Synbiotic Microencapsulation of Lactobacillus Strains from Mexican Fermented Beverages for Enhanced Probiotic Functionality
Abstract
1. Introduction
2. Results
2.1. Microencapsulation
2.2. Morphology of Microcapsules
2.3. Cell Viability During Storage
2.4. Probiotic Properties
2.4.1. Adhesion Properties: Auto-Aggregation and Hydrophobicity
2.4.2. Tolerance to Digestion Conditions
- Phenol
- pH
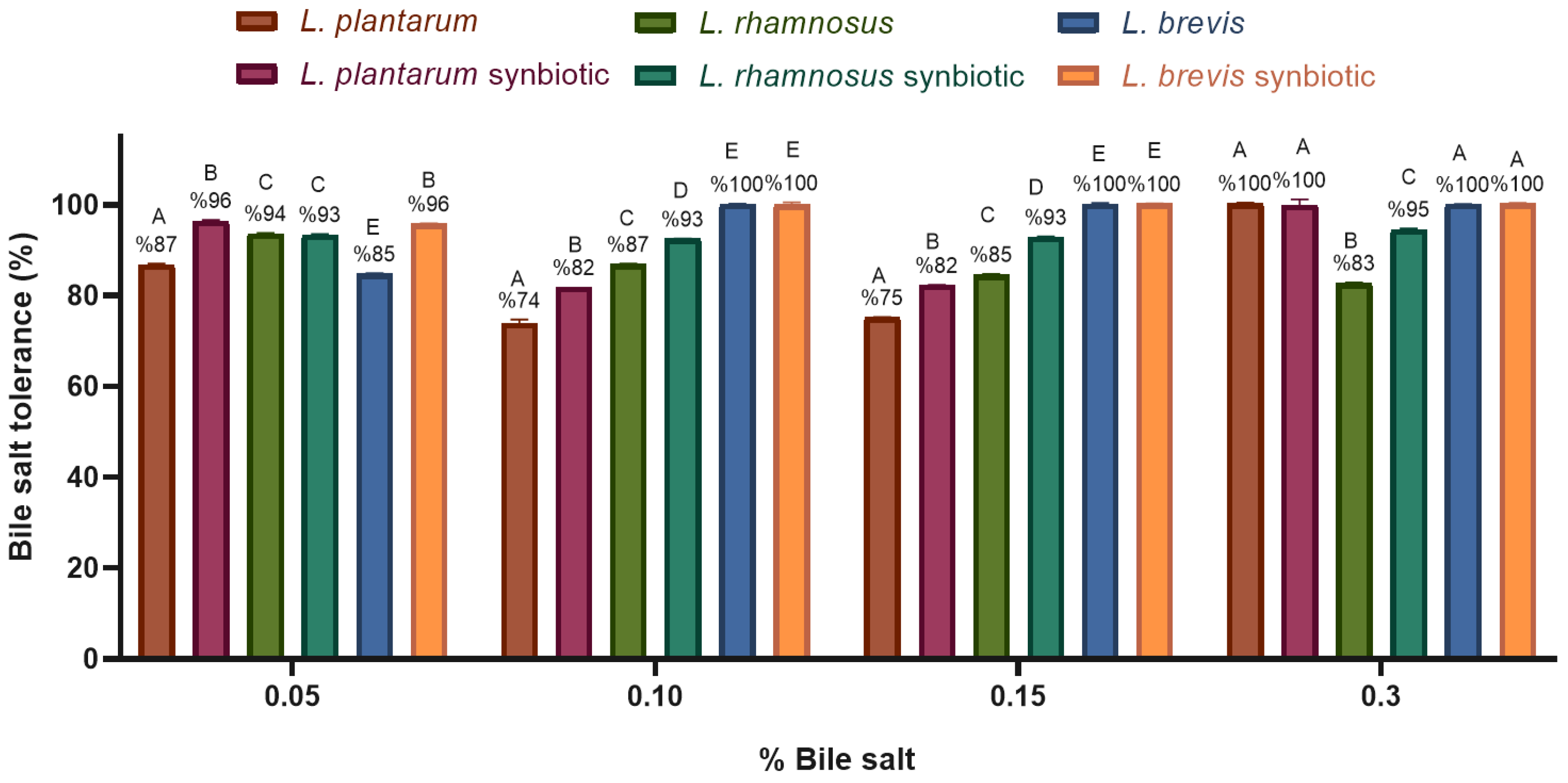
- Bile salt tolerance
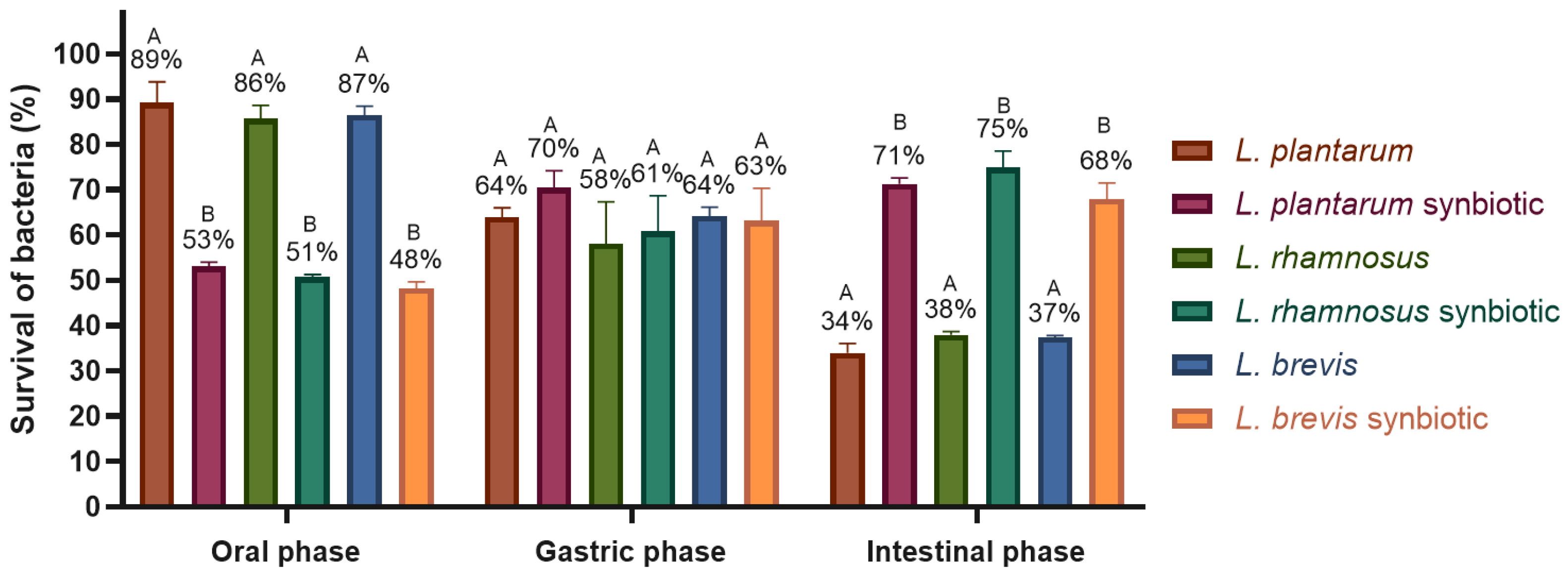
- In vitro digestion
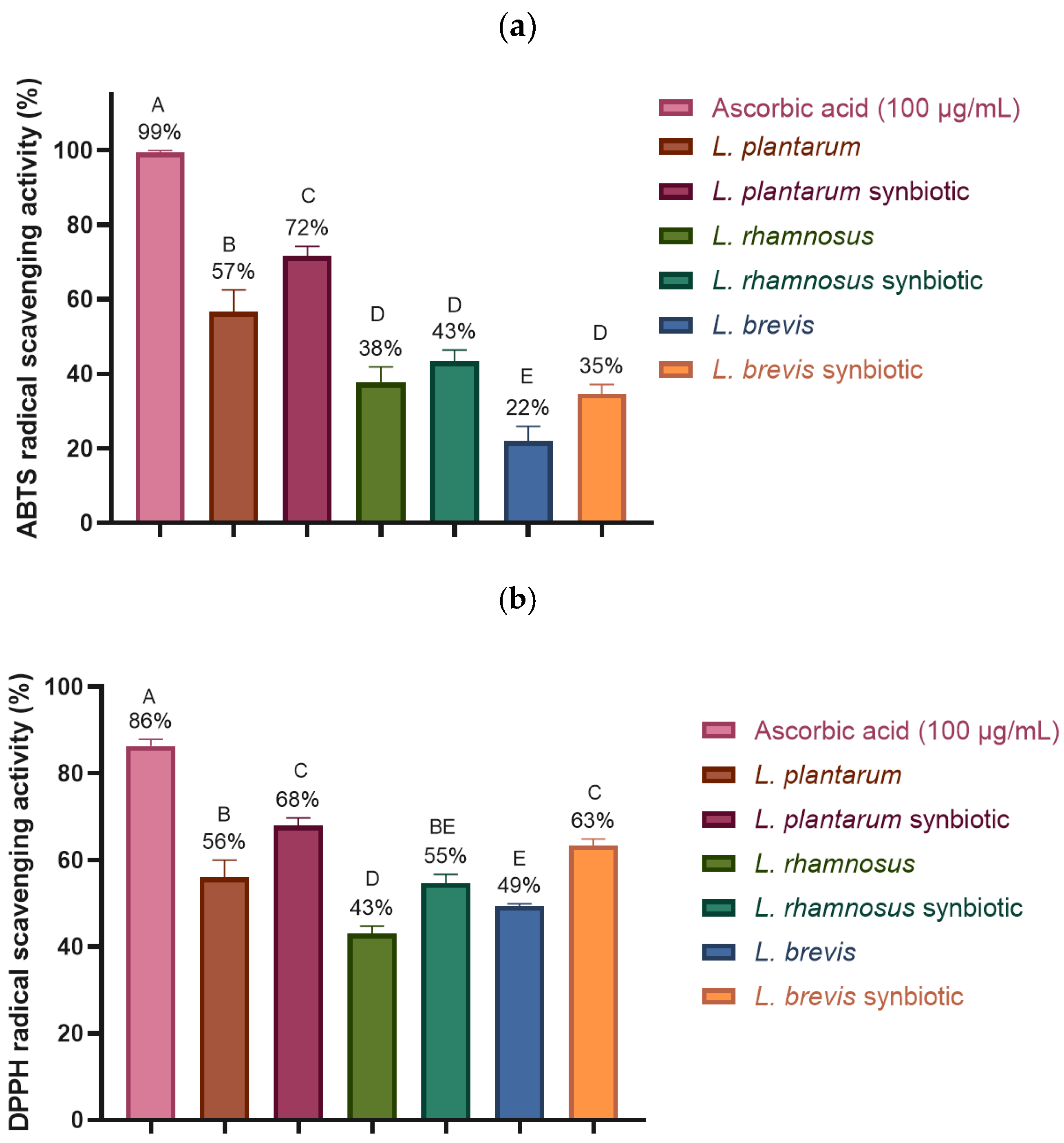
2.4.3. Antioxidant Activity: ABTS and DPPH Free Radical Scavenging
3. Discussion
4. Materials and Methods
4.1. Potential Probiotic Strains and Growth Conditions
4.2. Spray-Drying Microencapsulation
4.3. Scanning Electron Microscopy (SEM)
4.4. Adhesion Capacity
4.4.1. Hydrophobicity
4.4.2. Auto-Aggregation
4.5. Tolerance to Digestion Conditions
4.5.1. Phenol Resistance
4.5.2. pH Tolerance
4.5.3. Bile Salt Tolerance
4.5.4. In Vitro Digestion
4.6. Antioxidant Activity
4.6.1. ABTS Free Radical Scavenging
4.6.2. DPPH Free Radical Scavenging
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| Aw | Water activity |
| MRS | Man, Rogosa, and Sharpe medium |
| PBS | Phosphate buffered saline |
| GA | Gum arabic |
| MD | Maltodextrin |
| I | Inulin |
| SSF | Simulated salivary fluid |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
References
- Ramírez-Guzmán, K.N.; Torres-León, C.; Martinez-Medina, G.A.; De La Rosa, O.; Hernández-Almanza, A.; Alvarez-Perez, O.B.; Araujo, R.; González, L.R.; Londoño, L.; Ventura, J.; et al. Traditional Fermented Beverages in Mexico. In Fermented Beverages; The Science of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 605–635. