Improving Bitumen Properties with Chitosan: A Sustainable Approach to Road Construction
Abstract
1. Introduction
2. Results
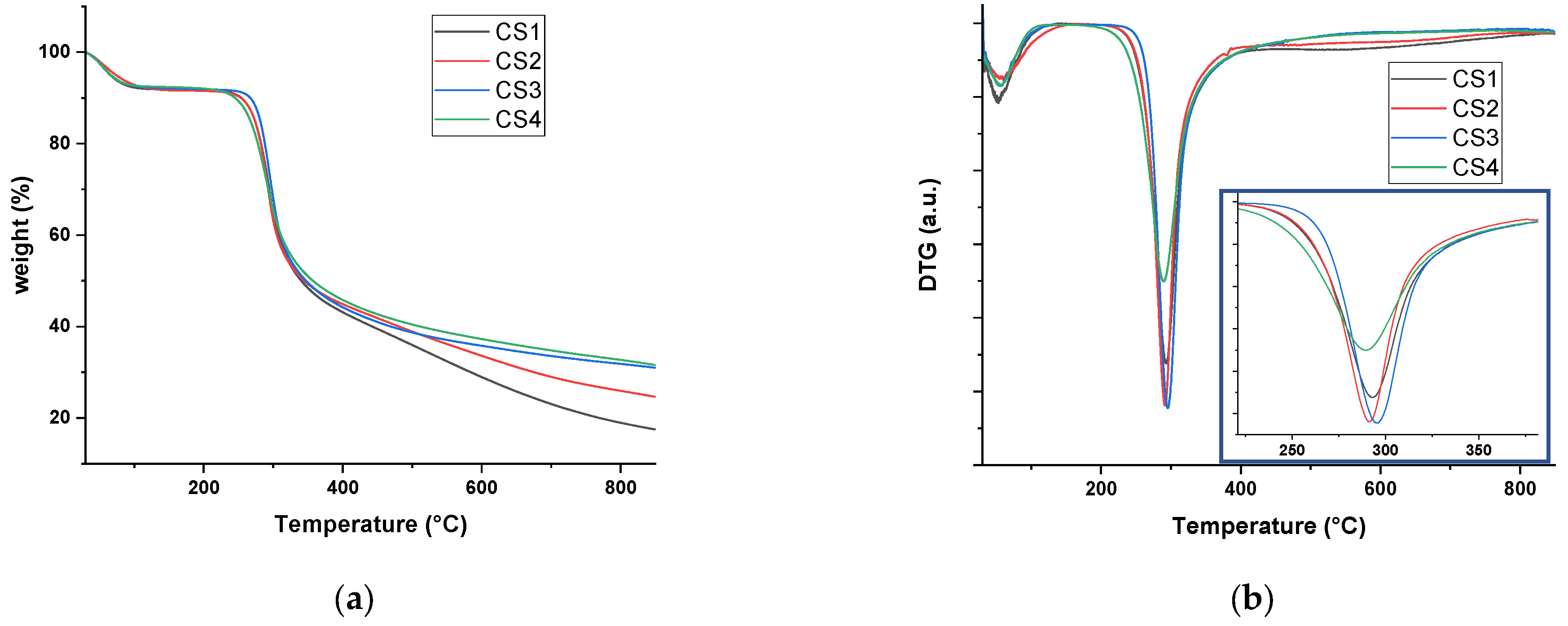
2.1. Thermal Stabilities of the Four CS Polymers
2.2. Rheological Modifying Effect
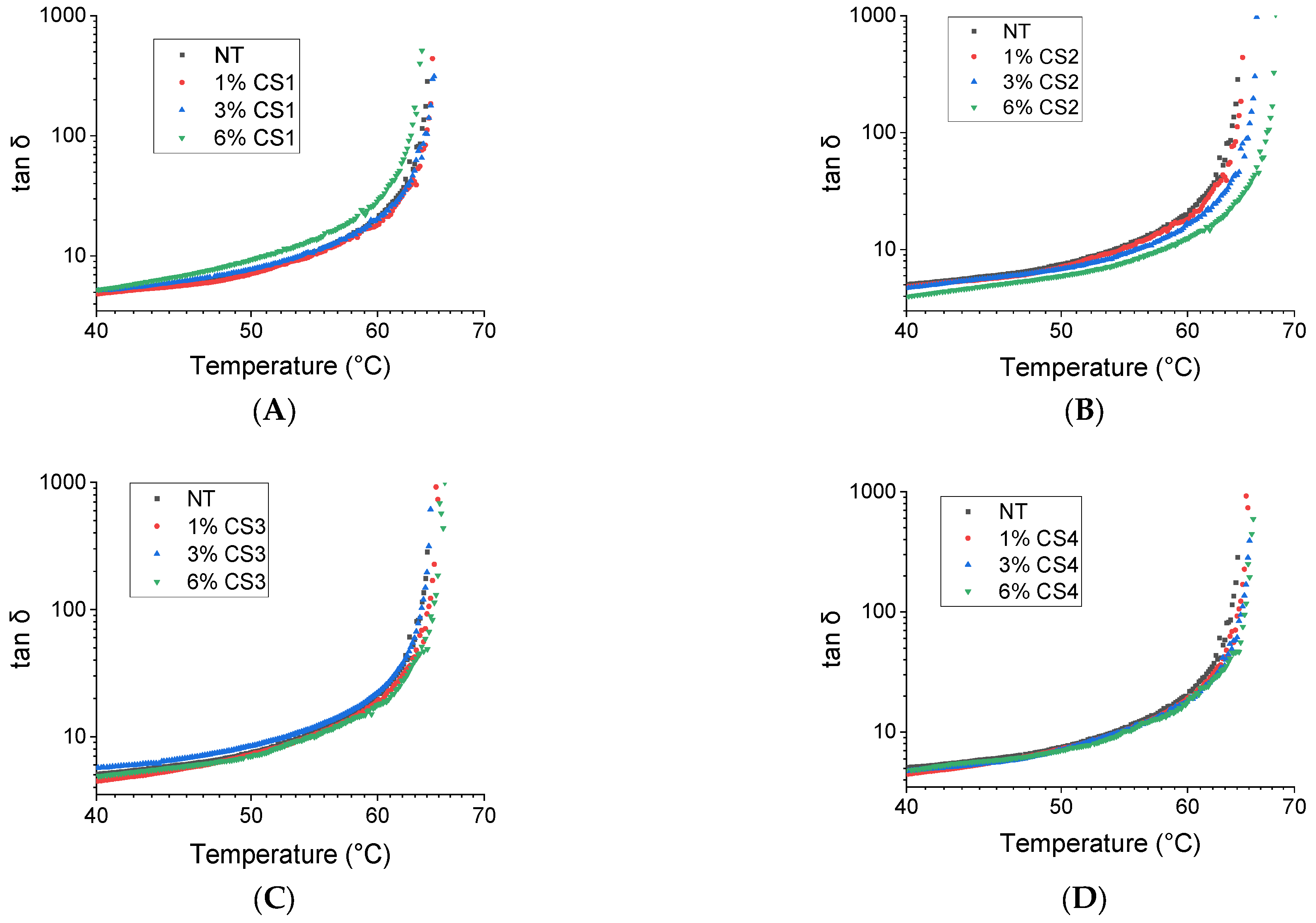
2.3. Antioxidant Effect
- (1)
- it is able to set the increase in transition temperature at a value of only 4.2 °C after a short period (75 min) of aging;
- (2)
- it sets the increase in transition temperature at a value of 13.2 °C upon a long period (225 min) of aging.
