Luminescence Efficiency Enhanced by Simple Substitutions on Donor and Acceptor in Radicals with Donor–Acceptor Structure
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure Characterization
2.2. Photophysical Properties
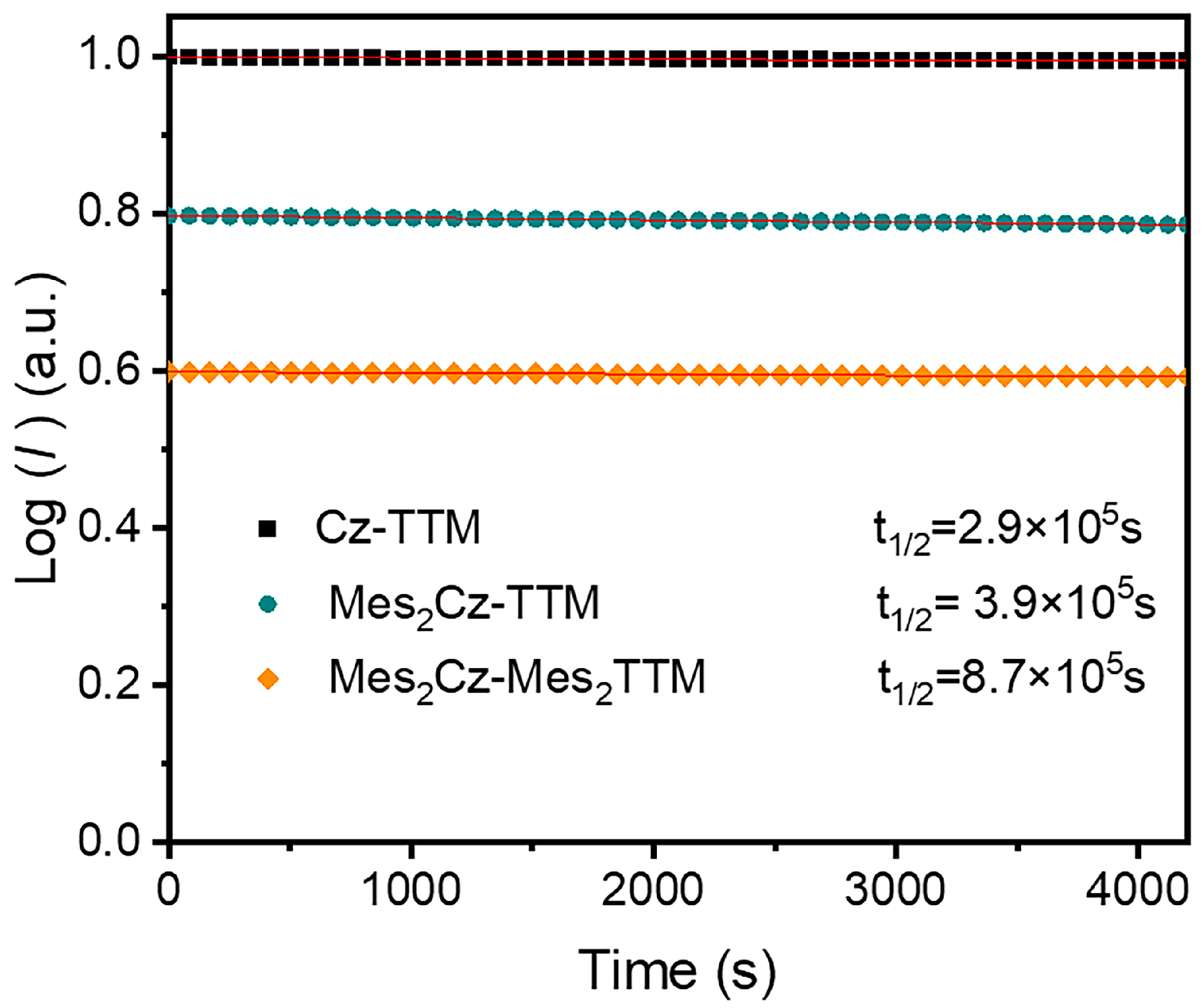
2.3. Stability
2.4. Electrochemistry
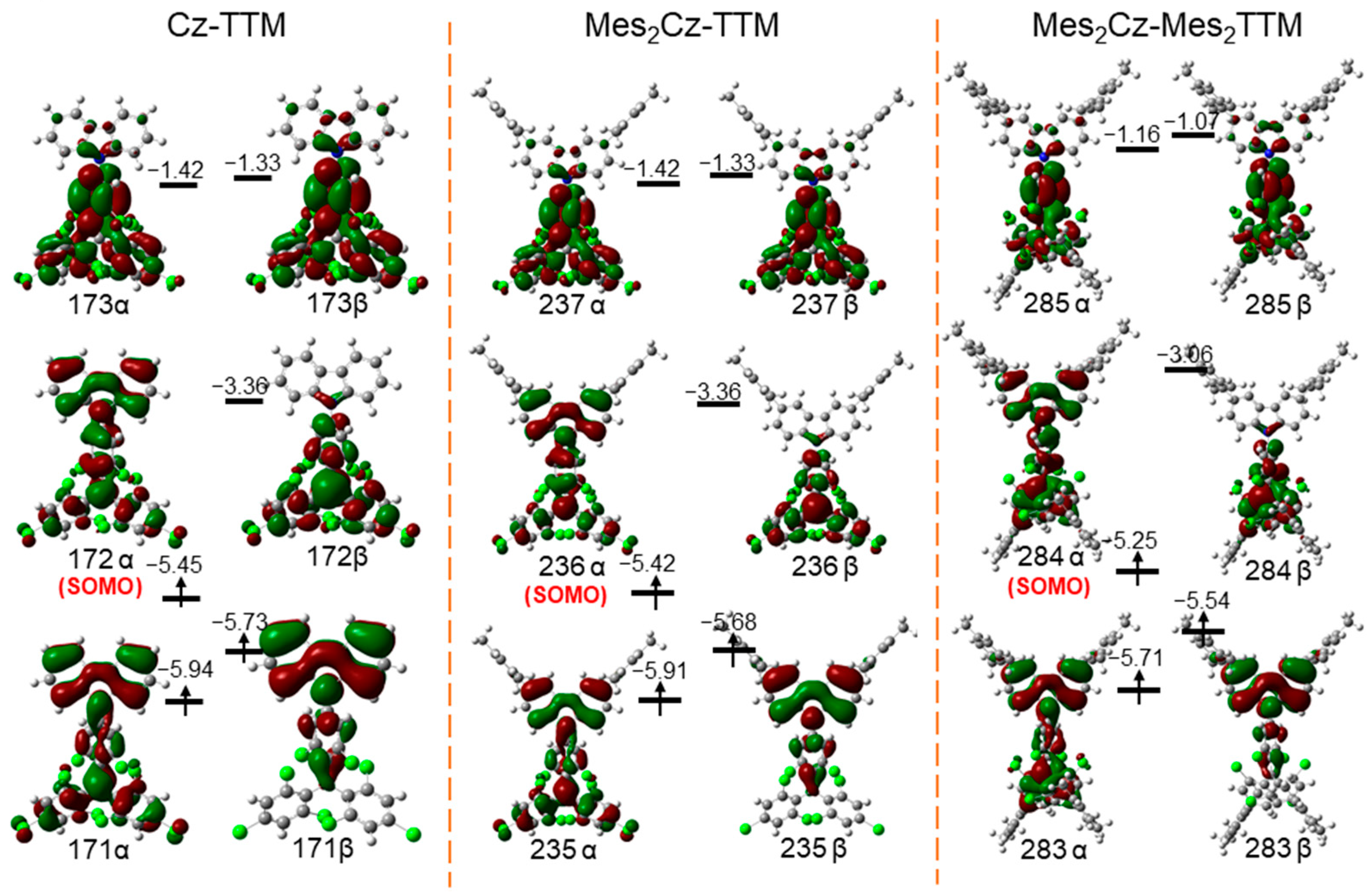
2.5. Theoretical Calculations
3. Materials and Methods
3.1. Synthesis of Compound Mes2Cz-TTM
3.2. Synthesis of Compound Mes2Cz-Mes2TTM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, W.; Li, X.; Baryshnikov, G.V.; Shan, X.; Siddique, F.; Qian, C.; Zhao, S.; Wu, H. Bright Free-Radical Emission in Ionic Liquids. Angew. Chem. Int. Ed. 2023, 62, e202305925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gong, J.; Alam, P.; Ma, C.; Wang, Y.; Guo, J.; Zeng, Z.; He, Z.; Sung, H.H.Y.; Williams, I.D.; et al. A Simple Approach to Achieve Organic Radicals with Unusual Solid-State Emission and Persistent Stability. CCS Chem. 2022, 4, 1912–1920. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, X.; Xie, Y.; Zhang, H.; Hu, L.; Chan, C.C.S.; Zhang, R.; Guo, J.; Kwok, R.T.K.; Lam, J.W.Y.; et al. A nonconjugated radical polymer with stable red luminescence in the solid state. Mater. Horiz. 2022, 9, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Qin, Y.; Zhou, Y.; Zheng, X.; Li, F.; Liu, S.; Ma, Y.; Zhao, Q.; Wong, W.Y. Photoactivated Circularly Polarized Luminescent Organic Radicals in Doped Amorphous Polymer. Angew. Chem. Int. Ed. 2024, 63, e202403660. [Google Scholar] [CrossRef]
