Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability
Abstract
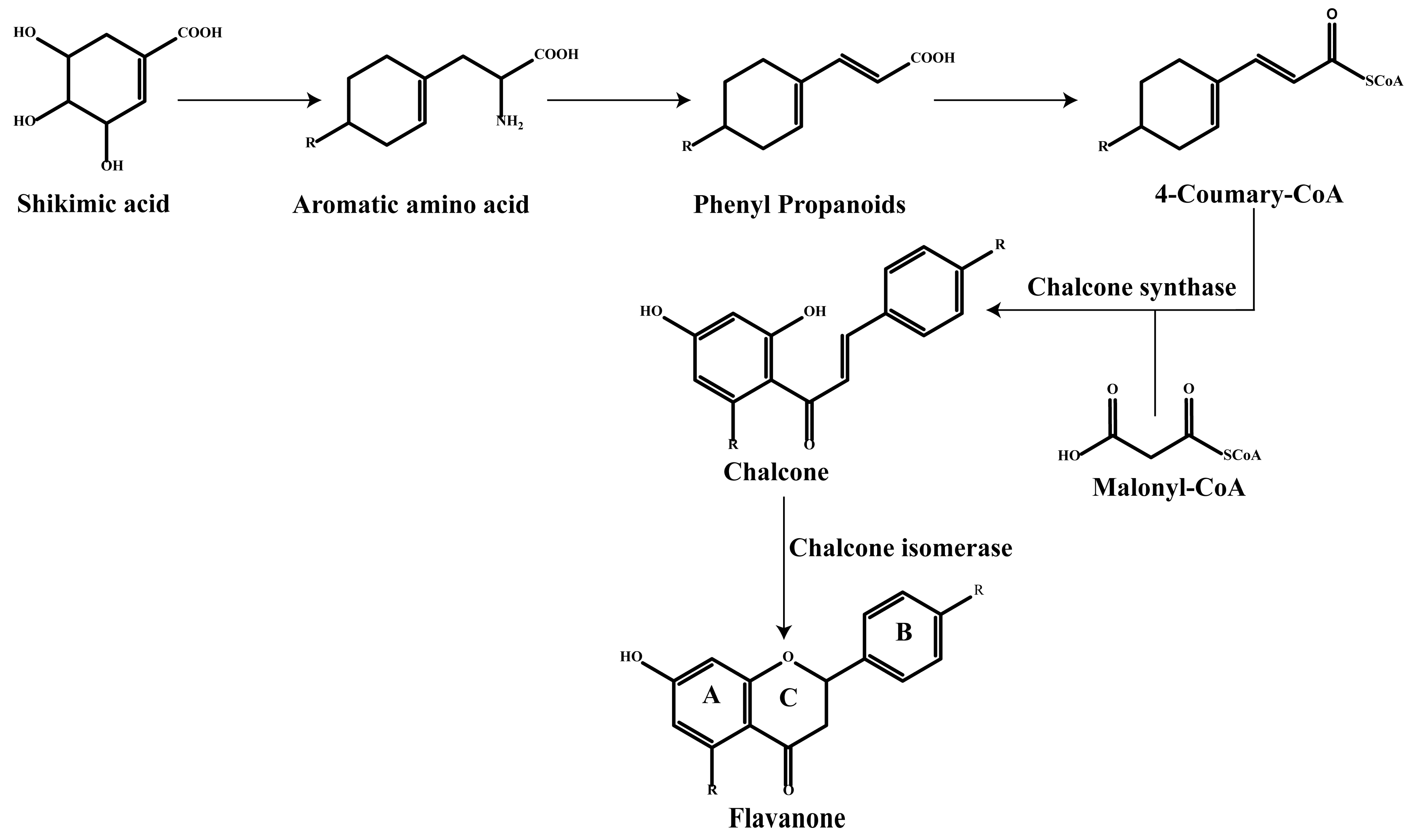
1. Introduction
2. Classification of Flavonoids
3. Efficacy of Flavonoids
3.1. Antioxidant
- The hydroxyl structure of flavonoids acts as a hydrogen atom donor to neutralize free radicals to directly remove ROS.
- The oxo group of flavonoids participates in the conjugated system to enhance electron delocalization.
- Activation of antioxidant enzymes activity.
- Inhibition of oxidase activity.
- Formation of metal chelates.
3.2. Anticancer
3.3. Anti-Inflammatory
3.4. Antimicrobial
4. Pharmacokinetics of Flavonoids
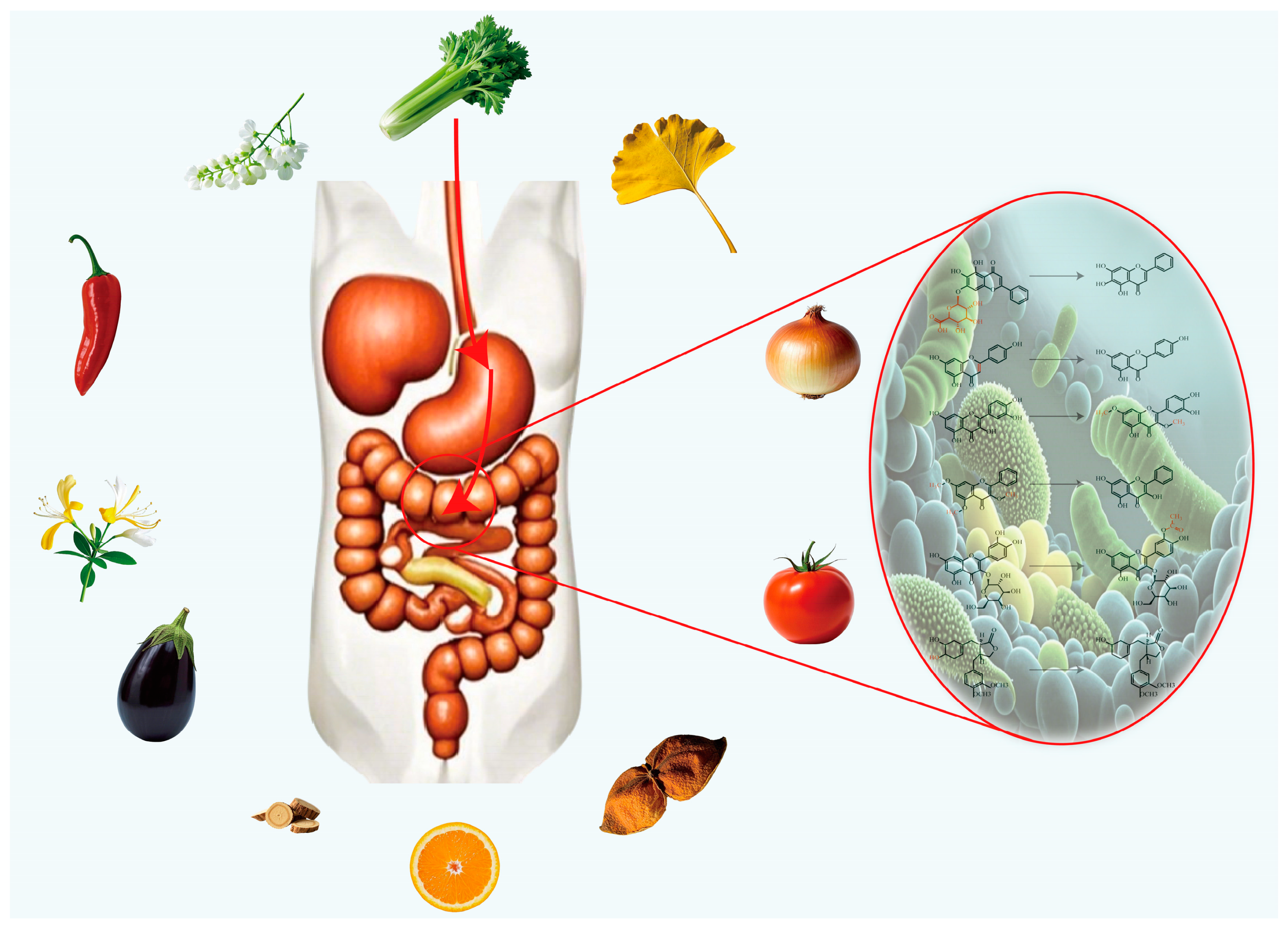
5. Flavonoids and Gut Microbiome
5.1. Hydrolysis Reaction
- Depending on the type of glycoside bond, flavonoid glycosides undergo hydrolysis in roughly the following order of difficulty C-glycosides, S-glycosides, O-glycosides, and N-glycosides.
- Among pyranosides, the hydrolysis rates are ranked from fast to slow as follows: pentose, methylpentose, hexose, heptose, glucuronide.
- Amino-glycosides are more difficult to hydrolyze than hydroxy-glycosides, which are more difficult to hydrolyze than deoxyglycosides: 2-aminoglycosides < 2-hydroxyglycosides < 3-deoxyglycosides < 2-deoxyglycosides < 2, 3-deoxyglycosides.
- Ketoglycosides are more easily hydrolyzed than aldosides.