2.4. Rigidity at Working Conditions
2.5. Adhesion to Stone
3. Materials and Methods
3.1. Materials
3.2. Bitumen Blend Preparation
3.3. Thermogravimetric Analysis
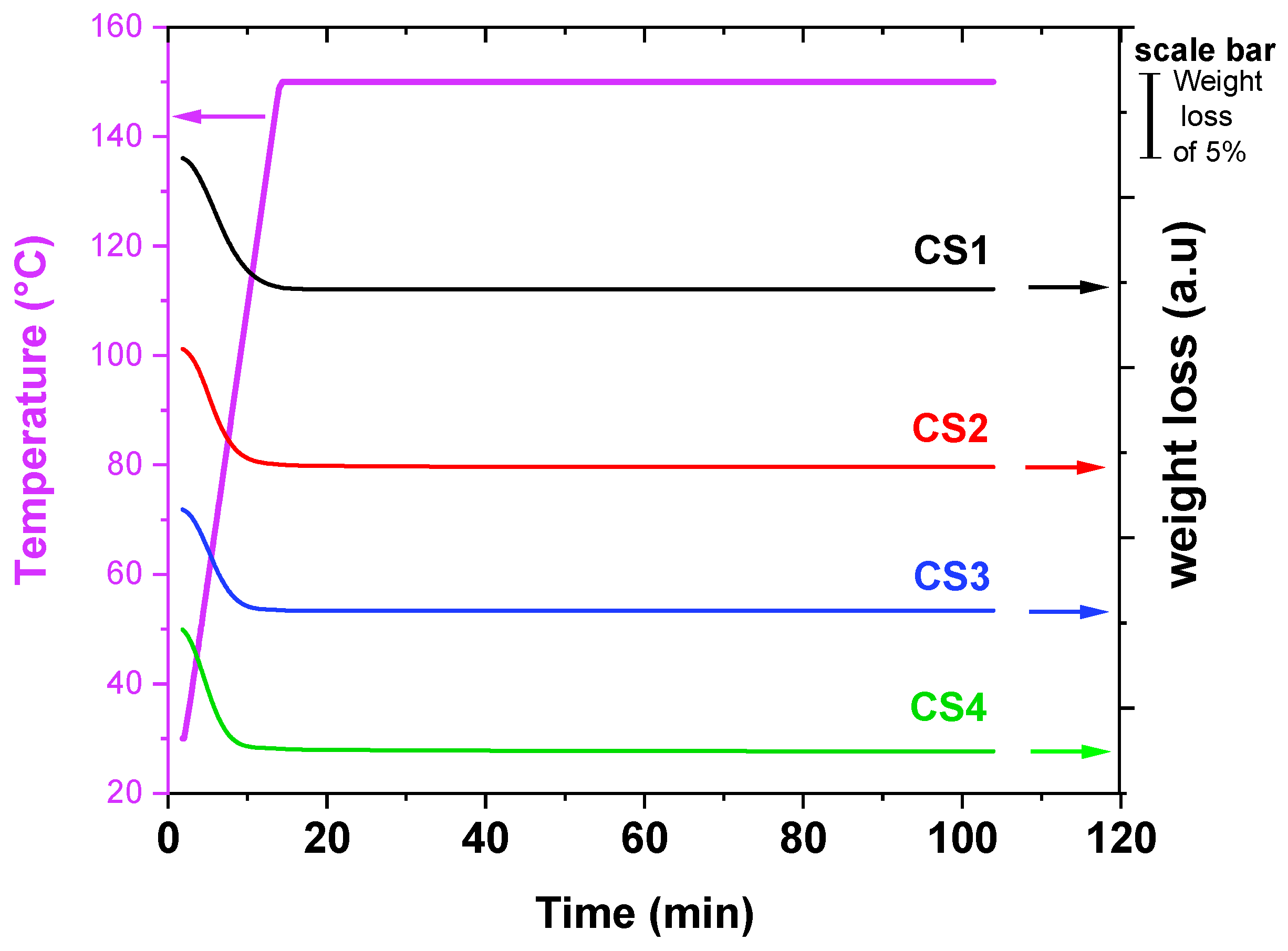
3.4. Aging Tests
3.5. Rheological Tests
4. Conclusions
- Best results in terms of rheological modifier were obtained for CS with a molecular weight of 310,000–375,000 kD and with a deacetylation degree ≥75% (free amine groups);
- A good antioxidant effect was shown by CS with high molecular weight (600,000–800,000 kD) and with a deacetylation degree >90% (free amine groups);
- No detrimental effect on the adhesion efficiency with the stones was observed, suggesting the possible use of CS as an additive;
- The findings suggest that CS can play a critical role in developing more durable, environmentally friendly, and cost-effective bitumen formulations, since CS is readily available from the waste shells of crustaceans obtained from seafood processing industries, making it a potentially sustainable and environmentally friendly material. The utilization of waste materials for CS extraction may present economic benefits by valorizing underutilized resources and reducing waste disposal costs; ultimately, it can represent a sustainable approach with significant potential to contribute to the circular economy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesueur, D. The Colloidal Structure of Bitumen: Consequences on the Rheology and on the Mechanisms of Bitumen Modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, O. Low-Temperature Rheology of Bitumen and Its Relationship with Chemical and Thermal Properties. Master’s Thesis, Aalto University, Espoo, Finland, 2015; p. 186. [Google Scholar]
- D’Melo, D.; Taylor, R. Constitution and Structure of Bitumens. In The Shell Bitumen; Hunter, R.N., Self, A., Read, J., Eds.; ICE Publishing: London, UK, 2015; pp. 47–62. ISBN 978-0-7277-5837-8. [Google Scholar]
- EN 12597:2000; Bitumen and Bituminous Binders-Terminology. European Committee for Standardization: Brussels, Belgium, 2000.
