Abstract
The Chamaenerion genus, particularly Chamaenerion angustifolium and Chamaenerion latifolium, is recognized for its rich phytochemical composition and extensive medicinal properties. These species are abundant in polyphenols, flavonoids, and tannins, which contribute to their potent antioxidant, antimicrobial, and anticancer activities. This review provides a comprehensive analysis of their phytochemical constituents, with an emphasis on how processing methods, including fermentation, influence bioactivity. Notably, fermentation enhances the levels of key bioactive compounds, such as oenothein B, gallic acid, and ellagic acid, thereby increasing their pharmacological potential. Additionally, this review evaluates the biological activities of Chamaenerion species in relation to their chemical composition, while also considering the limitations of current studies, such as the lack of in vivo or clinical trials. The literature for this review was sourced from scientific databases, including PubMed, Scopus, and ScienceDirect, covering research from 2010 to 2024. Future studies should focus on optimizing extraction methods, elucidating synergistic bioactivities, and conducting in-depth clinical trials to validate their efficacy and safety.
1. Introduction
Therapeutic plants are of major importance in human life due to their bioactive phytochemicals, which provide potential health benefits and possess commercial value [1,2,3].
The Onagraceae is a large family of flowering plants with around 650 species of trees, shrubs, and herbs spread throughout approximately 17 genera [4,5]. This family is divided into two subfamilies: Ludwigioideae, which primarily includes the genus Ludwigia; and Onagroideae, sometimes referred to as the willowherb or evening primrose family. Many species within Onagroideae are known for their therapeutic and dietary benefits [6]. Characteristically, Onagroideae species have two to four (rarely three) deciduous sepals, while Ludwigioideae often have three to four persistent sepals, allowing for clear differentiation between them [4]. Several well-known garden plants belong to this family, including evening primrose (Oenothera L.) and fuchsia (Fuchsia L.). Additionally, many Onagraceae species are valued for their medicinal applications. Many Onagraceae species, including Oenothera biennis, Epilobium angustifolium, and Ludwigia octovalvis, exhibit strong antioxidant, anti-inflammatory, and antimicrobial properties due to their rich flavonoid and polyphenol content. Additionally, compounds from Oenothera biennis and Oenothera paradoxa have demonstrated cytotoxic effects against prostate and breast cancer cells [7,8,9]. Among the genera of Onagraceae, Chamaenerion (including C. angustifolium and C. latifolium) stands out for its notable medicinal properties, which are closely linked to its natural habitat and distribution. The geographical range of these species is illustrated in Figure 1.
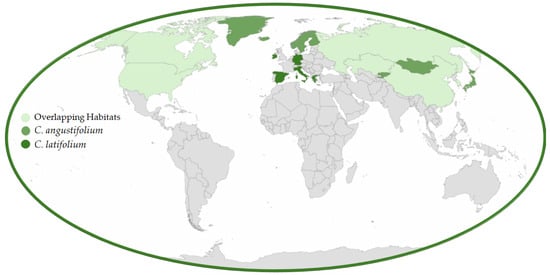
Figure 1.
Geographical distribution of Chamaenerion angustifolium and Chamaenerionlatifolium and their overlapping habitats.
In the last decade, the chemistry and biological activity of Chamaenerion species have been studied intensively, highlighting the significance of fireweed as an important medicinal plant widely utilized in the pharmaceutical, food, and cosmetic industries [10,11]. C. angustifolium, commonly known as willowherb or rosebay willowherb, Chamaenerion angustifolium (L.) Holub, Chamaenerion angustifolium (L.) Scop., Epilobium angustifolium L., is a perennial herbaceous plant widely distributed across various habitats in the Northern Hemisphere [3,11,12]. Traditional medicine has used fireweed plants to treat a variety of ailments, such as wound healing, infections, skin diseases, colds, urinary problems like prostatitis, gastric disorders, migraine headaches, and sleep disturbances [13,14]. In northern and eastern Europe, it is utilized as a food plant, particularly in the form of tea or as a traditional herbal remedy. The widespread appeal of this plant primarily stems from its anti-inflammatory, antioxidant, antibacterial, and anticancer properties [15]. It was also reported that fireweed extract demonstrates analgesic, anticholinesterase, and skin photoprotective properties, while recent studies have emphasized its wound-healing and cosmetic benefits [16,17,18].
The therapeutic potential of C. angustifolium lies in its rich polyphenolic profile, particularly tannins (ellagitannins), flavonoids, and phenolic acids [19,20,21]. The principal phenolic acids include gallic acid, caffeic acid, chlorogenic acid, rosmarinic acid, ellagic acid, p-coumaric acid, and cinnamic acid, while the predominant flavonoids consist of quercetin, myricetin, kaempferol, rutin, quercetin-3-O-glucoside, and hyperoside. Oenothein B, the most abundant ellagitannin, plays a central role in the plant’s medicinal properties [22,23], exhibiting antiandrogenic, antiproliferative, anticancer, antioxidant, anti-inflammatory, and immunomodulatory activities [24,25,26]. This synergistic interaction of polyphenols and ellagitannins underscores the plant’s extensive use in traditional medicine [27,28,29]. C. angustifolium also contains a smallquantity of essential oil, primarily composed of terpenes, such as limonene, bisabolene, and caryophyllene, as well as eugenol, linalool, pelargol, and terpineol [30].
C. latifolium, commonly known as arctic fireweed, alpine fireweed, dwarf fireweed, broad-leaved fireweed, river beauty, or Épilobe à feuilles larges, is a long-lived perennial herb native to arctic and alpine habitats throughout the Northern Hemisphere. Its distribution includes North America, Greenland, Iceland, and northern Russia, while it is largely absent from northern Europe [31,32]. In southern Asia, it occurs in the Himalayas, ranging from Afghanistan to western China [33,34], and is also found in Central Asian regions such as Altai, Tabagatai, Dzungarian Alatau, Zailiysky Alatau, Kyrgyz Alatau, Kungei Alatau, and the Western Tien Shan (Figure 1) [20]. This species can be distinguished from its regional counterpart, C. angustifolium Holub, by its shorter, decumbent to ascending, often branched stems (up to 40 cm) and compact, few-flowered racemes [33].
C. latifolium is noted for its complex chemical composition, which includes bioactive compounds such as terpenes, steroids, triterpenoids, phenolic acids, and flavonoids. The key constituents are phenolic compounds, like quercetin 3-glucoside, rutin, gallic acid, caffeic acid, and chlorogenic acid, which vary based on extraction methods [35]. Ethanol extracts (ChL-EtOH) have been shown to be very rich in phenolic compounds, with strong antibacterial activity against bacterial and fungal strains, and outstanding antioxidant qualities, according to studies using HPLC-UV-ESI/MS [20]. In particular, ChL-EtOH performed best in antioxidant assessments that showed significant DPPH scavenging and FRAP capabilities. Additionally, ChL-EtOH demonstrated remarkable antibacterial activity against the fungal strain Candida albicans as well as Gram-positive and Gram-negative bacteria [9]. These results, together with its chemical complexity, emphasise its pharmacological potential and suggest that it shares characteristics with C. angustifolium, which is a rich source of bioactive compounds with interesting pharmacological significance [20].
This review aims to provide a comprehensive analysis of the botanical characteristics, chemical compositions, and biological effects of C. angustifolium and C. latifolium, exploring potential benefits for human health.
2. Results
2.1. Taxonomic Classification and Botanical Description
The taxonomic classification of Chamaenerion species was obtained from the World Flora Online website (https://www.worldfloraonline.org/ accessed on 24 January 2025) and is outlined in Table 1.

Table 1.
Taxonomic classification of C. angustifolium and C. latifolium.
The perennial C. angustifolium Scop. (Figure 2) is characterized by scale-like white-pink leaves along its long rhizomes and stolons [23]. Its unbranched stems typically grow between 50 and 200 cm, either smooth or sparsely covered with short clinging hairs. The alternating leaves, densely arranged along the stem, measure 5–15 cm in length and 10–15 mm in width, featuring a linear-lanceolate shape that tapers at the base [31,32]. Dark green, glossy, and smooth, the leaves have prominent veins and margins that are either smooth or slightly serrated. As the stipules rise towards the top, they gradually become smaller, with the uppermost part often bristly [36,37,38]. The plant produces violet-pink flowers with rectangular petals, rounded at the tips, arranged in long terminal spike-like racemes. The lanceolate sepals, twice as long as the petals, are slightly hairy on the outer surface. The protandrous flowers bloom from June to September, beginning at the base of the raceme and progressing upward, with the style extending beyond the stamens [39].

Figure 2.
Chamaenerion angustifolium plant. Images retrieved with permission from https://fungi.su (accessed on 24 January 2025).
C. latifolium (Figure 3) is a perennial plant that grows between 10 and 50 cm tall, with a thick rhizome reaching up to 1.5 cm in diameter. Its stems are branched and can be either smooth or sparsely covered with hairs, particularly near the upper part [30,31]. The leaves, which can be bare or slightly hairy, have a grayish tint. They are sessile or have very short petioles, with the lower leaves arranged oppositely and the upper leaves alternately. These leaves are thick, broadly lanceolate, and wedge-shaped at the base, blunt at the tip, and have smooth edges, measuring 2–3.5 cm in length and 1–1.5 cm in width. They are lighter on the underside and lack prominent lateral veins [40,41].

Figure 3.
Chamaenerion latifolium plant. Images retrieved with permission from https://fungi.su (accessed on 24 January 2025).
2.2. Phytochemicals
2.2.1. Primary Metabolites
Primary metabolites (PMs) are the fundamental building blocks of life, essential for growth, development, and metabolic functions in all organisms. In plants like C. latifolium and C. angustifolium, these compounds, including carbohydrates, amino acids, fatty acids, and organic acids, are crucial for vital processes such as photosynthesis, respiration, and protein synthesis. They also serve as precursors to secondary metabolites, which contribute to the plant’s pharmacological properties [42,43]. The PMs found in C. latifolium and C. angustifolium are summarized in Table 2.
Carbohydrates are the most abundant PMs in Chamaenerion species, serving as the primary energy source and structural component. Glucose and galactose are particularly prominent, with glucose dominating the sugar profile. These sugars are vital for energy storage and transport, especially during flowering and seed development, reflecting the species’ adaptation to diverse environmental conditions. In C. angustifolium, glucose concentrations can reach up to 11.23 mg/g dry weight [44,45].
Amino acids are essential for protein biosynthesis, nitrogen transport, and stress response mechanisms. Uminska et al. analyzed the amino acid profile of C. angustifolium using GC-MS, identifying L-alanine (2.350–6.090 mg/g) and L-phenylalanine as the dominant amino acids, with moderate amounts of L-leucine, L-isoleucine, and L-valine. Interestingly, sulfur-containing amino acids are absent, potentially impacting the synthesis of specific sulfur-rich secondary metabolites. This strategic allocation of amino acids highlights their diverse structural and functional roles [46,47].
Fatty acids contribute to membrane integrity and bioactive lipid functions. Key compounds in Chamaenerion include linoleic acid, palmitic acid [48], and n-hexadecenoic acid [35]. The balanced fatty acid composition observed in both species underscores their adaptability to various ecological niches and provides precursors for secondary metabolites like lipophilic antioxidants.