- Aromatic glycosides are more easily hydrolyzed than aliphatic glycosides.
- Furanosides are more easily hydrolyzed than pyranosides.
5.2. Reduction Reaction
5.3. (De)Methylation, Acetylation, Dehydroxylation Reactions
6. Bioavailability of Flavonoids
6.1. Formulation Modification
| Type | Substance | Name | Administration | Dose | Animal/Cell Line | Disease | Pharmacokinetics (Compared with Pure Substances) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nanosuspensions | Baicalin | BG-NS and BG-MS | Oral administration | 85, 170, 340 and 680 mg/kg | Wistar rats | --- | AUC(0–t) increased by about 2.22 times and 1.37 times respectively; Vz/F reduced by about 2.27 times and 1.28 times respectively… | [176] |
| Luteolin | SLNC | Oral gavage | 20 mg/kg | Male Sprague Dawley rats | --- | Bioavailability increased by about 3.48 times | [177] | |
| Daidzein | F-A and F-B | Co-incubation | 50–400 μM | RG2 | Brain glioma | --- | [178] | |
| Naringenin | TPNS | Oral administration | 30 mg/kg | Male Sprague Dawley rats | --- | Cmax increased by about 2.1 times, AUMC0–∞ increased by about 3.76 times… | [179] | |
| Silybin | SPCs-NPs | Oral gavage | 50 mg/kg | Male Sprague Dawley rats | Liver protection | AUC0–∞ increased by about 124.70 times; CL reduced by about 124.58 times; Tmax increased by about 8.82 times… | [180] | |
| Kaempferol | TPGS-KAE-NSps | Oral gavage and vein injection | 15 mg/kg, ig; 5 mg/kg, iv | Female Balb/c mice | Breast cancer | Cmax increased by about 2.41 times; AUC0–t increased by about 4.83 times, T1/2 increased by about 2.64 times. | [181] | |
| Hydroxy genkwanin | HGK-NSps | vein injection | 10, 20, 40 mg/kg | Female NU/NU nude mice | Breast cancer | --- | [182] | |
| Naringenin | NRG-NS | Oral administration | 20 mg/kg | Female Wistar rats | --- | Cmax increased by about 2 times; AUC0–24 h increased by about 1.8 times; | [183] | |
| Baicalin | BCA-NS/NCCS | Oral administration | 10 mg/mL | Male Wister rats | --- | AUC(0–24) increased by 1.85 times; Cmax increased by about 1.90 times… | [184] | |
| Liposomes | Quercetin | Quercetin liposomes | Co-incubation | --- | RBL-2H3 | Allergic | Anti-allergic activity is higher than that of raw drug | [185] |
| Fisetin | --- | Intraperitoneal injection | 21 mg/kg | C57BL/6J mice | Lung cancer | Relative bioavailability increased by about 47 times | [186] | |
| Rutin | MP-LR | Oral administration | 16.15 mg | C57 BL/6N mice | Obesity | The RQ-AUC value (the lower the ratio indicates better fatty acid metabolism) is significantly lower than the raw material drug (p < 0.05) | [187] | |
| Rutin | RGD-RUT-LIPO and ABX-RUT-LIPO | Tail vein injection | 5 mg/kg (Rutin equivalent) | Male Sprague Dawley rats | Thrombus | Shortened clotting time; Relative bioavailability increased by about 3 times. | [188] | |
| Licochalcone A | LCA-Liposomes | Oral administration | 30, 60 mg/kg | Male Sprague Dawley rats | Renal injury | AUC0–24 increased by about 2.86 times; Cmax increased by about 2.49 times; MRT0–t increased by about 1.26 times; | [189] | |
| Solid lipid nanoparticles | Morin hydrate | MSN | Oral gavage | 50 mg/kg | Male Sprague Dawley rats | Cervical cancer | Cmax increased by about 2.95 times; AUC increased by about 3.10 times;MRT increased by about 2.04 times; | [190] |
| Hydroxysafflor yellow A | HSYA SLN | Oral administration | 20 mg/kg | Male Sprague Dawley rats | Nerve injury | Cmax increased by about 7.76 times; AUC increased by about 3.99 times; Oral absorption in rats increased by about 3.97 times. | [191] | |
| Baicalin | OX26-PEG-CSLN | vein injection | 4.42 mg/kg | Male Sprague Dawley rats | Cerebral ischemia reperfusion injury | AUC increased by about 5.69 times; Cmax increased by about 6.84 times; | [192] | |
| Naringenin | Nrg-SLNs | Oral administration | 6 mg/mL | Wistar rats | inflammation; Antioxidant | AUC0→∞ increased by about 17.44 times; MRT0→∞ increased by about 8.81 times; The overall bioavailability is increased by about 12 times; | [193] | |
| Naringenin | NRG-SLNs | intratracheal instillation | 20 mg/kg | Male Sprague Dawley rats | --- | Cmax increased by about 1.62 times; AUC0→∞ increased by about 3.66 times; MRT increased by about 3.33 times; Relative bioavailability increased by about 2.53 times; | [194] | |
| Puerarin | Pue-SLN | Oral gavage | 20 mg/kg | Sprague Dawley rats | --- | AUC0→∞ increased by about 3.00 times; MRT increased by about 1.79 times; CL reduced by about 3.21 times… | [195] | |
| Nanoemulsions | Breviscapine | --- | Oral administration | --- | Male Wister rats | --- | Cmax increased by about 2.87 times; AUC(0–t) ncreased by about 2.57 times; Relative bioavailability reached 249.70%; | [196] |
| Baicalein | BCL-NEs | Oral gavage | 25 mg/kg | Sprague Dawley rats | --- | AUC0–t increased by about 5.25 times; Cmax increased by about 7.66 times; Relative bioavailability reached 524.7% | [197] | |
| Acetylpuerarin | --- | Oral administration | 30 mg/kg | Wistar rats | Cerebral ischemic reperfusion injury | Cmax increased by about 2.89 times; AUC0–t increased by about 2.57 times; | [198] | |
| Luteolin | NECh-LUT | intranasal administration | 32 μg/kg | Male Wistar rats | Neuroblastoma | AUC0-∞increased by about 4.40 times; T1/2 increased by about 10 times; | [199] | |
| Fisetin | --- | intraperitoneal injection | 112.5 mg/kg | C57BL/6J mice | Lung cancer | Relative bioavailability increased by about 24 times | [200] | |
| Isoliquiritigenin | ISL-NE | Ocular instillation | 50 μL 0.2% (w/v) | Male New Zealand white rabbits | Corneal neovascularizaion | Cmax of tear, cornea, Conjunctiva Aqueous humor increased by about 8.70, 3.95, 298.75 and 1.88 times respectively; AUC0–8 h increased by about 5.76, 7.80, 356.57 and 2.13 times respectively… | [201] | |
| Hydrogel | Catechin | CA-NG4 | Transdermal administration | 5 mg (CA equivalent) | male Wistar rats | Antioxidant | AUC0–∞increased by about 10.33 times; Relative bioavailability increased to 894.73% | [202] |
| Metal-organic frameworks | Baicalin | PEG-FA@ZIF-8@BAN | vein injection | --- | female BALB/c mice | Breast cancer | Stronger tumor suppressor effect. | [203] |
| Nanoparticles | Epigallocatechin gallate | CE-HK NP | Intratumoral injection | 20 mg/kg and 40 mg/kg | Male BALB/c Nude | Liver cancer | Tumor suppression effect increased by about 2.77 times. | [204] |
| Metal nanoparticles | Hesperetin | Au-mPEG(5000)-S-HP NPs | Intraperitoneal injection(IP) | 1.5 mg/0.5 mL | Male, Wistar strain albino rats | Liver cancer | --- | [205] |
| Magnetic nanoparticles | Quercetin | Fe3O4@PCA-PEG-FA | Co-incubation | 50, 100, 200 μg/mL | MDA-MB-231 and HeLa | --- | --- | [206] |
| NA | Breviscapine | BVP-NS, BVP-LP, BVP-PLC | Oral gavage | 20 mg/kg | Male Sprague-Dawley rats | --- | The relative bioavailability increased to 245.97%, 237.51%, and 471.32, respectively; | [207] |
| Nanogel | Breviscapine | BRE-NG | intranasal administration | 3, 10, 50, 100 mg/mL | Male Sprague-Dawley rats | Cerebral ischemia reperfusion injury | Absolute bioavailability increased by about 142.80 times | [208] |
6.2. Structural Modification
6.2.1. Acetylation
6.2.2. Glycosylation
6.2.3. Methyl Etherification
6.2.4. Esterification
6.2.5. Acylation
7. Prospect
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, e27533. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Z.; Zhu, M.; Liu, K.; Farag, M.A.; Song, L.; Gao, F.; Tao, H. Biofortification of flavonoids in nuts along the agro-food chain for improved nutritional and health benefits, a comprehensive review and future prespectives. Food Chem. 2025, 464, 141754. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Maaiden, E.E.; Ullah, N.; Ezzariai, A.; Mazar, A.; Boukcim, H.; Hirich, A.; Nasser, B.; Qarah, N.; Kouisni, L.; Kharrassi, Y.E. Comparing antioxidant and cytoprotective effects: Quercetin glycoside vs. aglycone from Ephedra alata. Phytomedicine Plus 2024, 4, 100603. [Google Scholar] [CrossRef]
