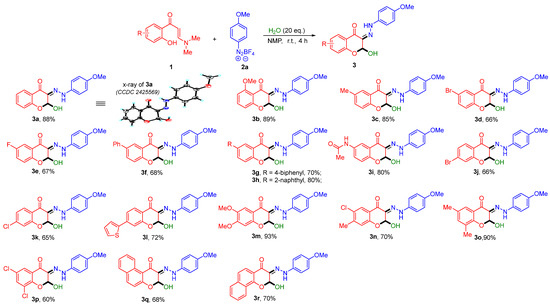
Scheme 2.
Scope of o-hydroxyaryl enaminones a,b. a Reaction conditions: 1 (0.3 mmol), 2 (0.45 mmol), and H2O (20 equiv.) in 3 mL of NMP, stirred at room temperature for 4.0 h. b Isolated yields.
Subsequently, the scope of aryldiazonium salts 2 was also explored in the reaction using o-HPEs 1a as a partner (Scheme 3). For various aryl diazonium salts bearing halogen (Cl and Br), OCF3, alkynyl, cyano, and methyl substituents at ortho, meta, or para-positions, the desired 2-hydroxy-3-hydrazono-chromone products (3s–3a′) could be furnished in excellent yields (78–88%). Moreover, 2-naphthyl- and 3-thienyl-substituted components were also compatible with this transformation, providing the corresponding compounds 3b′–3c′ in 81–85% yields. Notably, even diazonium salts bearing amide substituents on the benzene ring were well-tolerated, especially (−)-camphanic acid-derived diazonium salt, affording compounds 3d′–3e′ in 61–75% yields. This remarkable performance with amide-containing substrates further highlights the excellent substrate generality of this transformation, which can serve as a valuable tool for the synthesis of various functionalized compounds.

Scheme 3.
Scope of aryldiazonium salts a,b. a Reaction conditions: 1 (0.3 mmol), 2 (0.45 mmol), and H2O (20 equiv.) in 3 mL of NMP, stirred at room temperature for 4.0 h. b Isolated yields.
To further demonstrate the potential practicability of this method, a gram-scale synthesis and several further transformations were implemented (Scheme 4). First, a 5.0 mmol scale reaction was carried out under the standard reaction conditions to afford the desired product 3o in 70% (1.14 g) (Scheme 4a). Next, some further transformations of 2-hydroxy-3-hydrazono-chromones 3 were performed by benzylation and oxidation reactions. A bibenzylated product 4 in 15% yield was prepared by the reaction of 3o with benzyl bromide in the presence of K2CO3 (Scheme 4b). A chromane-2,4-dione derivative 5 with 59% yield could be synthetized by the treatment of 3o with trichloroisocyanuric acid (TCICA) and pyridine in ethyl acetate (EA) at room temperature (Scheme 4c).
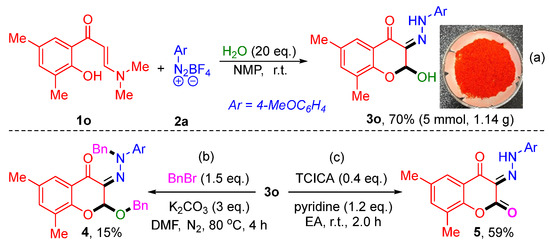
Scheme 4.
(a) Gram-scale synthesis; (b) Benzylation derivatization; (c) Oxidation derivatization.
To gain a deeper understanding of the reaction mechanism, a series of control experiments were conducted. Initially, the reaction of chromone 6 and p-methoxy diazonium salt 2a was carried out under the standard reaction conditions, and the target product 3a was not observed (Scheme 5a). Subsequently, o-HPE 1a and p-methoxy diazonium salt 2a were reacted in acetonitrile at room temperature for 30 min, leading to the formation of an unstable intermediate 7, and then treated with water (20 equiv.) under the standard reaction conditions, resulting in the target compound 3a in 80% yield (Scheme 5b). These results confirmed that the formation of 3a was achieved through the initial coupling of the diazonium salt with the o-HPE, followed by cyclization to form the chromone skeleton.
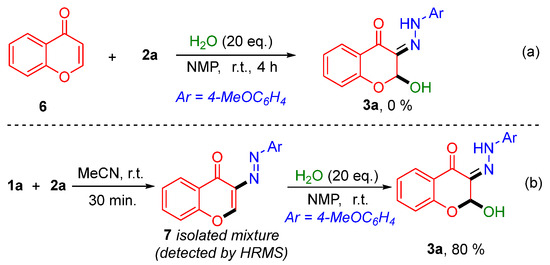
Scheme 5.
(a) Intermediate validation experiments; (b) Intermediate-capture experiments.
Based on the above experimental results and previous reports [51,52], a plausible mechanism for this cascade α,β-C(sp2)–H bond difunctionalization/chromone annulation reaction was proposed in Scheme 6. First, diazonium salts 2 are coupled with α-position of o-HPEs 1 to form intermediate A, then undergo intramolecular cyclization and deamination to yield intermediate C, which possesses the chromone skeleton. The nitrogen atom of intermediate C forms an intermolecular hydrogen bond with water, activating the N=N double bond. Finally, another molecule of water attacks the 2-position of the chromone skeleton to form the target compound 3.
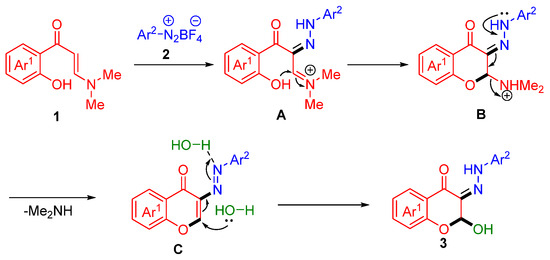
Scheme 6.
Proposed mechanism.
3. Materials and Methods
3.1. General Information
All the compounds were fully characterized by spectroscopic data. The NMR spectra were recorded on a DRX600 (1H: 600 MHz, 13C: 150 MHz) and an AV500 (1H: 500 MHz, 13C: 125 MHz), chemical shifts (δ) are expressed in ppm, J values are given in Hz, and deuterated DMSO-d6 and Pyridine-d6 were used as solvents. The reactions were monitored by thin-layer chromatography (TLC) using silica gel GF254. The melting points were determined on an XT-4A melting point apparatus and are uncorrected. HRMs were performed on an Agilent LC/MS TOF instrument (From Agilent Technologies Inc., Santa Clara, CA, USA).
All the chemicals and solvents were used as received without further purification, unless otherwise stated. Column chromatography was performed on silica gel (200–300 mesh).
o-Hydroxyphenylenaminones 1 were prepared according to the literature [57,58,59], and aryldiazonium salts 2 were prepared according to the literature [60,61]. Other reagents were purchased from Energy Chemical and Adamas-beta® (From Shanghai, China).
3.2. Synthetic Procedures
3.2.1. Synthetic Procedures of 2-Hydroxy-3-Hydrazono-Chromones 3
o-Hydroxyarylenaminones 1 (0.3 mmol), aryldiazonium salts 2 (0.45 mmol), H2O (20 equiv.), and NMP (3.0 mL) were charged into 10 mL Ace Glass pressure tubes, and the mixture was stirred at r.t. for 4.0 h until o-hydroxyarylenaminones were completely consumed. EtOAc (50 mL × 2) were added. The organic phase was washed with water (30 mL), dried over Na2SO4, concentrated, and purified by flash column chromatography to afford 2-hydroxy-3-hydrazono-chromones 3.
3.2.2. Synthetic Procedures of Bibenzylated 2-Hydroxy-3-Hydrazono-Chromone 4
2-Hydroxy-3-hydrazono-chromone 3o (0.3 mmol), benzyl bromide (0.45 mmol), potassium carbonate (3.0 equiv.), and DMF (5.0 mL) were charged into 10 mL Ace Glass pressure tubes, and the mixture was stirred at 80 °C for 4.0 h until 3o was completely consumed. EtOAc (5 mL × 2) were added. The organic phase was washed with water (10 mL), dried over Na2SO4, concentrated, and purified by flash column chromatography to afford bibenzylated 2-hydroxy-3-hydrazono-chromone 4.
