Efficient and Near-Zero Thermal Quenching Cr3+-Doped Garnet-Type Phosphor for High-Performance Near-Infrared Light-Emitting Diode Applications
Abstract
1. Introduction
2. Results and Discussion
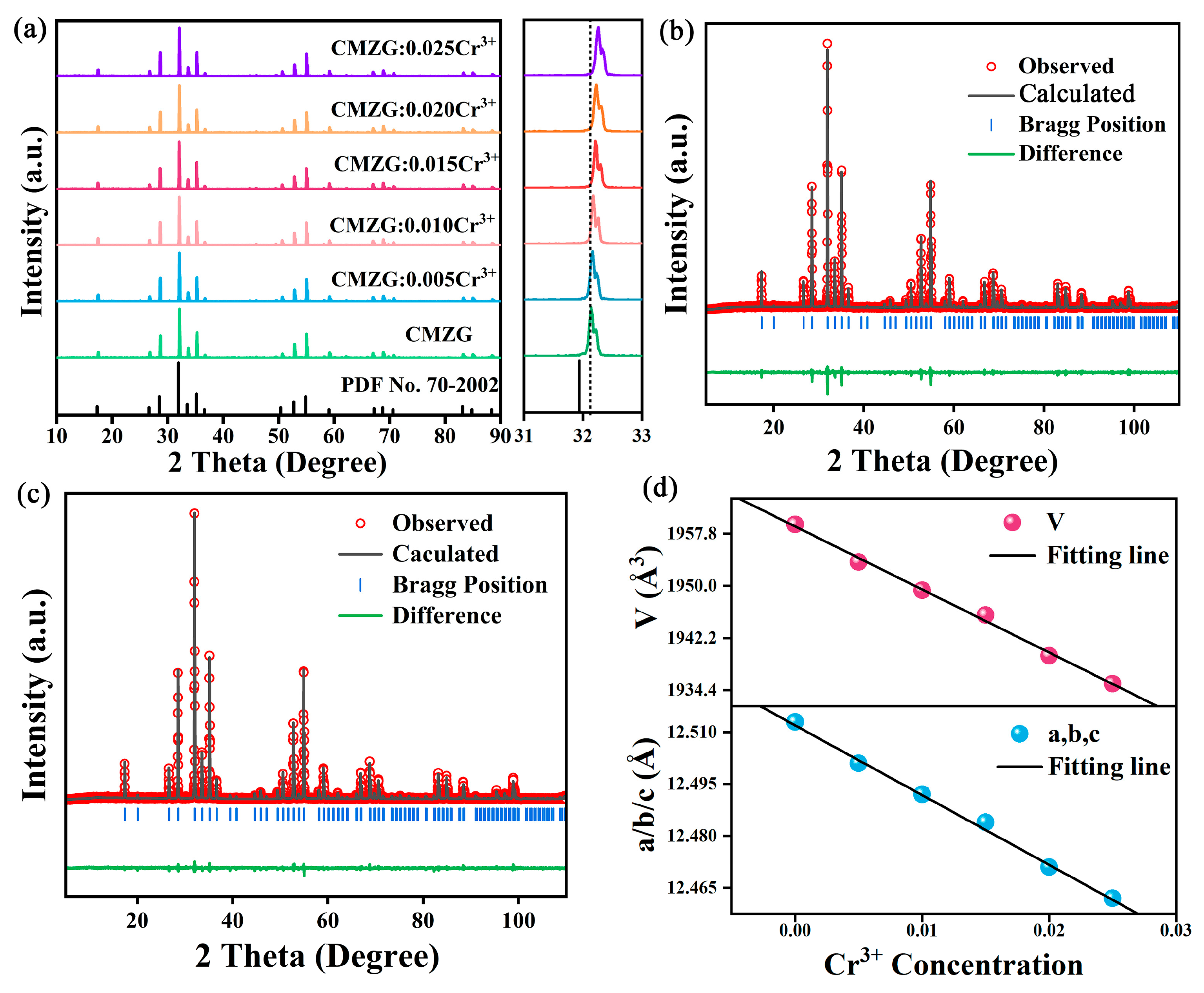
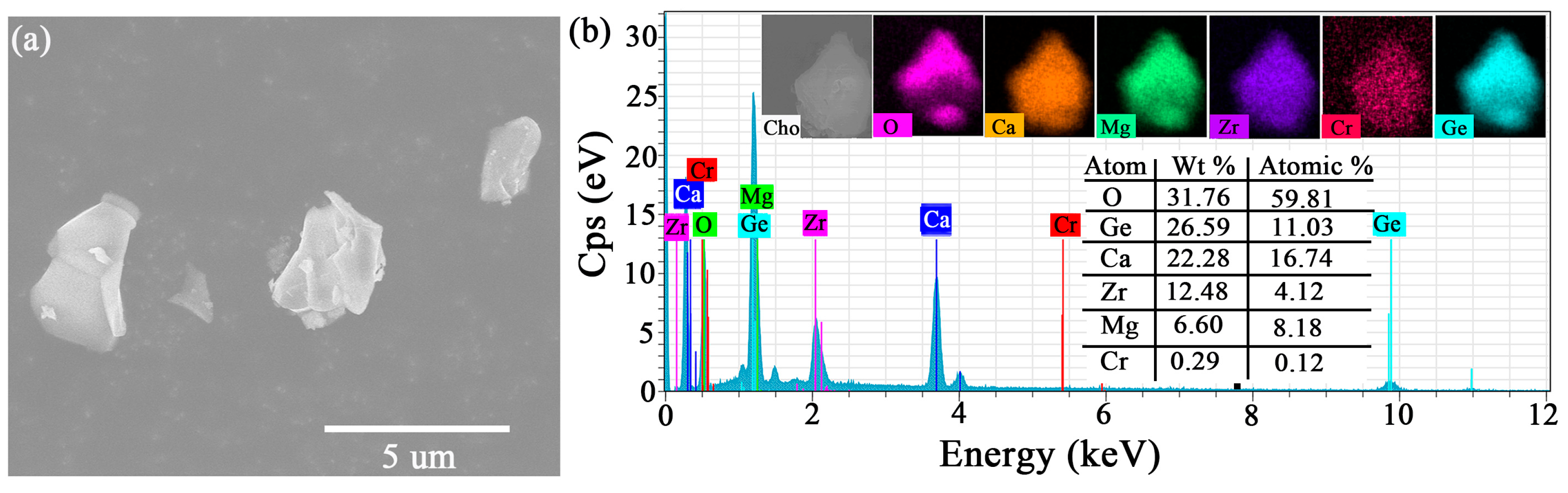
2.1. Crystal Structure
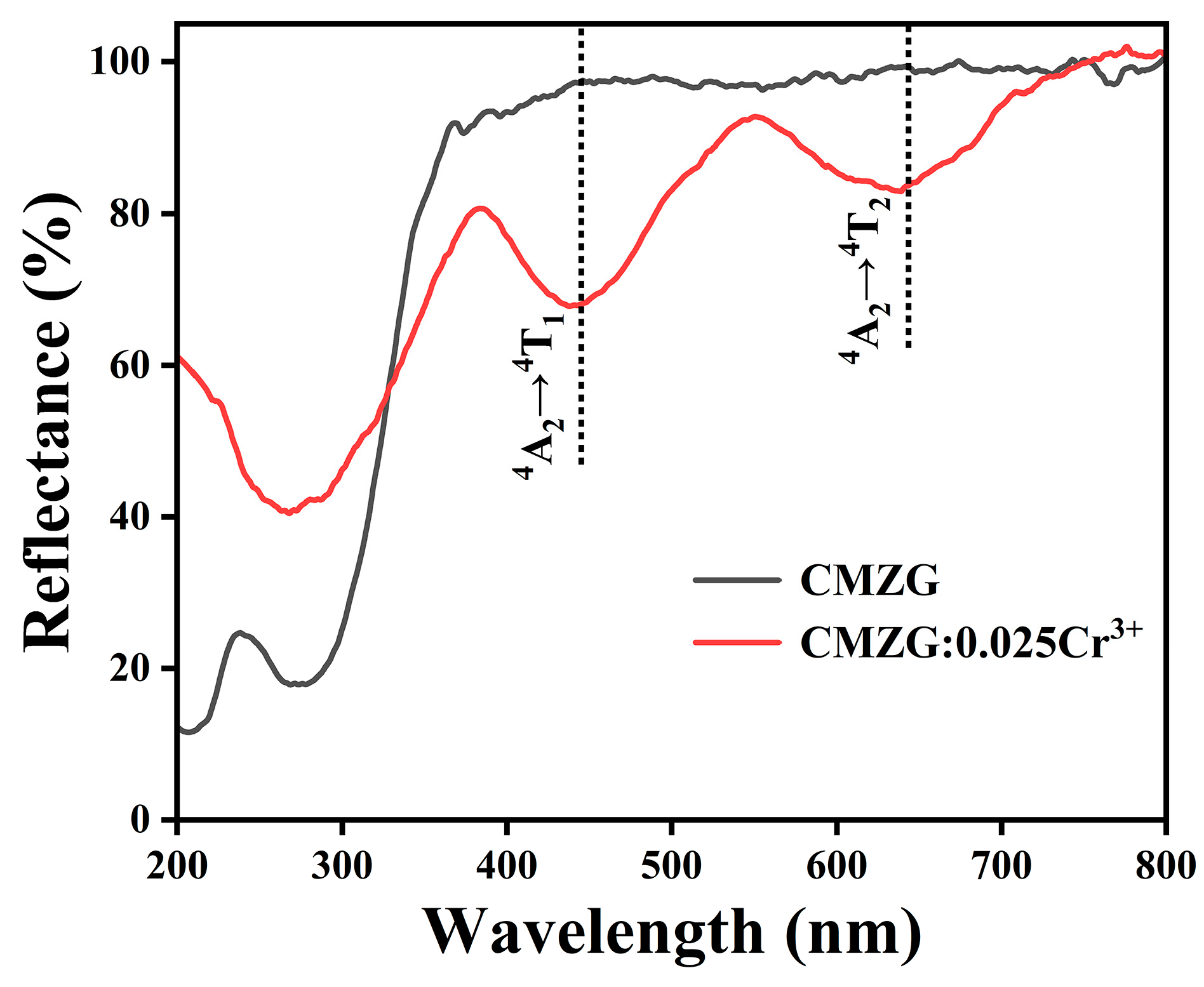
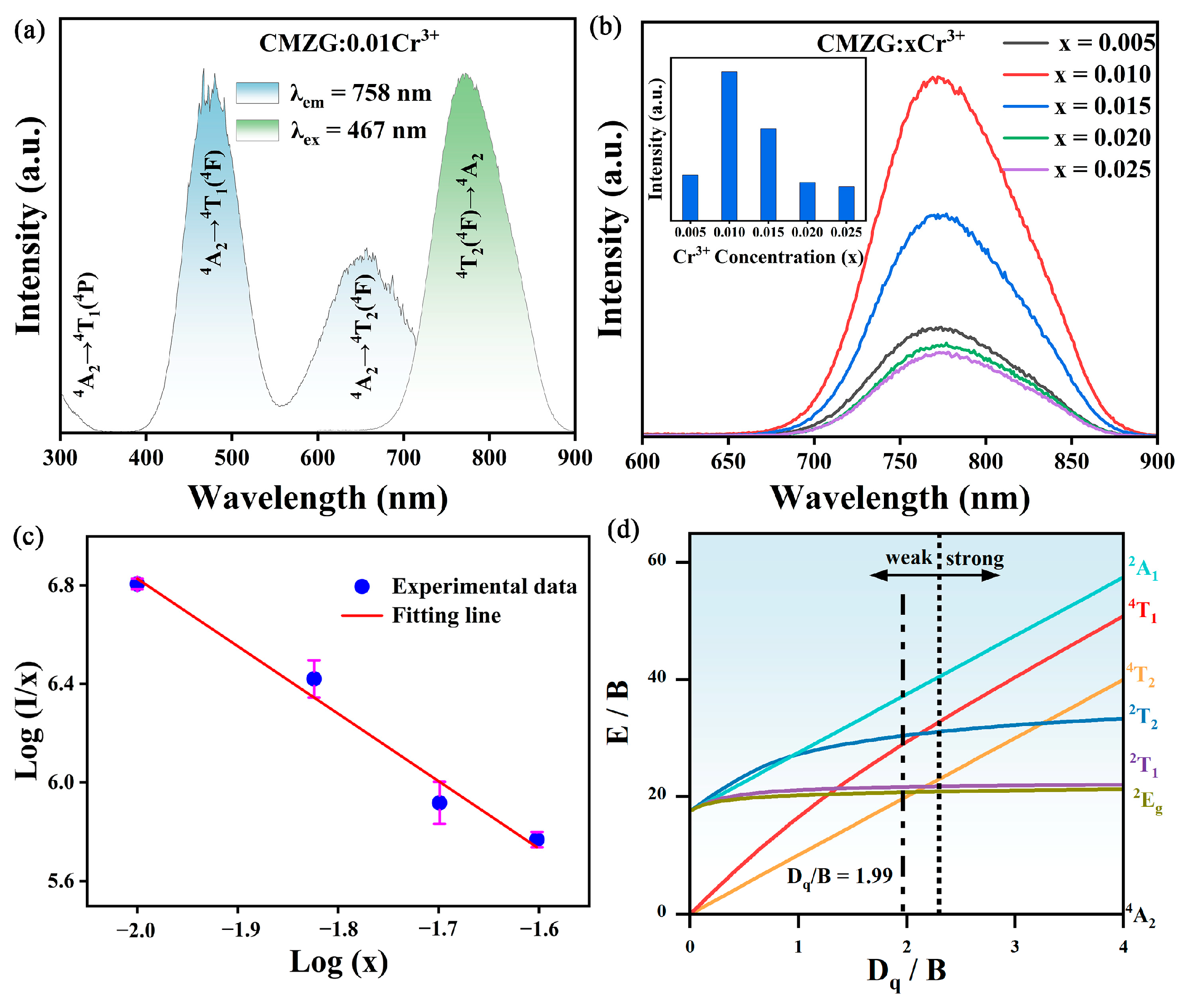
2.2. Optical Luminescence Properties
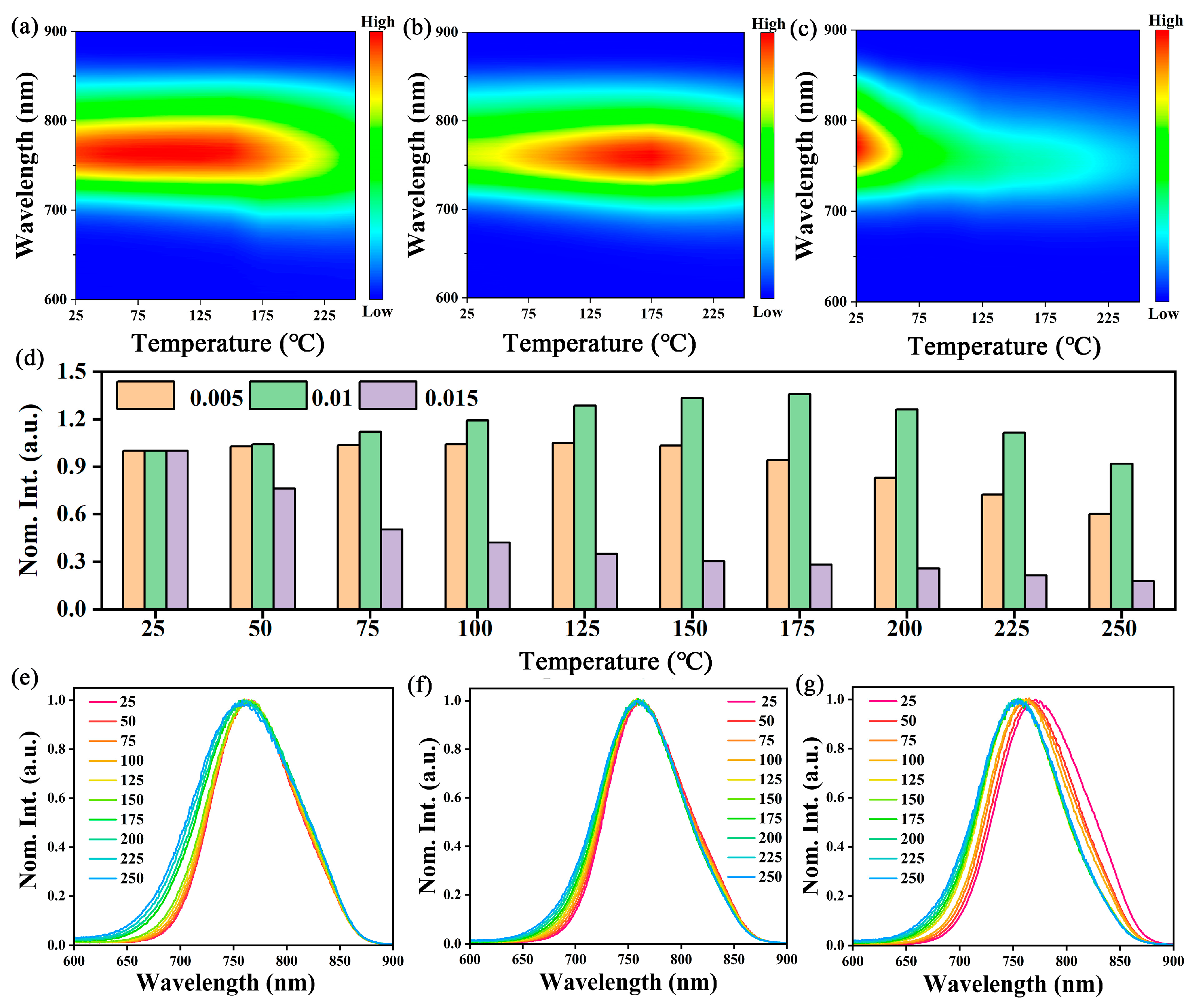
2.3. Thermal Stability
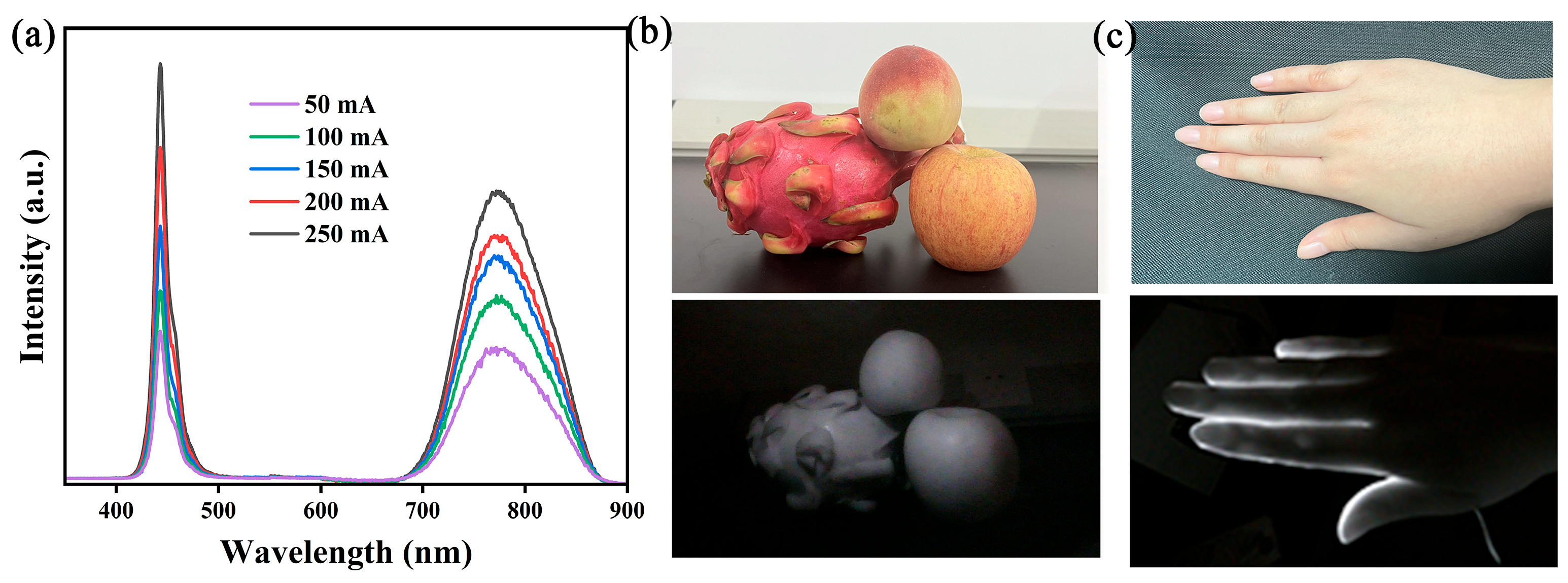
2.4. Potential Applications
3. Materials and Methods
3.1. Preparation of Materials
3.2. Characterization of Materials
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szymczak, M.; Runowski, M.; Lavín, V.; Marciniak, L. Highly Pressure-Sensitive, Temperature Independent Luminescence Ratio-metric Manometer Based on MgO:Cr3+ Nanoparticles. Laser Photonics Rev. 2023, 17, 2200801. [Google Scholar] [CrossRef]
- Wang, T.; Gao, B.; Li, J.; Wang, Z.; Li, P. Achieving Luminescence of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+ via [In3+] Substitution [Ga3+] and Its Application to NIR pc-LED in Non-Destructive Testing. Molecules 2023, 28, 8059. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Liang, Y.; Zhang, Y.; Chen, D.; Wang, X.-J. Broadband short-wave infrared light-emitting diodes based on Cr3+-doped LiScGeO4 phosphor. ACS Appl. Mater. Interfaces 2021, 13, 36011–36019. [Google Scholar] [CrossRef] [PubMed]
- Deren, P.J.; Watras, A.; Gagor, A.; Pazik, R. Weak Crystal Field in Yttrium Gallium Garnet (YGG) Submicrocrystals Doped with Cr3+. Cryst. Growth Des. 2012, 12, 4752–4757. [Google Scholar] [CrossRef]