- Mu, Y.; Liu, Y.; Tian, H.; Ou, D.; Gong, L.; Zhao, J.; Zhang, Y.; Huo, Y.; Yang, Z.; Chi, Z. Sensitive and Repeatable Photoinduced Luminescent Radicals from A Simple Organic Crystal. Angew. Chem. Int. Ed. 2021, 60, 6367–6371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.L.; Chen, C.; Ren, Y.Y.; Han, Y.F. A platform for blue-luminescent carbon-centered radicals. Nat. Commun. 2022, 13, 5367. [Google Scholar] [CrossRef]
- Abdurahman, A.; Hele, T.J.H.; Gu, Q.; Zhang, J.; Peng, Q.; Zhang, M.; Friend, R.H.; Li, F.; Evans, E.W. Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mater. 2020, 19, 1224–1229. [Google Scholar] [CrossRef]
- Zhu, Z.; Kuang, Z.; Shen, L.; Wang, S.; Ai, X.; Abdurahman, A.; Peng, Q. Dual Channel Emissions of Kasha and Anti-Kasha from a Single Radical Molecule. Angew. Chem. Int. Ed. 2024, 63, e202410552. [Google Scholar]
- Zhao, Y.; Abdurahman, A.; Zhang, Y.; Zheng, P.; Zhang, M.; Li, F. Highly Efficient Multifunctional Luminescent Radicals. CCS Chem. 2022, 4, 722–731. [Google Scholar] [CrossRef]
- Yuan, J.W.; Peng, Q.C.; Fu, J.C.; Yang, Q.; Gao, Z.Y.; Wang, Z.Y.; Li, K.; Zang, S.Q.; Tang, B.Z. Highly Efficient Stable Luminescent Radical-Based X-ray Scintillator. J. Am. Chem. Soc. 2023, 145, 27095–27102. [Google Scholar] [CrossRef]
- Yan, C.; An, D.; Chen, W.; Zhang, N.; Qiao, Y.; Fang, J.; Lu, X.; Zhou, G.; Liu, Y. Stable Diarylamine-Substituted Tris(2,4,6-trichlorophenyl)methyl Radicals: One-Step Synthesis, Near-Infrared Emission, and Redox Chemistry. CCS Chem. 2022, 4, 3190–3203. [Google Scholar] [CrossRef]
- Rui, X.; Ota, W.; Sato, T.; Furukori, M.; Nakayama, Y.; Hosokai, T.; Hisamura, E.; Nakamura, K.; Matsuda, K.; Nakao, K.; et al. Carbazole-Dendronized Luminescent Radicals. Angew. Chem. Int. Ed. 2023, 62, e202302550. [Google Scholar]