3.2.3. Synthetic Procedures of Chromane-2,4-Dione 5
2-Hydroxy-3-hydrazono-chromone 3o (0.3 mmol), trichloroisocyanuric acid (0.4 equiv.), pyridine (1.2 equiv.), and ethyl acetate (3.0 mL) were charged into 10 mL Ace Glass pressure tubes, and the mixture was stirred at r.t. for 2.0 h until 3o was completely consumed. EtOAc (5 mL × 2) were added. The organic phase was washed with water (10 mL), dried over Na2SO4, concentrated, and purified by flash column chromatography to afford chromane-2,4-dione 5.
3.3. Characterization of Products
2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3a). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 79 mg (88%); mp = 154–155 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.99 (s, 1H, OH), 7.88 (d, J = 7.4 Hz, 1H, NH), 7.77 (d, J = 5.5 Hz, 1H, ArH), 7.62 (t, J = 7.2 Hz, 1H, ArH), 7.43 (d, J = 8.5 Hz, 2H, ArH), 7.19–7.16 (m, 1H, ArH), 7.10 (d, J = 8.1 Hz, 1H, ArH), 6.98 (d, J = 8.6 Hz, 2H, ArH), 6.09 (d, J = 5.5 Hz, 1H, O-CH), 3.76 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 177.0, 156.8, 156.8, 136.5, 136.1, 129.3, 126.9, 122.6, 122.3, 119.2, 117.0, 117.0, 115.3, 115.3, 95.9, 55.8; HRMS (TOF ES+): m/z calcd for C16H14N2NaO4+ [(M+Na)+], 321.0846, found, 321.0858.
2-Hydroxy-5-methoxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3b). VPetroleum ether/VEthyl acetate = 5:1, Rf = 0.2; Red solid: 88 mg (89%); mp = 199–200 °C; 1H NMR (600 MHz, DMSO-d6): δ = 14.09 (s, 1H, OH), 7.67 (d, J = 5.7 Hz, 1H, NH), 7.50 (t, J = 8.3 Hz, 1H, ArH), 7.39 (d, J = 8.9 Hz, 2H, ArH), 6.95 (d, J = 8.9 Hz, 2H, ArH), 6.76 (d, J = 8.4 Hz, 1H, ArH), 6.64 (d, J = 8.2 Hz, 1H, ArH), 5.95 (d, J = 5.7 Hz, 1H, O-CH), 3.85 (s, 3H, ArOCH3), 3.74 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 176.8, 160.9, 157.9, 156.0, 136.2, 136.1, 129.2, 116.2, 116.2, 114.8, 114.8, 112.1, 110.8, 105.5, 95.4, 56.0, 55.4; HRMS (TOF ES+): m/z calcd for C17H16N2NaO5+ [(M+Na)+], 351.0951, found, 351.0960.
2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-6-methylchroman-4-one (3c). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 80 mg (85%); mp = 164–165 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.97 (s, 1H, OH), 7.69 (d, J = 5.7 Hz, 1H, NH), 7.64 (s, 1H, ArH), 7.42–7.40 (m, 3H, ArH), 7.00–6.96 (m, 3H, ArH), 6.04 (d, J = 5.6 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3), 2.31 (s, 3H, ArCH3); 13C NMR (150 MHz, DMSO-d6): δ = 176.8, 156.3, 154.4, 137.0, 135.7, 131.2, 129.1, 126.0, 121.6, 118.6, 116.5, 116.5, 114.9, 114.9, 95.4, 55.4, 20.2; HRMS (TOF ES+): m/z calcd for C17H16N2NaO4+ [(M+Na)+], 335.1002, found, 335.1006.
6-Bromo-2-hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3d). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 75 mg (66%); mp = 180–181 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.95 (s, 1H, OH), 7.91–7.89 (m, 1H, ArH), 7.88 (d, J = 5.9 Hz, 1H, NH), 7.76–7.73 (m, 1H, ArH), 7.45–7.42 (m, 2H, ArH), 7.10–7.08 (m, 1H, ArH), 6.97 (d, J = 9.3 Hz, 2H, ArH), 6.10 (d, J = 5.6 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 174.9, 156.7, 155.5, 138.3, 135.5, 128.4, 128.3, 123.6, 121.4, 116.9, 116.9, 114.9, 114.9, 113.9, 95.7, 55.4; HRMS (TOF ES+): m/z calcd for C16H13BrN2NaO4+ [(M+Na)+], 398.9951, found, 398.9987.
6-Fluoro-2-hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3e). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 64 mg (68%); mp = 160–161 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.97 (s, 1H, OH), 7.80 (d, J = 5.9 Hz, 1H, NH), 7.56–7.54 (m, 1H, ArH), 7.51–7.47 (m, 1H, ArH), 7.44 (d, J = 8.6 Hz, 2H, ArH), 7.17–7.15 (m, 1H, ArH), 6.98 (d, J = 8.9 Hz, 2H, ArH), 6.08 (d, J = 5.8 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 175.4, 157.1 (C–F, J = 238.5 Hz), 156.6, 152.7, 135.6, 128.5, 123.3 (C–F, J = 24.4 Hz), 122.6 (C–F, J = 6.5 Hz), 120.8 (C–F, J = 7.9 Hz), 116.8, 116.8, 114.9, 114.9, 111.3 (C–F, J = 23.6 Hz), 95.5, 55.4; 19F NMR (470 MHz, DMSO-d6) δ = -120.9; HRMS (TOF ES+): m/z calcd for C16H13FN2NaO4+ [(M+Na)+], 339.0752, found, 339.0756.
2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-6-phenylchroman-4-one (3f). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.1; Red solid: 76 mg (68%); mp = 185–186 °C; 1H NMR (500 MHz, DMSO-d6): δ = 14.04 (s, 1H, OH), 8.07 (d, J = 2.4 Hz, 1H,ArH), 7.92–7.90 (m, 1H, ArH), 7.82 (d, J = 5.8 Hz, 1H, NH), 7.67 (d, J = 9.0 Hz, 2H, ArH), 7.50–7.47 (m, 2H, ArH), 7.43 (d, J = 9.0 Hz, 2H, ArH), 7.39–7.36 (m, 1H, ArH), 7.20 (d, J = 8.5 Hz, 1H, ArH), 6.99–6.97 (m, 2H, ArH), 6.13 (d, J = 5.8 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3); 13C NMR (125 MHz, DMSO-d6): δ = 176.4, 156.4, 155.9, 138.9, 135.6, 134.4, 134.2, 129.1, 129.1, 128.9, 127.5, 126.4, 126.4, 123.9, 122.1, 119.4, 116.6, 116.6, 114.9, 114.9, 95.6, 55.4; HRMS (TOF ES+): m/z calcd for C22H18N2NaO4+ [(M+Na)+], 397.1159, found, 397.1156.
6-([1,1′-Biphenyl]-4-yl)-2-hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3g). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.1; Red solid: 95 mg (70%); mp = 225–226 °C; 1H NMR (500 MHz, Pyridine-d5): δ = 14.65 (s, 1H, OH), 8.56–8.55 (m, 1H, ArH), 7.95–7.92 (m, 1H, NH), 7.79–7.74 (m, 7H, ArH), 7.53–7.48 (m, 4H, ArH), 7.44–7.39 (m, 2H, ArH), 7.07–7.04 (m, 2H, ArH), 6.83 (s, 1H, O-CH), 3.69 (s, 3H, ArOCH3); 13C NMR (125 MHz, Pyridine-d5): δ = 178.4, 157.7, 157.7, 141.3, 140.7, 139.5, 137.0, 135.2, 135.0, 130.9, 129.9, 129.9, 128.4, 128.4, 128.3, 128.0, 128.0, 127.8, 127.8, 125.6, 120.5, 117.4, 117.4, 115.7, 115.7, 97.7, 55.9, 55.9; HRMS (TOF ES+): m/z calcd for C28H22N2NaO4+ [(M+Na)+], 473.1472, found, 473.1469.
2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-6-(naphthalen-2-yl)chroman-4-one (3h). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.1; Red solid: 102 mg (80%); mp = 187–188 °C; 1H NMR (600 MHz, DMSO-d6): δ = 14.07 (s, 1H, OH), 8.22 (s, 2H, ArH), 8.07–8.05 (m, 1H, ArH), 8.02 (d, J = 8.3 Hz, 2H, ArH), 7.94 (d, J = 7.8 Hz, 1H, NH), 7.87 (d, J = 5.9 Hz, 1H, ArH), 7.84 (d, J = 10.4 Hz, 1H, ArH), 7.55–7.52 (m, 2H, ArH), 7.43 (d, J = 6.7 Hz, 2H, ArH), 7.24 (d, J = 8.5 Hz, 1H, ArH), 6.98 (d, J = 8.9 Hz, 2H, ArH), 6.14 (d, J = 5.8 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 176.4, 156.5, 156.1, 136.3, 135.7, 134.7, 134.0, 133.4, 132.3, 128.9, 128.8, 128.3, 127.6, 126.6, 126.3, 125.0, 124.8, 124.2, 122.2, 119.6, 116.6, 116.6, 114.9, 114.9, 95.7, 55.4; HRMS (TOF ES+): m/z calcd for C26H20N2NaO4+ [(M+Na)+], 447.1315, found, 447.1313.