- Zhao, F.; Song, Z.; Liu, Q. Advances in Chromium-Activated Phosphors for Near-Infrared Light Sources. Laser Photonics Rev. 2022, 16, 2200380. [Google Scholar] [CrossRef]
- Duan, F.F.; Wang, L.; Shi, Q.F.; Guo, H.J.; Qiao, J.W.; Cui, C.E.; Huang, P. Suitable selection of high-energy state excitation to enhance the thermal stability of Eu3+ and the sensitivity of La2CaSnO6:Eu3+, Mn4+ temperature measuring materials. J. Mater. Chem. C 2023, 11, 14705–14713. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhou, Y.; Zhang, S.; Li, C.; Hu, D.; Xu, L.; Jiao, H. An Ultra-Broadband Near-Infrared Cr3+-Activated Gallogermanate Mg3Ga2GeO8 Phosphor as Light Sources for Food Analysis. ACS Appl. Electron. Mater. 2019, 1, 1046. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Y.; Yuan, L.; Wang, B.; Zhang, R.; Wu, H.; Yao, Q.; Hu, Y. Highly Efficient and Thermally Stable Broadband NIR Phosphors by Rationally Bridging Cr3+ − Yb3+ in LiScGe2O6 for Optical Bioimaging. Inorg. Chem. Front. 2023, 10, 860–868. [Google Scholar] [CrossRef]
- Butkute, S.; Turan, K.; Zarkov, A.; Kontrimaviciute, E.; Tautkus, S.; Ramanauskas, R.; Kareiva, A. Investigation of distribution of metals in Cr3+-doped Y3Ga5O12 and Gd3Sc2Ga3O12 using ICP-OES and EDX. Chemija 2017, 28, 154–159. [Google Scholar]
- Zhang, Y.; Gao, Z.X.; Li, Y.G.; Chen, S.Y.; Han, M.X.; Li, J.; Zhang, Q.H.; Shen, Y.; Deng, D.G.; Xu, S.Q. Broadband La2LiSbO6: Cr3+ Phosphors with Double Luminescent Centers for NIR pc-LEDs. Inorg. Chem. 2023, 62, 17371–17381. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wei, G.; Yang, Y.; He, S.; Li, J.; Shi, Y.; Li, R.; Zhang, J.; Li, P. Ultra-Broadband and high efficiency Near-Infrared Gd3ZnxGa5-2xGexO12: Cr3+ (x = 0–2.0) garnet phosphors via crystal field engineering. Chem. Eng. J. 2022, 437, 135346. [Google Scholar] [CrossRef]