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic Viability in Yoghurt: A Review of Influential Factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Chaluvadi, S.; Hotchkiss, A.T.; Yam, K.L. Gut Microbiota: Impact of Probiotics, Prebiotics, Synbiotics, Pharmabiotics, and Postbiotics on Human Health. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 515–523. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated Probiotic Cells: Relevant Techniques, Natural Sources as Encapsulating Materials and Food Applications—A Narrative Review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Spray Drying, Freeze Drying and Related Processes for Food Ingredient and Nutraceutical Encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier Masson SAS: Issy-les-Moulineaux, France, 2012; pp. 73–109. [Google Scholar] [CrossRef]
- Nedović, V.; Kalušević, A.; Manojlović, V.; Petrović, T.; Bugarski, B. Encapsulation Systems in the Food Industry. In Advances in Food Process Engineering Research and Applications; Yanniotis, S., Taoukis, P., Stoforos, N., Karathanos, V.T., Eds.; Springer: Boston, MA, USA, 2013; pp. 229–253. [Google Scholar]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2014, 7, 452–490. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK; American Pharmacists Association: Washington, DC, USA, 2009. [Google Scholar]
- Arepally, D.; Reddy, R.S.; Goswami, T.K. Encapsulation of: Lactobacillus acidophilus NCDC 016 Cells by Spray Drying: Characterization, Survival after in Vitro Digestion, and Storage Stability. Food Funct. 2020, 11, 8694–8706. [Google Scholar] [CrossRef] [PubMed]
