Abstract
The development of alternative adsorptive separation technologies is extremely significant for the separation of C2H2/CO2 and CO2/CH4 in the chemical industry. Emerging metal–organic frameworks (MOFs) have shown great potential as adsorbents for gas adsorption and separation. Herein, we synthesized two layered Zn-MOFs, UPC-96 and UPC-97, with 1,2,4,5-tetrakis(4-carboxyphenyl)-3,6-dimethylbenzene (TCPB-Me) as a ligand via the solvent regulation of the pH values. UPC-96 with a completely deprotonated ligand was obtained without the addition of acid, exhibiting two different channels with cross-sectional sizes of 11.6 × 7.1 and 8.3 × 5.2 Å2. In contrast, the addition of acid led to the partial deprotonation of the ligand and afforded UPC-97 two types of channels with cross-sectional sizes of 11.5 × 5.7 and 7.4 × 3.9 Å2. Reversible N2 adsorption isotherms at 77 K confirmed their permanent porosity, and the differentiated single-component C2H2, CO2, and CH4 adsorption isotherms indicated their potential in C2H2/CO2 and CO2/CH4 separation.
1. Introduction
In the face of energy shortages and environmental crises, increasing attention has been paid to cleaner and more economical energy resources such as natural gas, biogas, shale gas, and coalbed methane [1,2,3]. The main component of these gas resources is methane (CH4), and they usually contain impurity gas, such as carbon dioxide (CO2), which may lead to the corrosion of pipelines during conveyance [4,5,6]. Therefore, it is important to selectively capture CO2 and realize the efficient purification of CH4. Acetylene (C2H2) is a significant raw material in the petrochemical industry, which has been widely used as fuel in welding and to produce fundamental chemicals like polyvinyl chloride, butadiene, and acrylic acid derivatives, as well as synthetic materials like rubber, fiber, and plastic [7,8,9]. Nowadays, the main source of C2H2 is the partial combustion of CH4 and cracking of hydrocarbons, which inevitably generate CO2 as a by-product [10,11,12]. Due to having similar boiling points (189.3 K for C2H2 and 194.7 K for CO2) and the same kinetic diameter (3.3 Å), it is a big challenge to remove CO2 and acquire high-purity C2H2 that meets the needs of the chemical industry. Therefore, it is necessary to capture and remove CO2 for energy gas storage and industrial raw gas purification. Carbon capture and storage (CCS) covers a range of technologies that will be crucial in supporting these separations. CCS technologies have matured significantly, with over 30 commercial facilities operating globally as of 2023, and the total CO2 capture capacity of operational projects has exceeded 40 million tons per annum (Mtpa), with many more projects under development [13].
Traditional separation processes mainly include cryogenic distillation and solvent absorption. Cryogenic distillation is energy-intensive due to the huge amount of energy required to realize phase changes, and solvent absorption is environmentally unfriendly due to the use of massive amounts of toxic solvents [14]. In comparison, adsorbent-based gas separation represents an energy-efficient and environmentally friendly separation technology [15,16,17,18,19,20,21,22]. So far, various adsorbents have been researched and developed, among which metal–organic frameworks (MOFs) are outstanding due to their high specific surface area, tunable pore size, and facile modification with functional groups in comparison with traditional adsorbents like zeolite, molecular sieve, and activated carbon [23,24,25,26,27,28,29]. Hence, MOFs are regarded as efficient and adaptable adsorbents with low energy consumption, and great progress has been made in the application of gas separation [30,31,32,33,34,35,36]. As structure determines performance, the regulation of an MOF’s structure can effectively optimize the gas adsorption and separation performance. For example, Zhou et al. investigated the effect of solvent proton-supplying/accepting capacity on the structure and CO2 adsorption performance of Mg-MOF-74 through the regulation of the polarity of the reaction solvent [37]. The highest performing sample was synthesized in an N,N’-dimethylformamide and methanol environment with a high CO2 adsorption capacity of 7.38 mmol/g. Lewiński et al. reported the controlled construction of two-dimensional (2D) MOFs through solvent-templated growth and obtained a series of 2D Cu(II)–carboxylate MOFs with various stacking modes and distances with a diffusion-controlled MOF deposition approach in different solvents [38]. Further research on the gas adsorption properties demonstrated the crystal size had a remarkable effect on the nitrogen (N2) adsorption capacity, which may be related to the limited number of pore entrances in the bigger flake-shaped crystals. Jeong et al. developed a solvent-assisted reversible strategy to modulate the interpenetration form, in which interpenetration was accomplished with protic solvents of small molecular sizes, such as water, methanol, and ethanol, and non-interpenetration was achieved using pyridine with Lewis basicity [39]. Compared to the non-interpenetrated MOF-143(NI) with a large pore size, the interpenetrated MOF-14(I) with narrow pores exhibited a higher ethane and ethylene uptake capability, as well as higher levels of ethane selectivity. Therefore, studies on solvent regulation in MOFs to achieve the effective optimization of specific gas adsorption and separation performance are attractive, and there is still much room for exploration.