N-(2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-4-oxochroman-6-yl)acetamide (3i). VPetroleum ether/VEthyl acetate = 3:1, Rf = 0.2; Red solid: 85 mg (80%); mp > 250 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.97 (s, 1H, OH), 10.08 (s, 1H, ArNH), 8.14 (d, J = 2.7 Hz, 1H, N=NH), 7.74–7.72 (m, 1H, ArH), 7.70 (d, J = 5.9 Hz, 1H, ArH), 7.42 (d, J = 8.5 Hz, 2H, ArH), 7.05 (d, J = 8.8, 1H, ArH), 6.97 (d, J = 8.6 Hz, 2H, ArH), 6.04 (d, J = 5.8 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3), 2.05 (s, 3H, C-CH3); 13C NMR (150 MHz, DMSO-d6): δ = 176.5, 168.3, 156.4, 152.1, 135.7, 133.9, 129.0, 127.4, 121.7, 119.0, 116.6, 116.6, 115.9, 115.9, 114.9, 95.4, 55.4, 23.9; HRMS (TOF ES+): m/z calcd for C18H17N3NaO5+ [(M+Na)+], 378.1060, found, 378.1064.
7-Bromo-2-hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3j). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 75 mg (66%); mp = 190–191 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.96 (s, 1H, OH), 7.91 (d, J = 5.9 Hz, 1H, NH), 7.78 (d, J = 8.2 Hz, 1H, ArH), 7.43–7.42 (m, 2H, ArH), 7.38–7.35 (m, 2H, ArH), 6.97 (d, J = 9.1 Hz, 2H, ArH), 6.11 (d, J = 5.9 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 175.6, 156.8, 156.5, 135.6, 129.2, 128.5, 128.2, 125.5, 121.5, 121.2, 116.7,116.7, 114.9, 114.9, 96.1, 55.4; HRMS (TOF ES+): m/z calcd for C16H13BrN2NaO4+ [(M+Na)+], 398.9951, found, 398.9944.
7-Chloro-2-hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3k). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 65 mg (65%); mp = 183–184 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.96 (s, 1H, OH), 7.92 (d, J = 5.9 Hz, 1H, NH), 7.85 (d, J = 8.5 Hz, 1H, ArH), 7.41 (d, J = 8.6 Hz, 2H, ArH), 7.22 (d, J = 7.1 Hz, 2H, ArH), 6.96 (d, J = 8.5 Hz, 2H, ArH), 6.11 (d, J = 5.8 Hz, 1H, O-CH), 3.74 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 175.5, 157.0, 156.6, 140.2, 135.6, 128.4, 128.2, 122.7, 120.9, 118.6, 116.7, 116.7, 114.9, 114.9, 96.1, 55.4; HRMS (TOF ES+): m/z calcd for C16H13ClN2NaO4+ [(M+Na)+], 355.0456, found, 355.0460.
2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-7-(thiophen-2-yl)chroman-4-one (3l). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 82 mg (72%); mp = 197–198 °C; 1H NMR (500 MHz, DMSO-d6): δ = 14.04 (s, 1H, OH), 8.02 (d, J = 2.4 Hz, 1H, ArH), 7.92–7.90 (m, 1H, ArH), 7.83 (d, J = 5.9 Hz, 1H, NH), 7.55 (d, J = 5.1 Hz, 1H, ArH), 7.50 (d, J = 3.6 Hz, 1H, ArH), 7.43 (d, J = 9.0 Hz, 2H, ArH), 7.17–7.14 (m, 2H, ArH), 6.98 (d, J = 9.0 Hz, 2H, ArH), 6.12 (d, J = 5.9 Hz, 1H, O-CH), 3.76 (s, 3H, ArOCH3); 13C NMR (125 MHz, DMSO-d6): δ = 176.0, 156.5, 155.8, 142.1, 135.6, 133.0, 128.8, 128.7, 128.0, 125.6, 123.7, 122.4, 122.0, 119.6, 116.6, 116.6, 114.9, 114.9, 95.7, 55.4; HRMS (TOF ES+): m/z calcd for C20H16N2NaO4S+ [(M+Na)+], 403.0723, found, 403.0721.
2-Hydroxy-6,7-dimethoxy-3-(2-(4-methoxyphenyl)hydrazineylidene)chroman-4-one (3m). VPetroleum ether/VEthyl acetate = 5:1, Rf = 0.1; Red solid: 100 mg (93%); mp = 179–180 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.90 (s, 1H, OH), 7.71 (d, J = 6.0 Hz, 1H, NH), 7.34 (d, J = 8.6 Hz, 2H, ArH), 7.22 (s, 1H, ArH), 6.96 (d, J = 8.5 Hz, 2H, ArH), 6.65 (s, 1H, ArH), 6.01 (d, J = 5.8 Hz, 1H, O-CH), 3.85 (s, 3H, ArOCH3), 3.78 (s, 3H, ArOCH3), 3.74 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 175.8, 156.3, 156.0, 153.1, 144.7, 135.9, 129.0, 116.1, 116.1 114.9, 114.9, 113.7, 106.4, 101.5, 95.8, 56.3, 55.8, 55.4; HRMS (TOF ES+): m/z calcd for C18H18N2NaO6+ [(M+Na)+], 381.1057, found, 381.1061.
6-Chloro-2-hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-7-methylchroman-4-one (3n). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 73 mg (70%); mp = 183–184 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.92 (s, 1H, OH), 7.82 (d, J = 5.9 Hz, 1H, NH), 7.74 (s, 1H, ArH), 7.42 (d, J = 8.6 Hz, 2H, ArH), 7.14 (s, 1H, ArH), 6.97 (d, J = 8.6 Hz, 2H, ArH), 6.07 (d, J = 5.8 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3), 2.37 (s, 3H, ArCH3); 13C NMR (150 MHz, DMSO-d6): δ = 175.1, 156.5, 154.9, 144.3, 135.6, 128.4, 126.9, 125.6, 121.3, 121.2, 116.7, 116.7, 114.9, 114.9, 95.7, 55.4, 20.3; HRMS (TOF ES+): m/z calcd for C17H15ClN2NaO4+ [(M+Na)+], 369.0613, found, 369.0611.
2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-6,8-dimethylchroman-4-one (3o). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 88mg (90%); mp = 134–135 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.98 (s, 1H, OH), 7.62 (s, 1H, NH), 7.47 (s, 1H, ArH), 7.40 (d, J = 8.6 Hz, 2H, ArH), 7.29 (s, 1H, ArH), 6.96 (d, J = 8.6 Hz, 2H, ArH), 6.08 (s, 1H, O-CH), 3.74 (s, 3H, ArOCH3), 2.27 (s, 3H, ArCH3), 2.20 (s, 3H, ArCH3); 13C NMR (150 MHz, DMSO-d6): δ = 177.2, 156.3, 152.7, 137.8, 135.8, 130.5, 129.1, 127.3, 123.6, 121.4, 116.5, 116.5, 114.9, 114.9, 95.2, 55.4, 20.2, 15.6; HRMS (TOF ES+): m/z calcd for C18H18N2NaO4+ [(M+Na)+], 349.1159, found, 349.1169.
6,8-Dichloro-2-hydroxy-3-(2-phenylhydrazineylidene)chroman-4-one (3p). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 66 mg (60%); mp = 198–199 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.97 (s, 1H, OH), 8.15 (d, J = 6.1 Hz, 1H, ArH), 7.94 (d, J = 2.7 Hz, 1H, ArH), 7.74 (d, J = 2.6 Hz, 1H, NH), 7.46 (d, J = 8.6 Hz, 2H, ArH), 6.98 (d, J = 8.5 Hz, 2H, ArH), 6.24 (d, J = 6.0 Hz, 1H, O-CH), 3.76 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 173.9, 156.9, 150.8 135.4, 134.6, 127.7, 126.1, 124.4, 124.0, 124.0, 117.1, 117.1, 114.9, 114.9, 96.3, 55.4; HRMS (TOF ES+): m/z calcd for C16H12Cl2N2NaO4+ [(M+Na)+], 389.0066, found, 389.0069.