- Bartnik, M.; Facey, P.C. Chapter 8—Glycosides. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 101–161. [Google Scholar]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- Valenta, K.; Nevo, O.; Martel, C.; Chapman, C.A. Plant attractants: Integrating insights from pollination and seed dispersal ecology. Evol. Ecol. 2017, 31, 249–267. [Google Scholar] [CrossRef]
- Golovatskaya, I.F.; Medvedeva, Y.V.; Kadyrbaev, M.K.; Boyko, E.V. Specificity of Growth and Accumulation of Flavonoids in Plants and Cell Cultures of Lychnis chalcedonica Obtained from Explants of Different Organs. Russ. J. Plant Physiol. 2024, 71, 24. [Google Scholar] [CrossRef]
- Patil, J.R.; Mhatre, K.J.; Yadav, K.; Yadav, L.S.; Srivastava, S.; Nikalje, G.C. Flavonoids in plant-environment interactions and stress responses. Discov. Plants 2024, 1, 68. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Das, A.; Choudhury, S.; Gopinath, V.; Majeed, W.; Chakraborty, S.; Bhairavi, K.S.; Chowdhury, S.; Dubey, V.K.; Akhtar, M.S. Functions of Flavonoids in Plant, Pathogen, and Opportunistic Fungal Interactions. In Opportunistic Fungi, Nematode and Plant Interactions: Interplay and Mechanisms; Akhtar, M.S., Ed.; Springer Nature: Singapore, 2024; pp. 91–123. [Google Scholar]
- Zheng, X.; Zhang, X.; Zeng, F. Biological Functions and Health Benefits of Flavonoids in Fruits and Vegetables: A Contemporary Review. Foods 2025, 14, 155. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Sun, G.; Sun, T.; Fan, Y.; Bai, S.; Guo, S.; Huang, S.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C.; Guo, Q.; Gu, Y.; Shi, T.Q. Advances in Flavonoid and Derivative Biosynthesis: Systematic Strategies for the Construction of Yeast Cell Factories. ACS Synth. Biol. 2024, 13, 2667–2683. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. Int. J. Exp. Plant Biol. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef]
- Cruz, L.; Basílio, N.; Mateus, N.; de Freitas, V.; Pina, F. Natural and Synthetic Flavylium-Based Dyes: The Chemistry Behind the Color. Chem. Rev. 2022, 122, 1416–1481. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Osigwe, C.; Akah, P.; Nworu, C.; Okoye, F. Apigenin: A methanol fraction component of Newbouldia laevis leaf, as a potential antidiabetic agent. J. Phytopharm. 2017, 6, 38–44. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Li, S.-G.; Yang, R.; Lu, M.-M.; Wang, S.-M.; Meng, J. A new isoflavone from processed root barks of Paeonia suffruticosa and their procoagulant activity. Nat. Prod. Res. 2024, 11, 1–7. [Google Scholar] [CrossRef]
- Li, P.; Ding, W.; Chen, F.; Zhou, F.; Ruan, Z.; Li, J.; Wu, Y. Isoflavones relieve intestinal motility disorders in colitis rats by regulating 5-hydroxytryptamine and interstitial cells of Cajal. Food Biosci. 2024, 63, 105587. [Google Scholar] [CrossRef]
- Selepe, M.A. Isoflavone Derivatives as Potential Anticancer Agents: Synthesis and Bioactivity Studies. ChemMedChem 2024, 19, e202400420. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Targeting Cardiovascular Diseases by Flavonols: An Update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef]
- Felice, M.R.; Maugeri, A.; De Sarro, G.; Navarra, M.; Barreca, D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022, 23, 4411. [Google Scholar] [CrossRef]
- Dajas, F.; Andrés, A.C.; Florencia, A.; Carolina, E.; Felicia, R.M. Neuroprotective actions of flavones and flavonols: Mechanisms and relationship to flavonoid structural features. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 30–35. [Google Scholar] [CrossRef]
- Duan, Y.; Eduardo Melo Santiago, F.; Rodrigues Dos Reis, A.; de Figueiredo, M.A.; Zhou, S.; Thannhauser, T.W.; Li, L. Genotypic variation of flavonols and antioxidant capacity in broccoli. Food Chem. 2021, 338, 127997. [Google Scholar] [CrossRef]
- Yang, C.P.; Shie, P.H.; Huang, G.J.; Chien, S.C.; Kuo, Y.H. New Anti-inflammatory Flavonol Glycosides from Lindera akoensis Hayata. Molecules 2019, 24, 563. [Google Scholar] [CrossRef]
- Lea, M.A. Flavonol regulation in tumor cells. J. Cell. Biochem. 2015, 116, 1190–1194. [Google Scholar] [CrossRef]
- Wisetsai, A.; Choodej, S.; Ngamrojanavanich, N.; Pudhom, K. Fatty acid acylated flavonol glycosides from the seeds of Nephelium lappaceum and their nitric oxide suppression activity. Phytochemistry 2022, 201, 113262. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.L.; et al. Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X.; et al. Naringenin: A flavanone with anti-inflammatory and anti-infective properties. Biomed. Pharmacother. = Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytother. Res. PTR 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, S.; Li, S.M. Naturally occurring prenylated chalcones from plants: Structural diversity, distribution, activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2236–2260. [Google Scholar] [CrossRef]
- Tuli, H.S.; Aggarwal, V.; Parashar, G.; Aggarwal, D.; Parashar, N.C.; Tuorkey, M.J.; Varol, M.; Sak, K.; Kumar, M.; Buttar, H.S. Xanthohumol: A Metabolite with Promising Anti-Neoplastic Potential. Anti-Cancer Agents Med. Chem. 2022, 22, 418–432. [Google Scholar] [CrossRef]
- Sun, W.; Yue, J.; Xu, T.; Cui, Y.; Huang, D.; Shi, H.; Xiong, J.; Sun, W.; Yi, Q. Xanthohumol alleviates palmitate-induced inflammation and prevents osteoarthritis progression by attenuating mitochondria dysfunction/NLRP3 inflammasome axis. Heliyon 2023, 9, e21282. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O'Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274–3299. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress; Academic Press Inc.: Cambridge, MA, USA, 1985. [Google Scholar]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Syed Mohamad, S.N.A.; Khatib, A.; Latip, J. Antioxidant and Antidiabetic Effects of Flavonoids: A Structure-Activity Relationship Based Study. BioMed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, G.R.; Rabelo, A.C.; Meira, J.S.; Rossoni-Júnior, J.V.; Castro-Borges, W.; Guerra-Sá, R.; Batista, M.A.; Silveira-Lemos, D.D.; Souza, G.H.; Brandão, G.C.; et al. Baccharis trimera inhibits reactive oxygen species production through PKC and down-regulation p47 (phox) phosphorylation of NADPH oxidase in SK Hep-1 cells. Exp. Biol. Med. 2017, 242, 333–343. [Google Scholar] [CrossRef]
- Chlebda, E.; Magdalan, J.; Merwid-Ląd, A.; Trocha, M.; Kopacz, M.; Kuźniar, A.; Nowak, D.; Szeląg, A. Influence of water-soluble flavonoids, quercetin-5′-sulfonic acid sodium salt and morin-5′-sulfonic acid sodium salt, on antioxidant parameters in the subacute cadmium intoxication mouse model. Exp. Toxicol. Pathol. 2010, 62, 105–108. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Chen, W.; Xu, S.; Feng, X.; Zhang, L. Natural products: The role and mechanism in low-density lipoprotein oxidation and atherosclerosis. Phytother. Res. 2021, 35, 2945–2967. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free. Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Wang, S.; Fang, Y.; Yu, X.; Guo, L.; Zhang, X.; Xia, D. The flavonoid-rich fraction from rhizomes of Smilax glabra Roxb. ameliorates renal oxidative stress and inflammation in uric acid nephropathy rats through promoting uric acid excretion. Biomed. Pharmacother. 2019, 111, 162–168. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Wang, W.-Y.; Huang, C.-H.; Hu, M.-L. Pro-oxidative effect of β-carotene and the interaction with flavonoids on UVA-induced DNA strand breaks in mouse fibroblast C3H10T1/2 cells. J. Nutr. Biochem. 2005, 16, 729–735. [Google Scholar] [CrossRef]