- Shi, J.; Xu, W.; Yu, H.; Wang, X.; Jin, F.; Zhang, Q.; Zhang, H.; Peng, Q.; Abdurahman, A.; Wang, M. A Highly Luminescent Metallo-Supramolecular Radical Cage. J. Am. Chem. Soc. 2023, 145, 24081–24088. [Google Scholar] [CrossRef]
- Peng, Q.; Obolda, A.; Zhang, M.; Li, F. Organic Light-Emitting Diodes Using a Neutral pi Radical as Emitter: The Emission from a Doublet. Angew. Chem. Int. Ed. 2015, 54, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Mayorga Burrezo, P.; Jimenez, V.G.; Blasi, D.; Ratera, I.; Campana, A.G.; Veciana, J. Organic Free Radicals as Circularly Polarized Luminescence Emitters. Angew. Chem. Int. Ed. 2019, 58, 16282–16288. [Google Scholar] [CrossRef]
- Li, F.; Gillett, A.J.; Gu, Q.; Ding, J.; Chen, Z.; Hele, T.J.H.; Myers, W.K.; Friend, R.H.; Evans, E.W. Singlet and triplet to doublet energy transfer: Improving organic light-emitting diodes with radicals. Nat. Commun. 2022, 13, 2744. [Google Scholar] [CrossRef]
- Gamero, V.; Velasco, D.; Latorre, S.; López-Calahorra, F.; Brillas, E.; Juliá, L. [4-(N-Carbazolyl)-2,6-dichlorophenyl]bis(2,4,6-trichlorophenyl)methyl radical an efficient red light-emitting paramagnetic molecule. Tetrahedron Lett. 2006, 47, 2305–2309. [Google Scholar] [CrossRef]
- Cho, H.H.; Gorgon, S.; Hung, H.C.; Huang, J.Y.; Wu, Y.R.; Li, F.; Greenham, N.C.; Evans, E.W.; Friend, R.H. Efficient and Bright Organic Radical Light-Emitting Diodes with Low Efficiency Roll-Off. Adv. Mater. 2023, 35, e2303666. [Google Scholar] [CrossRef]
- Chang, X.; Arnold, M.E.; Blinder, R.; Zolg, J.; Wischnat, J.; van Slageren, J.; Jelezko, F.; Kuehne, A.J.C.; von Delius, M. A Stable Chichibabin Diradicaloid with Near-Infrared Emission. Angew. Chem. Int. Ed. 2024, 63, e202404853. [Google Scholar] [CrossRef]