Table 2.
Primary metabolites identified in the aerial parts of C. angustifolium and C. latifolium.
Table 2.
Primary metabolites identified in the aerial parts of C. angustifolium and C. latifolium.
| Compounds | Molecular Weight, g/mol | Plant | Identification Method | Extraction Method | Extract Type | Ref. |
|---|---|---|---|---|---|---|
| Fatty acids | ||||||
| n-Hexadecanoic | 256.43 | CL | GC-MS | Maceration | Hexane | [35] |
| Tetradecanoic | 228.37 | CL | GC-MS | Maceration | Hexane | [35] |
| Linoleic | 280.45 | CA | GC-MS | Maceration | Methanol | [35] |
| Palmitic | 256.43 | CA | C-MS | Reflux | Methanol | [44] |
| Capric | 172.26 | CA | C-MS | Reflux | MTBE | [44] |
| Myristic | 228.37 | CA | C-MS | Reflux | MTBE | [44] |
| Lauric | 200.32 | CA | C-MS | Reflux | MTBE | [44] |
| Pentadecanoic | 242.41 | CA | C-MS | Reflux | MTBE | [44] |
| Pentadecenic | 240.39 | CA | C-MS | Reflux | MTBE | [44] |
| Palmitoleic | 254.41 | CA | C-MS | Reflux | MTBE | [44] |
| Margaric | 270.46 | CA | C-MS | Reflux | MTBE | [44] |
| γ-Linolenic | 278.43 | CA | C-MS | Reflux | MTBE | [44] |
| Nonadecanoic | 298.5 | CA | C-MS | Reflux | MTBE | [44] |
| Tetracosanic | 368.63 | CA | C-MS | Reflux | MTBE | [44] |
| Heneicosanic | 326.57 | CA | C-MS | Reflux | MTBE | [44] |
| 2-Hydroxyoctacosanic | 440.74 | CA | C-MS | Reflux | MTBE | [44] |
| 2-Hydroxytriacontanic | 468.78 | CA | C-MS | Reflux | MTBE | [44] |
| Hexadecandioic | 286.35 | CA | C-MS | Reflux | MTBE | [44] |
| Octadecanedioic | 314.47 | CA | C-MS | Reflux | MTBE | [44] |
| Eicosandioic | 342.52 | CA | C-MS | Reflux | MTBE | [44] |
| Hexacosanic | 394.66 | CA | C-MS | Reflux | MTBE | [44] |
| 2-Hydroxyhexacosanic | 410.68 | CA | C-MS | Reflux | MTBE | [48] |
| 2-Hydroxytetracosanic | 382.63 | CA | C-MS | Reflux | MTBE | [44] |
| 2-Hydroxytricosanic | 368.61 | CA | C-MS | Reflux | MTBE | [44] |
| Pentacosanic | 396.66 | CA | C-MS | Reflux | MTBE | [44] |
| Triacontanic | 452.79 | CA | C-MS | Reflux | MTBE | [44] |
| Octacosanic | 424.73 | CA | C-MS | Reflux | MTBE | [44] |
| Nonacosanic | 438.76 | CA | C-MS | Reflux | MTBE | [44] |
| Heptacosanic | 410.71 | CA | C-MS | Reflux | MTBE | [44] |
| Behenic | 340.57 | CA | C-MS | Reflux | MTBE | [44] |
| Arachic | 312.52 | CA | C-MS | Reflux | MTBE | [44] |
| Tricosanic | 366.64 | CA | C-MS | Reflux | MTBE | [44] |
| Amino acids | ||||||
| L-Alanine | 89.09 | CA | GC-MS, | SPE | Methanol | [47,48] |
| L-Phenylalanine | 165.19 | CA | GC-MS | UAE | Methanol | [47,48] |
| L-Leucine | 131.18 | CA | GC-MS | UAE | Methanol | [47,48] |
| L-Isoleucine | 131.18 | CA | GC-MS | UAE | Methanol | [47,48] |
| L-Proline | 115.13 | CA | GC-MS | SPE | Methanol | [48] |
| L-Serine | 105.09 | CA | GC-MS | SPE | Methanol | [48] |
| L-Threonine | 119.12 | CA | GC-MS | SPE | Methanol | [48] |
| L-Phenylalanine | 165.19 | CA | GC-MS | SPE | Methanol | [48] |
| L-Aspartic acid | 133.1 | CA | GC-MS | SPE | Methanol | [48] |
| L-Glutamic acid | 147.13 | CA | GC-MS | SPE | Methanol | [48] |
| Carbohydrates | ||||||
| D-Glucose | 180.16 | CA | GC-MS | SPE | Methanol | [48] |
| D-Galactose | 180.16 | CA | GC-MS | SPE | Methanol | [48] |
| Myo-Inositol | 180.16 | CA | GC-MS | Reflux | Methanol | [44] |
| D-Mannose | 180.16 | CA | GC-MS | Reflux | Methanol | [44] |
| D-Arabinose | 150.13 | CA | GC-MS | Reflux | Methanol | [44] |
| D-Ribose | 150.13 | CA | GC-MS | Reflux | Methanol | [44] |
| Glucose | 180.16 | CL | PC | Maceration | Aqueous | [45] |
| Galactose | 180.16 | CL | PC | Maceration | Aqueous | [45] |
| Xylose | 150.13 | CL | PC | Maceration | Aqueous | [45] |
MTBE—Methyl tert-Butyl Ether; CL—C. latifolium; CA—C. angustifolium; PC—paper chromatography; SPE—stepwise percolation extraction; UAE—ultrasonic-assisted extraction.
2.2.2. Volatile and Lipophilic Constituents
Volatile and lipophilic compounds are integral to plants, contributing to their unique scents, facilitating ecological interactions, and enhancing their medicinal properties [49,50]. In C. angustifolium and C. latifolium, these compounds play a vital role in antioxidant, antimicrobial, and anti-inflammatory activities, making them a central focus in pharmacological research [9]. Advanced techniques, like gas chromatography–mass spectrometry (GC-MS), have allowed for the detailed exploration of these bioactive compounds, revealing their diversity and functional significance [51]. A detailed volatile and lipophilic contents of both plants islisted in Table 3.
The lipophilic fraction of Chamaenerion species is notably rich in long-chain hydrocarbons, esters, and triterpenoids. GC-MS analysis of hexane extracts from C. latifolium highlights a substantial presence of these classes of compounds. Among them, nonacosane and tetracosanol dominate as the primary alkanes and alcohols, accounting for 31.339% of the leaves and 48.158% of the stems. These long-chain hydrocarbons possess hydrophobic properties, making them valuable in promoting a skin barrier function in medicinal applications [35]. In C. angustifolium, pentacosanal stands out as a significant aldehyde, contributing 31.1% of the aldehyde fraction. This long-chain aldehyde is linked to anti-inflammatory properties [52].
Volatile compounds impart Chamaenerion species with their distinctive aromas, which play a crucial ecological role in attracting pollinators and deterring pests. Key volatiles identified in C. angustifolium include trans-2-hexenal, α-pinene, and linalool. Beyond their aromatic properties, these compounds exhibit noteworthy biological activities. Trans-2-hexenal, known for its fresh, green scent, enhances the plant’s defense mechanisms and demonstrates antimicrobial effects. Similarly, α-pinene and linalool are recognized for their anti-inflammatory and antioxidant properties, underscoring their therapeutic potential [23,30]. Cis-3-hexenol, often referred to as “leaf alcohol”, is particularly abundant in fresh samples, comprising 17.5% to 68.6% of the total volatiles [23]. Additionally, sesquiterpenes, such as α- and β-caryophyllenes, are prominent for their anti-inflammatory and anticancer activities, with α-caryophyllene contributing up to 52.3% of the total volatiles in C. angustifolium [53].
GC-MS has revolutionized the study of volatile and lipophilic compounds, enabling their precise identification and quantification [54]. Researchers employ various extraction methods, including maceration, percolation, and hydrodistillation, to isolate these compounds from Chamaenerion species. Non-polar solvents like hexane and methyl tert-butyl ether are highly effective for extracting lipophilic compounds, whereas hydrodistillation is ideal for volatile aromatics [55,56]. These methodological advancements have ensured the accurate characterization of the complex chemical profiles of Chamaenerion species.
The therapeutic potential of volatile and lipophilic compounds in Chamaenerion species is substantial. Alkanes like nonacosane and tetracosane enhance the skin barrier function, making them valuable in dermatological applications [57]. Meanwhile, sesquiterpenes, including α- and β-caryophyllenes, exhibit potent anti-inflammatory and anticancer activities, with α-caryophyllene constituting a significant portion of the total volatiles [58]. These findings highlight the importance of Chamaenerion species as a natural source of bioactive compounds with diverse pharmacological applications.

Table 3.
Volatile and lipophilic components of C. angustifolium and C. latifolium identified using GC-MS.
Table 3.
Volatile and lipophilic components of C. angustifolium and C. latifolium identified using GC-MS.
| Compounds | Molecular Weight, g/mol | Plant | Plant Part | Extraction Method | Extract Type | Ref. |
|---|---|---|---|---|---|---|
| Sesquiterpene | ||||||
| Caryophyllenes (α) | 893.51 | CA | Leaves | SPME | Methanol (aq.) | [23,53] |
| Caryophyllenes (β) | 907.49 | CA | Leaves | SPME, | Methanol (aq.) | [23,53] |
| Phenylpropanoids | ||||||
| Anethole | 148.20 | CA | Leaves | SPME | Methanol (aq.) | [23,53] |
| Monoterpene Hydrocarbon | ||||||
| α-Pinene | 136.23 | CA | Flowers | Hydrodistillation | EO | [30] |
| Camphene | 136.23 | CA | Flowers | Hydrodistillation | EO | [30] |
| Linalyl propionate | 210.31 | CA | Flowers | Hydrodistillation | EO | [30] |
| Terpineol | 154.25 | CA | Flowers | Hydrodistillation | EO | [30] |
| Oxygenated Monoterpene | ||||||
| Linalool | 154.25 | CA | Flowers | Hydrodistillation | EO | [30] |
| Eugenol | 164.20 | CA | Flowers | Hydrodistillation | EO | [30] |
| Alkanes | ||||||
| Tricosane | 324.63 | CA | Aerial parts | Percolation | Lipophilic | [52] |
| Tetradecane | 198.39 | CA | Aerial parts | Percolation | Lipophilic | [52] |
| Hexadecane | 226.44 | CA | Aerial parts | Percolation | Lipophilic | [52] |
| Heptadecane | 240.47 | CA | Aerial parts | Percolation | Lipophilic | [52] |
| Pentadecane | 212.42 | CA | Aerial parts | Percolation | Lipophilic | [52] |
| Tetracosane | 338.65 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Pentacosane | 352.69 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Hexacosane | 366.70 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| n-Octacosane | 394.77 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Nonacosane | 408.60 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Hentriacontane | 436.85 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Ester | ||||||
| β-Amyrenyl acetate | 468.80 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Icosylhexadecanoate | 536.96 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Bis(2-ethylhexyl) phthalate | 390.55 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Alcohols | ||||||
| n-Tetracosanol-1 | 354.65 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Cis-3-Hexenol | 100.16 | CA | Aerial parts | SPME | Methanol (aq.) | [23] |
| Aldehydes | ||||||
| Nonacosanal | 422.77 | CL | Leaves and Stems | Maceration | Hexane | [35] |
| Pentacosanal | 366.66 | CA | Aerial parts | Percolation | Lipophilic | [52] |
| Tricosanal | 338.60 | CA | Aerial parts | Percolation | Lipophilic | [52] |
| Trans-2-Hexenal | 98.14 | CA | Leaves | SPME | Methanol (aq.) | [23] |
| Benzacetaldehyde | 120.15 | CA | Flowers | Hydrodistillation | EO | [30] |
| Triterpenoids | ||||||
| α-Amyrin | 426.72 | CL, CA | Leaves | Maceration | Hexane | [35,52] |
| β-Amyrenol | 426.72 | CL, CA | Steams | Maceration | Hexane | [35,52] |
SPME—solid-phase microextraction; CL—C. latifolium; CA—C. angustifolium; EO—essential oil.