- Elnour, A.A.M.; Abdurahman, N.H.; Musa, K.H.; Rasheed, Z. Prebiotic Potential of Gum Arabic for Gut Health. Int. J. Health Sci. 2023, 17, 4. [Google Scholar]
- Nunes, G.L.; de Araújo Etchepare, M.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; Barin, J.S.; de Moares Flores, É.M.; de Bona da Silva, C.; de Menezes, C.R. Inulin, Hi-Maize, and Trehalose as Thermal Protectants for Increasing Viability of Lactobacillus acidophilus Encapsulated by Spray Drying. LWT 2018, 89, 128–133. [Google Scholar] [CrossRef]
- Romano, N.; Mobili, P.; Zuñiga-Hansen, M.E.; Gómez-Zavaglia, A. Physico-Chemical and Structural Properties of Crystalline Inulin Explain the Stability of Lactobacillus plantarum during Spray-Drying and Storage. Food Res. Int. 2018, 113, 167–174. [Google Scholar] [CrossRef]
- Bustamante, M.; Laurie-Martínez, L.; Vergara, D.; Campos-Vega, R.; Rubilar, M.; Shene, C. Effect of Three Polysaccharides (Inulin, and Mucilage from Chia and Flax Seeds) on the Survival of Probiotic Bacteria Encapsulated by Spray Drying. Appl. Sci. 2020, 10, 4623. [Google Scholar] [CrossRef]
- Russo, M.I.; Abeijón-Mukdsi, M.C.; Santacruz, A.; Ross, R.; Malo, A.L.; Gauffin-Cano, P.; Medina, R.B. Spray Dried Lactobacilli Maintain Viability and Feruloyl Esterase Activity during Prolonged Storage and under Gastrointestinal Tract Conditions. J. Food Sci. Technol. 2022, 59, 1202–1210. [Google Scholar] [CrossRef]
- Xavier dos Santos, D.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved Probiotic Survival to in Vitro Gastrointestinal Stress in a Mousse Containing Lactobacillus acidophilus La-5 Microencapsulated with Inulin by Spray Drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Zhu, Z.; Luan, C.; Zhang, H.; Zhang, L.; Hao, Y. Effects of Spray Drying on Lactobacillus plantarum BM-1 Viability, Resistance to Simulated Gastrointestinal Digestion, and Storage Stability. Dry. Technol. 