Herein, we synthesized two kinds of 2D layered Zn-MOFs, UPC-96 and UPC-97, with methyl modified tetracarboxylic 1,2,4,5-tetrakis(4-carboxyphenyl)-3,6-dimethylbenzene (TCPB-Me) as a ligand. The solvent regulation of the pH value led to different coordination modes of the ligand and thus afforded two distinct frameworks. The difference in the porosity of UPC-96 and UPC-97 resulted in obviously differential levels of adsorption and separation performance. UPC-96 performed better than UPC-97 in terms of Brunauer–Emmett–Teller (BET) surface area, pore volume, adsorption capacity, enthalpy, and selectivity.
2. Results and Discussion
2.1. Crystal Structure
Colorless rod-shaped crystals of UPC-96 were obtained by a solvothermal reaction following the method in Section 3.3. Single-crystal X-ray diffraction (SCXRD) measurements indicate that UPC-96 crystallizes in the monoclinic P21/c space group. The asymmetric unit contains one Zn2+ ion, one half of a completely deprotonated ligand, one water molecule, and one N,N’-dimethylacetamide (DMA) molecule. Each Zn2+ ion is coordinated with three oxygen atoms from two different ligands, one oxygen atom from the water molecule, and one oxygen atom from the DMA molecule (Figure 1a). Each ligand adopts two coordination modes to link four Zn2+ ions, affording a 2D layered framework with an sql topology (Figure 1b). The adjacent three layers further form a 2D layered unit by a weak intermolecular interaction (Figure 1c) through O–H···O hydrogen bonds (H···O, 1.834 Å) between the water molecules and the uncoordinated oxygen atom from the ligand to generate a three-dimensional (3D) framework with two channels (Figure 1d). After the removal of the coordinated solvents, interlayer channel I exhibits a cross-sectional size of 11.6 × 7.1 Å2, and intralayer channel II exhibits a cross-sectional size of 8.3 × 5.2 Å2.
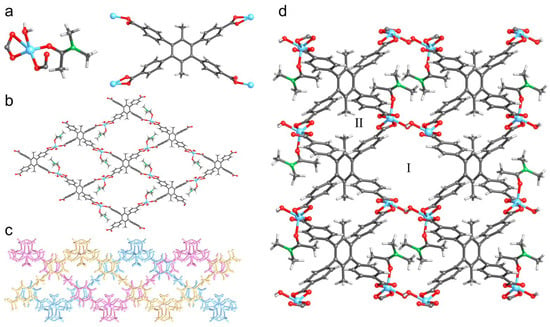
Figure 1.
Crystal structure of UPC-96: (a) Coordination mode of Zn2+ and ligand; (b) Single layered framework with sql topology; (c) 2D layered unit with three layers; (d) 3D framework with two channels. Zn, sky-blue; C, gray; N, green; O, red; and H, pale.