- Forni, C.; Rossi, M.; Borromeo, I.; Feriotto, G.; Platamone, G.; Tabolacci, C.; Mischiati, C.; Beninati, S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules 2021, 26, 3583. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A. Targeting the RAS upstream and downstream signaling pathway for cancer treatment. Eur. J. Pharmacol. 2024, 979, 176727. [Google Scholar] [PubMed]
- Gao, Y.; Hou, J.; Fei, X.; Ren, L.; Lu, R.; Liu, P.; Liu, S.; Zhu, C.; Wang, X.; Pan, Y. TAGLN2 targeted control of ARPC5-mediated activation of the MEK/ERK signaling pathway influences the proliferation, invasion, and metastasis of pancreatic cancer cells. Cell. Signal. 2024, 120, 111227. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Z.; Song, X.-F.; Liao, Y.-J.; Xiahou, J.-H.; Li, Y.; Zhang, Z.-H. Targeting Nrf2 signaling pathways in the role of bladder cancer: From signal network to targeted therapy. Biomed. Pharmacother. 2024, 176, 116829. [Google Scholar] [CrossRef]
- Liang, J.-L.; Jin, X.-K.; Deng, X.-C.; Huang, Q.-X.; Zhang, S.-M.; Chen, W.-H.; Zhang, X.-Z. Targeting activation of cGAS-STING signaling pathway by engineered biomaterials for enhancing cancer immunotherapy. Mater. Today 2024, 78, 251–296. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, T.; Chen, J.; Tang, X.; Yuan, Y.; Hu, D.; Zhang, Y.; Li, Y.; Zou, J. Aberrant angiogenic signaling pathways: Accomplices in ovarian cancer progression and treatment. Cell. Signal. 2024, 120, 111240. [Google Scholar] [CrossRef]
- Khan, I.R.; Sadida, H.Q.; Hashem, S.; Singh, M.; Macha, M.A.; Al-Shabeeb Akil, A.S.; Khurshid, I.; Bhat, A.A. Therapeutic implications of signaling pathways and tumor microenvironment interactions in esophageal cancer. Biomed. Pharmacother. 2024, 176, 116873. [Google Scholar] [CrossRef]
- Chu, J.; Yuan, C.; Zhou, L.; Zhao, Y.; Wu, X.; Yan, Y.; Liu, Y.; Liu, X.; Jing, L.; Dong, T.; et al. JianPiTongLuo (JPTL) Recipe regulates anti-apoptosis and cell proliferation in colorectal cancer through the PI3K/AKT signaling pathway. Heliyon 2024, 10, e35490. [Google Scholar] [CrossRef]
- Jin, X.; Wang, S.; Luo, L.; Yan, F.; He, Q. Targeting the Wnt/β-catenin signal pathway for the treatment of gastrointestinal cancer: Potential for advancement. Biochem. Pharmacol. 2024, 227, 116463. [Google Scholar] [CrossRef]
- Napolitano, S.; Martini, G.; Ciardiello, D.; Del Tufo, S.; Martinelli, E.; Troiani, T.; Ciardiello, F. Targeting the EGFR signalling pathway in metastatic colorectal cancer. Lancet Gastroenterol. Hepatol. 2024, 9, 664–676. [Google Scholar] [CrossRef]
- Yang, M.H.; Basappa, B.; Deveshegowda, S.N.; Ravish, A.; Mohan, A.; Nagaraja, O.; Madegowda, M.; Rangappa, K.S.; Deivasigamani, A.; Pandey, V.; et al. A novel drug prejudice scaffold-imidazopyridine-conjugate can promote cell death in a colorectal cancer model by binding to β-catenin and suppressing the Wnt signaling pathway. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Bharathiraja, P.; Yadav, P.; Sajid, A.; Ambudkar, S.V.; Prasad, N.R. Natural medicinal compounds target signal transduction pathways to overcome ABC drug efflux transporter-mediated multidrug resistance in cancer. Drug Resist. Updates 2023, 71, 101004. [Google Scholar] [CrossRef] [PubMed]
- Hemmati-Dinarvand, M.; Ahmadvand, H.; Seghatoleslam, A. Nitazoxanide and Cancer Drug Resistance: Targeting Wnt/β-catenin Signaling Pathway. Arch. Med. Res. 2022, 53, 263–270. [Google Scholar] [CrossRef]
- Morris, M.E.; Zhang, S. Flavonoid–drug interactions: Effects of flavonoids on ABC transporters. Life Sci. 2006, 78, 2116–2130. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, L.; Zhang, S.; Yang, J.; Zhu, A.; Sun, J.; Kalvakolanu, D.V.; Cong, X.; Zhang, J.; Tang, J.; et al. Taxifolin inhibits melanoma proliferation/migration impeding USP18/Rac1/JNK/β-catenin oncogenic signaling. Phytomedicine 2024, 123, 155199. [Google Scholar] [CrossRef]
- Manigandan, K.; Manimaran, D.; Jayaraj, R.L.; Elangovan, N.; Dhivya, V.; Kaphle, A. Taxifolin curbs NF-κB-mediated Wnt/β-catenin signaling via up-regulating Nrf2 pathway in experimental colon carcinogenesis. Biochimie 2015, 119, 103–112. [Google Scholar] [CrossRef]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019, 8, 2252–2267. [Google Scholar] [CrossRef]
- Desai, V.; Jain, A.; Shaghaghi, H.; Summer, R.; Lai, J.C.K.; Bhushan, A. Combination of Biochanin A and Temozolomide Impairs Tumor Growth by Modulating Cell Metabolism in Glioblastoma Multiforme. Anticancer. Res. 2019, 39, 57–66. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Fuster, J.J.; Zuriaga, M.A.; Fuster, V. Inflammation as a Driver of Disease. In Encyclopedia of Cell Biology, 2nd ed.; Bradshaw, R.A., Hart, G.W., Stahl, P.D., Eds.; Academic Press: Oxford, UK, 2023; pp. 495–501. [Google Scholar]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef]
- Murata, T.; Ishiwa, S.; Lin, X.; Nakazawa, Y.; Tago, K.; Funakoshi-Tago, M. The citrus flavonoid, nobiletin inhibits neuronal inflammation by preventing the activation of NF-κB. Neurochem. Int. 2023, 171, 105613. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression-3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Van der Heiden, K.; Cuhlmann, S.; Luong Le, A.; Zakkar, M.; Evans, P.C. Role of nuclear factor kappaB in cardiovascular health and disease. Clin. Sci. 2010, 118, 593–605. [Google Scholar] [CrossRef]
- Karin, M.; Greten, F.R. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, Q.; Xie, Y.; Zhang, Q.; Zhou, Y.; Sun, H.; Qian, R.; Wang, L.; Chen, X.; Gao, Y.; et al. Green tea polyphenol alleviates silica particle-induced lung injury by suppressing IL-17/NF-κB p65 signaling-driven inflammation. Phytomedicine 2024, 135, 156238. [Google Scholar] [CrossRef]
- Wu, D.-G.; Yu, P.; Li, J.-W.; Jiang, P.; Sun, J.; Wang, H.-Z.; Zhang, L.-D.; Wen, M.-B.; Bie, P. Apigenin potentiates the growth inhibitory effects by IKK-β-mediated NF-κB activation in pancreatic cancer cells. Toxicol. Lett. 2014, 224, 157–164. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Jia, Z.; Battino, M.; Miron, A.; Yu, Z.; Cao, H.; Xiao, J. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Crit. Rev. Food Sci. Nutr. 2018, 58, 2908–2924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, J.; Peng, L.; Zhang, Q.; Rong, X.; Luo, Y.; Li, J. Flavonoids: Potential therapeutic agents for cardiovascular disease. Heliyon 2024, 10, e32563. [Google Scholar] [CrossRef] [PubMed]
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zeng, F.; Wang, M.; Guo, S.; Tang, Z.; Itagaki, K.; Lin, Y.; Shen, X.; Cao, Y.; Duan, J.-A.; et al. Antimicrobial activities of lavandulylated flavonoids in Sophora flavences against methicillin-resistant Staphylococcus aureus via membrane disruption. J. Adv. Res. 2024, 57, 197–212. [Google Scholar] [CrossRef]
- Das, T.; Kutty, S.K.; Kumar, N.; Manefield, M. Pyocyanin facilitates extracellular DNA binding to Pseudomonas aeruginosa influencing cell surface properties and aggregation. PLoS ONE 2013, 8, e58299. [Google Scholar] [CrossRef]
- Casilag, F.; Lorenz, A.; Krueger, J.; Klawonn, F.; Weiss, S.; Häussler, S. The LasB Elastase of Pseudomonas aeruginosa Acts in Concert with Alkaline Protease AprA To Prevent Flagellin-Mediated Immune Recognition. Infect. Immun. 2016, 84, 162–171. [Google Scholar] [CrossRef]
- Tao, J.; Yan, S.; Wang, H.; Zhao, L.; Zhu, H.; Wen, Z. Antimicrobial and antibiofilm effects of total flavonoids from Potentilla kleiniana Wight et Arn on Pseudomonas aeruginosa and its potential application to stainless steel surfaces. LWT 2022, 154, 112631. [Google Scholar] [CrossRef]
- Noor Mohammadi, T.; Maung, A.T.; Sato, J.; Sonoda, T.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Mechanism for antibacterial action of epigallocatechin gallate and theaflavin-3,3′-digallate on Clostridium perfringens. J. Appl. Microbiol. 2019, 126, 633–640. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Khadke, S.K.; Yamano, A.; Woo, J.-T.; Lee, J. Antimicrobial and antibiofilm activities of prenylated flavanones from Macaranga tanarius. Phytomedicine 2019, 63, 153033. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Donovan, J.L.; Manach, C.; Faulks, R.M.; Kroon, P.A. Absorption and Metabolism of Dietary Plant Secondary Metabolites. In Plant Secondary Metabolites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 303–351. [Google Scholar]