- Paliukaitė, M.; Vaitkus, A.; Zofka, A. Evaluation of Bitumen Fractional Composition Depending on the Crude Oil Type and Production Technology. In Proceedings of the 9th International Conference “Environmental Engineering 2014”, Vilnius, Lithuania, 22–23 May 2014; Vilnius Gediminas Technical University Press “Technika”: Vilnius, Lithuania, 2014. [Google Scholar]
- Read, J.; Witheoak, D. The Shell Bitumen Handbook, 5th ed.; Thomas Telford Publishing: London, UK, 2003. [Google Scholar]
- Glazachev, A.O.; Ivanova, O.V.; Pavlov, S.Y.; Salov, A.S.; Akhmetshin, R.M. Synergetic Improvement of Technological Characteristics of Highway Road Surfaces by Bitumen Microdispersed Emulsions. Nanotechnologies Constr. 2024, 16, 463–472. [Google Scholar] [CrossRef]
- Liyanage, C.S.; Gonapinuwala, S.T.; Fernando, C.A.N.; de Croos, M.D.S.T. A Simple and Effective Method to Extract Chitosan from Crustacean Shell Waste. J. Aquat. Food Prod. Technol. 2023, 32, 396–415. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and Chitosan Derived from Crustacean Waste Valorization Streams Can Support Food Systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Policastro, D.; Giorno, E.; Scarpelli, F.; Godbert, N.; Ricciardi, L.; Crispini, A.; Candreva, A.; Marchetti, F.; Xhafa, S.; De Rose, R.; et al. New Zinc-Based Active Chitosan Films: Physicochemical Characterization, Antioxidant, and Antimicrobial Properties. Front. Chem. 2022, 10, 884059. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Malinowski, S.; Woszuk, A.; Wróbel, M.; Makowska, M.; Franus, W.; Zofka, A. Bitumen Binders Modified with Chemically-Crosslinked Chitosan. Road Mater. Pavement Des. 2023, 24, 3–18. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-Based Nanocarriers for Encapsulation and Delivery of Curcumin: A Review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef]
- Rodríguez, M.S.; Albertengo, L.A.; Agulló, E. Emulsification Capacity of Chitosan. Carbohydr. Polym. 2002, 48, 271–276. [Google Scholar] [CrossRef]
- Payet, L.; Terentjev, E.M. Emulsification and Stabilization Mechanisms of O/W Emulsions in the Presence of Chitosan. Langmuir 2008, 24, 12247–12252. [Google Scholar] [CrossRef]
- Mallawarachchi, D.R.; Amarasinghe, A.D.U.S.; Prashantha, M.A.B. Suitability of Chitosan as an Emulsifier for Cationic Bitumen Emulsions and Its Behaviour as an Additive to Bitumen Emulsion. Constr. Build. Mater. 2016, 102, 1–6. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Muhammad, Y.; Li, J.Q.; Ye, Y.; Li, J.; Li, Z.; Pei, R. Effect of Glutaraldehyde-Chitosan Crosslinked Graphene Oxide on High Temperature Properties of SBS Modified Asphalt. Constr. Build. Mater. 2022, 357, 129387. [Google Scholar] [CrossRef]