Colorless lamellar crystals of UPC-97 were obtained under similar solvothermal conditions except for the acidic pH value. SCXRD measurements reveal that UPC-97 crystallizes in the triclinic P-1 space group. The asymmetric unit contains two Zn2+ ions, two partially deprotonated ligands, and four DMA molecules. Each Zn2+ ion adopts a distorted octahedron coordination geometry with four oxygen atoms from two different ligands and two oxygen atoms from two different DMA molecules (Figure 2a). Due to the acidic condition, the ligand is partially deprotonated and links two Zn2+ ions to form an infinite S-shaped chain (Figure 2b). Adjacent chains are arranged in parallel in opposite directions to afford a layered unit (Figure 2c), which is further connected by O–H···O hydrogen bonds (H···O, 1.813–1.853 Å) between the deprotonated and protonated carboxyl groups from different chains to form a 3D framework with two channels (Figure 2d). After the removal of the coordinated DMA molecules, interlayer channels I and II are observed with cross-sectional sizes of 11.5 × 5.7 and 7.4 × 3.9 Å2, respectively.
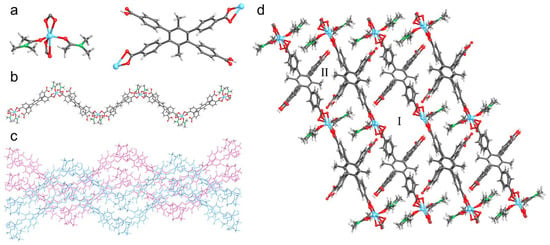
Figure 2.
Crystal structure of UPC-97: (a) Coordination mode of Zn2+ and ligand; (b) Infinite S-shaped chain; (c) Parallel layered unit of chains in opposite directions; (d) 3D framework with two channels. Zn, sky-blue; C, gray; N, green; O, red; and H, pale.
2.2. Structure Characterization
The powder X-ray diffraction (PXRD) patterns of UPC-96 and UPC-97 were collected at room temperature to confirm the phase purity (Figure 3). The PXRD patterns of the as-synthesized samples match well with the simulated patterns solved with SCXRD, indicating the correct structures are obtained with good crystallinity and phase purity values. In addition, an elemental analysis further confirms their chemical purity. Anal. calcd for UPC-96 (C22H22NO6Zn): C, 57.22; H, 4.80; and N, 3.03%; found: C, 57.44; H, 4.73; and N, 3.16%. Anal. calcd for UPC-97 (C88H84N4O20Zn2): C, 64.12; H, 5.13; and N, 3.39%; found: C, 63.93; H, 5.07; and N, 3.46%.
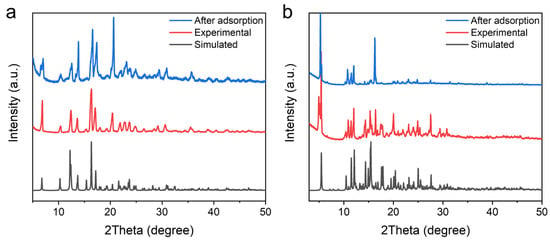
Figure 3.
PXRD patterns of (a) UPC-96 and (b) UPC-97.
Considering that the morphology may significantly impact the adsorption performance, scanning electron microscopy (SEM) images were taken as shown in Figure 4. UPC-96 and UPC-97 both exhibited a uniform flaky morphology, providing surface texture and different levels of microporosity.
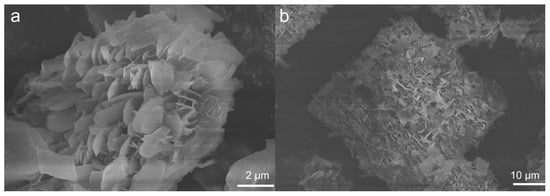
Figure 4.
SEM images of (a) UPC-96 and (b) UPC-97.