- Merkininkaite, G.; Zabiliute-Karaliune, A.; Jüstel, T.; Klimkevicius, V.; Sakirzanovas, S.; Katelnikovas, A. Ce3+→ Cr3+ energy transfer in Y3Al3MgSiO12 garnet host and application in horticultural lighting. Ceram. Int. 2023, 49, 16796. [Google Scholar] [CrossRef]
- He, S.; Zhang, L.; Wu, H.; Wu, H.; Pan, G.; Hao, Z.; Zhang, X.; Zhang, L.; Zhang, H.; Zhang, J. Efficient Super Broadband NIR Ca2LuZr2Al3O12: Cr3+, Yb3+ Garnet Phosphor for pc-LED Light Source toward NIR Spectroscopy Applications. Adv. Opt. Mater. 2020, 8, 1901684. [Google Scholar] [CrossRef]
- Malysa, B.; Meijerink, A.; Jüstel, T. Temperature dependent Cr3+ photoluminescence in garnets of the type X3Sc2Ga3O12 (X = Lu, Y, Gd, La). J. Lumin. 2018, 202, 523–531. [Google Scholar] [CrossRef]
- Nie, W.; Yao, L.; Chen, G.; Wu, S.; Liao, Z.; Han, L.; Ye, X. A novel Cr3+-doped Lu2CaMg2Si3O12 garnet phosphor with broadband emission for near-infrared applications. Dalton Trans. 2021, 50, 8446–8456. [Google Scholar] [CrossRef] [PubMed]
- Boiko, V.; Dai, Z.F.; Li, J.; Hreniak, D. Effect of Nd concentration on persistent luminescence of Y3Al2Ga3O12:Ce3+, Cr3+,Nd3+ ceramics for the near-infrared region. J. Lumin. 2022, 250, 119115. [Google Scholar] [CrossRef]
- Mao, N.; Liu, S.; Song, Z.; Yu, Y.; Liu, Q. A broadband near-infrared phosphor Ca3Y2Ge3O12: Cr3+ with garnet structure. J. Alloys Compd. 2021, 863, 158699. [Google Scholar] [CrossRef]