- Abdurahman, A.; Wang, J.; Zhao, Y.; Li, P.; Shen, L.; Peng, Q. A Highly Stable Organic Luminescent Diradical. Angew. Chem. Int. Ed. 2023, 62, e202300772. [Google Scholar] [CrossRef]
- Velasco, D.; Castellanos, S.; López, M.; López-Calahorra, F.; Brillas, E.; Juliá, L. Red organic light-emitting radical adducts of carbazole and tris (2, 4, 6-trichlorotriphenyl) methyl radical that exhibit high thermal stability and electrochemical amphotericity. J. Org. Chem. 2007, 72, 7523–7532. [Google Scholar] [CrossRef]
- Ballester, M.; Castaner, J.; Riera, J.; Ibanez, A.; Pujadas, J. Inert carbon free radicals. 2. Monofunctionalized tetradecachlorotriphenylmethyl radicals and related compounds. J. Org. Chem. 1982, 47, 259–264. [Google Scholar] [CrossRef]
- Heckmann, A.; Dümmler, S.; Pauli, J.; Margraf, M.; Köhler, J.; Stich, D.; Lambert, C.; Fischer, I.; Resch-Genger, U. Highly fluorescent open-shell NIR dyes: The time-dependence of back electron transfer in triarylamine-perchlorotriphenylmethyl radicals. J. Phys. Chem. C 2009, 113, 20958–20966. [Google Scholar] [CrossRef]
- Guo, H.; Peng, Q.; Chen, X.K.; Gu, Q.; Dong, S.; Evans, E.W.; Gillett, A.J.; Ai, X.; Zhang, M.; Credgington, D.; et al. High stability and luminescence efficiency in donor-acceptor neutral radicals not following the Aufbau principle. Nat. Mater. 2019, 18, 977–984. [Google Scholar] [CrossRef]
- Matsuoka, R.; Kimura, S.; Kusamoto, T. Solid-State Room-Temperature Near-Infrared Photoluminescence of a Stable Organic Radical. ChemPhotoChem 2021, 5, 669–673. [Google Scholar] [CrossRef]