2016, 34, 177–184. [Google Scholar] [CrossRef]
- Paéz, R.; Lavari, L.; Vinderola, G.; Audero, G.; Cuatrin, A.; Zaritzky, N.; Reinheimer, J. Effect of Heat Treatment and Spray Drying on Lactobacilli Viability and Resistance to Simulated Gastrointestinal Digestion. Food Res. Int. 2012, 48, 748–754. [Google Scholar] [CrossRef]
- Rosolen, M.D.; Bordini, F.W.; de Oliveira, P.D.; Conceição, F.R.; Pohndorf, R.S.; Fiorentini, Â.M.; da Silva, W.P.; Pieniz, S. Symbiotic Microencapsulation of Lactococcus lactis subsp. Lactis R7 Using Whey and Inulin by Spray Drying. LWT 2019, 115, 108411. [Google Scholar] [CrossRef]
- Avila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. Rhamnosus by Spray-Drying Using Two Prebiotics Colloids to Enhance the Viability. Carbohydr. Polym. 2014, 102, 423–430. [Google Scholar] [CrossRef]
- Hernández-Delgado, N.C.; Torres-Maravilla, E.; Mayorga-Reyes, L.; Martín, R.; Langella, P.; Pérez-Pastén-Borja, R.; Sánchez-Pardo, M.E.; Bermúdez-Humarán, L.G. Microorganisms Antioxidant and Anti-Inflammatory Properties of Probiotic Candidate Strains Isolated during Fermentation of Agave (Agave angustifolia Haw). Microorganisms 2021, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Lenoir, M.; Mayorga-Reyes, L.; Allain, T.; Sokol, H.; Langella, P.; Sánchez-Pardo, M.E.; Bermúdez-Humarán, L.G. Identification of Novel Anti-Inflammatory Probiotic Strains Isolated from Pulque. Appl. Microbiol. Biotechnol. 