2.2.3. Polyphenolic Compounds
Polyphenolic compounds are a diverse group of secondary metabolites known for their potent antioxidant, anti-inflammatory, and anticancer properties [59,60]. These compounds, including phenolic acids, flavonoids, and tannins, play a critical role in the therapeutic potential of plants. In C. angustifolium and C. latifolium, polyphenols are abundant and diverse, contributing significantly to the pharmacological activities of these species [61]. Advanced analytical techniques, such as high-performance liquid chromatography (HPLC) coupled with UV detection, diode array detection, and multi-stage mass spectrometry, have enabled precise profiling of these bioactive compounds, providing valuable insights into their biological roles and variations under different conditions [62].
As summarized in Table 4 and Figure 4, the polyphenolic composition of C. angustifolium and C. latifolium includes key compounds, such as oenothein B, quercetin, chlorogenic acid, caffeic acid, ellagic acid, and gallic acid, which contribute significantly to their antioxidant and anti-inflammatory activities [30].
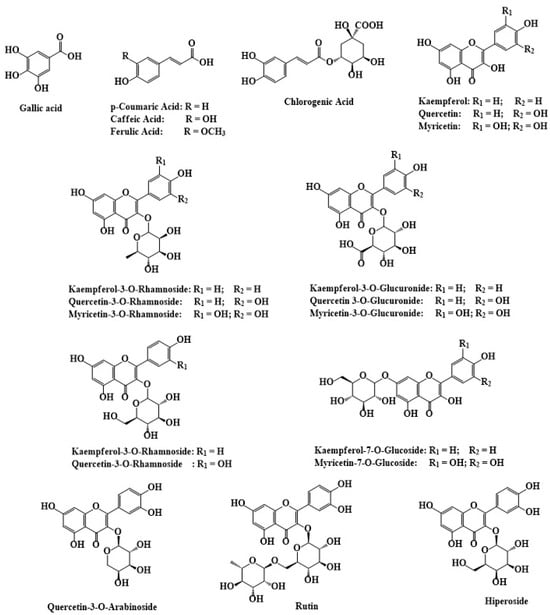
Figure 4.
The chemical structures of polyphenolic compounds identified in C. angustifolium and C. latifolium. The structures were drawn using ChemDraw Ultra 12.0 software.
The composition and concentration of polyphenols are influenced by various factors including the growth stage, environmental conditions, and extraction methods [63,64]. Research by Gryszczyńska et al. on C. angustifolium demonstrated that bioactive compound concentrations vary depending on harvest time, with oenothein B, sterols, flavonoids, and polyphenolic acids reaching higher levels during the flowering period. Similarly, their study identified phenolic metabolites, with gallic acid, oenothein B, and quercetin 3-O-arabinoside as the dominant compounds. These results highlight the crucial role of harvest timing in maximizing the medicinal value of C. angustifolium cultivated ex vitro [65]. Further elucidating the polyphenolic profile, Maruška et al. (2014) investigated the flavonoid composition and antioxidant activity of C. angustifolium across different vegetation stages, applying HPLC with UV detection and DPPH radical scavenging analysis, identifying key flavonoids such as hyperoside, myricetin, quercetin, quercetin-3-O-arabinoside, myricetin-7-O-glucoside, kaempferol, kaempferol-7-O-glycosides, and kaempferol-3-O-glucoside [66]. The flavonoid concentration and antioxidant activity peaked during massive blooming, correlating with high levels of myricetin, its glycosides, and hyperoside, highlighting blooms as primary flavonoid storage sites. This phase also enhances the medicinal potential of key flavonoids, such as quercetin (antioxidant, anti-inflammatory, cardioprotective, and anticancer), kaempferol (antiproliferative and pro-apoptotic), myricetin (antibacterial), and kaempferol-7-O-glucoside (antiviral against HIV-1), emphasizing the importance of selective harvesting at full bloom for the optimal yield [65,66,67]. Recent studies highlight the biological significance of isocoumarins, structural isomers of key flavonoids like quercetin, kaempferol, and myricetin. Ramanan et al. found that 3-aryl isocoumarins inhibit 5-LOX and mPGES1, demonstrating strong anti-inflammatory activity [68]. Their structural similarity to flavonoids suggests potential synergies, warranting further research.

Table 4.
HPLC profile of polyphenolic compounds in the aerial parts of C. angustifolium and C. latifolium.
Table 4.
HPLC profile of polyphenolic compounds in the aerial parts of C. angustifolium and C. latifolium.
| Compounds | Molecular Weight, g/mol | Plant | Identification Method | Extraction Method | Extract Type | Ref. |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| Gallic acid | 170.12 | CA CL | HPLC-DAD-MSn, HPLC-UV, HPLC-UV-ESI/MS | Hydrodistillation, Reflux | Methanol (aq.), Methanol, Ethanol | [9,30,53] |
| Chlorogenic acid | 354.31 | CL | HPLC-UV, HPLC-DAD, HPLC-UV-ESI/MS | Hydrodistillation, Reflux | Methanol (aq.), Methanol, Ethanol | [9,30,53] |
| Caffeic Acid | 180.16 | CL | HPLC-UV-ESI/MS | Reflux | Ethanol | [9] |
| Ellagic Acid | 302.20 | CA | HPLC-UV-ESI/MS | UAE | Methanol | [67] |
| p-Coumaric Acid | 164.04 | CL | HPLC-UV-ESI/MS | Reflux | Ethanol | [9] |
| Ferulic Acid | 194.18 | CA | HPLC-UV-ESI/MS | UAE | Methanol | [67] |
| Flavonoids | ||||||
| Rutin | 610.52 | CA, CL | HPLC-UV, HPLC-UV-ESI/MS | UAE, Reflux | Methanol (aq.), Ethanol | [9,67] |
| Quercitin | 302.24 | CL, CA | HPLC-UV | Reflux, SPME | Methanol (aq.) | [23,66] |
| Quercetin-3-O-Glucoside | 464.38 | CL | HPLC-UV-ESI/MS | Reflux | Ethanol | [9] |
| Quercetin-3-O-Arabinoside | 434.35 | CA | HPLC-DAD-MSn | Hydrodistillation | Methanol | [66] |
| Quercetin 3-O-Glucuronide | 478.36 | CL, CA | HPLC-DAD, UPLC-MS/MS | SPE, Reflux | Methanol | [30,53,65] |
| Quercetin-3-O-Rhamnoside | 448.38 | CA | HPLC-DAD, HPLC-MS/MS | UAE, | Methanol | [67] |
| Myricetin | 318.24 | CL, CA | HPLC-UV-ESI/MS, HPLC-UV | Reflux, SPME | Ethanol, Methanol (aq.) | [9,23] |
| Myricetin-3-O-Rhamnoside | 464.38 | CA | HPLC-UV-ESI/MS | UAE | Methanol | [67] |
| Myricetin-3-O-Glucuronide | 494.36 | CA | HPLC-UV-ESI/MS | UAE | Methanol | [67] |
| Myricetin-7-O-Glucoside | 480.38 | CA | HPLC-DAD-MSn | UAE | Methanol | [67] |
| Kaempferol | 286.24 | CL | HPLC-UV | SPME | Methanol (aq.) | [23] |
| Kaempferol-3-O-Glucuronide | 462.36 | CA | HPLC-UV-ESI/MS | UAE | Methanol | [67] |
| Kaempferol-3-O-Rhamnoside | 432.38 | CA | HPLC-UV-ESI/MS | UAE | Methanol | [65] |
| Kaempferol-7-O-Glucoside | 448.38 | CA | HPLC-DAD-MSn | SPE | Methanol | [67] |
| Kaempferol-3-O-Glucoside | 448.48 | CA | HPLC-DAD-MSn | SPE | Methanol | [66] |
| Hyperoside | 464.38 | CL | HPLC-UV | SPME | Methanol (aq.), | [23] |
| Tannins | ||||||
| Oenothein B | 1569.10 | CA | HPLC-DAD-MSn, HPLC-DAD, HPLC-UV | SPE, UAE, SPME, | Methanol, Methanol (aq.) | [23,65,67] |
CL—C. latifolium; CA—C. angustifolium; SPME—solid-phase microextraction; UAE—ultrasonic-assisted extraction; SPE—solid-phase extraction.
In alignment with these findings, Kaškonienė et al. conducted a detailed HPLC analysis of polyphenolic compounds in C. angustifolium, focusing on the effects of drying [23]. Oenothein B was identified as the primary polyphenolic compound, abundant in both fresh and dried samples, but drying reduced its concentration approximately five-fold. Notably, Oenothein B exhibits a range of biological activities, including anti-tumor potential, the ability to decrease tumor growth in vivo, and macrophage activation. Additionally, it demonstrates anti-HIV, anti-inflammatory, antiprostate hyperplasia, and immunomodulatory properties [65]. Clinical studies have confirmed its therapeutic potential. A randomized, double-blind, placebo-controlled trial on Epilobium angustifolium extract (500 mg daily for six months) demonstrated significant improvements in symptoms of benign prostatic hyperplasia, including reduced post-void residual urine volume and nocturia, with good tolerability [69]. Another 12-week clinical trial in Japan showed that eucalyptus extract containing oenothein B significantly reduced the visceral fat area, waist circumference, body weight, and BMI in overweight individuals compared to a placebo [70]. Rutin levels similarly decreased by 2.2 times with drying, while quercetin and gallic acid showed stability [67]. Rutin is highly significant in scientific research due to its extensive pharmacological potential. Numerous reviews have highlighted its diverse bioactivities, including anti-inflammatory, antidiabetic, cardiovascular, hepatoprotective, anticancer, and neuroprotective effects. Additionally, glycosylated isocoumarins, which are structural isomers of rutin, have been synthesized for similar bioactive properties. Aidhen and Kasireddy synthesized 3-glycosylated isocoumarins using Julia olefination and Meinwald rearrangement, demonstrating their relevance as bioactive flavonoid analogs [12,20,70,71]. Chlorogenic acid, however, demonstrated sensitivity to preparation, as it was absent in some dried samples, indicating drying’s impact on specific polyphenols and suggesting fresh samples retain a more robust polyphenolic profile [66,67]. Chlorogenic acid possesses strong antioxidant properties and has been studied for its protective effects against UV-induced skin damage [17].