- Kimura, S.; Tanushi, A.; Kusamoto, T.; Kochi, S.; Sato, T.; Nishihara, H. A luminescent organic radical with two pyridyl groups: High photostability and dual stimuli-responsive properties, with theoretical analyses of photophysical processes. Chem. Sci. 2018, 9, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Kusamoto, T.; Kimura, S.; Kato, K.; Teki, Y.; Nishihara, H. Magnetoluminescence in a Photostable, Brightly Luminescent Organic Radical in a Rigid Environment. Angew. Chem. Int. Ed. 2018, 57, 12711–12715. [Google Scholar] [CrossRef]
- Kato, K.; Kimura, S.; Kusamoto, T.; Nishihara, H.; Teki, Y. Luminescent Radical-Excimer: Excited-State Dynamics of Luminescent Radicals in Doped Host Crystals. Angew. Chem. Int. Ed. 2019, 58, 2606–2611. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Highly photostable luminescent open-shell (3,5-dihalo-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radicals: Significant effects of halogen atoms on their photophysical and photochemical properties. RSC Adv. 2015, 5, 64802–64805. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Enhanced luminescent properties of an open-shell (3,5-dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl radical by coordination to gold. Angew. Chem. Int. Ed. 2015, 54, 3731–3734. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Luminescence, Stability, and Proton Response of an Open-Shell (3,5-Dichloro-4-pyridyl)bis(2,4,6-trichlorophenyl)methyl Radical. Angew. Chem. 2014, 126, 12039–12042. [Google Scholar] [CrossRef]
- Wu, C.; Lu, C.; Yu, S.; Zhang, M.; Zhang, H.; Zhang, M.; Li, F. Highly Efficient Near-Infrared Luminescent Radicals with Emission Peaks over 750 nm. Angew. Chem. Int. Ed. 2024, 63, e202412483. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, L.; Cui, Z.; Ai, X. Improving the Luminescence and Stability of Carbon-Centered Radicals by Kinetic Isotope Effect. Molecules 2023, 28, 4805. [Google Scholar] [CrossRef]