- Bai, B.; Dang, P.P.; Huang, D.Y.; Lian, H.Z.; Lin, J. Broadband near-infrared emitting Ca2LuScGa2Ge2O12:Cr3+ phosphors: Luminescence properties and application in light-emitting diodes. Inorg. Chem. 2020, 59, 13481–13488. [Google Scholar] [CrossRef]
- Zhao, F.; Cai, H.; Song, Z.; Liu, Q. Structural confinement for Cr3+ activators toward efficient near-infrared phosphors with suppressed concentration quenching. Chem. Mater. 2021, 33, 3621–3630. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, R.; Yang, S.; Bu, H.; Zhao, J. Enhancing the Luminescence of La3Mg2NbO9:Mn4+ Phosphor through H3BO3 and Charge Compensator Co-Doping for Use in Plant Growth Lamps. Molecules 2024, 29, 1402. [Google Scholar] [CrossRef]
- Xu, T.; Yuan, L.; Chen, Y.; Zhao, Y.; Ding, L.; Liu, J.; Xiang, W.; Liang, X. Y3Al5O12:Ce3+ single crystal and red-emitting Y3Al5O12:Cr3+ single crystal for high power W-LEDs. Opt. Mater. 2019, 91, 30–34. [Google Scholar] [CrossRef]
- Liu, G.; Liu, L.; Yuan, F.; Huang, Y.; Zhang, L.; Lin, Z. Cr-doped Ca3NbGa3Si2O14: A promising near-infrared tunable laser crystal. J. Lumin. 2020, 228, 117583. [Google Scholar] [CrossRef]
- Lemanski, K.; Deren, P.J.; Gagor, A.; Strek, W. Spectroscopic investigations of Gd3Sc2Ga3O12 garnet doped with Cr3+ and Nd3+ ions. J. Rare. Earth. 2009, 27, 560–563. [Google Scholar] [CrossRef]
- Liang, H.; Wu, S.; Li, Y.; Zuo, J.; Song, B.; Peng, J.; Ye, X. A highly efficient and broadband near-infrared-emitting garnet phosphor for plant lighting realized by cation co-substitution in lutetium aluminum garnet. J. Alloys Compd. 2023, 937, 168374. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer between inequivalent Eu2+ ions. J. Solid State Chem. 1986, 62, 207–211. [Google Scholar] [CrossRef]