The efficient extraction of polyphenols depends on the choice of solvent and method. Ethanol has been identified as the most effective solvent for isolating polyphenols from C. latifolium species, yielding high concentrations of gallic acid, quercetin 3-glucoside, and rutin. By contrast, ethyl acetate extracts tend to favor selective isolation of specific compounds like myricetin, albeit at lower overall yields [9]. Advances in extraction technology, such as ultrasound-assisted extraction and solid-phase microextraction, have further enhanced the recovery and analysis of these bioactive compounds, paving the way for their utilization in pharmacological applications [72].
The polyphenolic composition of C. angustifolium and C. latifolium underscores their significance as a natural source of bioactive compounds. Seasonal and environmental factors, along with extraction methods, profoundly influence the yield and efficacy of these compounds [73].
2.2.4. Impact of Fermentation on the Chemical Composition of C. angustifolium
Fermentation is a widely recognized process in food and pharmaceutical industries, known for enhancing the bioavailability and functionality of bioactive compounds in plant materials [74]. In the case of C. angustifolium, fermentation significantly alters its chemical profile, particularly increasing the concentration of polyphenols, flavonoids, and specific antioxidants [21].
The chemical composition of non-fermented and fermented C. angustifolium leaves highlights the significant impact of fermentation on bioactive compound concentrations (Table 5). Jarine et al. observed a significant increase in total polyphenolic content throughout both aerobic and anaerobic fermentation, peaking after 48 h of aerobic fermentation. Ellagic acid is the primary phenolic acid in rosebay willowherb leaves, with its content significantly increasing after 48 h of aerobic solid-state fermentation, escalating from 1246.56 mg to 2588.25 mg per 100 g dry weight in the fermented samples [75]. During fermentation, p-coumaric acid exhibited a decrease after 24 and 48 h of aerobic fermentation but showed a significant increase after 72 h compared to unfermented leaves. Gallic acid concentrations in non-fermented leaves started at 29.14 mg/100 g DW and rose sharply during fermentation, especially in aerobic conditions, to reach 135.20 mg/100 g DW after 24 h [76]. Lasinkas and his team reported that solid-state fermented leaves had elevated levels of benzoic acid, quercetin, and oenothein B, with oenothein B steadily increasing over two years of fermentation. A particularly noticeable rise in oenothein B was recorded after 24 h of fermentation [77].
The physiological activity of fireweed leaves is shaped by various factors, including solid-state fermentation and agricultural practices such as natural, organic, and biodynamic methods [78]. Biodynamic farming enhances soil and plant health through fermented preparations. Organic farming relies on natural compost and excludes synthetic inputs, while natural farming minimizes interventions like compost or preparations [79]. Among these practices, non-fermented samples showed the highest chlorogenic acid content (47.37 mg/100 g DW), while organic leaves achieved the peak concentration of quercetin-3-O-rutinoside (79.19 mg/100 g DW). Biodynamic farming excelled in producing the highest levels of lutein and beta-carotene, at 35.59 and 15.90 mg/100 g DW, respectively [80].
Carotenoids, vital for human health and abundant in plant-based foods, are highly recommended for inclusion in the daily diet. However, chlorophyll A and B degrade during fermentation due to oxidative or enzymatic processes, with chlorophyll B declining from 172.43 to 156.98 mg/100 g DW after 24 h, highlighting the impact of fermentation on pigment stability [80].
Moreover, proteins and fibers exhibit a modest increase with fermentation, especially after 48 h. This may result from structural changes in plant cells, enhancing digestibility. Non-fermented leaves contain higher sugar levels (7.08 mg/100 g DW). Fermentation significantly reduces the sugar content (4.23 mg/100 g DW after 48 h), as sugars are metabolized by microbes, indicating fermentation’s potential for reducing caloric content. Non-fermented leaves contain moderate levels of vitamin C (247.19 mg/100 g DW). Fermented samples showed a sharp increase in these levels (534.70 mg/100 g DW after 48 h), likely due to microbial synthesis or better preservation under acidic conditions [81].
Fermentation profoundly impacts the chemical composition of C. angustifolium leaves, enhancing the concentration of polyphenols, flavonoids, and specific antioxidants while reducing the sugar levels. These transformations not only improve the nutritional value of the leaves but also amplify their medicinal potential [81,82].

Table 5.
Changes in the chemical composition of C. angustifolium during fermentation.
Table 5.
Changes in the chemical composition of C. angustifolium during fermentation.
| Classes | Components | Fermentation Status | Time (h) | Concentration (mg/100 g DW) | Ref. |
|---|---|---|---|---|---|
| Phenolic Acids | Gallic Acid | Non-Fermented | 0 | 29.14 | [76] |
| Fermented(Aerobic) | 24 | 135.20 | [76] | ||
| Chlorogenic Acid | Non-Fermented | 0 | 56.79 | [80] | |
| Fermented (natural) | 24 | 47.37 | [80] | ||
| p-Coumaric Acid | Non-Fermented | 0 | 213.81 | [76] | |
| Fermented (Anaerobic) | 72 | 255.73 | [76] | ||
| Ellagic Acid | Non-Fermented) | 0 | 1246.56 | [75] | |
| Fermented (Aerobic | 48 | 2588.25 | [75] | ||
| Benzoic Acid | Non-Fermented | 0 | 3.00 | [77] | |
| Fermented | 48 | 29.81 | [77] | ||
| Tannins | Oenothein B | Non-Fermented | 0 | 1442.22 | [77] |
| Fermented | 24 | 1753.65 | [77] | ||
| Flavonoids | Myricetin | Non-Fermented | 0 | 11.31 | [75] |
| Fermented (Aerobic) | 48 | 25.83 | [75] | ||
| Quercetin-3-O-Rutinoside | Non-Fermented | 0 | 20.85 | [80] | |
| Fermented (organic) | 24 | 79.19 | [80] | ||
| Quercetin-3-O-Glucoside | Non-Fermented | 0 | 55.61 | [80] | |
| Fermented | 24 | 66.20 | [80] | ||
| Quercetin | Non-Fermented | 0 | 2.45 | [21] | |
| Fermented | 24 | 10.65 | [21] | ||
| Luteolin | Non-Fermented | 0 | 6.33 | [77] | |
| Fermented | 24 | 2.40 | [77] | ||
| Kaempferol | Non-Fermented | 0 | 3.87 | [77] | |
| Fermented | 24 | 2.70 | [77] | ||
| Carotenoids | Lutein | Non-Fermented | 0 | 33.16 | [80] |
| Fermented (Biodynamic) | 24 | 35.59 | [80] | ||
| Zeaxanthin | Non-Fermented | 0 | 14.89 | [80] | |
| Fermented | 48 | 17.66 | [80] | ||
| Beta-Carotene | Non-Fermented) | 0 | 15.28 | [80] | |
| Fermented (Biodynamic | 24 | 15.90 | [80] | ||
| Chlorophylls | Chlorophyll B | Non-Fermented | 0 | 172.43 | [80] |
| Fermented | 24 | 156.98 | [80] | ||
| Chlorophyll A | Non-Fermented | 0 | 153.97 | [80] | |
| Fermented (Biodynamic) | 24 | 127.33 | [80] | ||
| Carbohydrates | Total Sugars | Non-Fermented | 0 | 7.08 | [81] |
| Fermented | 48 | 4.23 | [81] | ||
| Organic acids | Vitamin C | Non-Fermented | 0 | 247.19 | [81] |
| Fermented | 48 | 534.70 | [81] |
2.2.5. Sterols
Sterols are a vital component of the lipophilic extracts in C. angustifolium, showcasing significant bioactive properties and medicinal potential [83]. Analytical techniques, such as GC-MS and HPLC-DAD-MS/MS, have enabled the comprehensive profiling of sterols, including the identification of key compounds like β-sitosterol, campesterol [52,65,67], and stigmasterol [83], which are listed in Table 6. β-Sitosterol, constituting 63% of the lipophilic fraction in C. angustifolium, is well-known for its cholesterol-lowering properties, making this plant a promising candidate for cardiovascular health supplements [84]. Additionally, its sterol-rich composition supports applications in functional foods and nutraceuticals aimed at regulating lipid metabolism and hormonal balance. Studies by Gryszczyńska et al. further highlight that in vitro cultivation significantly enhances sterol concentrations, with stigmasterol being the most abundant sterol in both in vitro and field-grown samples [52,65,67] (Figure 5).

Table 6.
Sterols identified in C. angustifolium extracts.

Figure 5.
The chemical structures of sterols identified in C. angustifolium. The structures were drawn using ChemDraw Ultra 12.0 software.
2.2.6. Tentatively Isolated and Identified Secondary Metabolites from C. angustifolium
Frolova and her team conducted an in-depth analysis of the lipophilic components of C. angustifolium, with a particular focus on pomolic acid, a bioactive compound exhibiting significant therapeutic potential. Pomolic acid was extracted using methyl tert-butyl ether (MTBE) and further purified through chromatography and recrystallization with a hexane–diethyl ether mixture, yielding 0.04% with a purity of 95%, as confirmed usingchromatography–mass spectrometry (C-MS). In addition, pomolic acid showed notable cytotoxic activity against cancer cells and demonstrated potential in other medicinal applications, including antiviral and anti-inflammatory activities [48].
Moreover, Movsumov and his team performed an in-depth study of the biologically active compounds found in C. angustifolium cultivated in Azerbaijan. Aerial parts of C. angustifolium were collected and air-dried before being extracted with 80% ethanol. The extract was subsequently partitioned with chloroform and ethyl acetate, followed by chromatography using alumina columns, and the structures of the isolated compounds were confirmed using advanced techniques such as nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy. Their research led to the identification of several notable compounds including β-sitosterol, ursolic acid, ellagic acid, kaempferol, and quercetin. Among the identified compounds, β-sitosterol is a plant sterol, while ursolic acid is a pentacyclic triterpenoid. Ellagic acid and the flavonoids kaempferol and quercetin were also detected, each with significant biological activities [85]. The chemical structures of secondary metabolites isolated from C. angustifolium are given in Figure 6.
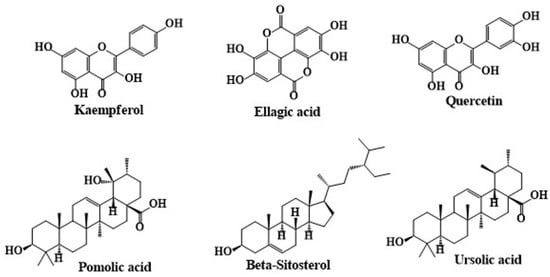
Figure 6.
The chemical structures of secondary metabolites isolated from C. angustifolium. The structures were drawn using ChemDraw Ultra 12.0 software.
2.3. Biological Activity
2.3.1. Diverse Biological Activities of Isolated Compounds from C. angustifolium
C. angustifolium, a widely recognized medicinal plant, serves as a valuable reservoir of biologically active compounds including pomolic acid, β-sitosterol, ursolic acid, ellagic acid, kaempferol, and quercetin. Among these, quercetin stands out due to its well-documented antioxidant properties, which stem from its ability to neutralize free radicals and prevent lipid peroxidation [42,80,81]. Ulusoy and Sanlier explored the metabolism and bioavailability of quercetin, emphasizing its role in health protection through oxidative stress reduction [86]. Beyond its antioxidant capacity, quercetin has demonstrated significant anti-inflammatory potential by modulating key inflammatory pathways. By inhibiting the release of pro-inflammatory cytokines, such as TNF-α and IL-6, it emerges as a promising therapeutic agent for chronic inflammatory disorders, including rheumatoid arthritis and inflammatory bowel disease [87]. Alizadeh etal. highlighted that the poor water solubility of quercetin poses challenges for its bioavailability, but various delivery systems have been explored to enhance its therapeutic efficacy [88].