3-Hydroxy-2-(2-(4-methoxyphenyl)hydrazineylidene)-2,3-dihydro-1H-benzo[f]chromen-1-one (3q). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.1; Red solid: 71 mg (68%); mp = 187–188 °C; 1H NMR (600 MHz, DMSO-d6): δ =14.04 (s, 1H, OH), 8.31 (d, J = 8.3 Hz, 1H, ArH), 8.01 (d, J = 6.1 Hz, 1H, NH), 7.97 (d, J = 8.1 Hz, 1H, ArH), 7.86 (d, J = 8.6 Hz, 1H, ArH), 7.73–7.71 (m, 1H, ArH), 7.66–7.62 (m, 2H, ArH), 7.43 (d, J = 9.0 Hz, 2H, ArH), 6.98 (d, J = 8.9 Hz, 2H, ArH), 6.33 (d, J = 6.0 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 176.5, 156.3, 154.4, 136.9, 135.8, 129.9, 128.7, 128.2, 126.8, 124.9, 123.4, 121.8, 121.2, 116.5, 116.5, 116.3, 114.9, 114.9, 96.2, 55.4; HRMS (TOF ES+): m/z calcd for C20H16N2NaO4+ [(M+Na)+], 371.1002, found, 371.1010.
2-Hydroxy-3-(2-(4-methoxyphenyl)hydrazineylidene)-2,3-dihydro-4H-benzo[h]chromen-4-one (3r). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 73 mg (70%); mp = 190–191 °C; 1H NMR (600 MHz, DMSO-d6): δ =14.03 (s, 1H, OH), 8.31 (d, J = 8.3 Hz, 1H, ArH), 8.01 (d, J = 6.2 Hz, 1H, NH), 7.97 (d, J = 8.2 Hz, 1H, ArH), 7.86 (d, J = 8.6 Hz, 1H, ArH), 7.72 (t, J = 7.5 Hz, 1H, ArH), 7.66–7.63 (m, 2H, ArH), 7.44 (d, J = 6.9 Hz, 2H, ArH), 6.98 (d, J = 8.8 Hz, 2H, ArH), 6.34 (d, J = 6.1 Hz, 1H, O-CH), 3.75 (s, 3H, ArOCH3); 13C NMR (150 MHz, DMSO-d6): δ = 176.5, 156.3, 154.4, 136.9, 135.8, 129.9, 128.7, 128.2, 126.8, 124.9, 123.4, 121.8, 121.2, 116.5, 116.5, 116.3, 114.9, 114.9, 96.2, 55.4; HRMS (TOF ES+): m/z calcd for C20H16N2NaO4+ [(M+Na)+], 371.1002, found, 371.1011.
2-Hydroxy-3-(2-phenylhydrazineylidene)chroman-4-one (3s). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 78 mg (86%); mp = 170–171 °C; 1H NMR (500 MHz, DMSO-d6): δ = 13.78 (s, 1H, OH), 7.90–7.88 (m, 1H, ArH), 7.84 (d, J = 5.7 Hz, 1H, NH), 7.65–7.61 (m, 1H, ArH), 7.46–7.44 (m, 2H, ArH), 7.40–7.37 (m, 2H, ArH), 7.20–7.16 (m, 1H, ArH), 7.12–7.08 (m, 2H, ArH), 6.10 (d, J = 5.8 Hz, 1H, O-CH); 13C NMR (125 MHz, DMSO-d6): δ = 177.3, 156.6, 142.2, 136.4, 129.9, 129.6, 129.6, 126.6, 124.0, 122.3, 121.8, 118.8, 115.1, 115.1, 95.5; HRMS (TOF ES+): m/z calcd for C15H13N2O3 + [(M+H)+], 269.0921, found, 269.0926.
3-(2-(4-Bromophenyl)hydrazineylidene)-2-hydroxychroman-4-one (3t). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 81 mg (78%); mp = 161–162 °C; 1H NMR (500 MHz, DMSO-d6): δ = 13.64 (s, 1H, OH), 7.89 (s, 2H, ArH), 7.64 (s, 1H, NH), 7.54 (s, 2H, ArH), 7.43 (s, 2H, ArH), 7.14 (d, J = 35.4 Hz, 2H, ArH), 6.08 (s, 1H, O-CH); 13C NMR (125 MHz, DMSO-d6): δ = 177.4, 156.7, 141.8, 136.6, 132.3, 132.3, 130.5, 126.7, 122.3, 121.8, 118.9, 117.1, 117.1, 115.6, 95.6; HRMS (TOF ES+): m/z calcd for C15H11BrN2NaO3+ [(M+Na)+], 368.9845, found, 368.9847.
2-Hydroxy-3-(2-(4-(trifluoromethoxy)phenyl)hydrazineylidene)chroman-4-one (3u). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 91 mg (86%); mp = 173–174 °C; 1H NMR (500 MHz, DMSO-d6): δ = 13.65 (s, 1H, OH), 7.90–7.87 (m, 2H, ArH), 7.66–7.62 (m, 1H, NH), 7.56 (d, J = 8.8 Hz, 2H, ArH), 7.37 (d, J = 8.5 Hz, 2H, ArH), 7.18 (t, J = 7.5 Hz, 1H, ArH), 7.11 (d, J = 8.3 Hz, 1H, ArH), 6.09 (d, J = 5.3 Hz, 1H, O-CH); 13C NMR (125 MHz, DMSO-d6): δ = 177.4, 156.7, 144.2, 141.6, 136.6, 130.6, 126.7, 122.5, 122.5, 122.3, 121.8, 120.2 (C-F, J = 256.0 Hz), 118.9, 116.4, 116.4, 95.6; 19F NMR (470 MHz, DMSO-d6) δ = -57.1; HRMS (TOF ES+): m/z calcd for C16H11F3N2NaO4+ [(M+Na)+], 375.0563, found, 375.0563.
3-(2-(4-Ethynylphenyl)hydrazineylidene)-2-hydroxychroman-4-one (3v). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.1; Red solid: 70 mg (80%); mp = 179–180 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.66 (s, 1H, OH), 7.91 (d, J = 5.8 Hz, 1H, NH), 7.89–7.87 (m, 1H, ArH), 7.66–7.63 (m, 1H, ArH), 7.48–7.45 (m, 4H, ArH), 7.19–7.17 (m, 1H, ArH), 7.11 (d, J = 8.3 Hz, 1H, ArH), 6.09 (d, J = 5.8 Hz, 1H, O-CH), 4.15 (s, 1H, C≡CH); 13C NMR (150 MHz, DMSO-d6): δ = 177.5, 156.8, 142.7, 136.7, 133.2, 133.2, 130.9, 126.7, 122.4, 121.8, 119.0, 116.7, 115.2, 115.2, 95.6, 83.6, 80.6; HRMS (TOF ES+): m/z calcd for C17H12N2NaO3+ [(M+Na)+], 315.0740, found, 315.0746.
3-(2-(3-Bromophenyl)hydrazineylidene)-2-hydroxychroman-4-one (3w). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 90 mg (87%); mp = 191–192 °C; 1H NMR (500 MHz, DMSO-d6): δ = 13.54 (s, 1H, OH), 7.89–7.87 (m, 2H, ArH), 7.70 (s, 1H, NH), 7.64 (t, J = 7.9 Hz, 1H, ArH), 7.45 (d, J = 8.1 Hz, 1H, ArH), 7.33–7.29 (m, 1H, ArH), 7.24 (d, J = 7.9 Hz, 1H, ArH), 7.19–7.16 (m, 1H, ArH), 7.11 (d, J = 8.4 Hz, 1H, ArH), 6.10 (d, J = 5.8 Hz, 1H, O-CH); 13C NMR (125 MHz, DMSO-d6): δ = 177.5, 156.7, 144.1, 136.6, 131.4, 130.9, 126.7, 126.1, 122.5, 122.3, 121.7, 118.9, 117.4, 114.2, 95.5; HRMS (TOF ES+): m/z calcd for C15H12N2BrO3+ [(M+H)+], 347.0026, found, 347.0031.