- Zhang, H.; Hassan, Y.I.; Liu, R.; Mats, L.; Yang, C.; Liu, C.; Tsao, R. Molecular Mechanisms Underlying the Absorption of Aglycone and Glycosidic Flavonoids in a Caco-2 BBe1 Cell Model. ACS Omega 2020, 5, 10782–10793. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Walgren, R.A.; Lin, J.-T.; Kinne, R.K.H.; Walle, T. Cellular Uptake of Dietary Flavonoid Quercetin 4′-β-Glucoside by Sodium-Dependent Glucose Transporter SGLT111This study was supported by National Institutes of Health Grant GM55561. J. Pharmacol. Exp. Ther. 2000, 294, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Mellon, F.; Barron, D.; Sarrazin, G.; Morgan, M.R.A.; Williamson, G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free. Radic. Res. 2001, 35, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Swanson, H. Flavonoids, Inflammation and Cancer; World Scientific: Singapore, 2015. [Google Scholar]
- Koster, H.; Halsema, I.; Scholtens, E.; Knippers, M.; Mulder, G.J. Dose-dependent shifts in the sulfation and glucuronidation of phenolic compounds in the rat in vivo and in isolated hepatocytes. The role of saturation of phenolsulfotransferase. Biochem. Pharmacol. 1981, 30, 2569–2575. [Google Scholar] [CrossRef]
- Piskula, M.K. Soy Isoflavone Conjugation Differs in Fed and Food-Deprived Rats. J. Nutr. 2000, 130, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free. Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2024, 54, 1002–1016. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Hsiu, S.-L.; Hou, Y.-C.; Chen, H.-Y.; Chao, P.-D.L. Degradation of Flavonoid Aglycones by Rabbit, Rat and Human Fecal Flora. Biol. Pharm. Bull. 2003, 26, 747–751. [Google Scholar] [CrossRef]
- Simons, A.L.; Renouf, M.; Hendrich, S.; Murphy, P.A. Human Gut Microbial Degradation of Flavonoids: Structure−Function Relationships. J. Agric. Food Chem. 2005, 53, 4258–4263. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free. Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Liang, X.; Wei, X.H.; Chen, F.L.; Tang, Q.F.; Tan, X.M. Comparative metabolism of the eight main bioactive ingredients of gegen qinlian decoction by the intestinal flora of diarrhoeal and healthy piglets. Biomed. Chromatogr. BMC 2019, 33, e4421. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nishihata, T.; Jin, J.S.; Ma, C.M.; Komatsu, K.; Iwashima, M.; Hattori, M. The C-glucosyl bond of puerarin was cleaved hydrolytically by a human intestinal bacterium strain PUE to yield its aglycone daidzein and an intact glucose. Chem. Pharm. Bull. 2011, 59, 23–27. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hong, S.; Yang, P.; Sun, Y.; Wang, Y.; Zhang, P.; Jiang, W.; Gu, Y. Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria. Nat. Commun. 2021, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Engst, W.; Elsinghorst, P.W.; Furtmann, N.; Bajorath, J.; Gütschow, M.; Blaut, M. Chalcone Isomerase from Eubacterium ramulus Catalyzes the Ring Contraction of Flavanonols. J. Bacteriol. 2016, 198, 2965–2974. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhskiy, L.; Ershov, P.; Yablokov, E.; Shkel, T.; Grabovec, I.; Mezentsev, Y.; Gnedenko, O.; Usanov, S.; Shabunya, P.; Fatykhava, S.; et al. Human Lanosterol 14-Alpha Demethylase (CYP51A1) Is a Putative Target for Natural Flavonoid Luteolin 7,3′-Disulfate. Molecules 2021, 26, 2237. [Google Scholar] [CrossRef]
- Cui, M.Y.; Lu, A.R.; Li, J.X.; Liu, J.; Fang, Y.M.; Pei, T.L.; Zhong, X.; Wei, Y.K.; Kong, Y.; Qiu, W.Q.; et al. Two types of O-methyltransferase are involved in biosynthesis of anticancer methoxylated 4′-deoxyflavones in Scutellaria baicalensis Georgi. Plant Biotechnol. J. 2022, 20, 129–142. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. New York Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Kim, B.G.; Jung, B.R.; Lee, Y.; Hur, H.G.; Lim, Y.; Ahn, J.H. Regiospecific flavonoid 7-O-methylation with Streptomyces avermitilis O-methyltransferase expressed in Escherichia coli. J. Agric. Food Chem. 2006, 54, 823–828. [Google Scholar] [CrossRef]
- Burapan, S.; Kim, M.; Han, J. Demethylation of Polymethoxyflavones by Human Gut Bacterium, Blautia sp. MRG-PMF1. J. Agric. Food Chem. 2017, 65, 1620–1629. [Google Scholar] [CrossRef]
- Yang, J.; Qian, D.; Guo, J.; Jiang, S.; Shang, E.-X.; Duan, J.-A.; Xu, J. Identification of the major metabolites of hyperoside produced by the human intestinal bacteria using the ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. J. Ethnopharmacol. 2013, 147, 174–179. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, F.; Liu, M.Y.; Zhao, Y.K.; Wang, D.M.; Hao, Q.H.; Wang, X.L. Isolation and Characterization of a Human Intestinal Bacterium Eggerthella sp. AUH-JLD49s for the Conversion of (-)-3′-Desmethylarctigenin. J. Agric. Food Chem. 2017, 65, 4051–4056. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Premathilaka, R.; Rashidinejad, A.; Golding, M.; Singh, J. Oral delivery of hydrophobic flavonoids and their incorporation into functional foods: Opportunities and challenges. Food Hydrocoll. 2022, 128, 107567. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Rahaman, S.T.; Mondal, S. Flavonoids: A vital resource in healthcare and medicine. Pharm. Pharmacol. Int. J. 2020, 8, 91–104. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230s–242s. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of Cocoa Procyanidins in the Gastric Milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef]
- Zong, G.; Fei, S.; Liu, X.; Li, J.; Gao, Y.; Yang, X.; Wang, X.; Shen, Y. Crystal structures of rhamnosyltransferase UGT89C1 from Arabidopsis thaliana reveal the molecular basis of sugar donor specificity for UDP-β-l-rhamnose and rhamnosylation mechanism. Plant J. Cell Mol. Biol. 2019, 99, 257–269. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Liu, Y.; Zhao, L.; Pei, J. An efficient preparation and biocatalytic synthesis of novel C-glycosylflavonols kaempferol 8-C-glucoside and quercetin 8-C-glucoside through using resting cells and macroporous resins. Biotechnol. Biofuels Bioprod. 2022, 15, 129. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131 (Suppl. S4), 1362s–1375s. [Google Scholar] [CrossRef]
- Mao, S.; Ren, Y.; Ye, X.; Tian, J. The metabolites of flavonoids with typical structure enhanced bioactivity through gut microbiota. Food Biosci. 2024, 59, 104165. [Google Scholar] [CrossRef]
- Zhou, M.; Ma, J.; Kang, M.; Tang, W.; Xia, S.; Yin, J.; Yin, Y. Flavonoids, gut microbiota, and host lipid metabolism. Eng. Life Sci. 2024, 24, 2300065. [Google Scholar] [CrossRef] [PubMed]
- Makino, R.; Takano, K.; Kita, K.; Nishimukai, M. Influence of long-term feeding of high-fat diet on quercetin and fat absorption from the small intestine in lymph duct-cannulated rats. Biosci. Biotechnol. Biochem. 2018, 82, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Favari, C.; Rinaldi de Alvarenga, J.F.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors driving the inter-individual variability in the metabolism and bioavailability of (poly)phenolic metabolites: A systematic review of human studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef]
- Sime, F.B.; Roberts, M.S.; Roberts, J.A. Optimization of dosing regimens and dosing in special populations. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015, 21, 886–893. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Aquino-Martinez, R.; Evered, A.H.; Cross, T.-W.L.; Everhart, J.; Ulland, T.K.; Kay, C.D.; Bolling, B.W.; Bäckhed, F.; et al. Gut microbes modulate the effects of the flavonoid quercetin on atherosclerosis. NPJ Biofilms Microbiomes 2025, 11, 12. [Google Scholar] [CrossRef]