Kaempferol, another flavonol present in C. angustifolium, has been shown to possess strong antibacterial and antifungal activities. Research conducted by Periferakis et al. demonstrated that kaempferol effectively suppresses the growth of pathogenic microorganisms, including Staphylococcus aureus, Escherichia coli, and Candida spp., reinforcing its potential as a natural antimicrobial agent [89]. In addition to its antimicrobial effects, kaempferol exhibits anti-inflammatory properties through the inhibition of NF-κB activation and the downregulation of COX-2 expression, as described by Alam et al. [90].
Ellagic acid, a polyphenolic compound, has gained considerable attention for its anticancer properties. As demonstrated by Čižmáriková et al., ellagic acid disrupts cancer cell signaling by targeting multiple molecular pathways, thereby inhibiting cell proliferation, angiogenesis, and mechanisms that allow cancer cells to evade apoptosis. Additionally, its chemopreventive role is linked to its ability to enhance the activity of detoxification enzymes and facilitate DNA repair [91].
Ursolic acid, a pentacyclic triterpenoid found in C. angustifolium, also exhibits a broad spectrum of biological activities. It functions as a potent inhibitor of inflammatory cytokines and enzymes, such as COX-2 and iNOS, making it a viable candidate for managing inflammatory disorders [92]. Mlala et al. further elaborated on its antimicrobial activity, noting its effectiveness against Bacillus cereus, Escherichia coli, and Klebsiella pneumoniae, supporting its potential use in antimicrobial therapies [93]. Another notable bioactive compound is β-sitosterol, a plant sterol that contributes to cardiovascular health by lowering cholesterol levels through the inhibition of intestinal cholesterol absorption. In addition to its cardioprotective role, β-sitosterol has been linked to immune system modulation, as it enhances T-cell proliferation and reduces inflammation [94].
Finally, pomolic acid, a pentacyclic triterpenoid isolated from C. angustifolium, has been extensively studied for its therapeutic potential, particularly in anticancer, antiviral, and anti-inflammatory applications. Research by Martins et al. demonstrated that pomolic acid effectively induces apoptosis and inhibits multidrug resistance mechanisms in prostate cancer cells [95]. Additionally, it exhibits significant cytotoxic effects against lymphocytic leukemia cells and HIV, with an efficacy comparable to 5-fluorouracil. Its mechanism of action includes the stimulation of AMP-dependent protein kinase, the suppression of cancer cell proliferation, and a reduction ininflammation. Importantly, the Ames test and SOS chromotest confirmed its lack of genotoxicity and mutagenicity, supporting its clinical safety profile [48]. Recent studies further highlight pomolic acid’s selective cytotoxicity against glioma cells, including U-87 MG and primary glioma cell lines, demonstrating dose-dependent tumor inhibition [96]. Beyond its anticancer effects, pomolic acid has also shown promise in renal fibrosis treatment. In an in vivo renal fibrosis model, it significantly reduced fibrosis through cadherin and α-SMA modulation while suppressing collagen deposition and extracellular matrix accumulation [97].
2.3.2. Bioactivity of C. angustifolium and C. latifolium Extracts
Antioxidant Properties
The increasing use of plant species for phytotherapeutic applications has driven a surge in studies on their antioxidant properties, as these compounds play a vital role in counteracting oxidative stress [98]. Medicinal plants, abundant in bioactive molecules like phenolic compounds, flavonoids, and terpenoids, are recognized for their strong antioxidant potential [99,100,101,102]. Natural antioxidants have gained prominence as safer and healthier alternatives to synthetic ones [103,104,105].
C. angustifolium, a traditional medicinal plant, is distinguished by its abundant phenolic acids, flavonoids, and ellagitannins, which enhance its noteworthy antioxidant qualities [80]. Research indicates that growing methods and solid-phase fermentation (SSF) significantly affect the bioactive chemical composition in fireweed leaves. Evidence indicates that specific SSF parameters enhance the accumulation of certain bioactive compounds in fireweed [80,106]. For example, Lasinskas et al. found that natural fireweed samples exhibited high antioxidant activity (1319.16 M Trolox eq./g D.M.) [76]. Jariene et al. reported that the antioxidant activity initially declined after 24 h of SSF under aerobic (19.23%) and anaerobic (11.14%) conditions but increased after 48 h to levels higher than those of unfermented leaves (324.56 mM TEAC/100 g DW) under aerobic (15.50%) and anaerobic (14.27%) conditions [75].
Additional research by Lasinskas et al. demonstrated annual fluctuations in the antioxidant activity of C. angustifolium leaves obtained from a biodynamic farm in Lithuania. In 2017, fermented leaves exhibited more antioxidant activity than unfermented leaves; conversely, in 2018, the reverse was noted. A significant association was observed between the antioxidant activity, quantified in mg 100 g−1 DW Trolox equivalents, and the total polyphenol content, with variations dependent on fermentation length [76].
Ecotypes of C. angustifolium cultivated in Lithuania showed notable differences in radical scavenging capacity, according to Vilma Kaškonienė et al. [23]. Key bioactive components, such as rutin, caffeic acid, 3,4-dihydroxybenzoic acid, oenothein B, and chlorogenic acid, were found in their study, which also demonstrated a radical scavenging activity ranging from 110.9 to 174.2 mg/g in analyzed samples. These polyphenolic compounds are vital for scavenging free radicals, reducing oxidative stress, and protecting cells from damage due to their potent antioxidant properties. The potent antioxidant capacity of these extracts increases the likelihood of many health benefits, including anti-inflammatory and disease-preventive qualities against oxidative stress [3,107]. The greatest radical scavenging activity was demonstrated by oenothein B in an HPLC-DPPH assay [23].
Maruška et al. evaluated the seasonal radical scavenging activity of C. angustifolium in relation to its flavonoid content [66,82]. Leaves harvested at the peak of the blooming phase exhibited the highest flavonoid concentrations (8.71–11.12 mg per 100 g D.M.) and radical scavenging activity [66]. The antioxidant capacity of C. angustifolium leaves has been thoroughly investigated, with applications in food preservation and pharmaceutical development examined [108,109].
The antioxidant potential of C. latifolium extracts was evaluated usingDPPH radical scavenging and FRAP tests, with both demonstrating significant antioxidant activity. The ethanol extract showed superior activity, with low IC50 values of 21.31 ± 0.65 μg/mL (DPPH) and 18.13 ± 0.15 μg/mL (FRAP), underscoring its potential as a natural antioxidant source [9].
Antimicrobial Activities
Antimicrobial resistance poses a serious global health threat, driving the search for novel therapeutic alternatives [110]. Increasingly, researchers are turning to plant-derived natural compounds due to their diverse bioactive properties and unique mechanisms of action [111,112,113,114]. Medicinal plants produce a wide range of secondary metabolites, including alkaloids, terpenoids, and phenolic compounds, which have demonstrated potentc antimicrobial activity [115]. The antibacterial properties of C. latifolium extracts have also been assessed using the disc diffusion method against Gram-positive (Staphylococcus aureus, Bacillus cereus), Gram-negative (Escherichia coli, Klebsiella pneumoniae), and fungal (Candida albicans) pathogens. Ethanol extracts exhibited broad activity, with inhibition zone diameters (IZD) ranging from 8.53 to 14.27 mm, whereas ethyl acetate extracts were ineffective against bacteria but inhibited Candida albicans (IZD: 8.58 mm) [9]. The antimicrobial effects of plant-derived compounds are often linked to their ability to disrupt cellular membranes, inhibit enzymatic activity, and interfere with essential cellular processes [116]. Additionally, the synergistic interactions between phytochemicals in plant extracts enhance antimicrobial potency and may reduce the risk of resistance development. Several studies highlight the potential of crude plant extracts to work synergistically with conventional antibiotics, improving their efficacy against multidrug-resistant bacteria [117].
Anticancer and Cytotoxic Activities
Plant extracts have been widely investigated for their ability to inhibit cancer cell proliferation [118,119]. Maruška et al. reported a dose-dependent suppression of C. angustifolium aqueous extracts on breast cancer cell lines (MCF7, MDA-MB-468, and MDA-MB-231), with the most effective concentration ranging from 0.266 to 0.443 mg/mL [66]. The fraction with the highest content of oenothein B (91% phenolics) exhibited the strongest cytotoxic effects, whereas the water-based and third fractions showed comparatively lower activity. Intermediate activity was observed in oenothein B fractions 2 and 3, with the MDA-MB-468 cell line demonstrating the highest sensitivity, indicating the potential of oenothein B in breast cancer therapy [66]. Oenotheins B have been identified as major bioactive constituents in various medicinal plants, particularly within the Onagraceae, Lythraceae, and Myrtaceae families [120]. These macrocyclic ellagitannins are often accompanied by structurally related oligomers, further contributing to their diverse pharmacological properties. Among the most notable biological effects of oenothein B are its antitumor, antioxidant, anti-inflammatory, immunomodulatory, and antimicrobial activities, contributing to its significant health benefits [121]. Traditionally, tannin-rich medicinal plants containing oenothein B have been widely used as folk remedies for various ailments, including gastrointestinal disorders, wound healing, skin conditions, and haemostatic purposes [120], while in vitro and in vivo studies indicate promising anticancer potential. However, further clinical trials are necessary to validate these effects in humans, as factors such as bioavailability, metabolism, and potential side effects must be thoroughly assessed before therapeutic applications can be established.
3. Methods
3.1. Search Strategy
A comprehensive literature review was conducted to gather relevant scientific data on C. angustifolium and C. latifolium. The search encompassed main articles published between 2010 and 2024, sourced from major scientific databases including PubMed, Google Scholar, and ScienceDirect. To ensure a thorough and systematic approach, a combination of keywords and Medical Subject Headings (MeSHs) terms was utilized. The search strategy incorporated specific terms related to the plants of interest, such as “Chamaenerion angustifolium” and “Chamaenerionlatifolium”. Furthermore, additional terms were included to cover various aspects of their phytochemical composition, biological properties, and pharmacological potential. These included “phytochemicals”, “bioactive compounds”, “phytochemical content”, “taxonomic classification”, “botanical description”, “biological properties, activities, or effects”, “pharmacological properties, activities, or effects”, “antioxidant”, “anticancer” or “antiproliferative”, “antidiabetic”, and “antibacterial and antifungal”.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria were (I) studies explicitly investigating C. angustifolium and C. latifolium, including their phytochemical profiles, biological activities, and pharmacological applications; (II) research detailing the identification and quantification of polyphenols, flavonoids, tannins, sterols, and volatile compounds using advanced analytical techniques such as HPLC and GC-MS; (III) studies evaluating antioxidant, antimicrobial, anti-inflammatory, anticancer, and other pharmacological properties; (IV) investigations examining the effects of various extraction techniques (maceration, ultrasonic-assisted extraction, solid-phase extraction) and processing methods (fermentation) on the bioactive compound profile.