- Ai, X.; Chen, Y.; Feng, Y.; Li, F. A Stable Room-Temperature Luminescent Biphenylmethyl Radical. Angew. Chem. Int. Ed. 2018, 57, 2869–2873. [Google Scholar] [CrossRef] [PubMed]
- Murto, P.; Li, B.; Fu, Y.; Walker, L.E.; Brown, L.; Bond, A.D.; Zeng, W.; Chowdhury, R.; Cho, H.H.; Yu, C.P.; et al. Steric Control of Luminescence in Phenyl-Substituted Trityl Radicals. J. Am. Chem. Soc. 2024, 146, 13133–13141. [Google Scholar] [CrossRef]
- Murto, P.; Chowdhury, R.; Gorgon, S.; Guo, E.; Zeng, W.; Li, B.; Sun, Y.; Francis, H.; Friend, R.H.; Bronstein, H. Mesitylated trityl radicals, a platform for doublet emission: Symmetry breaking, charge-transfer states and conjugated polymers. Nat. Commun. 2023, 14, 4147. [Google Scholar] [CrossRef]
- Lu, C.; Cho, E.; Cui, Z.; Gao, Y.; Cao, W.; Bredas, J.L.; Coropceanu, V.; Li, F. Towards Efficient and Stable Donor-Acceptor Luminescent Radicals. Adv. Mater. 2023, 35, e2208190. [Google Scholar] [CrossRef]
- Liu, C.H.; Hamzehpoor, E.; Sakai-Otsuka, Y.; Jadhav, T.; Perepichka, D.F. A Pure-Red Doublet Emission with 90% Quantum Yield: Stable, Colorless, Iodinated Triphenylmethane Solid. Angew. Chem. Int. Ed. 2020, 59, 23030–23034. [Google Scholar] [CrossRef]
- Hattori, Y.; Kitajima, R.; Ota, W.; Matsuoka, R.; Kusamoto, T.; Sato, T.; Uchida, K. The simplest structure of a stable radical showing high fluorescence efficiency in solution: Benzene donors with triarylmethyl radicals. Chem. Sci. 2022, 13, 13418–13425. [Google Scholar] [CrossRef]