- Liu, H.; Wu, B.; Pan, G.; He, L.; Li, Z.; Fan, M.; Jian, L.; Chen, M.; Wang, K.; Huang, C. Metabolism and pharmacokinetics of mangiferin in conventional rats, pseudo-germ-free rats, and streptozotocin-induced diabetic rats. Drug Metab. Dispos. Biol. Fate Chem. 2012, 40, 2109–2118. [Google Scholar] [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Li, Z.; Liu, Y.; Yang, W.; Zhang, T.; Guo, P.; Liu, Z.; Qi, D.; Pi, J. The Flavonoid Components of Scutellaria baicalensis: Biopharmaceutical Properties and their Improvement using Nanoformulation Techniques. Curr. Top. Med. Chem. 2023, 23, 17–29. [Google Scholar]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Joma, N.; Bielawski, P.B.; Saini, A.; Kakkar, A.; Maysinger, D. Nanocarriers for natural polyphenol senotherapeutics. Aging Cell 2024, 23, e14178. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Sysak, S.; Czarczynska-Goslinska, B.; Szyk, P.; Koczorowski, T.; Mlynarczyk, D.T.; Szczolko, W.; Lesyk, R.; Goslinski, T. Metal Nanoparticle-Flavonoid Connections: Synthesis, Physicochemical and Biological Properties, as Well as Potential Applications in Medicine. Nanomaterials 2023, 13, 1531. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, C.; Guo, M.; Zhu, F.; Yu, Z.; Zhang, W.; Li, W.; Zhang, Y.; Tian, W. Circadian Rhythm-Dependent Therapy by Composite Targeted Polyphenol Nanoparticles for Myocardial Ischemia-Reperfusion Injury. ACS Nano 2024, 18, 28154–28169. [Google Scholar] [CrossRef]
- Li, J.; Jiang, X.; Shang, L.; Li, Z.; Yang, C.; Luo, Y.; Hu, D.; Shen, Y.; Zhang, Z. L-EGCG-Mn nanoparticles as a pH-sensitive MRI contrast agent. Drug Deliv. 2021, 28, 134–143. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Yu, H.; Tian, H.; Chen, X. Multifunctional Theranostic Nanoparticles Derived from Fruit-Extracted Anthocyanins with Dynamic Disassembly and Elimination Abilities. ACS Nano 2018, 12, 8255–8265. [Google Scholar] [CrossRef]
- Fu, S.; Cai, Z.; Gu, H.; Lui, S.; Ai, H.; Song, B.; Wu, M. Rutin-coated ultrasmall manganese oxide nanoparticles for targeted magnetic resonance imaging and photothermal therapy of malignant tumors. J. Colloid Interface Sci. 2024, 670, 499–508. [Google Scholar] [CrossRef]
- Guo, C.; Sun, J.; Dong, J.; Cai, W.; Zhao, X.; Song, B.; Zhang, R. A natural anthocyanin-based multifunctional theranostic agent for dual-modal imaging and photothermal anti-tumor therapy. J. Mater. Chem. B 2021, 9, 7447–7460. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, H.; Wang, T.; Zhang, H.; Yang, Z.; Yang, P.; Li, Y.; Ma, X.; Gu, Z. Epicatechin-assembled nanoparticles against renal ischemia/reperfusion injury. J. Mater. Chem. B 2022, 10, 6965–6973. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.P.; Swain, S.; Sa, N.; Pilla, S.N.; Behera, A.; Sahu, P.K.; Chandra Si, S. Photocatalysis of environmental organic pollutants and antioxidant activity of flavonoid conjugated gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 282, 121699. [Google Scholar] [CrossRef]
- Hassani, S.; Maghsoudi, H.; Fattahi, F.; Malekinejad, F.; Hajmalek, N.; Sheikhnia, F.; Kheradmand, F.; Fahimirad, S.; Ghorbanpour, M. Flavonoids nanostructures promising therapeutic efficiencies in colorectal cancer. Int. J. Biol. Macromol. 2023, 241, 124508. [Google Scholar] [CrossRef] [PubMed]
- Wen, E.; Cao, Y.; He, S.; Zhang, Y.; You, L.; Wang, T.; Wang, Z.; He, J.; Feng, Y. The mitochondria-targeted Kaempferol nanoparticle ameliorates severe acute pancreatitis. J. Nanobiotechnology 2024, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- Aiello, P.; Consalvi, S.; Poce, G.; Raguzzini, A.; Toti, E.; Palmery, M.; Biava, M.; Bernardi, M.; Kamal, M.A.; Perry, G.; et al. Dietary flavonoids: Nano delivery and nanoparticles for cancer therapy. Semin. Cancer Biol. 2021, 69, 150–165. [Google Scholar] [CrossRef]
- Jain, A.K.; Thanki, K.; Jain, S. Co-encapsulation of Tamoxifen and Quercetin in Polymeric Nanoparticles: Implications on Oral Bioavailability, Antitumor Efficacy, and Drug-Induced Toxicity. Mol. Pharm. 2013, 10, 3459–3474. [Google Scholar] [CrossRef]
- Sharma, T.; Singh, D.; Mahapatra, A.; Mohapatra, P.; Sahoo, S.; Sahoo, S.K. Advancements in clinical translation of flavonoid nanoparticles for cancer treatment. OpenNano 2022, 8, 100074. [Google Scholar] [CrossRef]
- Shi-Ying, J.; Jin, H.; Shi-Xiao, J.; Qing-Yuan, L.; Jin-Xia, B.; Chen, H.G.; Rui-Sheng, L.; Wei, W.; Hai-Long, Y. Characterization and evaluation in vivo of baicalin-nanocrystals prepared by an ultrasonic-homogenization-fluid bed drying method. Chin. J. Nat. Med. 2014, 12, 71–80. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Cheng, M.; Liu, Q.; Liu, W.; Gao, C.; Feng, J.; Jin, Y.; Tu, L. Improving Oral Bioavailability of Luteolin Nanocrystals by Surface Modification of Sodium Dodecyl Sulfate. AAPS PharmSciTech 2021, 22, 133. [Google Scholar] [CrossRef]
- Uğur Kaplan, A.B.; Öztürk, N.; Çetin, M.; Vural, İ.; Öznülüer Özer, T. The Nanosuspension Formulations of Daidzein: Preparation and In Vitro Characterization. Turk. J. Pharm. Sci. 2022, 19, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Pooja, D.; Ravuri, H.G.; Gunukula, A.; Kulhari, H.; Sistla, R. Fabrication of surfactant-stabilized nanosuspension of naringenin to surpass its poor physiochemical properties and low oral bioavailability. Phytomedicine 2018, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Zhang, C.; Liu, Y.; Nie, H.; Zhou, J.; Ding, Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur. J. Pharm. Sci. 2020, 144, 105212. [Google Scholar] [CrossRef]
- He, W.; Zhang, J.; Ju, J.; Wu, Y.; Zhang, Y.; Zhan, L.; Li, C.; Wang, Y. Preparation, characterization, and evaluation of the antitumor effect of kaempferol nanosuspensions. Drug Deliv. Transl. Res. 2023, 13, 2885–2902. [Google Scholar] [CrossRef]
- Ao, H.; Li, Y.; Li, H.; Wang, Y.; Han, M.; Guo, Y.; Shi, R.; Yue, F.; Wang, X. Preparation of hydroxy genkwanin nanosuspensions and their enhanced antitumor efficacy against breast cancer. Drug Deliv. 2020, 27, 816–824. [Google Scholar] [CrossRef]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and Evaluation of Naringenin Nanosuspensions for Bioavailability Enhancement. AAPS PharmSciTech 2017, 18, 3151–3162. [Google Scholar] [CrossRef]
- Xie, J.; Luo, Y.; Liu, Y.; Ma, Y.; Yue, P.; Yang, M. Novel redispersible nanosuspensions stabilized by co-processed nanocrystalline cellulose-sodium carboxymethyl starch for enhancing dissolution and oral bioavailability of baicalin. Int. J. Nanomed. 2019, 14, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, R.; Huang, H. Anti-Allergic Effects of Quercetin and Quercetin Liposomes in RBL-2H3 Cells. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 692–701. [Google Scholar] [CrossRef]
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y.M.; Ramos Romano, M.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal encapsulation of the natural flavonoid fisetin improves bioavailability and antitumor efficacy. Int. J. Pharm. 2013, 444, 146–154. [Google Scholar] [CrossRef]
- Li, Z.; Liang, S.; Sun, H.; Bao, C.; Li, Y. Antilipogenesis Effect of Rutin-Loaded Liposomes Using a Microneedle Delivery System. ACS Appl. Mater. Interfaces 2023, 15, 54294–54303. [Google Scholar] [CrossRef]
- Priya, V.; Singh, S.K.; Revand, R.; Kumar, S.; Mehata, A.K.; Sushmitha, P.; Mahto, S.K.; Muthu, M.S. GPIIb/IIIa Receptor Targeted Rutin Loaded Liposomes for Site-Specific Antithrombotic Effect. Mol. Pharm. 2023, 20, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Z.; Yang, Y.; Adu-Frimpong, M.; Chen, L.; Ji, H.; Toreniyazov, E.; Wang, Q.; Yu, J.; Xu, X. Preparation, characterization, pharmacokinetics, and antirenal injury activity studies of Licochalcone A-loaded liposomes. J. Food Biochem. 2022, 46, e14007. [Google Scholar] [CrossRef]