3-(2-(2-Hydroxy-4-oxochroman-3-ylidene)hydrazineyl)benzonitrile (3x). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.1; Yellow solid: 77 mg (88%); mp = 208–209 °C; 1H NMR (500 MHz, DMSO-d6): δ = 13.51 (s, 1H, OH), 7.93 (d, J = 5.7 Hz, 1H, NH), 7.90–7.88 (m, 2H, ArH), 7.81–7.78 (m, 1H, ArH), 7.67–7.63 (m, 1H, ArH), 7.58–7.54 (m, 1H, ArH), 7.51–7.49 (m, 1H, ArH), 7.20–7.17 (m, 1H, ArH), 7.12 (d, J = 8.3 Hz, 1H, ArH), 6.11 (d, J = 5.6 Hz, 1H, O-CH); 13C NMR (125 MHz, DMSO-d6): δ = 177.7, 156.9, 143.4, 136.8, 131.4, 130.8, 126.9, 126.7, 122.4, 121.7, 119.7, 119.0, 118.7, 118.0, 112.2, 95.6; HRMS (TOF ES+): m/z calcd for C16H12N3O3+ [(M+H)+], 294.0873, found, 294.0880.
3-(2-(3,5-Dimethylphenyl)hydrazineylidene)-2-hydroxychroman-4-one (3y). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 76 mg (86%); mp = 167–168 °C; 1H NMR (500 MHz, DMSO-d6): δ= 13.80 (s, 1H, OH), 7.89–7.86 (m, 1H, ArH), 7.81 (d, J = 5.8 Hz, 1H, NH), 7.64–7.60 (m, 1H, ArH), 7.19–7.16 (m, 1H, ArH), 7.10 (d, J = 8.3 Hz, 1H, ArH), 7.05 (s, 2H, ArH), 6.74 (s, 1H, ArH), 6.09 (d, J = 5.7 Hz, 1H, O-CH), 2.27 (s, 6H, ArCH3); 13C NMR (125 MHz, DMSO-d6): δ = 177.2, 156.5, 142.1, 138.9, 138.9, 136.3, 129.7, 126.6, 125.8, 122.2, 121.8, 118.8, 112.8, 112.8, 95.5, 21.0, 21.0; HRMS (TOF ES+): m/z calcd for C17H17N2O3+ [(M+H)+], 297.1234, found, 297.1239.
2-Hydroxy-3-(2-(2-methylnaphthalen-1-yl)hydrazineylidene)chroman-4-one (3z). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 71 mg (84%); mp = 206–207 °C; 1H NMR (500 MHz, DMSO-d6): δ = 14.09 (s, 1H, OH), 7.91 (d, J = 7.8 Hz, 1H, ArH), 7.86 (d, J = 5.8 Hz, 1H, NH), 7.64–7.61 (m, 2H, ArH), 7.30–7.23 (m, 2H, ArH), 7.19–7.16 (m, 1H, ArH), 7.11 (d, J = 8.3 Hz, 1H, NH), 7.04–7.01 (m, 1H, ArH), 6.12 (d, J = 5.8 Hz, 1H, O-CH), 2.32 (s, 3H, ArCH3); 13C NMR (125 MHz, DMSO-d6): δ = 177.5, 156.6, 140.0, 136.5, 130.9, 130.8, 127.4, 126.7, 123.9, 123.5, 122.3, 121.8, 118.8, 113.3, 95.4, 16.5; HRMS (TOF ES+): m/z calcd for C16H15N2O3+ [(M+H)+], 283.1077, found, 283.1083.
3-(2-(2-Chlorophenyl)hydrazineylidene)-2-hydroxychroman-4-one (3a’). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Yellow solid: 79 mg (87%); mp = 208–209 °C; 1H NMR (500 MHz, DMSO-d6): δ = 14.07 (s, 1H, OH), 7.95 (d, J = 5.9 Hz, 1H, NH), 7.93–7.91 (m, 1H, ArH), 7.74 (d, J = 8.3 Hz, 1H, ArH), 7.67–7.64 (m, 1H, ArH), 7.52 (d, J = 8.0 Hz, 1H, ArH), 7.41 (t, J = 7.9 Hz, 1H, ArH), 7.20–7.17 (m, 1H, ArH), 7.13–7.09 (m, 2H, ArH), 6.12 (d, J = 5.9 Hz, 1H, O-CH); 13C NMR (125 MHz, DMSO-d6): δ = 178.1, 156.8, 138.5, 136.9, 132.3, 129.7, 128.7, 126.9, 124.5, 122.4, 121.6, 119.0, 118.9, 115.0, 95.3; HRMS (TOF ES+): m/z calcd for C15H12ClN2O3+ [(M+H)+], 303.0531, found, 303.0538.
2-Hydroxy-3-(2-(naphthalen-2-yl)hydrazineylidene)chroman-4-one (3b’). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.2; Red solid: 81 mg (85%); mp = 177–178 °C; 1H NMR (500 MHz, DMSO-d6): δ = 14.00 (s, 1H, OH), 7.95 (d, J = 9.0 Hz, 1H, NH), 7.92–7.86 (m, 5H, ArH), 7.72 (d, J = 8.9 Hz, 1H, ArH), 7.66–7.63 (m, 1H, ArH), 7.51–7.48 (m, 1H, ArH), 7.42–7.39 (m, 1H, ArH), 7.21–7.18 (m, 1H, ArH), 7.12 (d, J = 8.3 Hz, 1H, ArH), 6.16 (d, J = 5.7 Hz, 1H, O-CH); 13C NMR (125 MHz, DMSO-d6): δ =177.3, 156.6, 140.0, 136.5, 133.7, 130.4, 130.4, 129.6, 127.9, 127.3, 127.0, 126.7, 124.9, 122.3, 121.9, 118.9, 115.8, 110.9, 95.6; HRMS (TOF ES+): m/z calcd for C19H15N2O3+ [(M+H)+], 319.1077, found, 319.1086.
Methyl-3-(2-(2-hydroxy-4-oxochroman-3-ylidene)hydrazineyl)thiophene-2-carboxylate (3c’). VPetroleum ether/VEthyl acetate = 10:1, Rf = 0.1; Red solid: 81 mg (81%); mp = 202–203 °C; 1H NMR (500 MHz, DMSO-d6): δ = 14.49 (s, 1H, OH), 7.97 (d, J = 5.8 Hz, 1H, NH), 7.92–7.90 (m, 2H, ArH), 7.67–7.63 (m, 1H, ArH), 7.46 (d, J = 5.5 Hz, 1H, ArH), 7.20–7.17 (m, 1H, ArH), 7.11 (d, J = 8.3 Hz, 1H, ArH), 6.06 (d, J = 5.9 Hz, 1H, O-CH), 3.88 (s, 3H, O-CH3); 13C NMR (125 MHz, DMSO-d6): δ = 177.5, 162.7, 156.8, 148.9, 136.9, 134.1, 132.2, 126.9, 122.4, 121.8, 119.0, 118.3, 106.7, 95.4, 52.2; HRMS (TOF ES+): m/z calcd for C15H13N2O5S+ [(M+H)+], 333.0540, found, 333.0545.
N-(4-(2-(2-Hydroxy-4-oxochroman-3-ylidene)hydrazineyl)phenyl)acetamide (3d’). VPetroleum ether/VEthyl acetate = 3:1, Rf = 0.2; Red solid: 73 mg (75%); mp = 230–231 °C; 1H NMR (600 MHz, DMSO-d6): δ = 13.93 (s, 1H, OH), 10.00 (s, 1H, Ar-NH), 7.88–7.86(m, 1H, ArH), 7.81 (d, J = 5.7 Hz, 1H, N=NH), 7.63–7.62 (m, 1H, ArH), 7.61–7.58 (m, 2H, ArH), 7.41–7.39 (m, 2H, ArH), 7.19–7.16 (m, 1H, ArH), 7.10 (d, J = 8.2 Hz, 1H, ArH), 6.08 (d, J = 5.8 Hz, 1H, O-CH), 2.03 (s, 3H, C-CH3); 13C NMR (150 MHz, DMSO-d6): δ = 176.9, 168.3, 156.5, 137.5, 136.3, 136.0, 129.4, 126.6, 122.3, 121.9, 120.2, 120.2, 118.8, 115.6, 115.6, 95.5, 24.1; HRMS (TOF ES+): m/z calcd for C17H15N3NaO4+ [(M+Na)+], 348.0955, found, 348.0962.