The exclusion criteria were (I) short communications, letters, editorials, conference abstracts, and other publications lacking detailed experimental and methodological data; (II) research without a clear focus on Chamaenerion species; (III) articles in languages other than English or Russian without a translation.
3.3. Data Extraction and Analysis
Relevant studies were selected based on their methodology, experimental design, and reported findings. Extracted data included (I) study type (in vitro research); (II) plant parts used and quantity of material analyzed; (III) analytical techniques applied; (IV) phytochemical composition and identified bioactive compounds; (VI) biological activities and corresponding assays.
Discrepancies in data interpretation were resolved through discussion among the authors.
3.4. Addressing Publication Bias
To minimize potential publication bias, the following strategies were implemented:
- Comprehensive database search: inclusion of multiple scientific databases.
- Evaluation of publication trends: analysis of temporal publication patterns to identify biases.
- Assessing methodological consistency: evaluation of study designs and experimental procedures to ensure data reliability.
3.5. Study Selection and Quality Assessment
Two independent reviewers (A.K. and Y.T.) assessed the studies for relevance and quality. Disagreements were resolved through discussion. Quality assessment was based on clarity of research objectives, appropriateness of study design, reliability of analytical methods, and consistency of reported results.
3.6. Screening and Selection of Relevant Studies
An extensive database search initially identified 2010 studies. After screening based on the established inclusion and exclusion criteria, 52 articles were selected for data extraction and results analysis. The discussion of these findings is provided in the subsequent section.
4. Conclusions and Future Perspectives
This review underscores the exceptional phytochemical diversity and pharmacological potential of C. angustifolium and C. latifolium. These species are notable for their abundance of bioactive compounds, including polyphenols, flavonoids, ellagitannins, and volatile constituents, which are closely associated with their pronounced antioxidant, anti-inflammatory, antimicrobial, and anticancer activities. Utilizing advanced analytical methodologies, such as GC-MS and HPLC, researchers have gained valuable insights into their chemical compositions, laying a solid foundation for their diverse therapeutical applications.
A strong correlation exists between the biological activities of Chamaenerion species and their chemical composition. Polyphenols such as oenothein B and flavonoids like quercetin contribute significantly to their antioxidant and anti-inflammatory effects. Ellagitannins exhibit potent anticancer potential, while volatile compounds like α-pinene and linalool enhance antimicrobial effects. The influence of factors such as fermentation, environmental conditions, and extraction techniques has been extensively investigated, revealing significant modifications in the composition and bioavailability of these bioactive compounds. Among these factors, fermentation stands out as it enhances the concentration of polyphenols and flavonoids, thereby amplifying the antioxidant and anti-inflammatory properties of Chamaenerion species.
Nevertheless, despite considerable advancements, notable gaps in the existing knowledge persist. Future research should prioritize the following:
Optimizing extraction and processing techniques to maximize the yield, stability, and efficacy of bioactive compounds.
Elucidating the synergistic interactions of Chamaenerion compounds within complex biological pathways.
Conducting rigorous clinical trials to confirm their therapeutic efficacy and safety for human health.
The integration of traditional medicinal knowledge with contemporary scientific approaches presents an exciting avenue for fully realizing the medicinal potential of Chamaenerion species. Continued interdisciplinary investigations will not only enhance our understanding of their chemical and pharmacological attributes but also facilitate the development of innovative health-promoting products, addressing the rising global demand for natural and sustainable therapeutic solutions.
Author Contributions
Conceptualization, A.K. and Y.T.; investigation, all authors; original draft preparation, A.K., M.I. and Y.T.; review and editing, A.K., M.I. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant no. AP13268729, project PI—Y.T.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-LOX | 5-Lipoxygenase |
| α-SMA | Alpha-Smooth Muscle Actin |
| AMP | Adenosine Monophosphate |
| BMI | Body Mass Index |
| CA; C. angustifolium | Chamaenerion angustifolium |
| CL; C. latifolium | Chamaenerion latifolium |
| COX-2 | Cyclooxygenase-2 |
| C-MS | Chromatography-Mass Spectrometry |
| DNA | Deoxyribonucleic Acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DM | Dry Mass |
| DW | Dry Weight |
| FRAP | Ferric Reducing Antioxidant Power |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| HPLC-DAD | High-Performance Liquid Chromatography with Diode Array Detection |
| HPLC-DAD-MSn | High-Performance Liquid Chromatography with Diode Array Detection and Multi-Stage Mass Spectrometry |
| HPLC-UV | High-Performance Liquid Chromatography with Ultraviolet Detection |
| HPLC-UV-ESI/MS | High-Performance Liquid Chromatography with Ultraviolet Detection and Electrospray Ionization Mass Spectrometry |
| IC50 | Half-Maximal Inhibitory Concentration |
| IL-6 | Interleukin-6 |
| iNOS | Inducible Nitric Oxide Synthase |
| IR | Infrared Spectroscopy |
| IZD | Inhibition Zone Diameters |
| MCF7 | Michigan Cancer Foundation-7 (Human Breast Cancer Cell Line) |
| MDA-MB-231 | Human Triple-Negative Breast Cancer Cell Line |
| MDA-MB-468 | Human Triple-Negative Breast Cancer Cell Line |
| MeSH | Medical Subject Headings |
| mPGES1 | Microsomal Prostaglandin E Synthase-1 |
| MTBE | Methyl tert-butyl ether |
| NMR | Nuclear Magnetic Resonance |
| NF-κB | Nuclear Factor Kappa B |
| PC | Paper Chromatography |
| PMs | Primary Metabolites |
| Ref. | References |
| SPME | Solid-Phase Microextraction |
| SSF | Solid-Phase Fermentation |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| T-cell | T Lymphocyte (a type of immune cell) |
| TNF-α | Tumor Necrosis Factor Alpha |
| U-87 MG | Human Glioblastoma Cell Line |
| UAE | Ultrasonic-Assisted Extraction |
References
- Ostrovska, H.; Oleshchuk, O.; Vannini, S.; Cataldi, S.; Albi, E.; Codini, M.; Moulas, A.; Marchyshyn, S.; Beccari, T.; Ceccarini, M.R. Epilobium angustifolium L.: A Medicinal Plant with Therapeutic Properties. EuroBiotech J. 2017, 1, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kalle, R.; Belichenko, O.; Kuznetsova, N.; Kolosova, V.; Prakofjewa, J.; Stryamets, N.; Mattalia, G.; Šarka, P.; Simanova, A.; Prūse, B.; et al. Gaining Momentum: Popularization of Epilobium angustifolium as Food and Recreational Tea on the Eastern Edge of Europe. Appetite 2020, 150, 104638. [Google Scholar] [CrossRef]
- Adamczak, A.; Dreger, M.; Seidler-Łożykowska, K.; Wielgus, K. Fireweed (Epilobium angustifolium L.): Botany, Phytochemistry and Traditional Uses. A Review. Herba Pol. 2019, 65, 51–63. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Shawky, E.M.; Elgindi, M.R.; Ibrahim, H.A.; Baky, M.H. The potential and outgoing trends in traditional, phytochemical, economical, and ethnopharmacological importance of family Onagraceae: A comprehensive review. J. Ethnopharmacol. 2021, 281, 114450. [Google Scholar] [CrossRef]
- Wagner, W.L.; Hoch, P.C. Onagraceae. In Steyermark’s Flora of Missouri; Yatskievych, G., Ed.; Missouri Botanical Garden Press: St. Louis, MO, USA, 2013. [Google Scholar]
- Stolarczyk, M.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. herbs induce apoptosis in human hormone-dependent prostate cancer cells by activating the mitochondrial pathway. J. Pharm. Pharmacol. 2013, 65, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Perużyńska, M.; Nowak, A.; Birger, R.; Ossowicz-Rupniewska, P.; Konopacki, M.; Rakoczy, R.; Kucharski, Ł.; Wenelska, K.; Klimowicz, A.; Droździk, M.; et al. Anticancer properties of bacterial cellulose membrane containing ethanolic extract of Epilobium angustifolium L. Front. Bioeng. Biotechnol. 2023, 11, 1133345. [Google Scholar] [CrossRef]
- Kozhantayeva, A.; Tursynova, N.; Kolpek, A.; Aibuldinov, Y.; Tursynova, A.; Mashan, T.; Mukazhanova, Z.; Ibrayeva, M.; Zeinuldina, A.; Nurlybayeva, A. Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium L. Pharmaceuticals 2024, 17, 996. [Google Scholar] [CrossRef]
- Sõukand, R.; Mattalia, G.; Kolosova, V.; Stryamets, N.; Prakofjewa, J.; Belichenko, O.; Kuznetsova, N.; Minuzzi, S.; Keedus, L.; Prūse, B. Inventing a herbal tradition: The complex roots of the current popularity of Epilobium angustifolium in Eastern Europe. J. Ethnopharmacol. 2020, 247, 112254. [Google Scholar] [CrossRef]
- Gorbachev, V.; Nikitin, I.; Velina, D.; Klokonos, M.; Mutallibzoda, S.; Tefikova, S.; Orlovtseva, O.; Ivanova, N.; Posnova, G.; Bychkova, T. Rosebay Willowherb (Chamerion angustifolium) in food products: Evaluation of the residual anti-radical activity of polyphenol compounds and N-acetylcysteine. Curr. Nutr. Food Sci. 2024, 20, 220–226. [Google Scholar] [CrossRef]
- Paniagua-Zambrana, N.Y.; Jan, H.A.; Bussmann, R.W. Epilobium angustifolium L. Epilobium collinum C. C. Gmelin Epilobium hirsutum L. Epilobium montanum L. Epilobium palustre L. Epilobium parviflorum Schreber Onagraceae. In Ethnobotany of the Mountain Regions of Eastern Europe. Ethnobotany of Mountain Regions; Bussmann, R.W., Paniagua-Zambrana, N.Y., Kikvidze, Z., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and pharmacological importance of plant secondary metabolites in modern medicine. In Bioorganic Phase in Natural Food: An Overview; Springer: Berlin/Heidelberg, Germany, 2018; pp. 135–156. [Google Scholar]
- Nowak, A.; Zielonka-Brzezicka, J.; Perużyńska, M.; Klimowicz, A. Epilobium angustifolium L. as a potential herbal component of topical products for skin care and treatment—A review. Molecules 2022, 27, 3536. [Google Scholar] [CrossRef] [PubMed]