- Perumal, D.; Laad, M.; Sangita, S. Structural, Morphological, Rheological Study of Chitosan Modified Bitumen Composite. Eng. Res. Express 2023, 5, 025024. [Google Scholar] [CrossRef]
- De Britto, D.; Campana-Filho, S.P. Kinetics of the Thermal Degradation of Chitosan. Thermochim. Acta 2007, 465, 73–82. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced Physico-Chemical Characterization of Chitosan by Means of TGA Coupled on-Line with FTIR and GCMS: Thermal Degradation and Water Adsorption Capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Subhy, A. Advanced Analytical Techniques in Fatigue and Rutting Related Characterisations of Modified Bitumen: Literature Review. Constr. Build. Mater. 2017, 156, 28–45. [Google Scholar] [CrossRef]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1989; ISBN 0444871403. [Google Scholar]
- Verma, M.; Saboo, N. Use of Antioxidants to Retard Aging of Bitumen: A Review. Environ. Sci. Pollut. Res. 2024, 31, 48839–48863. [Google Scholar] [CrossRef]
- Somé, C.; Pavoine, A.; Chailleux, E.; Andrieux, L.; DeMarco, L.; Philippe, D.S.; Stephan, B. Rheological Behavior of Vegetable Oil-Modified Asphaltite Binders and Mixes. In Proceedings of the 6th Eurasphalt & Eurobitume Congress, Prague, Czech Republic, 1–3 June 2016; Czech Technical University: Prague, Czech Republic, 2016. [Google Scholar]
- Porto, M.; Caputo, P.; Loise, V.; De Filpo, G.; Oliviero Rossi, C.; Calandra, P. Polysaccharides-Reinforced Bitumens: Specificities and Universality of Rheological Behavior. Appl. Sci. 2019, 9, 5564. [Google Scholar] [CrossRef]
- Calandra, P.; Quaranta, S.; Apolo Miranda Figueira, B.; Caputo, P.; Porto, M.; Oliviero Rossi, C. Mining Wastes to Improve Bitumen Performances: An Example of Circular Economy. J. Colloid Interface Sci. 2022, 614, 277–287. [Google Scholar] [CrossRef]
- Caputo, P.; Calandra, P.; Policicchio, A.; Conte, G.; Agostino, R.G.; Pochylski, M.; Abe, A.; Oliviero Rossi, C. Char from Pyrolysis of Waste Tires to Increase Bitumen Performances. Appl. Sci. 2023, 14, 30. [Google Scholar] [CrossRef]
- Caputo, P.; Algieri, V.; Maiuolo, L.; De Nino, A.; Sicilia, E.; Ponte, F.; Calandra, P.; Oliviero Rossi, C. Waste Additives as Biopolymers for the Modification of Bitumen: Mechanical Performance and Structural Analysis Characterization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131079. [Google Scholar] [CrossRef]