- Van Uitert, L.G. Characterization of energy transfer interactions between rare earth ions. J. Electrochem. Soc. 1967, 114, 1048–1053. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Liu, Q.; Guo, Y.; Liu, F.; Wang, B.; Wei, S. Energy Conversion and Transfer in the Luminescence of CeSc3(BO3)4:Cr3+ Phosphor. Materials 2023, 16, 1231. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Chen, W.; Xia, Z. Crystallization of Na2SrGe6O14:Cr3+, Yb3+ Glass Ceramics Enabling a Watt-Level Output Power NIR-I/NIR-II Lighting Source. Laser Photonics Rev. 2024, 18, 2300784. [Google Scholar] [CrossRef]
- Kim, I.W.; Kaur, S.; Yadav, A.; Rao, A.S.; Saravanakumar, S.; Rao, J.L.; Singh, V. Structural, luminescence and EPR properties of deep red emitting MgY2Al4SiO12:Cr3+ garnet phosphor. J. Lumin. 2020, 220, 116975. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, J.; Zheng, J.; Zhao, X.; Suo, H.; Guo, C. Ab Initio Two-Sites Occupancy and Broadband Near-Infrared Emission of Cr3+ in Li2MgZrO4. Mater. Chem. Front. 2021, 5, 4334–4342. [Google Scholar] [CrossRef]
- Zhong, C.; Xu, Y.; Wu, X.; Yin, S.; Zhang, X.; Zhou, L.; You, H. Near-infrared Sr7NaGa(PO4)6:Cr3+,Ln3+ (Ln = Nd, Er, and Yb) phosphors with different energy transfer paths: Photoluminescence enhancement and versatility. J. Mater. Chem. C 2023, 11, 3375. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, X.; Ma, X.; Su, Z.; Liu, B.; Wang, Y. Highly Efficient and Thermally Stable Broadband NIR Phosphors with Superlong Afterglow Performance and their Multifunctional Applications. Laser Photonics Rev. 2024, 2400541. [Google Scholar] [CrossRef]