- Tita, B.; Abdel-Haq, H.; Vitalone, A.; Mazzanti, G.; Saso, L. Analgesic properties of Epilobium angustifolium, evaluated by the hot plate test and the writhing test. Farmaco 2001, 56, 341–343. [Google Scholar] [CrossRef]
- Ruszová, E.; Cheel, J.; Pávek, S.; Moravcová, M.; Hermannová, M.; Matějková, I.; Spilková, J.; Velebný, V.; Kubala, L. Epilobium angustifolium extract demonstrates multiple effects on dermal fibroblasts in vitro and skin photo-protectionin vivo. Gen. Physiol. Biophys. 2013, 32, 347. [Google Scholar]
- Majtan, J.; Bucekova, M.; Jesenak, M. Natural Products and Skin Diseases. Molecules 2021, 26, 4489. [Google Scholar] [CrossRef]
- Kozhantayeva, A.; Tashenov, Y.; Tosmaganbetova, K.; Tazhkenova, G.; Mashan, T.; Bazarkhankyzy, A.; Iskakova, Z.; Sapiyeva, A.; Gabbassova, A. Circaea lutetiana (L) plant and its chemical composition. Rasayan J. Chem. 2022, 15, 1653–1659. [Google Scholar] [CrossRef]
- Kozhantayeva, A.; Rakhmadiyeva, S.; Gulmira, O. Investigation of polyphenolic compounds of Chamaenerion latifolium (L.) plant. Rasayan J. Chem. 2020, 13, 2474–2482. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Kulaitiene, J.; Najman, K.; Hallmann, E. Studies of the variability of polyphenols and carotenoids in different methods fermented organic leaves of willowherb (Chamerion angustifolium (L.) Holub). Appl. Sci. 2020, 10, 5254. [Google Scholar] [CrossRef]
- Jürgenson, S.; Matto, V.; Raal, A. Vegetational variation of phenolic compounds in Epilobium angustifolium. Nat. Prod. Res. 2012, 26, 1951–1953. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Stankevičius, M.; Drevinskas, T.; Akuneca, I.; Kaškonas, P.; Bimbiraitė-Survilienė, K.; Maruška, A.; Ragažinskienė, O.; Kornyšova, O.; Briedis, V. Evaluation of phytochemical composition of fresh and dried raw material of introduced Chamerion angustifolium L. using chromatographic, spectrophotometric and chemometric techniques. Phytochemistry 2015, 115, 184–193. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Armonavičius, D.; Stankevičius, M.; Maruška, A. Extraction of bioactive compounds and influence of storage conditions of raw material Chamaenerion angustifolium (L.) Holub using different strategies. Molecules 2024, 29, 5530. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, T.; Kumar, D. A comprehensive review and recent advancement in the application of tannins for treating Parkinson disease. Pharmacol. Res.-Mod. Chin. Med. 2024, 12, 100499. [Google Scholar] [CrossRef]
- Molnar, M.; Jakovljević Kovač, M.; Pavić, V. A comprehensive analysis of diversity, structure, biosynthesis, and extraction of biologically active tannins from various plant-based materials using deep eutectic solvents. Molecules 2024, 29, 2615. [Google Scholar] [CrossRef]
- Ibáñez, F.; Mujica, V. Enhancing ellagitannin production in pecans and strawberry fruits through pre-harvest biotic stresses. Curr. Food Sci. Technol. Rep. 2024, 2, 27–35. [Google Scholar] [CrossRef]
- Tsarev, V.N.; Bazarnova, N.G.; Dubenskii, M.M. Chamerion angustifolium L.: Chemical composition and biological activity (review). Khimiya Rastit. Syr’ya 2016, 4, 15–26. [Google Scholar]
- Brinker, S.R. Discovery of Chamaenerion latifolium (L.) Holub (Onagraceae) in the Great Lakes Region. Great Lakes Bot. 2019, 55, 3–9. [Google Scholar]
- Pavlov, N.V. Flora of Kazakhstan; Publishing House of the Academy of Sciences of the Kazakh SSR: Alma-Ata, Kazakhstan, 1956; Volume 5. [Google Scholar]
- Komarov, V.L. Flora of the USSR; Nauka: Leningrad-Moscow, Russia, 1949; Volume 15, 742p. [Google Scholar]
- Wan, L.; Xing, Z.; Chang, X.; Liu, J.; Zhang, G. Research on light response curve fitting model of four Chamaenerion plants on the Serzilla Mountains. Am. J. Plant Sci. 2018, 9, 1630–1645. [Google Scholar] [CrossRef][Green Version]
- Iskakova, Z.; Kozhantayeva, A.; Tazhkenova, G.; Mashan, T.; Tosmaganbetova, K.; Tashenov, Y. Investigation of chemical constituents of Chamaenerion latifolium L. Antiinflamm. Antiallergy Agents Med. Chem. 2022, 21, 173–178. [Google Scholar] [CrossRef]
- Fredskild, B. Distribution and occurrence of Onagraceae in Greenland. Nord. J. Bot. 1984, 4, 475–480. [Google Scholar]
- Kadam, P.; Patil, M.; Yadav, K. A review on phytopharmacopial potential of Epilobium angustifolium. Pharmacogn. J. 2018, 10. [Google Scholar] [CrossRef]
- Van Andel, J.; Bos, W.; Ernst, W. An experimental study on two populations of Chamaenerion angustifolium (L.) Scop. (= Epilobium angustifolium L.) occurring on contrasting soils, with particular reference to the response to bicarbonate. New Phytol. 1978, 81, 763–772. [Google Scholar]
- Raven, P.H. The generic subdivision of Onagraceae, tribe Onagreae. Brittonia 1964, 16, 276–288. [Google Scholar]
- Güven, S.; Makbul, S.; Mertayak, F.; Coşkunçelebi, K. Anatomical properties of Epilobium and Chamaenerion from a taxonomical perspective in Turkey. Protoplasma 2021, 258, 827–847. [Google Scholar]
- Kundakçi, S.; Makbul, S.; Gültepe, M.; Güzel, M.E.; Okur, S.; Coşkunçelebi, K. Improvements in the phylogeny of Epilobium and Chamaenerion inferred from nrDNA and cpDNA data focusing on Türkiye. Turk. J. Bot. 2023, 47, 152–168. [Google Scholar]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant metabolomics: An overview of the role of primary and secondary metabolites against different environmental stress factors. Life 2023, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, C.O.; Palai, S.; Ekwuabu, C.P.; Egbuna, C.; Adetunji, J.B.; Ehis-Eriakha, C.B.; Kesh, S.S.; Mtewa, A.G. General principles of primary and secondary plant metabolites: Biogenesis, metabolism, and extraction. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, Netherlands, 2021; pp. 3–23. [Google Scholar]
- Slobodianiuk, L.; Budniak, L.; Feshchenko, H.; Sverstiuk, A.; Palaniza, Y. Quantitative analysis of fatty acids and monosaccharides composition in Chamerion angustifolium L. by GC/MS method. Pharmacia 2022, 69, 167–174. [Google Scholar]
- Kozhantayeva, A.; Rakhmadiyeva, S. Chemical sciences. Annali d’Italia 2020, 6, 6. [Google Scholar]
- Mishra, S.; Levengood, H.; Fan, J.; Zhang, C. Plants under stress: Exploring physiological and molecular responses to nitrogen and phosphorus deficiency. Plants 2024, 13, 3144. [Google Scholar] [CrossRef]
- Uminska, K.; Gudžinskas, Z.; Georgiyants, V. Amino acid profiling in wild Chamaenerion angustifolium populations applying chemometric analysis. J. Appl. Pharm. Sci. 2023, 13, 171–180. [Google Scholar]
- Frolova, T.S.; Sal’Nikova, O.I.; Dudareva, T.A.; Kukina, T.P.; Sinitsyna, O.I. Isolation of pomolic acid from Chamaenerion angustifolium and the evaluation of its potential genotoxicity in bacterial test systems. Russ. J. Bioorg. Chem. 2014, 40, 82–88. [Google Scholar]
- Rowan, D.D. Volatile metabolites. Metabolites 2011, 1, 41–63. [Google Scholar] [CrossRef]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function, and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [PubMed]
- Costa, R.; Dugo, P.; Santi, L.; Dugo, G.; Mondello, L. Advances of modern gas chromatography and hyphenated techniques for analysis of plant extracts. Curr. Org. Chem. 2010, 14, 1752–1768. [Google Scholar]
- Kukina, T.P.; Frolova, T.S.; Sal’nikova, O.I. Neutral constituents of Chamaenerion angustifolium leaves. Chem. Nat. Compd. 2014, 50, 233–236. [Google Scholar]
- Kaškonienė, V.; Maruška, A.; Akuņeca, I.; Stankevičius, M.; Ragažinskienė, O.; Bartkuvienė, V.; Kornyšova, O.; Briedis, V.; Ugenskienė, R. Screening of antioxidant activity and volatile compounds composition of Chamerion angustifolium (L.) Holub ecotypes grown in Lithuania. Nat. Prod. Res. 2016, 30, 1373–1381. [Google Scholar] [CrossRef]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics 2018, 14, 152. [Google Scholar]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications, and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Marčac Duraković, N.; Cegledi, E.; Elez Garofulić, I.; Balbino, S.; Pedisić, S.; Jokić, S.; Dragović-Uzelac, V.; Repajić, M. Recovery of fennel non-polar bioactives via supercritical carbon dioxide extraction. Processes 2024, 12, 1764. [Google Scholar] [CrossRef]
- Figueiredo, C.R.; Matsuo, A.L.; Pereira, F.V.; Rabaca, A.N.; Farias, C.F.; Girola, N.; Massaoka, M.H.; Azevedo, R.A.; Scutti, J.A.B.; Arruda, D.C. Pyrostegia venusta heptane extract containing saturated aliphatic hydrocarbons induces apoptosis on B16F10-Nex2 melanoma cells and displays antitumor activity in vivo. Pharmacogn. Mag. 2014, 10 (Suppl. S2), S363. [Google Scholar] [CrossRef] [PubMed]
- Sabulal, B.; Dan, M.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [PubMed]
- Bhatti, M.Z.; Ismail, H.; Kayani, W.K. Plant secondary metabolites: Therapeutic potential and pharmacological properties. In Secondary Metabolites-Trends and Reviews; IntechOpen: London, UK, 2022. [Google Scholar]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; anti-inflammatory/antioxidant effects of polyphenols: A comparative review on the parental compounds and their metabolites. Food Rev. Int. 2021, 37, 759–811. [Google Scholar] [CrossRef]
- Sehaki, C.; Jullian, N.; Ayati, F.; Fernane, F.; Gontier, E. A review of Pistacia lentiscus polyphenols: Chemical diversity and pharmacological activities. Plants 2023, 12, 279. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L. Analytical strategies for fingerprinting of antioxidants, nutritional substances, and bioactive compounds in foodstuffs based on high performance liquid chromatography–mass spectrometry: An overview. Foods 2020, 9, 1734. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Álvarez, J.A.; Ayuso, J.; Barbero, G. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 1: Pressurized Liquid Extraction. Agronomy 2020, 10, 183. [Google Scholar] [CrossRef]
- Gryszczyńska, A.; Dreger, M.; Piasecka, A.; Piotr, K.; Witaszak, N.; Sawikowska, A.; Ożarowski, M.; Opala, B.; Łowicki, Z.; Pietrowiak, A.; et al. Qualitative and quantitative analyses of bioactive compounds from ex vitro Chamaenerion angustifolium (L.) (Epilobium angustifolium) herb in different harvest times. Ind. Crops Prod. 2018, 123, 208–220. [Google Scholar]