- Karamchedu, S.; Tunki, L.; Kulhari, H.; Pooja, D. Morin hydrate loaded solid lipid nanoparticles: Characterization, stability, anticancer activity, and bioavailability. Chem. Phys. Lipids 2020, 233, 104988. [Google Scholar] [CrossRef]
- Zhao, B.; Gu, S.; Du, Y.; Shen, M.; Liu, X.; Shen, Y. Solid lipid nanoparticles as carriers for oral delivery of hydroxysafflor yellow A. Int. J. Pharm. 2018, 535, 164–171. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; He, Q.; Liu, X.; Okeke, C.I.; Tong, L.; Guo, L.; Yang, H.; Zhang, Q.; Zhao, H.; et al. Effect of Baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia-reperfusion in rats. Int. J. Pharm. 2015, 489, 131–138. [Google Scholar] [CrossRef]
- Zaheer, Y.; Ali, M.A.; Rehman, M.; Iftikhar, M.; Anwar, S.; Ali, A.; Mobeen, A.; Iqbal, M.; Iqbal, S.; Younis, M.R.; et al. Naringenin loaded solid lipid nanoparticles alleviate oxidative stress and enhance oral bioavailability of naringenin. Colloids Surf. B Biointerfaces 2024, 247, 114423. [Google Scholar] [CrossRef]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 911–925. [Google Scholar]
- Luo, C.-F.; Yuan, M.; Chen, M.-S.; Liu, S.-M.; Zhu, L.; Huang, B.-Y.; Liu, X.-W.; Xiong, W. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int. J. Pharm. 2011, 410, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, H.; Guan, S. Enhancement of the oral bioavailability of breviscapine by nanoemulsions drug delivery system. Drug Dev. Ind. Pharm. 2015, 41, 177–182. [Google Scholar] [CrossRef]
- Yin, J.; Xiang, C.; Wang, P.; Yin, Y.; Hou, Y. Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. Int. J. Nanomed. 2017, 12, 2923–2931. [Google Scholar] [CrossRef]
- Sun, D.; Wei, X.; Xue, X.; Fang, Z.; Ren, M.; Lou, H.; Zhang, X. Enhanced oral absorption and therapeutic effect of acetylpuerarin based on D-α-tocopheryl polyethylene glycol 1000 succinate nanoemulsions. Int. J. Nanomed. 2014, 9, 3413–3423. [Google Scholar]
- Diedrich, C.; Camargo Zittlau, I.; Schineider Machado, C.; Taise Fin, M.; Maissar Khalil, N.; Badea, I.; Mara Mainardes, R. Mucoadhesive nanoemulsion enhances brain bioavailability of luteolin after intranasal administration and induces apoptosis to SH-SY5Y neuroblastoma cells. Int. J. Pharm. 2022, 626, 122142. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Luo, Q.; Shi, J.; Xu, H.; Zhang, J. Preparation and in vitro and in vivo evaluation of an isoliquiritigenin-loaded ophthalmic nanoemulsion for the treatment of corneal neovascularization. Drug Deliv. 2022, 29, 2217–2233. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Mukherjee, P.K.; Kar, A.; Bahadur, S.; Al-Dhabi, N.A.; Duraipandiyan, V. Enhancement of photoprotection potential of catechin loaded nanoemulsion gel against UVA induced oxidative stress. J. Photochem. Photobiol. B Biol. 2016, 160, 318–329. [Google Scholar] [CrossRef]
- Mi, X.; Hu, M.; Dong, M.; Yang, Z.; Zhan, X.; Chang, X.; Lu, J.; Chen, X. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int. J. Nanomed. 2021, 16, 8337–8352. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Sun, Q.; Yang, H.; Tang, B.; Pu, H.; Li, H. Honokiol nanoparticles based on epigallocatechin gallate functionalized chitin to enhance therapeutic effects against liver cancer. Int. J. Pharm. 2018, 545, 74–83. [Google Scholar] [CrossRef]
- Krishnan, G.; Subramaniyan, J.; Chengalvarayan Subramani, P.; Muralidharan, B.; Thiruvengadam, D. Hesperetin conjugated PEGylated gold nanoparticles exploring the potential role in anti-inflammation and anti-proliferation during diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian J. Pharm. Sci. 2017, 12, 442–455. [Google Scholar] [CrossRef]
- Mashhadi Malekzadeh, A.; Ramazani, A.; Tabatabaei Rezaei, S.J.; Niknejad, H. Design and construction of multifunctional hyperbranched polymers coated magnetite nanoparticles for both targeting magnetic resonance imaging and cancer therapy. J. Colloid Interface Sci. 2017, 490, 64–73. [Google Scholar] [CrossRef]
- Song, Z.; Yin, J.; Xiao, P.; Chen, J.; Gou, J.; Wang, Y.; Zhang, Y.; Yin, T.; Tang, X.; He, H. Improving Breviscapine Oral Bioavailability by Preparing Nanosuspensions, Liposomes and Phospholipid Complexes. Pharmaceutics 2021, 13, 132. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xie, J.; Zheng, Q.; Yue, P.; Chen, L.; Hu, P.; Yang, M. Nose-to-Brain Delivery by Nanosuspensions-Based in situ Gel for Breviscapine. Int. J. Nanomed. 2020, 15, 10435–10451. [Google Scholar] [CrossRef]
- Sajid, M.; Channakesavula, C.N.; Stone, S.R.; Kaur, P. Synthetic Biology towards Improved Flavonoid Pharmacokinetics. Biomolecules 2021, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Isika, D.K.; Sadik, O.A. Selective Structural Derivatization of Flavonoid Acetamides Significantly Impacts Their Bioavailability and Antioxidant Properties. Molecules 2022, 27, 8133. [Google Scholar] [CrossRef] [PubMed]
- Isika, D.K.; Özkömeç, F.N.; Çeşme, M.; Sadik, O.A. Synthesis, biological and computational studies of flavonoid acetamide derivatives. RSC Adv. 2022, 12, 10037–10050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, D.; Zhang, Y.; Li, J.; Wu, Z.; Wang, Z.; Wang, D. Investigation of the pro-apoptotic effects of arbutin and its acetylated derivative on murine melanoma cells. Int. J. Mol. Med. 2018, 41, 1048–1054. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.J.; Lee, M.J.; Ho, C.T.; Yang, C.S. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab. Dispos. Biol. Fate Chem. 2006, 34, 2111–2116. [Google Scholar] [CrossRef]
- Li, X.-F.; Yuan, T.; Xu, H.; Xin, X.; Zhao, G.; Wu, H.; Xiao, X. Whole-Cell Catalytic Synthesis of Puerarin Monoesters and Analysis of Their Antioxidant Activities. J. Agric. Food Chem. 2019, 67, 299–307. [Google Scholar] [CrossRef]
- Xiao, C.; Li, J.; Dong, X.; He, X.; Niu, X.; Liu, C.; Zhong, G.; Bauer, R.; Yang, D.; Lu, A. Anti-oxidative and TNF-α suppressive activities of puerarin derivative (4AC) in RAW264.7 cells and collagen-induced arthritic rats. Eur. J. Pharmacol. 2011, 666, 242–250. [Google Scholar] [CrossRef]
- Yuan, D.; Guo, Y.; Pu, F.; Yang, C.; Xiao, X.; Du, H.; He, J.; Lu, S. Opportunities and challenges in enhancing the bioavailability and bioactivity of dietary flavonoids: A novel delivery system perspective. Food Chem. 2024, 430, 137115. [Google Scholar] [CrossRef]
- Dai, J.; Liang, K.; Zhao, S.; Jia, W.; Liu, Y.; Wu, H.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S.; et al. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2018, 115, E5896–E5905. [Google Scholar] [CrossRef]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Yang, C.S. Bioavailability and stability issues in understanding the cancer preventive effects of tea polyphenols. J. Sci. Food Agric. 2006, 86, 2256–2265. [Google Scholar] [CrossRef]
- Lotito, S.B.; Zhang, W.J.; Yang, C.S.; Crozier, A.; Frei, B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free. Radic. Biol. Med. 2011, 51, 454–463. [Google Scholar] [CrossRef]
- Ha, T.; Kim, M.K.; Park, K.S.; Jung, W.; Choo, H.; Chong, Y. Structural Modification of (-)-Epigallocatechin Gallate (EGCG) Shows Significant Enhancement in Mitochondrial Biogenesis. J. Agric. Food Chem. 2018, 66, 3850–3859. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The catechol-O-methyltransferase inhibitor, tolcapone, increases the bioavailability of unmethylated (-)-epigallocatechin-3-gallate in mice. J. Funct. Foods 2015, 17, 183–188. [Google Scholar] [CrossRef]
- Calla, B. Diverse defenses: O-methylated flavonoids contribute to the maize arsenal against fungal pathogens. Plant Physiol. 2022, 188, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhang, M.; Li, X.-F.; Zhao, G. Biocatalytic Synthesis of Lipophilic Baicalin Derivatives as Antimicrobial Agents. J. Agric. Food Chem. 2019, 67, 11684–11693. [Google Scholar] [CrossRef]