N-(4-(2-(2-Hydroxy-4-oxochroman-3-ylidene)hydrazineyl)phenyl)-4,7,7-trimethyl-3-oxo-2-oxabicyclo[2.2.1]heptane-1-carboxamide (3e’). VPetroleum ether/VEthyl acetate = 5:1, Rf = 0.2; Red solid: 86 mg (61%); mp = 230–231 °C; 1H NMR (500 MHz, DMSO-d6): δ = 13.88 (s, 1H, OH), 9.88 (s, 1H, C-NH), 7.88 (d, J = 7.8 Hz, 1H, ArH), 7.84 (d, J = 5.7 Hz, 1H, N=NH), 7.73 (d, J = 8.4 Hz, 2H, ArH), 7.62 (s, 1H, ArH)), 7.42 (d, J = 8.5 Hz, 2H, ArH), 7.19–7.16 (m, 1H, ArH), 7.10 (d, J = 8.4 Hz, 1H, ArH), 6.08 (s, 1H, O-CH), 2.03–1.87 (m, 3H, C-CH2), 1.58 (s, 1H C-CH2), 1.05–1.02 (m, 6H, C-CH3), 0.90 (s, 3H, C-CH3); 13C NMR (125 MHz, DMSO-d6): δ = 178.2, 177.1, 165.3, 156.6, 138.6, 136.4, 134.3, 129.7, 126.6, 122.3, 122.3, 121.9, 121.4, 118.9, 115.2, 115.2, 95.5, 92.0, 54.7, 53.7, 30.1, 28.5, 16.7, 16.5, 9.7.; HRMS (TOF ES+): m/z calcd for C25H26N3O6+ [(M+H)+], 464.1816, found, 464.1822.
3-(2-Benzyl-2-(4-methoxyphenyl)hydrazineylidene)-2-(benzyloxy)-6,8-dimethylchroman-4-one (4). VPetroleum ether/VEthyl acetate = 5:1, Rf = 0.2; Red solid: 23 mg (15%); mp = 140–141 °C; 1H NMR (600 MHz, DMSO-d6): δ = 7.66 (d, J = 8.4 Hz, 2H, ArH), 7.53 (d, J = 7.5 Hz, 2H, ArH), 7.45–7.42 (m, 2H, ArH), 7.40–7.37 (m, 1H, ArH), 7.33–7.27 (m, 6H, ArH), 7.13 (s, 1H, ArH), 7.08 (d, J = 8.5 Hz, 2H, ArH), 6.61 (s, 1H, O-CH), 5.89 (d, J = 12.0 Hz, 1H, N-CH2), 5.76 (d, J = 12.0 Hz, 1H, N-CH2), 4.75 (d, J = 3.3 Hz, 2H, N-CH2), 3.83 (s, 3H, ArOCH3), 2.23 (d, J = 13.7 Hz, 6H, ArCH3); 13C NMR (150 MHz, DMSO-d6): δ = 161.0, 151.9, 148.7, 146.9, 137.5, 137.2, 134.1, 130.5, 128.6, 128.6, 128.4, 128.3, 128.3, 128.2, 128.2, 128.1, 128.1, 127.8, 127.8, 125.8, 124.0, 124.0, 122.1, 118.7, 114.7, 114.7, 93.7, 77.3, 69.6, 55.6, 20.5, 15.2; HRMS (TOF ES+): m/z calcd for C32H30N2O4Na+ [(M+Na)+], 529.2098, found, 529.2111.
3-(2-(4-Methoxyphenyl)hydrazineylidene)-6,8-dimethylchromane-2,4-dione (5). VPetroleum ether/VEthyl acetate = 5:1, Rf = 0.2; Red solid: 57 mg (59%); mp = 133–134 °C; 1H NMR (600 MHz, DMSO-d6): δ = 8.42 (s, 1H, NH), 7.32 (s, 2H, ArH), 7.13–7.10 (m, 2H, ArH), 6.88 (d, J = 9.1 Hz, 2H, ArH), 3.69 (s, 3H, ArOCH3), 2.34 (s, 3H, CH3), 2.14 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6): δ = 182.5, 160.2, 155.7, 143.4, 136.0, 134.8, 133.7, 131.2, 130.5, 128.6, 121.7, 116.4, 116.4, 114.7, 114.7, 55.3, 20.3, 16.0; HRMS (TOF ES+): m/z calcd for C18H16N2O4Na+ [(M+Na)+], 347.1002, found, 347.0993.
4. Conclusions
In summary, we have developed a novel method for the simultaneous functionalization of the α- and β-positions of o-hydroxyaryl enaminones, utilizing water as a nucleophile and diazonium salts as an electrophilic reagent, to efficiently construct a 2,3-disubstituted chromone skeleton. This approach features a broad substrate scope, is environmentally friendly, and operates efficiently without the need for any metal catalysts. The scalability of the reaction was demonstrated through gram-scale experiments, highlighting its potential for industrial synthesis applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30061194/s1. Figure S1: X-Ray crystal structure of 3a; Table S1: Crystal data and structure refinement for 3a; Figures S2–S69: 1H NMR and 13C NMR spectra.
Author Contributions
X.W. and M.P. contributed equally to this article; conceptualization, F.Y. and L.C.; methodology, F.Y. and L.C.; validation, F.Y., X.W., and M.P.; investigation, X.W., M.P., Y.W., S.S., and Y.X.; resources, F.Y. and L.C.; data curation, F.Y., X.W., and M.P.; writing—original draft preparation, L.C.; writing—review and editing, F.Y. and L.C.; supervision, F.Y.; project administration, F.Y.; and funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by financial support from the National Natural Science Foundation of China (21961018), and the Plan of Funding Out-standing Young Talents of Yunnan Province.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gaspar, A.; Matos, M.J.; Uriarte, J.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.M.; Pinto, D.; Silva, A. Chromones: A promising ring system for new anti-inflammatory drugs. ChemMedChem 2016, 11, 2252–2260. [Google Scholar] [CrossRef]
- Reis, J.; Gaspar, A.; Milhazes, N.; Borges, F. Chromone as a privileged scaffold in drug discovery: Recent advances. J. Med. Chem. 2017, 60, 7941–7957. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Bao, J.; Liu, K.-S.; Zhang, X.-Y.; He, F.; Wang, Y.-F.; Nong, X.-H.; Qi, S.-H. Cytotoxic dihydrothiophene-condensed chromones from the marine-derived fungus penicillium oxalicum. Planta Med. 2013, 79, 1474–1479. [Google Scholar] [CrossRef]
- Nishijima, S.; Sugaya, K.; Miyazato, M.; Shimabukuro, S.; Morozumi, M.; Ogawa, Y. Activation of the rostral pontine reticular formation increases the spinal glycine level and inhibits bladder contraction in rats. J. Urol. 2005, 173, 1812–1816. [Google Scholar] [CrossRef]
- al-Rashida, M.; Batool, G.; Sattar, A.; Ejaz, S.A.; Khan, S.; Lecka, J.; Sévigny, J.; Hameed, A.; Iqbal, J. 2-Alkoxy-3-(sulfonylarylaminomethylene)-chroman-4-ones as potent and selective inhibitors of ectonucleotidases. Eur. J. Med. Chem. 2016, 115, 484–494. [Google Scholar] [CrossRef]
- Cortés, I.; Cordisco, E.; Kaufman, T.S.; Sortino, M.A.; Svetaz, L.A.; Bracca, A.B.J. First total synthesis of chromanone A, preparation of related compounds and evaluation of their antifungal activity against Candida albicans, a biofilm forming agent. RSC Adv. 2021, 11, 19587–19597. [Google Scholar] [CrossRef]
- Łazarenkow, A.; Nawrot-Madranka, J.; Brzezińska, E.; Krajewska, U.; Rόżalski, M. Synthesis, preliminary cytotoxicity evaluation of new 3-formylchromone hydrazones and phosphorohydrazone derivatives of coumarin and chromone. Med. Chem. Res. 2012, 21, 1861–1868. [Google Scholar] [CrossRef]
- Micek, I.; Nawrot, J.; Seraszek-Jaros, A.; Jenerowicz, D.; Schroeder, G.; Spizewski, T.; Suchan, A.; Pawlaczyk, M.; Gornowicz-Porowska, J. Taxifolin as a promising ingredient of cosmetics for adult skin. Antioxidants 2021, 10, 1625. [Google Scholar] [CrossRef]
- Lei, J.; Li, Y.; He, L.-J.; Luo, Y.-F.; Tang, D.-Y.; Yan, W.; Lin, H.-K.; Li, H.; Chen, Z.-Z.; Xu, Z.-G. Expeditious access of chromone analogues via a Michael addition-driven multicomponent reaction. Org. Chem. Front. 2020, 7, 987–992. [Google Scholar] [CrossRef]
- Tong, Q.; Xiu, R.-F.; Chen, J.-H.; Zhang, Y.; Cui, B.-D.; Wan, N.-W.; Chen, Y.-Z.; Han, W.-Y. Toward the generation of 2-amino-3-formyl difunctionalized chromones via Pd-enabled rearrangement strategy. ACS Catal. 2023, 13, 12692–12699. [Google Scholar] [CrossRef]