2016, 100, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Leylak, C.; Özdemir, K.S.; Gurakan, G.C.; Ogel, Z.B. Optimisation of Spray Drying Parameters for Lactobacillus acidophilus Encapsulation in Whey and Gum Arabic: Its Application in Yoghurt. Int. Dairy. J. 2021, 112, 104865. [Google Scholar] [CrossRef]
- Broeckx, G.; Vandenheuvel, D.; Henkens, T.; Kiekens, S.; van den Broek, M.F.L.; Lebeer, S.; Kiekens, F. Enhancing the Viability of Lactobacillus rhamnosus GG after Spray Drying and during Storage. Int. J. Pharm. 2017, 534, 35–41. [Google Scholar] [CrossRef]
- Huang, S.; Vignolles, M.L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray Drying of Probiotics and Other Food-Grade Bacteria: A Review. Trends Food Sci. Technol. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Kalita, D.; Saikia, S.; Gautam, G.; Mukhopadhyay, R.; Mahanta, C.L. Characteristics of Synbiotic Spray Dried Powder of Litchi Juice with Lactobacillus plantarum and Different Carrier Materials. LWT 2018, 87, 351–360. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.T. Effect of Drying Methods (Electrospraying, Freeze Drying and Spray Drying) on Survival and Viability of Microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis labrusca var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Akanny, E.; Bourgeois, S.; Bonhommé, A.; Commun, C.; Doleans-Jordheim, A.; Bessueille, F.; Bordes, C. Development of Enteric Polymer-Based Microspheres by Spray-Drying for Colonic Delivery of Lactobacillus rhamnosus GG. Int. J. Pharm. 2020, 584, 119414. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Moumita, S.; Goderska, K.; Johnson, E.M.; Das, B.; Indira, D.; Yadav, R.; Kumari, S.; Jayabalan, R. Evaluation of the Viability of Free and Encapsulated Lactic Acid Bacteria Using In-Vitro Gastro Intestinal Model and Survivability Studies of Synbiotic Microcapsules in Dry Food Matrix during Storage. LWT 2017, 77, 460–467. [Google Scholar] [CrossRef]