- Orucu, H.; Collins, J.; Di Bartolo, B. Photoluminescence spectroscopy of Cr3+ in CaY2Mg2Ge3O12. J. Appl. Phys. 2010, 108, 103101. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Yuan, C.; Leniec, G.; Miao, L.; Sun, P.; Liu, Z.; Luo, Z.; Dong, R.; Jiang, J. Thermally Stable CaLu2Mg2Si3O12:Cr3+ Phosphors for NIR LEDs. Adv. Opt. Mater. 2021, 9, 2100388. [Google Scholar] [CrossRef]
- Wang, T.Z.; Liu, G.C.; Xia, Z.G. Chemical unit co-substitution enabling broadband and tunable near-infrared emission in garnet-type Lu3Sc2Ga3O12:Cr3+ phosphors. Microstructures 2022, 2, 2022020. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Peng, J.; Wang, Y.; He, S.; Li, J.; Li, R.; Wei, G.; Yang, Y.; Li, P. Achieving the ultra-broadband near-infrared La3SnGa5O14:Cr3+ phosphor via multiple lattice sites occupation for biological nondestructive detection and night-vision technology. Mater. Today Adv. 2022, 16, 100305. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, Y.; Wang, G.; Xu, L.; Jia, H.; Sun, X. La3Ga5SiO14:Cr3+: A broadband near-infrared phosphor induced by the two-site occupation of Cr3+. Ceram. Int. 2023, 49, 18000. [Google Scholar] [CrossRef]
- Feofilov, S.P.; Kulinkin, A.B.; Rodnyi, P.A.; Khanin, V.M.; Meijerink, A. Disorder response of 3d3 ions zero-phonon lines in the luminescence spectra of Yttrium-Aluminum-Gallium garnet solid solution ceramics. J. Lumin. 2018, 200, 196–199. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Chen, W.; Mao, Y. Near-Infrared-Emitting K2CaP2O7:Cr3+ with Outstanding Thermal Stability. ACS Appl. Opt. Mater. 2023, 1, 1595. [Google Scholar]