- Hattori, Y.; Kitajima, R.; Baba, A.; Yamamoto, K.; Matsuoka, R.; Kusamoto, T.; Uchida, K. Effects of hydrocarbon substituents on highly fluorescent bis(4-phenylphenyl)pyridylmethyl radical derivatives. Mater. Adv. 2023, 4, 5149–5159. [Google Scholar] [CrossRef]
- Gou, Q.; Guan, J.; Zhang, L.; Ai, X. Phenyl Derivatives Modulate the Luminescent Properties and Stability of CzBTM-Type Radicals. Molecules 2024, 29, 2900. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Arnold, M.; Kittel, Y.; Blinder, R.; Jelezko, F.; Kuehne, A.J.C. 2,7-Substituted N-Carbazole Donors on Tris(2,4,6-trichlorophenyl)methyl Radicals with High Quantum Yield. Adv. Opt. Mater. 2022, 10, 2102101. [Google Scholar] [CrossRef]
- Ai, X.; Evans, E.W.; Dong, S.; Gillett, A.J.; Guo, H.; Chen, Y.; Hele, T.J.H.; Friend, R.H.; Li, F. Efficient radical-based light-emitting diodes with doublet emission. Nature 2018, 563, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Xiaotian, R.; Nakamura, K.; Furukori, M.; Hosokai, T.; Anraku, K.; Nakao, K.; Albrecht, K. Photostability of luminescent tris(2,4,6-trichlorophenyl)methyl radical enhanced by terminal modification of carbazole donor. Chem. Commun. 2022, 58, 13443–13446. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Matsuda, K.; Xiaotian, R.; Furukori, M.; Miyata, S.; Hosokai, T.; Anraku, K.; Nakao, K.; Albrecht, K. Effects of halogen atom substitution on luminescent radicals: A case study on tris(2,4,6-trichlorophenyl)methyl radical-carbazole dyads. Faraday Discuss. 2024, 250, 192–201. [Google Scholar] [CrossRef]