- Sánchez, L.; Tromps, J. Caracterización in Vitro de Bacterias Ácido Lácticas Con Potencial Probiótico In Vitro Characterization of Acid Lactic Bacterias with Probiotic Potential. Rev. Salud Anim. 2014, 36, 124–129. [Google Scholar]
- Reale, A.; Di Renzo, T.; Rossi, F.; Zotta, T.; Iacumin, L.; Preziuso, M.; Parente, E.; Sorrentino, E.; Coppola, R. Tolerance of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus Strains to Stress Factors Encountered in Food Processing and in the Gastro-Intestinal Tract. LWT 2015, 60, 721–728. [Google Scholar] [CrossRef]
- Yadav, R.; Puniya, A.K.; Shukla, P. Probiotic Properties of Lactobacillus plantarum RYPR1 from an Indigenous Fermented Beverage Raabadi. Front. Microbiol. 2016, 7, 1683. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Chen, Z.; Zhou, Q.; Wei, F.; Li, P.; Gu, Q. Antioxidant Properties and Molecular Mechanisms of Lactiplantibacillus plantarum ZJ316: A Potential Probiotic Resource. LWT 2023, 187, 115269. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S.; Kim, Y.G.; Jeong, Y.; Kim, J.E.; Paek, N.S.; Kang, C.H. Antioxidant and Probiotic Properties of Lactobacilli and Bifidobacteria of Human Origins. Biotechnol. Bioprocess. Eng. 2020, 25, 421–430. [Google Scholar] [CrossRef]