- Maruška, A.; Ragažinskiene, O.; Vyšniauskas, O.; Kaškoniene, V.; Bartkuviene, V.; Kornyšova, O.; Briedis, V.; Ramanauskiene, K. Flavonoids of willow herb (Chamerion angustifolium (L.) Holub) and their radical scavenging activity during vegetation. Adv. Med. Sci. 2014, 59, 136–141. [Google Scholar] [CrossRef]
- Dreger, M.; Seidler-Łożykowska, K.; Szalata, M.; Adamczak, A.; Wielgus, K. Phytochemical variability during vegetation of Chamerion angustifolium (L.) Holub genotypes derived from in vitro cultures. Plant Cell Tissue Organ Cult. 2021, 147, 619–633. [Google Scholar] [CrossRef]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar]
- Esposito, C.; Santarcangelo, C.; Masselli, R.; Buonomo, G.; Nicotra, G.; Insolia, V.; D’Avino, M.; Caruso, G.; Buonomo, A.R.; Sacchi, R.; et al. Epilobium angustifolium L. Extract with High Content in Oenothein B on Benign Prostatic Hyperplasia: A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Biomed. Pharmacother. 2021, 138, 111414. [Google Scholar]
- Nakajima, S.; Abe, T.; Aoshima, Y.; Haruta, T.; Tagami, T.; Tomimatsu, A.; Sakai, H.; Miyashita, M.; Fujioka, M.; Yokoyama, S. Anti-Obesity Effects of Eucalyptus Leaf Extract Containing Oenothein B: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Funct. Foods Health Dis. 2021, 11, 180–193. [Google Scholar]
- Sudarshan, K.; Aidhen, I.S. Convenient synthesis of 3-glycosylated isocoumarins. Eur. J. Org. Chem. 2017, 1, 34–38. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Jariene, E.; Lasinskas, M.; Danilcenko, H.; Vaitkeviciene, N.; Slepetiene, A.; Najman, K.; Hallmann, E. Polyphenols, antioxidant activity, and volatile compounds in fermented leaves of medicinal plant rosebay willowherb (Chamerion angustifolium (L.) Holub). Plants 2020, 9, 1683. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Kulaitiene, J.; Adamaviciene, A.; Hallmann, E. The impact of solid-phase fermentation on flavonoids, phenolic acids, tannins, and antioxidant activity in Chamerion angustifolium (L.) Holub (fireweed) leaves. Plants 2023, 12, 277. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Hallmann, E.; Najman, K. Effect of different durations of solid-phase fermentation for fireweed (Chamerion angustifolium (L.) Holub) leaves on the content of polyphenols and antioxidant activity in vitro. Molecules 2020, 25, 1011. [Google Scholar] [CrossRef]
- Ponzio, C.; Gangatharan, R.; Neri, D. Organic and Biodynamic Agriculture: A Review in Relation to Sustainability. Int. J. Plant Soil Sci. 2013, 2, 95–110. [Google Scholar]
- Santoni, M.; Ferretti, L.; Migliorini, P.; Vazzana, C.; Pacini, G.C. A review of scientific research on biodynamic agriculture. Org. Agric. 2022, 12, 373–396. [Google Scholar]
- Lasinskas, M.; Jariene, E.; Kulaitiene, J.; Vaitkeviciene, N.; Jakiene, E.; Skiba, D.; Hallmann, E. Studies of the variability of biologically active compounds and antioxidant activity in organically, biodynamically, and naturally grown and fermented fireweed (Chamerion angustifolium (L.) Holub) leaves. Plants 2023, 12, 2345. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jariene, E.; Vaitkeviciene, N.; Kulaitiene, J.; Trumbeckaite, S.; Velicka, A.; Hallmann, E. The variability of proximate composition, sugars, and vitamin C in natural, organic, and biodynamic, and fermented leaves of fireweed (Chamerion angustifolium (L.) Holub). Horticulturae 2023, 9, 1245. [Google Scholar] [CrossRef]
- Lasinskas, M.; Hallmann, E.; Najman, K. Flavonoids, phenolic acids, and tannin quantities and their antioxidant activity in fermented fireweed leaves grown in different systems. Plants 2024, 13, 1922. [Google Scholar] [CrossRef]
- Dreger, M.; Gryszczyńska, A.; Szalata, M.; Wielgus, K. Content of sterols in in vitro propagated Chamerion angustifolium (L.) Holub plants. Herba Pol. 2022, 68, 33. [Google Scholar]
- Mishra, B.; Mishra, A.K.; Kumar, S.; Mandal, S.K.; NSV, L.; Kumar, V.; Baek, K.-H.; Mohanta, Y.K. Antifungal Metabolites as Food Bio-Preservative: Innovation, Outlook, and Challenges. Metabolites 2022, 12, 12. [Google Scholar] [CrossRef]
- Movsumov, I.S.; Yusifova, D.Y.; Suleimanov, T.A.; Mahiou-Leddet, V.; Herbette, G.; Baghdikian, B.; Garayev, E.E.; Ollivier, E.; Garayev, E.A. Biologically active compounds from Chamaenerion angustifolium and Stachys annua growing in Azerbaidzhan. Chem. Nat. Compd. 2016, 52, 324–325. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Savadkouhi, N.; Ebrahimzadeh, M.A. Drug design strategies that aim to improve the low solubility and poor bioavailability conundrum in quercetin derivatives. Expert Opin. Drug Discov. 2023, 18, 1117–1132. [Google Scholar] [PubMed]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef]
- Čižmáriková, M.; Michalková, R.; Mirossay, L.; Mojžišová, G.; Zigová, M.; Bardelčíková, A.; Mojžiš, J. Ellagic Acid and Cancer Hallmarks: Insights from Experimental Evidence. Biomolecules 2023, 13, 1653. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 19721. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Salazar, J.R.; Loza-Mejía, M.A.; Soto-Cabrera, D. Chemistry, Biological Activities and In Silico Bioprospection of Sterols and Triterpenes from Mexican Columnar Cactaceae. Molecules 2020, 25, 1649. [Google Scholar] [CrossRef]
- Martins, C.D.A.; Rocha, G.D.G.; Gattass, C.R.; Takiya, C.M. Pomolic Acid Exhibits Anticancer Potential against a Docetaxel-Resistant PC3 Prostate Cell Line. Oncol. Rep. 2019, 42, 328–338. [Google Scholar] [CrossRef]
- Frolova, T.S.; Lipeeva, A.V.; Baev, D.S.; Tsepilov, Y.A.; Sinitsyna, O.I. Apoptosis as the Basic Mechanism of Cytotoxic Action of Ursolic and Pomolic Acids in Glioma Cells. Mol. Biol. 2017, 51, 705–711. [Google Scholar]
- Park, J.-H.; Jang, K.M.; An, H.J.; Kim, J.-Y.; Gwon, M.-G.; Gu, H.; Park, B.; Park, K.-K. Pomolic Acid Ameliorates Fibroblast Activation and Renal Interstitial Fibrosis through Inhibition of SMAD-STAT Signaling Pathways. Molecules 2018, 23, 2236. [Google Scholar] [CrossRef]
- Formagio, A.S.N.; Kassuya, C.A.L.; Neto, F.F.; Volobuff, C.R.F.; Iriguchi, E.K.K.; Vieira, M.D.C.; Foglio, M.A. The flavonoid content and antiproliferative, hypoglycaemic, anti-inflammatory and free radical scavenging activities of Annona dioica St. Hill. BMC Complement. Altern. Med. 2013, 13, 14. [Google Scholar]
- Roy, A.; Gupta, N.; Yadav, P.; Varma, A. Flavonoids: A bioactive compound from medicinal plants and its therapeutic applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Processb 2011, 89, 217–233. [Google Scholar]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M. Medicinal plants. Plants 2021, 10, 1355. [Google Scholar] [PubMed]
- Meenakumari, K.; Bupesh, G.; Phukan, M.M. Determination of in vitro antioxidant activity of the leaves extracts of Ehretia pubescens. Int. J. Res. Pharm. Sci. 2020, 11, 6262–6267. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms: A potential source of bioactive molecules for antioxidant applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural sweeteners: The relevance of food naturalness for consumers, food security aspects, sustainability, and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 6285. [Google Scholar] [CrossRef]
- Vaitkeviciene, N.; Jariene, E.; Kulaitiene, J.; Lasinskas, M.; Blinstrubiene, A.; Hallmann, E. Effect of solid-state fermentation on vitamin C, photosynthetic pigments, and sugars in willow herb (Chamerion angustifolium (L.) Holub) leaves. Plants 2022, 11, 3300. [Google Scholar] [CrossRef]
- Vlase, A.-M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2023, 12, 91. [Google Scholar] [CrossRef]
- Kowalik, K.; Polak-Berecka, M.; Prendecka-Wróbel, M.; Pigoń-Zając, D.; Niedźwiedź, I.; Szwajgier, D.; Baranowska-Wójcik, E.; Waśko, A. Biological activity of an Epilobium angustifolium L. (Fireweed) infusion after in vitro digestion. Molecules 2022, 27, 1006. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Smirnov, V.; Kvashninova, E.; Khlopin, V.; Vityazev, F.; Golovchenko, V. Isolation, chemical characterization and antioxidant activity of pectic polysaccharides of fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 7290. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Ashraf, M.V.; Pant, S.; Khan, M.A.H.; Shah, A.A.; Siddiqui, S.; Jeridi, M.; Alhamdi, H.W.S.; Ahmad, S. Phytochemicals as antimicrobials: Prospecting Himalayan medicinal plants as source of alternate medicine to combat antimicrobial resistance. Pharmaceuticals 2023, 16, 881. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in alternative strategies to combat antimicrobial resistance: Focus on antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Mani, J.; Johnson, J.; Hosking, H.; Schmidt, L.; Batley, R.; du Preez, R.; Broszczak, D.; Walsh, K.; Neilsen, P.; Naiker, M. Bioassay-guided fractionation of Pittosporum angustifolium and Terminalia ferdinandiana with liquid chromatography mass spectroscopy and gas chromatography mass spectroscopy exploratory study. Plants 2024, 13, 807. [Google Scholar] [CrossRef]
- Kochuthressia, K.P.; Britto, S.J. In Vitro Antimicrobial Evaluation of Kaempferia galanga L. Rhizome Extract. Am. J. Biotechnol. Mol. Sci. 2012, 2, 1. [Google Scholar] [CrossRef]
- Pharmacology, C.; Ternopil, I.H.; Medical, N.; Botany, M.; Ternopil, I.H.; Medical, N.; National, I.H.T. Study of antibacterial and antifungal properties of the lyophilized extract of fireweed (Chamaenerion angustifolium L.). Pharmacol. J. 2021, 2, 1464–1472. [Google Scholar]
- Wylie, M.R.; Merrell, D.S. The Antimicrobial Potential of the Neem Tree Azadirachta indica. Front. Pharmacol. 2022, 13, 1. [Google Scholar]
- Al-Sahlany, T.G.; Altemimi, A.B.; Al-Manhel, A.J.A.; Niamah, A.K.; Lakhssassi, N.; Ibrahim, S.A. Purification of bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Nguyen, T.T.; Ma, P.C.; Ta, Q.T.H.; Duong, T.-H.; Vo, V.G. Potential antimicrobial and anticancer activities of an ethanol extract from Bouea macrophylla. Molecules 2020, 25, 1996. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and Biological Significance of Oenothein B and Related Ellagitannin Oligomers with Macrocyclic Structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kośmider, A.; Piwowarski, J. Oenothein B’s Contribution to the Anti-Inflammatory and Antioxidant Activity of Epilobium sp. Phytomedicine 2011, 18, 557–560. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).