- Zhang, M.; Xin, X.; Zhao, G.; Zou, Y.; Li, X.-F. In vitro absorption and lipid-lowering activity of baicalin esters synthesized by whole-cell catalyzed esterification. Bioorganic Chem. 2022, 120, 105628. [Google Scholar] [CrossRef]
- Fernando, W.; Clark, R.F.; Rupasinghe, H.P.V.; Hoskin, D.W.; Coombs, M.R.P. Phloridzin Docosahexaenoate Inhibits Spheroid Formation by Breast Cancer Stem Cells and Exhibits Cytotoxic Effects against Paclitaxel-Resistant Triple Negative Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 14577. [Google Scholar] [CrossRef]
- Arumuggam, N.; Melong, N.; Too, C.K.; Berman, J.N.; Rupasinghe, H.V. Phloridzin docosahexaenoate, a novel flavonoid derivative, suppresses growth and induces apoptosis in T-cell acute lymphoblastic leukemia cells. Am. J. Cancer Res. 2017, 7, 2452–2464. [Google Scholar]
- Fernando, W.; Coyle, K.; Marcato, P.; Vasantha Rupasinghe, H.P.; Hoskin, D.W. Phloridzin docosahexaenoate, a novel fatty acid ester of a plant polyphenol, inhibits mammary carcinoma cell metastasis. Cancer Lett. 2019, 465, 68–81. [Google Scholar] [CrossRef]
- Mantso, T.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Pappa, A.; Rupasinghe, H.P.V.; Panayiotidis, M.I. Novel Docosahexaenoic Acid Ester of Phloridzin Inhibits Proliferation and Triggers Apoptosis in an In Vitro Model of Skin Cancer. Antioxidants 2018, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.N.; Ziaullah; Rupasinghe, H.P.V. Long Chain Fatty Acid Esters of Quercetin-3-O-glucoside Attenuate H2O2-induced Acute Cytotoxicity in Human Lung Fibroblasts and Primary Hepatocytes. Molecules 2016, 21, 452. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Gorain, B.; Mitra Mazumder, P. Recent advancements in drug delivery system of flavonoids with a special emphasis on the flavanone naringenin: Exploring their application in wound healing and associated processes. Inflammopharmacology 2024, 33, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Li, C.; Dai, T.; Chen, J.; Chen, M.; Liang, R.; Liu, C.; Du, L.; McClements, D.J. Modification of flavonoids: Methods and influences on biological activities. Crit. Rev. Food Sci. Nutr. 2023, 63, 10637–10658. [Google Scholar] [CrossRef]
- Baky, M.H.; Elshahed, M.; Wessjohann, L.; Farag, M.A. Interactions between dietary flavonoids and the gut microbiome: A comprehensive review. Br. J. Nutr. 2022, 128, 577–591. [Google Scholar] [CrossRef]
- Di Gioia, D.; Strahsburger, E.; Lopez de Lacey, A.M.; Bregola, V.; Marotti, I.; Aloisio, I.; Biavati, B.; Dinelli, G. Flavonoid bioconversion in Bifidobacterium pseudocatenulatum B7003: A potential probiotic strain for functional food development. J. Funct. Foods 2014, 7, 671–679. [Google Scholar] [CrossRef]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Mahomud, M.S.; Islam, M.N.; Hossen, D.; Wazed, M.A.; Yasmin, S.; Sarker, M.S.H. Innovative probiotic yogurt: Leveraging green banana peel for enhanced quality, functionality, and sensory attributes. Heliyon 2024, 10, e38781. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Abd El-Aziz, N.M.; Mehany, T.; Simal-Gandara, J. Taro leaves extract and probiotic lactic acid bacteria: A synergistic approach to improve antioxidant capacity and bioaccessibility in fermented milk beverages. LWT 2023, 187, 115280. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, 2, e93. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Shi, J.; Zhang, Y. Effects of derivatization and probiotic transformation on the antioxidative activity of fruit polyphenols. Food Chem. X 2024, 23, 101776. [Google Scholar] [CrossRef]
- Pereira, E.P.R.; Ferreira, B.M.; Freire, L.; Neri-Numa, I.A.; Guimarães, J.T.; Rocha, R.S.; Pastore, G.M.; Cruz, A.G.; Sant’ana, A.S. Enhancing the functionality of yogurt: Impact of exotic fruit pulps addition on probiotic viability and metabolites during processing and storage. Food Res. Int. 2024, 196, 115057. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Majeed, O.S.; Gaaz, T.S.; Akpoghelie, P.O.; Isoje, E.F.; Igbuku, U.A.; Owheruo, J.O.; Opiti, R.A.; Garba, Y.; et al. A review on probiotics and dietary bioactives: Insights on metabolic well-being, gut microbiota, and inflammatory responses. Food Chem. Adv. 2025, 6, 100919. [Google Scholar] [CrossRef]


| Subtype | Structure Backbone | Chemical Characterization | Common Biological Sources | Representatives | Applications | Ref. |
|---|---|---|---|---|---|---|
| Flavone | 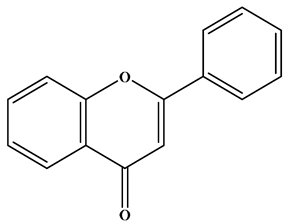 | There is a double bond between the C2 and C3 positions; a ketone group is at the C4 position; and the C2 position is connected to the B ring. | Celery, tea, red peppers, and oranges | Apigenin; Luteolin | Cancer, cardiovascular disease, neuroinflammation inflammation, anti-diabetic, antibacterial, antioxidant, and antiviral, etc. | [26,27,28] |
| Isoflavone |  | There is a double bond between the C2 and C3 positions; a ketone group is at the C4 position; and the C3 position is connected to the B ring. | Soybeans, and soy-derived products | Genistein; Daidzein | Antioxidant, diarrhea relief, procoagulant activity, and anticancer, etc. | [29,30,31] |
| Flavonol | 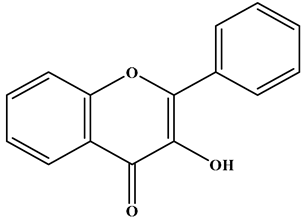 | There is a double bond between the C2 and C3 positions; a ketone group is at the C4 position; a hydroxyl group is connected to the C3 position; and the C2 position is connected to the B ring. | Apples, cherries, plums, apricots, berries, onions, kale, and leeks | Quercetin; Myricetin; Kaempferol | Cardiovascular diseases, anticancer, antioxidation, and neuroprotection, etc. | [32,33,34,35] |
| Flavanol | 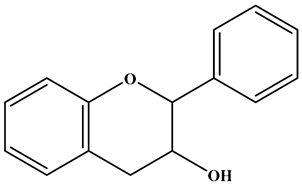 | There is no double bond between the C2 and C3 positions; there is no ketone group at the C4 position; a hydroxyl group is connected to the C3 position; the C2 position is connected to the B ring. | Broccoli, onions, asparagus, apples, and tea | Epicatechin; Epigallocatechin | Antioxidant, anticancer, and anti-inflammatory, etc. | [36,37,38] |
| Flavanone | 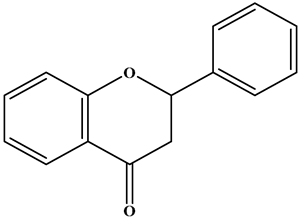 | There is no double bond between the C2 and C3 positions; there is a ketone group at C4 position; and the C2 position is connected to the B ring. | Citrus Fruits | Hesperidin; Naringin; Paclitaxel | Anticancer, anti-inflammatory, antibacterial, antioxidant, antiviral, and lipid-lowering, etc. | [2,39,40,41] |
| Chalcone | 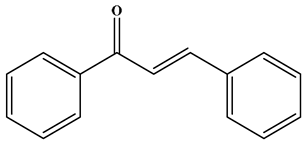 | No C ring (open-chain flavonoids). | Leguminosae, Moraceae, Zingiberaceae, and Cannabaceae | Xanthohumol; Corylifolinin | Antioxidant, antibacterial, anti-inflammatory, antiviral, and anticancer, etc. | [42,43,44] |
| Anthocyanidin | 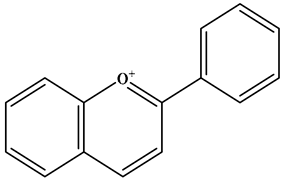 | 2-phenylbenzopyranyl cation structure; there is a double bond between the C1 and C2; there is a double bond between the C3 and C4. The C2 position is connected to the B ring. | Blueberries, red cabbage, tomatoes, purple sweet potatoes, and eggplant | Delphinidin; Cyanidin; Petunidin; Peonidin; Malvidin; Pelargonidin | Eye health, cardiovascular disease, antiobesity, antidiabetes, antibacterial, anticancer activity and neurodegenerative diseases, etc. | [25,45,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. https://doi.org/10.3390/molecules30051184
Hu L, Luo Y, Yang J, Cheng C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules. 2025; 30(5):1184. https://doi.org/10.3390/molecules30051184
Chicago/Turabian StyleHu, Lei, Yiqing Luo, Jiaxin Yang, and Chunsong Cheng. 2025. "Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability" Molecules 30, no. 5: 1184. https://doi.org/10.3390/molecules30051184
APA StyleHu, L., Luo, Y., Yang, J., & Cheng, C. (2025). Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules, 30(5), 1184. https://doi.org/10.3390/molecules30051184