- Mounir, M.; Ibijbijen, A.; Farih, K.; Rabetafika, H.N.; Razafindralambo, H.L. Synbiotics and Their Antioxidant Properties, Mechanisms, and Benefits on Human and Animal Health: A Narrative Review. Biomolecules 2022, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Garfias Noguez, C.; Ramírez Damián, M.; Ortiz Moreno, A.; Karina Márquez Flores, Y.; Alamilla Beltrán, L.; Márquez Lemus, M.; Bermúdez Humarán, L.G.; Elena Sánchez Pardo, M.; Adolfo López Mateos, P.; Wilfrido Massieu, A. Microencapsulation and Probiotic Characterization of Lactiplantibacillus plantarum LM-20: Therapeutic Application in A Murine Model of Ulcerative Colitis. Nutrients 2025, 17, 749. [Google Scholar] [CrossRef]
- García-Hernández, Y.; Pérez-Sánchez, T.; Boucourt, R.; Balcázar, J.L.; Nicoli, J.R.; Moreira-Silva, J.; Rodríguez, Z.; Fuertes, H.; Nuñez, O.; Albelo, N.; et al. Isolation, Characterization and Evaluation of Probiotic Lactic Acid Bacteria for Potential Use in Animal Production. Res. Vet. Sci. 2016, 108, 125–132. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic Characterization of Lactic Acid Bacteria Isolated from Fermented Foods and Beverage of Ladakh. LWT 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Vizoso Pinto, M.G.; Franz, C.M.A.P.; Schillinger, U.; Holzapfel, W.H. Lactobacillus spp. with in Vitro Probiotic Properties from Human Faeces and Traditional Fermented Products. Int. J. Food Microbiol. 2006, 109, 205–214. [Google Scholar] [CrossRef]
- Castillo Arroyo, P.L.; Betancur Hurtado, C.A.; Pardo Pérez, E. Characterization of Microorganisms with Probiotic Potential Isolated from Brahman Calf Manure in Sucre, Colombia. Rev. Investig. Vet. Peru 2018, 29, 438–448. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food-an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]

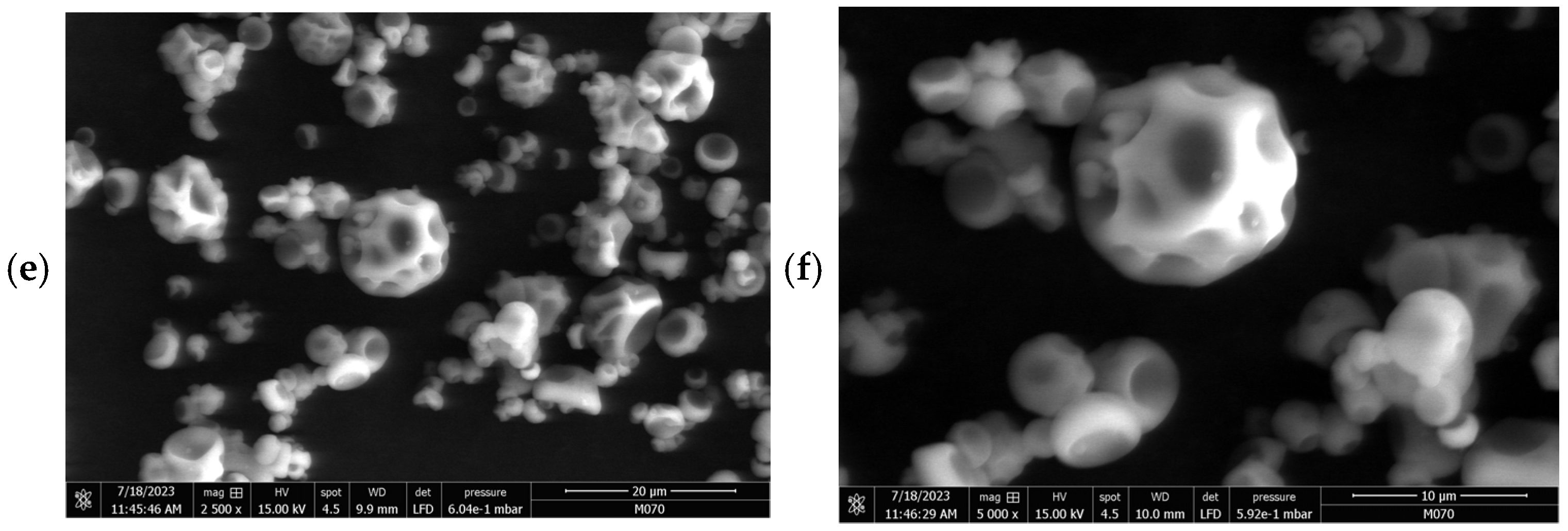
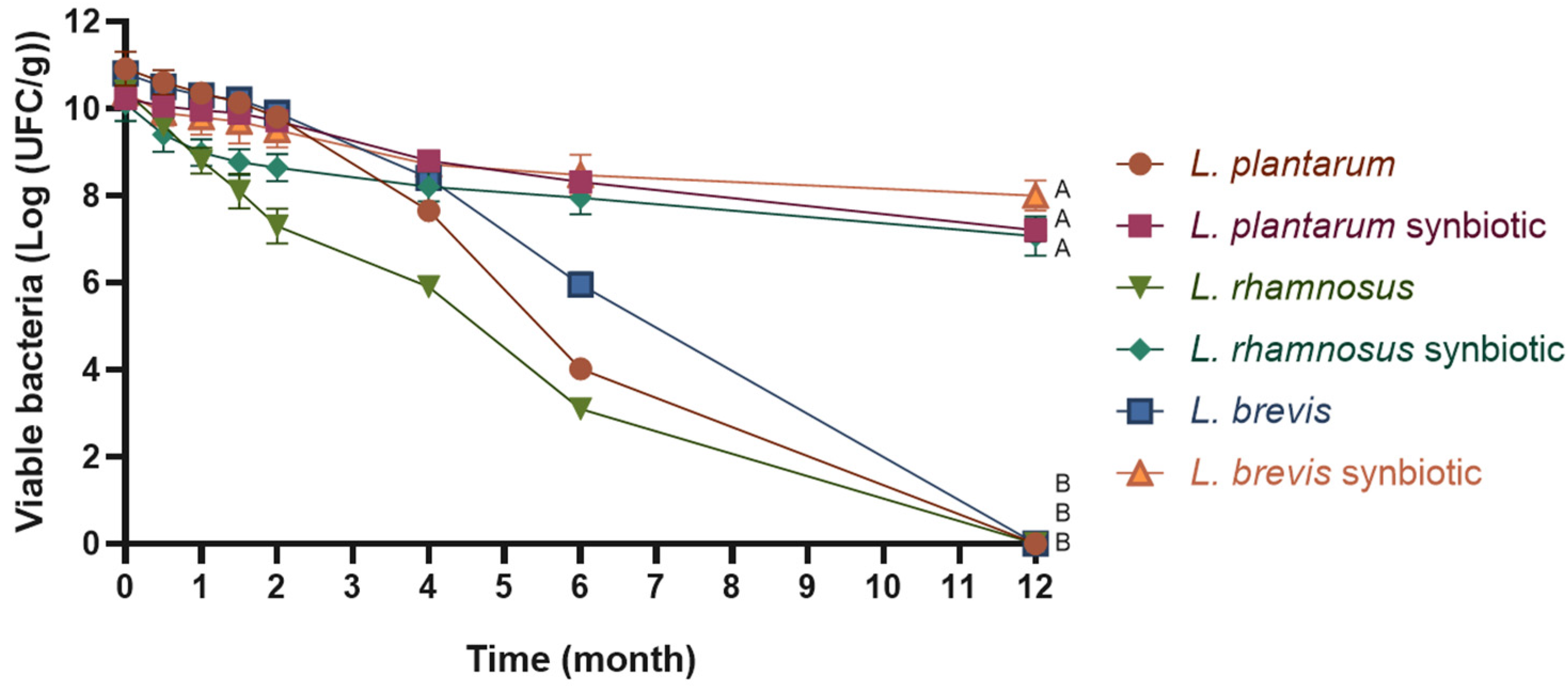