- Ling, C.; Hanz, A.; Bahia, H. Measuring Moisture Susceptibility of Cold Mix Asphalt with a Modified Boiling Test Based on Digital Imaging. Constr. Build. Mater. 2016, 105, 391–399. [Google Scholar] [CrossRef]
- Paliukaite, M.; Vorobjovas, V.; Bulevičius, M.; Andrejevas, V. Evaluation of Different Test Methods for Bitumen Adhesion Properties. Transp. Res. Procedia 2016, 14, 724–731. [Google Scholar] [CrossRef]
- Zhang, J.; Apeagyei, A.K.; Airey, G.D.; Grenfell, J.R.A. Influence of Aggregate Mineralogical Composition on Water Resistance of Aggregate-Bitumen Adhesion. Int. J. Adhes. Adhes. 2015, 62, 45–54. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Gu, F.; Xu, T.; Wang, H. Impact of Minerals and Water on Bitumen-Mineral Adhesion and Debonding Behaviours Using Molecular Dynamics Simulations. Constr. Build. Mater. 2018, 171, 214–222. [Google Scholar] [CrossRef]
- Vinet-Cantot, J.; Gaudefroy, V.; Delfosse, F.; Chailleux, E.; Crews, E. Stripping at bitumen aggregate interface: A laboratory methodology to assess loss of chemical adhesion. Energy Fuels 2019, 33, 2641–2650. [Google Scholar] [CrossRef]
- Petrauskas, D.; Ullah, S. Manufacture and Storage of Bitumens. In The Shell Bitumen Handbook; ICE Publishing: London, UK, 2015; ISBN 978-0-7277-5837-8. [Google Scholar]
- Loise, V.; Calandra, P.; Abe, A.A.; Porto, M.; Oliviero Rossi, C.; Davoli, M.; Caputo, P. Additives on Aged Bitumens: What Probe to Distinguish between Rejuvenating and Fluxing Effects? J. Mol. Liq. 2021, 339, 116742. [Google Scholar] [CrossRef]
- Shaffie, E.; Arshad, A.K.; Alisibramulisi, A.; Ahmad, J.; Hashim, W.; Abd Rahman, Z.; Jaya, R.P. Effect of Mixing Variables on Physical Properties of Modified Bitumen Using Natural Rubber Latex. Int. J. Civ. Eng. Technol. 2018, 9, 1812–1821. [Google Scholar]
- ASTM-D2872-04; Standard Test Method for Effect of Heat and Air on a Moving Film of Asphalt Binder (Rolling Thin-Film Oven Test). ASTM International: West Conshohocken, PA, USA, 2004. [CrossRef]
- EN 12607-1:2007; Bitumen and Bituminous Binders. Determination of the Resistance to Hardening Under Influence of Heat and Air. RTFOT Method. British Standards Institution: Milton Keynes, UK, 2007. [CrossRef]