- Looker, J.H.; McMechan, J.H.; Mader, J.W. An amine solvent modification of the Kostanecki-Robinson reaction. Application to the synthesis of flavonols. J. Org. Chem. 1978, 43, 2344–2347. [Google Scholar] [CrossRef]
- Wen, S.-S.; Wang, J.; Luo, Y.-M.; Yang, H. Synthesis of functionalized chromones via organocatalysis. Tetrahedron 2014, 70, 9314–9320. [Google Scholar] [CrossRef]
- Zhou, C.; Dubrovsky, A.V.; Larock, R.C. Diversity-oriented synthesis of 3-iodochromones and heteroatom analogues via ICl-induced cyclization. J. Org. Chem. 2006, 71, 1626–1632. [Google Scholar] [CrossRef]
- Stangier, M.; Messinis, A.M.; Oliveira, J.C.A.; Yu, H.; Ackermann, L. Rhodaelectro-catalyzed access to chromones via formyl C–H activation towards peptide electro-labeling. Nat. Commun. 2021, 12, 4736–4743. [Google Scholar] [CrossRef]
- Irgashev, R.A.; Sosnovskikh, V.Y.; Kalinovich, N.; Kazakova, O.; Rcschenthaler, G.-V. Methyl 2-methoxytetrafluoropropionate as a synthetic equivalent of methyl trifluoropyruvate in the Claisen condensation. The first synthesis of 2-(trifluoroacetyl)chromones and 5-aryl-2-hydroxy-2-(trifluoromethyl)furan-3(2H)-ones. Tetrahedron Lett. 2009, 50, 4903–4905. [Google Scholar] [CrossRef]
- Shen, C.; Li, W.; Yin, H.; Spannenberg, A.; Skrydstrup, T.; Wu, X.-F. Palladium-catalyzed carbonylative synthesis of 2,3-disubstituted chromones. Adv. Synth. Catal. 2016, 358, 466–479. [Google Scholar] [CrossRef]
- Mao, L.-L.; Liu, Y.; Wan, J.-P. An update on the advances in chromone and the derivatives synthesis based on the key chromone annulation of o-hydroxyaryl enaminones. Chin. Chem. Lett. 2024, 110784, in press. [Google Scholar] [CrossRef]
- Fu, L.; Wan, J.-P. Recent advances in the C3-functionalized chromones synthesis by the featured tandem C-H elaboration and chromone annulation of enaminones. Asian J. Org. Chem. 2019, 8, 767–776. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.; Wan, J.-P. Transition metal-free synthesis of 3-trifluoromethyl chromones via tandem C–H trifluoromethylation and chromone annulation of enaminones. Org. Chem. Front. 2020, 7, 2770–2775. [Google Scholar] [CrossRef]
- Thota, P.; Sheelam, K.; Kottawar, S.; Shivakumar, K.; Kaliyaperumal, M.; Yennam, S.; Behera, M. Langlois reagent mediated tandem cyclization of o-hydroxyaryl enaminones for the synthesis of 3-(trifluoromethyl)chromones. Synlett 2022, 33, 1660–1664. [Google Scholar]
- Song, S.; Wang, W.; Sun, J.; Luo, C.; Wu, C.; Li, J. Photo- and electro-induced perfluoroalkylation/cyclization of o-hydroxyaryl enaminones: Synthesis of perfluoroalkyl chromones. Green Synth. Catal. 2024, in press. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Chen, L.; Liu, D.; Sun, Y.; Chai, D.; Chen, X.-B.; Yu, F. Synthesis of functionalized 3-(1H-isochromen)-chromones via Ag2O-catalyzed cascade cyclization reaction of o-hydroxyarylenaminones with o-alkynylbenzaldehydes. Adv. Synth. Catal. 2022, 364, 4440–4446. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, M.; Qiu, Y.; Peng, M.; Zhang, Z.; Song, S.; Chen, X.-B.; Yu, F. Silver-catalyzed cascade bis-heteroannulation reaction of enynones and o-hydroxyphenyl enaminones: Access to highly functionalized 3-furylmethyl chromones. Adv. Synth. Catal. 2024, 366, 2363–2369. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Sun, J.; Zhao, X.-J.; He, Y.-H. Electrochemical-induced solvent-tuned selective C(sp3)–H bond activation towards the synthesis of C3-functionalized chromone derivatives. Chem. Commun. 2024, 60, 5050–5053. [Google Scholar] [CrossRef]
- Zhu, Z.-Q.; Hu, J.-Y.; Xie, Z.-B.; Tang, J.; Le, Z.-G. Visible-light-enabled photosensitizer- and additive-free decarboxylative coupling cyclization of enaminone with N-arylglycine for 3-aminoalkyl chromones. Adv. Synth. Catal. 2022, 364, 2169–2173. [Google Scholar] [CrossRef]
- Cheng, D.; Pu, Y.; Wang, M.; Shen, Y.; Shen, J.; Xu, X.; Yan, J. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)-mediated tandem oxidative-coupling/annulation of o-hydroxyaryl enaminones with cycloheptatriene. Synthesis 2021, 53, 1372–1378. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, M.; Deng, Z.; Yan, X.; Xu, X.; Yan, J. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone mediated tandem oxidative-coupling/annulation of enaminones with 1,3-diarylpropenes for the synthesis of 3-allylchromones. Eur. J. Org. Chem. 2019, 28, 4589–4592. [Google Scholar] [CrossRef]
- Fu, L.; Xu, Z.; Wan, J.-P.; Liu, Y. The domino chromone annulation and a transient halogenation-mediated C–H alkenylation toward 3-vinyl chromones. Org. Lett. 2020, 22, 9518–9523. [Google Scholar] [CrossRef]
- Mkrtchyan, S.; Iaroshenko, V.O. Visible-light-mediated arylation of ortho-hydroxyarylenaminones: Direct access to isoflavones. Chem. Commun. 2020, 56, 2606–2609. [Google Scholar] [CrossRef]
- Mkrtchyan, S.; Iaroshenko, V.O. Arylation of ortho-hydroxyarylenaminones by sulfonium salts and arenesulfonyl chlorides: An access to isoflavones. J. Org. Chem. 2021, 86, 4896–4916. [Google Scholar] [CrossRef]
- Mkrtchyan, S.; Jakubczyk, M.; Lanka, S.; Yar, M.; Mahmood, T.; Ayub, K.; Sillanpää, M.; Thomas, C.M.; Iaroshenko, V.O. Mechanochemical Ni-catalysed arylation of ortho-hydroxyarylenaminones: Synthesis of isoflavones. Adv. Synth. Catal. 2022, 364, 3512–3521. [Google Scholar] [CrossRef]
- Mkrtchyan, S.; Purohit, V.B.; Iaroshenko, V.O. Nanocellulose as convenient reaction media for the FeCl3 mediated mechanochemical synthesis of 3-acylchromones. ACS Sustain. Chem. Eng. 2023, 11, 13877–13884. [Google Scholar] [CrossRef]
- Mkrtchyan, S.; Purohit, V.B.; Khutsishvili, S.; Nociarová, J.; Yar, M.; Mahmood, T.; Ayub, K.; Budzák, S.; Skoršepa, M.; Iaroshenko, V.O. Mechanochemical defluorinative acylation of ortho-hydroxyarylenaminones by CF3-compounds: Synthesis of 3-acylchromones. Adv. Synth. Catal. 2023, 365, 2026–2035. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Zheng, Y.; Qian, Y.-E.; Guan, J.-P.; Lu, W.-D.; Yuan, C.-P.; Xiao, J.-A.; Chen, K.; Xiang, H.-Y.; Yang, H. Photoredox-catalyzed cascade of o-hydroxyarylenaminones to access 3-aminated chromones. J. Org. Chem. 2022, 87, 1477–1484. [Google Scholar] [CrossRef]
- Hu, W.; Diao, X.; Yuan, J.; Liang, W.; Liang, W.; Yang, W.; Yang, L.; Ma, J.; Zhang, S. Photoredox-catalyzed tandem cyclization of enaminones with N-sulfonylaminopyridinium salts toward the synthesis of 3-sulfonaminated chromones. J. Org. Chem. 2024, 89, 644–655. [Google Scholar] [CrossRef]