- Lim, J.X.; Wang, H.; Kang, X.; Lü, W.; Lim, H.S.; Zhu, Z.; Pan, Q.; Sakundarini, N.; Chuah, Y.D. Simultaneously Possessing High Quantum Efficiency and Thermal Robustness in a Near-Infrared Emitting ZnAlB(1-x) GaxO4:Cr3+ Phosphor. Laser Photonics Rev. 2024, 2400750. [Google Scholar] [CrossRef]
- Li, H.; Jiao, J.K.; Xiang, X.F.; Wu, J.Z.; Hu, W.B.; Xie, J.Y.; Huang, S.Q.; Zhang, H.Z.; Zhu, J. Directly Identifying Multiple Cr3+ Emitting Centers for Broad Near-Infrared Emission in an Efficient and Near-Zero Thermal Quenching Garnet-Type Phosphor. Adv. Opt. Mater. 2024, 12, 2302391. [Google Scholar] [CrossRef]
- Zou, X.; Wang, X.; Zhang, H.; Kang, Y.; Yang, X.; Zhang, X.; Molokeev, M.; Lei, B. A highly efficient and suitable spectral profile Cr3+-doped garnet near-infrared emitting phosphor for regulating photomorphogenesis of plants. Chem. Eng. J. 2022, 428, 132003. [Google Scholar] [CrossRef]
- Tan, T.; Wang, S.; Su, J.; Yuan, W.; Wu, H.; Pang, R.; Wang, J.; Li, C.; Zhang, H. Design of a Novel Near-Infrared Luminescence Material Li2Mg3TiO6:Cr3+ with an Ultrawide Tuning Range Applied to Near-Infrared Light-Emitting Diodes. ACS Sustain. Chem. Eng. 2022, 10, 3839. [Google Scholar] [CrossRef]
- Zeng, L.; Zhong, J.; Lin, W.; Zhao, W. Achieving a Tunable and Ultra-Broadband Near-Infrared Emission in the Ga2−2xZnxGexO3:Cr3+ Phosphor. Dalton Trans. 2022, 51, 16740–16747. [Google Scholar] [CrossRef]