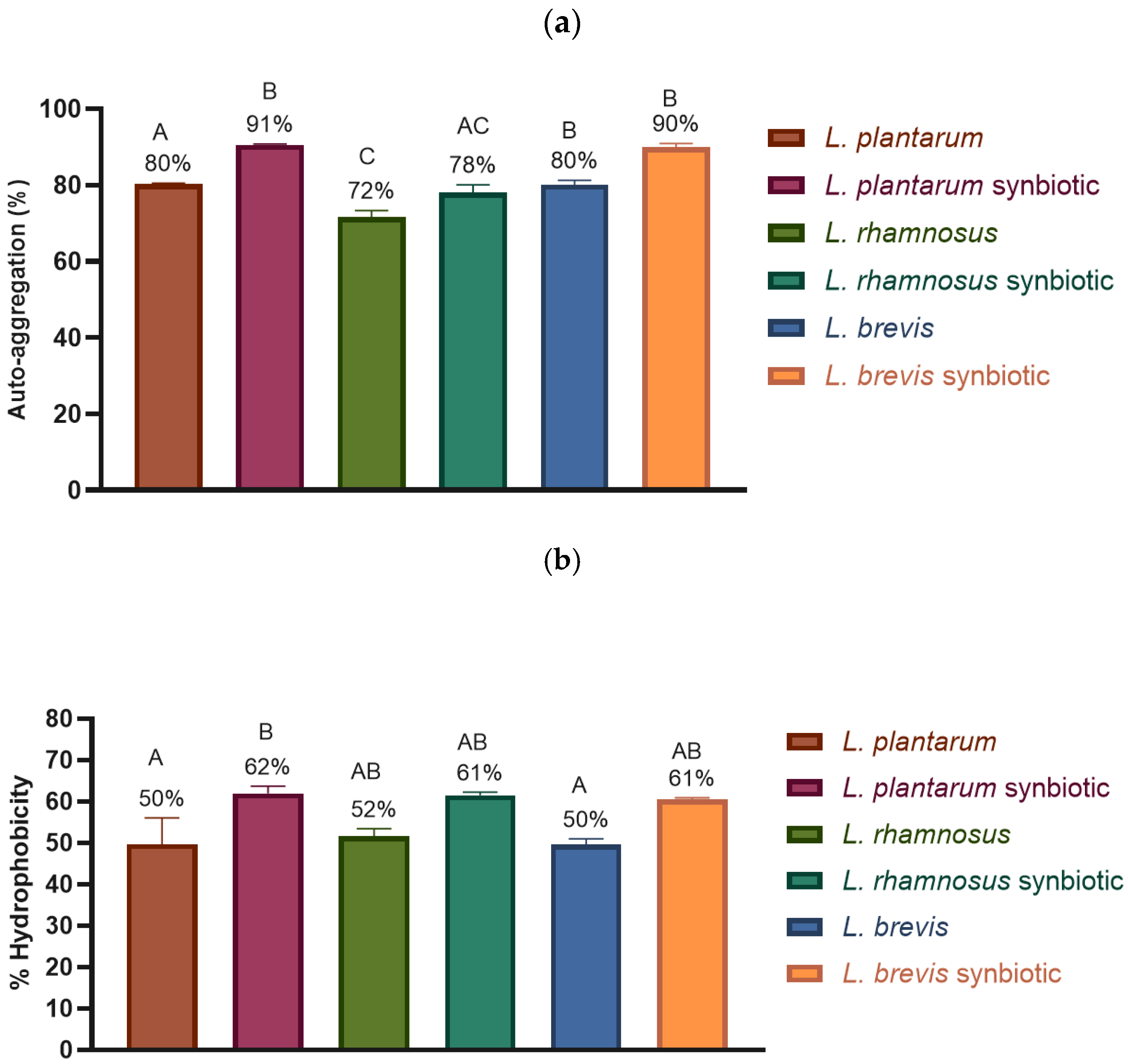
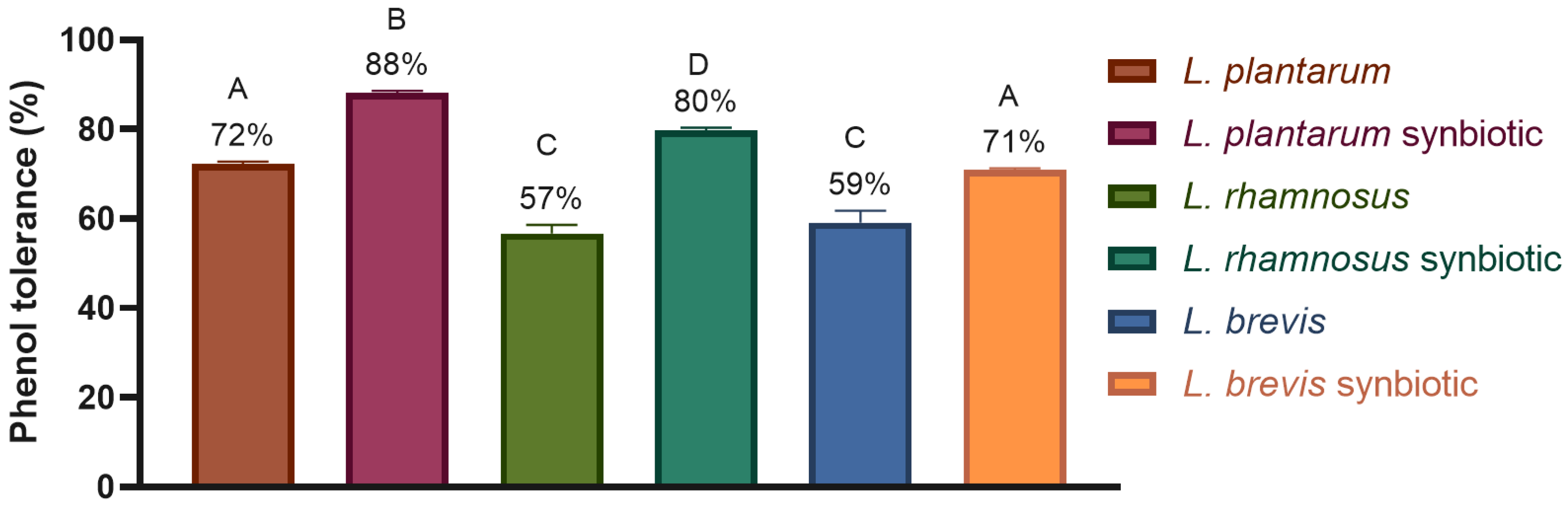

| Strain | L. rhamnosus LM07 | L. plantarum LM19 | L. brevis LBH1070 | |
|---|---|---|---|---|
| Moisture content (%) | 5.1 ± 0.9 | 5.0 ± 0.6 | 5.4 ± 0.7 | |
| Water activity (aw) | 0.324 ± 0.115 | 0.312 ± 0.130 | 0.317 ± 0.122 | |
| Survival of bacteria | Log (CFU/g) | 10.11 ± 0.40 | 10.23 ± 0.24 | 10.36 ± 0.36 |
| (%) | 91.9 ± 3.6 | 93.0 ± 2.8 | 94.2 ± 4.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Damián, M.; Garfias-Noguez, C.; Bermúdez-Humarán, L.G.; Sánchez-Pardo, M.E. Synbiotic Microencapsulation of Lactobacillus Strains from Mexican Fermented Beverages for Enhanced Probiotic Functionality. Molecules 2025, 30, 1185. https://doi.org/10.3390/molecules30051185
Ramírez-Damián M, Garfias-Noguez C, Bermúdez-Humarán LG, Sánchez-Pardo ME. Synbiotic Microencapsulation of Lactobacillus Strains from Mexican Fermented Beverages for Enhanced Probiotic Functionality. Molecules. 2025; 30(5):1185. https://doi.org/10.3390/molecules30051185
Chicago/Turabian StyleRamírez-Damián, Morayma, Cynthia Garfias-Noguez, Luis G. Bermúdez-Humarán, and María Elena Sánchez-Pardo. 2025. "Synbiotic Microencapsulation of Lactobacillus Strains from Mexican Fermented Beverages for Enhanced Probiotic Functionality" Molecules 30, no. 5: 1185. https://doi.org/10.3390/molecules30051185
APA StyleRamírez-Damián, M., Garfias-Noguez, C., Bermúdez-Humarán, L. G., & Sánchez-Pardo, M. E. (2025). Synbiotic Microencapsulation of Lactobacillus Strains from Mexican Fermented Beverages for Enhanced Probiotic Functionality. Molecules, 30(5), 1185. https://doi.org/10.3390/molecules30051185