- Sims, I. The Shell Bitumen Handbook. Proc. Inst. Civ. Eng.-Constr. Mater. 2016, 169, 44. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, S.; Xu, S.; Dong, N.; Yu, H. Rheological Behavior of Warm Mix Asphalt Modified with Foaming Process and Surfactant Additive. Crystals 2021, 11, 410. [Google Scholar] [CrossRef]
- Malinowski, S. Chemical structure analysis of chitosan-modified road bitumen after de-icing salt treatment. Mater. Struct. 2024, 57, 227. [Google Scholar] [CrossRef]

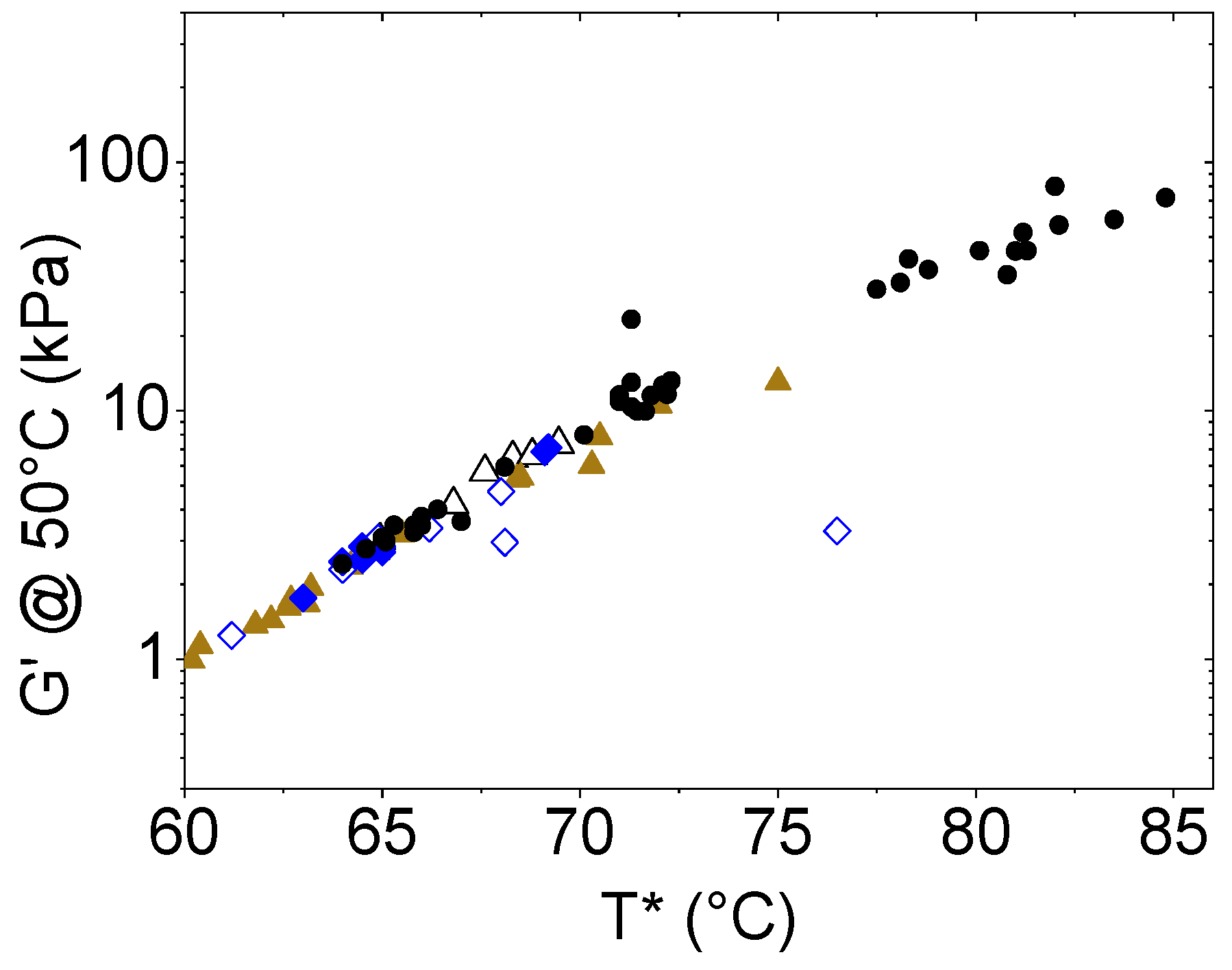

| Sample | T* (°C) | ΔT*1 = T*75min − T*unaged | ΔT*2 = T225min − Tunaged |
|---|---|---|---|
| Neat Bitumen (NT) | 64.6 | 5.5 | 16.4 |
| 1wt% CS1 | 65.1 | 6.55 | 15.0 |
| 3 wt% CS1 | 66.4 | 4.9 | 11.7 |
| 6 wt% CS1 | 64.0 | 7.45 | 14.8 |
| 1 wt% CS2 | 65.1 | 7.0 | 18.4 |
| 3 wt% CS2 | 65.0 | 6.0 | 17.0 |
| 6 wt% CS2 | 68.1 | 4.2 | 13.2 |
| 1 wt% CS3 | 65.3 | 6.8 | 19.5 |
| 3 wt% CS3 | 66.0 | 5.8 | 14.8 |
| 6 wt%CS3 | 67.0 | 4.3 | 14.2 |
| 1 wt% CS4 | 65.8 | 6.4 | 16.3 |
| 3 wt% CS4 | 65.8 | 5.5 | 12.5 |
| 6 wt% CS4 | 66.0 | 5.0 | 11.5 |
| Modifier | Molecular Weight (kD) | Deacetylation Degree |
|---|---|---|
| CS1 | 50,000–190,000 | ≥75% |
| CS2 | 310,000–375,000 | ≥75% |
| CS3 | 100,000–300,000 | >90% |
| CS4 | 600,000–800,000 | >90% |
| Property | Value |
|---|---|
| origin | Saudi Arabia |
| penetration grade | 50/70 |
| saturates content (wt%) | 3.8 |
| aromatics content (wt%) | 51.3 |
| resins content (wt%) | 21.5 |
| asphaltenes content (wt%) | 23.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, P.; Oliviero Rossi, C.; Calandra, P.; Policastro, D.; Giorno, E.; Godbert, N.; Aiello, I. Improving Bitumen Properties with Chitosan: A Sustainable Approach to Road Construction. Molecules 2025, 30, 1170. https://doi.org/10.3390/molecules30051170
Caputo P, Oliviero Rossi C, Calandra P, Policastro D, Giorno E, Godbert N, Aiello I. Improving Bitumen Properties with Chitosan: A Sustainable Approach to Road Construction. Molecules. 2025; 30(5):1170. https://doi.org/10.3390/molecules30051170
Chicago/Turabian StyleCaputo, Paolino, Cesare Oliviero Rossi, Pietro Calandra, Debora Policastro, Eugenia Giorno, Nicolas Godbert, and Iolinda Aiello. 2025. "Improving Bitumen Properties with Chitosan: A Sustainable Approach to Road Construction" Molecules 30, no. 5: 1170. https://doi.org/10.3390/molecules30051170
APA StyleCaputo, P., Oliviero Rossi, C., Calandra, P., Policastro, D., Giorno, E., Godbert, N., & Aiello, I. (2025). Improving Bitumen Properties with Chitosan: A Sustainable Approach to Road Construction. Molecules, 30(5), 1170. https://doi.org/10.3390/molecules30051170