- Srivastava, B.B.; Gupta, S.K.; Mohan, S.; Abraham, A.; Portales, A.; Mao, Y.B. Cr3+ assisted energy transfer for Mn2+ doped zinc gallogermanate with narrow and bright green emission. Mater. Lett. 2021, 297, 129964. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Q.; Yao, L.; Dong, Y.; Jiang, J. Highly Efficient and Thermally Stable Cr3+-Activated Silicate Phosphors for Broad-band Near-Infrared LED Applications. Chem. Eng. J. 2020, 383, 123108. [Google Scholar] [CrossRef]
- Wang, S.; Pang, R.; Tan, T.; Wu, H.; Wang, Q.; Li, C.; Zhang, S.; Tan, T.; You, H.; Zhang, H. Achieving High Quantum Efficiency Broadband NIR Mg4Ta2O9:Cr3+ Phosphor Through Lithium-Ion Compensation. Adv. Mater. 2023, 35, 2300124. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z. Efficient and Near-Zero Thermal Quenching Cr3+-Doped Garnet-Type Phosphor for High-Performance Near-Infrared Light-Emitting Diode Applications. Molecules 2024, 29, 4253. https://doi.org/10.3390/molecules29174253
Yang Z. Efficient and Near-Zero Thermal Quenching Cr3+-Doped Garnet-Type Phosphor for High-Performance Near-Infrared Light-Emitting Diode Applications. Molecules. 2024; 29(17):4253. https://doi.org/10.3390/molecules29174253
Chicago/Turabian StyleYang, Zaifa. 2024. "Efficient and Near-Zero Thermal Quenching Cr3+-Doped Garnet-Type Phosphor for High-Performance Near-Infrared Light-Emitting Diode Applications" Molecules 29, no. 17: 4253. https://doi.org/10.3390/molecules29174253
APA StyleYang, Z. (2024). Efficient and Near-Zero Thermal Quenching Cr3+-Doped Garnet-Type Phosphor for High-Performance Near-Infrared Light-Emitting Diode Applications. Molecules, 29(17), 4253. https://doi.org/10.3390/molecules29174253