- Kandula, V.; Thota, P.K.; Mallesham, P.; Raghavulu, K.; Chatterjee, A.; Yennam, S.; Behera, M. Selectfluor-mediated tandem cyclization of enaminones for the synthesis of 3-fluorochromones. Synlett 2019, 30, 2295–2299. [Google Scholar] [CrossRef]
- Lin, Y.; Jin, J.; Wang, C.; Wan, J.-P.; Liu, Y. Electrochemical C–H halogenations of enaminones and electron-rich arenes with sodium halide (NaX) as halogen source for the synthesis of 3-halochromones and haloarenes. J. Org. Chem. 2021, 86, 12378–12385. [Google Scholar] [CrossRef]
- Zhang, T.; Yao, W.; Wan, J.-P.; Liu, Y. Transition-metal-free C(sp2)−H dithiocarbamation and chromone annulation cascade for 3-dithiocarbamyl chromone synthesis. Adv. Synth. Catal. 2021, 363, 4811–4816. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, Z.; Yang, H.; Liu, D.; Sun, Y.; Xu, Y.; Yu, F.; Yan, S.-J. Transition-metal-free C(sp2)−H phosphorothiolation/cyclization of o-hydroxyarylenaminones: Access to S-3-chromon phosphorothioates. Adv. Synth. Catal. 2022, 364, 1602–1606. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, J.; Liu, Y.; Wan, J.-P.; Chen, F.-E. Transition metal-free tunable synthesis of 3-(trifluoromethylthio) and 3-trifluoromethylsulfinyl chromones via domino C-H bond functionalization and chromone annulation of enaminones. Chin. Chem. Lett. 2024, 35, 109683–109686. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Zhang, J.-R.; Huang, G.-B.; Zhou, Y.-H.; Chen, Y.-Y.; Xu, Y.-L. Visible light-promoted selenylation/cyclization of enaminones toward the formation of 3-selanyl-4H-chromen-4-ones. Adv. Synth. Catal. 2021, 363, 1656–1661. [Google Scholar] [CrossRef]
- Cen, K.; Liu, Y.; Yu, J.; Zeng, Z.; Hou, Q.; He, G.; Ouyang, M.; Wang, Q.; Wang, D.; Zhao, F.; et al. Electrocatalytic cascade selenylation/cyclization/deamination of 2-hydroxyaryl enaminones: Synthesis of 3-selenylated chromones under mild conditions. J. Org. Chem. 2024, 89, 8632–8640. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Wan, J.-P.; Liu, Y. Synthesis of chromone-3-phosphonates via Arbuzov-type C−P cross coupling from o-hydroxyphenyl enaminones and phosphites. Adv. Synth. Catal. 2024, 366, 3670–3675. [Google Scholar] [CrossRef]
- Guo, Y.; Xiang, Y.; Wei, L.; Wan, J.-P. Thermoinduced free-radical C-H acyloxylation of tertiary enaminones: Catalyst-free synthesis of acyloxyl chromones and enaminones. Org. Lett. 2018, 20, 3971–3974. [Google Scholar] [CrossRef]
- Engelhart, C.A.; Aldrich, C.C. Synthesis of chromone, quinolone, and benzoxazinone sulfonamide nucleosides as conformationally constrained inhibitors of adenylating enzymes required for siderophore biosynthesis. J. Org. Chem. 2013, 78, 7470–7481. [Google Scholar] [CrossRef]
- Wan, J.-P.; Zhong, S.; Guo, Y.; Wei, L. Iodine-mediated domino C(sp2)–H sulfonylation/annulation of enaminones and sulfonyl hydrazines for the synthesis of 3-sulfonyl chromones. Eur. J. Org. Chem. 2017, 2017, 4401–4404. [Google Scholar] [CrossRef]
- Luo, T.; Wan, J.-P.; Liu, Y. Toward C2-nitrogenated chromones by copper-catalyzed β-C(sp2)–H N-heteroarylation of enaminones. Org. Chem. Front. 2020, 7, 1107–1112. [Google Scholar] [CrossRef]
- Lin, Y.; Wan, J.-P.; Liu, Y. Cascade in situ iodination, chromone annulation, and cyanation for site-selective synthesis of 2-cyanochromones. J. Org. Chem. 2023, 88, 4017–4023. [Google Scholar] [CrossRef]
- Qian, Y.-E.; Zeng, L.; Zhao, Q.-L.; Xiao, J.-A.; Chen, K.; Xiang, H.-Y.; Yang, H. TBN-triggered, manipulable annulations of o-hydroxyarylenaminones for divergent syntheses of oximinochromanones and oximinocoumaranones. Chem. Commun. 2021, 57, 12285–12288. [Google Scholar] [CrossRef]
- Song, S.; Peng, M.; Zhang, Z.; Hu, H.; Wei, Y.; Yan, S.-J.; Wang, Y.; Yu, F. Divergent synthesis of 2-chromonyl-3-hydrazono-chromones and 2-alkoxy-3-hydrazono-chromones through switchable annulation reactions of o-hydroxyphenylenaminones with aryldiazonium salts. Org. Lett. 2024, 26, 4980–4985. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Z.; Peng, M.; Xia, X.; Dong, S.; Wang, Y.-C.; Yu, F. Selective synthesis of pyridazin-fused chromones and 3-pyridazinyl chromones through intermolecular chromone annulation of o-hydroxyphenylenaminones with aryldiazonium salts. Org. Chem. Front. 2024, 11, 3906–3912. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Peng, M.; Song, S.; Wei, Y.; Hu, H.; Wang, X.; Yu, F. Pd(II)-catalyzed regioselective ring opening/[3+2] annulation reaction of enaminones with cyclopropenones: Divergent synthesis of γ-butenolides and γ-lactams. Chem. Commun. 2024, 60, 14968–14971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, L.; Liu, Z.; Huang, J.; Yu, F. Unprecedented chemoselective Ru(III)-catalyzed [3+2] annulation of enaminones with iodonium ylides for the synthesis of functionalized 3a,7a-dihydroxy hexahydro-4H-indol-4-ones. Org. Chem. Front. 2023, 10, 5660–5666. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L.; Sun, H.; Liu, Z.; Yan, S.-J.; Yu, F. Rh(III)-Catalyzed [3+2] annulation/Pinacol rearrangement reaction of enaminones with iodonium ylides: Direct synthesis of 2-spirocyclo-pyrrol-3-ones. Org. Lett. 2023, 25, 7214–7219. [Google Scholar] [CrossRef] [PubMed]
- Tingoli, M.; Mazzella, M.; Panunzi, B.; Tuzi, A. L-Proline-catalyzed activation of methyl ketones or active methylene compounds and DMF-DMA for syntheses of (2E)-3-dimethylamino-2-propen-1-ones. Eur. J. Org. Chem. 2012, 2012, 399–404. [Google Scholar]
- Das, B.; Venkateswarlu, K.; Majhi, V.; Reddy, M.R.; Reddy, K.N.; Yerra Rao, K.; Ravikumar, K.; Sridhar, B. Highly efficient, mild and chemo- and stereoselective synthesis of enaminones and enamino esters using silica supported perchloric acid under solvent-free conditions. J. Mol. Catal. A Chem. 2006, 246, 276–281. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, R.; Wan, J.-P. Water-promoted synthesis of enaminones. Mechanism investigation and application in multicomponent reactions. Synth. Commun. 2013, 43, 2475–2483. [Google Scholar] [CrossRef]
- Wu, J.; Gu, Y.; Leng, X.; Shen, Q. Copper-promoted sandmeyer difluoromethylthiolation of aryl and heteroaryl diazonium salts. Angew. Chem. Int. Ed. 2015, 54, 7648–7652. [Google Scholar] [CrossRef]
- Wu, P.; He, Y.; Wang, H.; Zhou, Y.-G.; Yu, Z. Copper(II)-catalyzed C-H nitrogenation/annulation cascade of ketene N,S-acetals with aryldiazonium salts: A direct access to N2-substituted triazole and triazine derivatives. Org. Lett. 2020, 22, 310–315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).