- Lu, C.; Cho, E.; Wan, K.; Wu, C.; Gao, Y.; Coropceanu, V.; Brédas, J.L.; Li, F. Achieving Nearly 100% Photoluminescence Quantum Efficiency in Organic Radical Emitters by Fine-Tuning the Effective Donor-Acceptor Distance. Adv. Funct. Mater. 2024, 34, 2314811. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.02; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
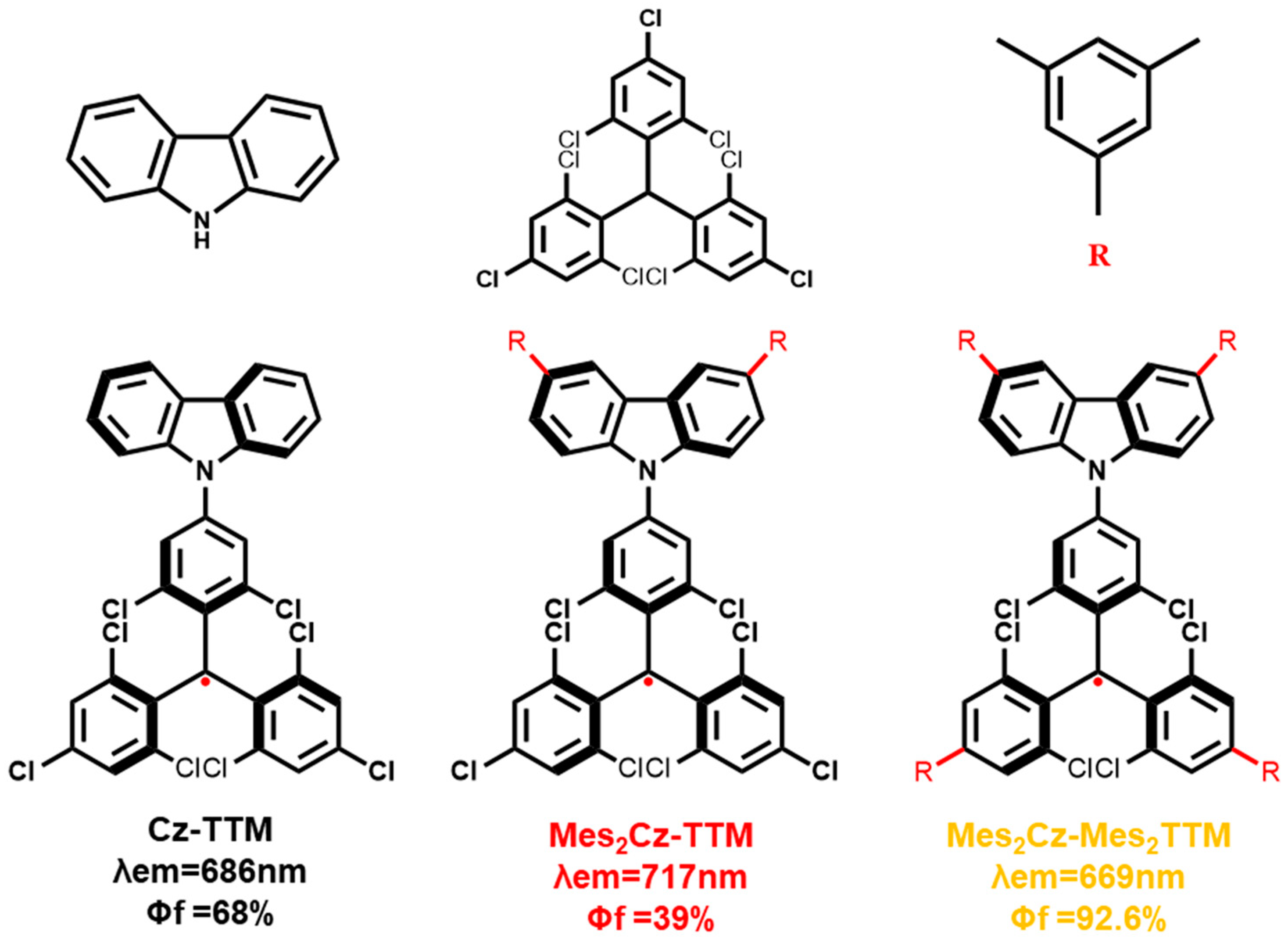
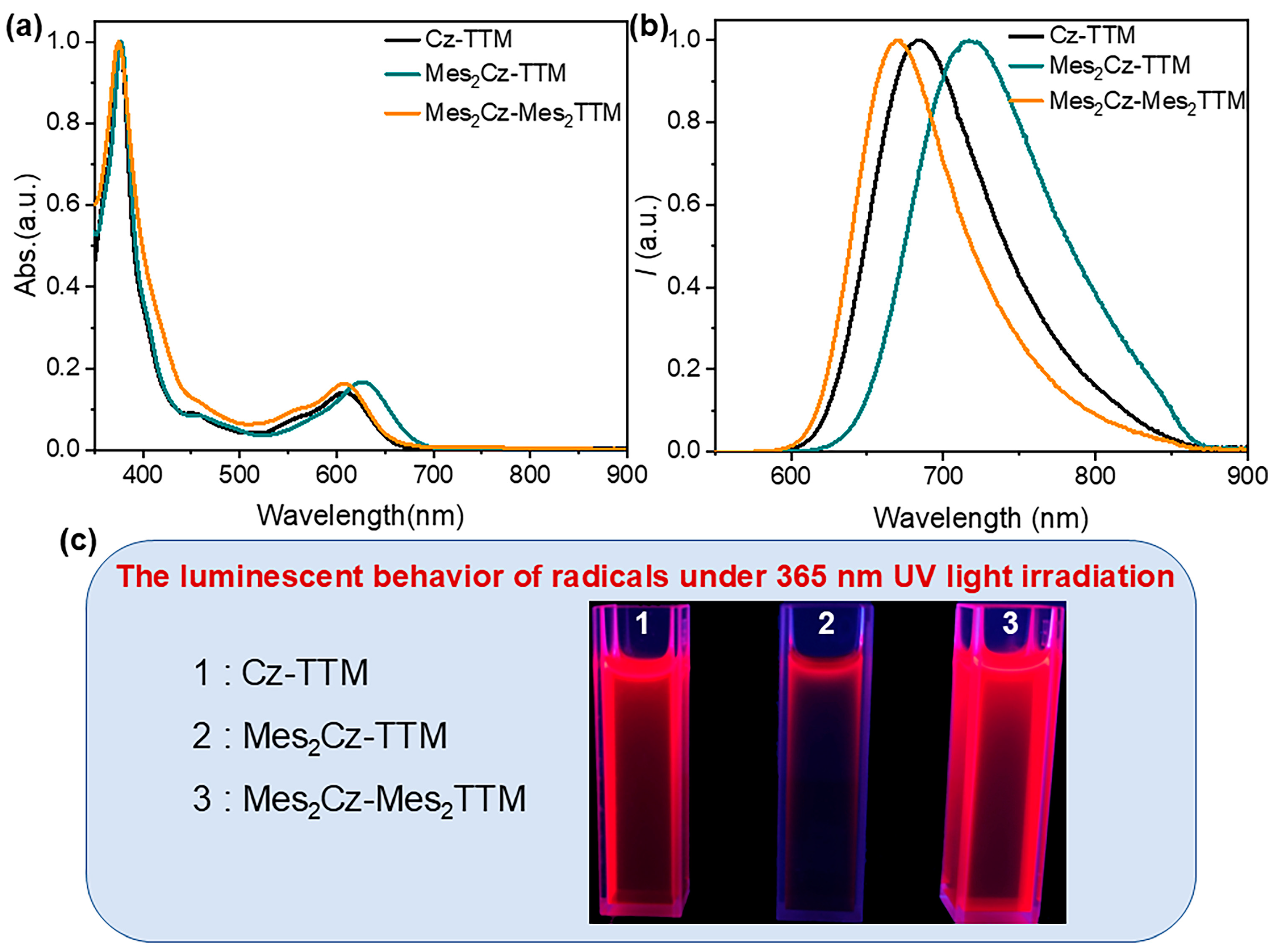
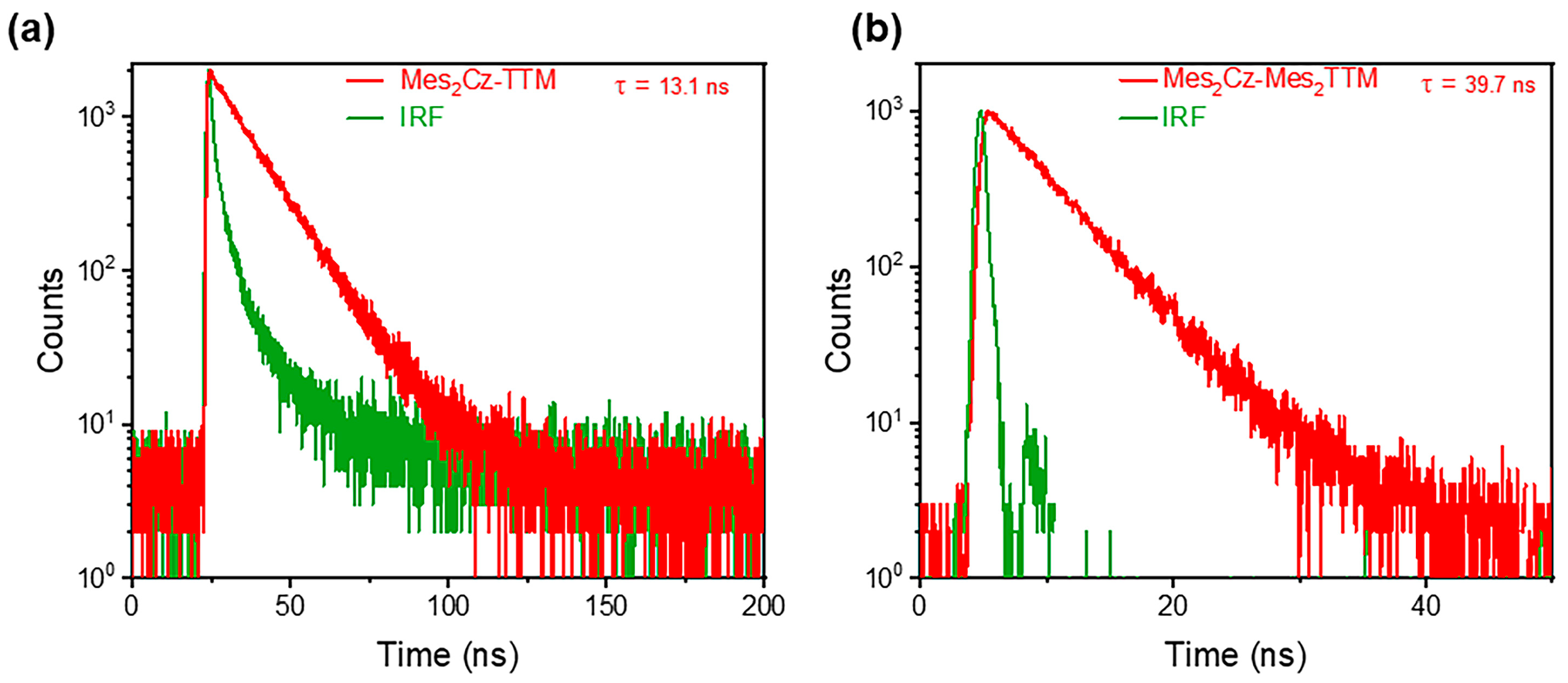

| Radicals | λa (nm) [a] | λf (nm) [a] | Φf (%) [b] | τ (ns) [c] | kr (s−1) | knr (s−1) |
|---|---|---|---|---|---|---|
| Cz-TTM | 376,608 | 686 | 68 | 24.9 | 27 × 106 | 13.2 × 106 |
| Mes2Cz-TTM | 377,626 | 717 | 39 | 13.1 | 29 × 106 | 47 × 106 |
| Mes2Cz-Mes2TTM | 375,610 | 669 | 92.6 | 39.7 | 34 × 106 | 2.8 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Guan, J.; Zhang, L.; Ai, X. Luminescence Efficiency Enhanced by Simple Substitutions on Donor and Acceptor in Radicals with Donor–Acceptor Structure. Molecules 2025, 30, 1191. https://doi.org/10.3390/molecules30061191
Gao S, Guan J, Zhang L, Ai X. Luminescence Efficiency Enhanced by Simple Substitutions on Donor and Acceptor in Radicals with Donor–Acceptor Structure. Molecules. 2025; 30(6):1191. https://doi.org/10.3390/molecules30061191
Chicago/Turabian StyleGao, Shuang, Jiahao Guan, Lintao Zhang, and Xin Ai. 2025. "Luminescence Efficiency Enhanced by Simple Substitutions on Donor and Acceptor in Radicals with Donor–Acceptor Structure" Molecules 30, no. 6: 1191. https://doi.org/10.3390/molecules30061191
APA StyleGao, S., Guan, J., Zhang, L., & Ai, X. (2025). Luminescence Efficiency Enhanced by Simple Substitutions on Donor and Acceptor in Radicals with Donor–Acceptor Structure. Molecules, 30(6), 1191. https://doi.org/10.3390/molecules30061191