To analyze the thermal stability of UPC-96 and UPC-97, a thermogravimetric analysis (TGA) was performed under a N2 atmosphere with a heating rate of 10 °C/min from 40 °C to 900 °C (Figure 5). According to the results of the TGA curves before and after activation, the weight loss of UPC-96 before 240 °C corresponds to the release of coordinated solvent molecules, and UPC-96 remains stable till 400 °C, at which point the framework collapses. In contrast, UPC-97 releases the coordinated solvent molecules before 100 °C and maintains stability until 400 °C, at which point the framework collapses. These investigations confirm their good thermostability.
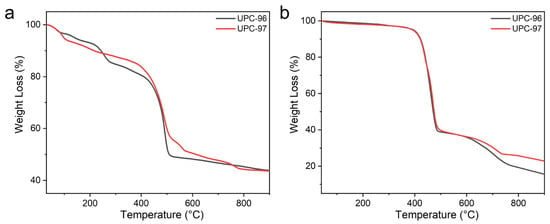
Figure 5.
TGA curves of UPC-96 and UPC-97 (a) before and (b) after activation.
Fourier transform infrared (FT-IR) spectra of UPC-96 and UPC-97 were recorded with KBr pellets (Figure 6). The broad absorption band of UPC-96 at 3400 cm−1 is assigned to the hydroxyl stretching vibration of water molecules, which is not observed for UPC-97 without coordinated water molecules. The strong absorption bands of UPC-96 at 1605 and 1390 cm−1 and those of UPC-97 at 1605 and 1400 cm−1 correspond to the asymmetric and symmetric carboxyl stretching vibrations, respectively.
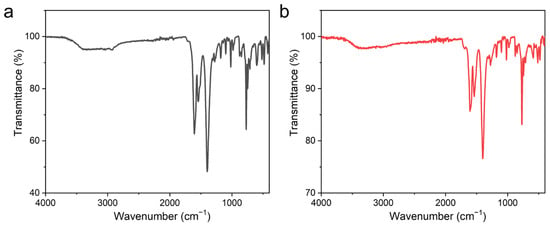
Figure 6.
FT-IR spectra of (a) UPC-96 and (b) UPC-97.
2.3. Gas Adsorption and Separation
As mentioned previously, both UPC-96 and UPC-97 possess two kinds of channels with potential for gas adsorption and separation. To confirm the porosity of UPC-96 and UPC-97, N2 adsorption/desorption isotherms were collected at 77 K (Figure 7a). UPC-96 exhibited a reversible type I isotherms with a maximum uptake of 133.9 cm3/g, which is much higher than that of UPC-97 (56.8 cm3/g). The calculated BET surface area of UPC-96 reached 220.2 m2/g with a pore volume of 0.19 cm3/g, whereas a negligible BET surface area of 37.6 m2/g and pore volume of 0.07 cm3/g were observed for UPC-97, indicating the significant pore structure difference by solvent regulation. The corresponding pore size distribution was determined by the nonlocal density functional theory (DFT) method (Figure 7b), with the pore size mainly concentrated at 6.0 and 5.5 Å for UPC-96 and UPC-97, respectively.
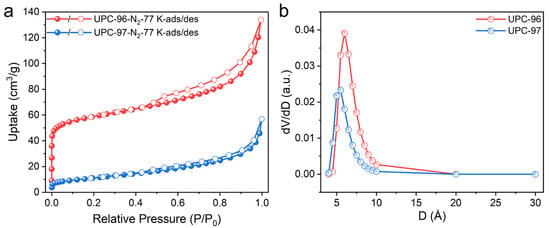
Figure 7.
(a) N2 adsorption/desorption isotherms and (b) pore size distribution of UPC-96 and UPC-97.
Considering the ideal porosity and suitable pore size, the potential of UPC-96 and UPC-97 in C2H2/CO2 and CO2/CH4 separation was investigated. Single-component adsorption/desorption isotherms of C2H2, CO2, and CH4 were measured at 273 and 298 K (Figure 8). As expected, the adsorption capacity follows the order of C2H2 > CO2 > CH4, and UPC-96 presents a much higher uptake than UPC-97. The maximum quantities of C2H2 adsorbed reached 49.7 and 44.1 cm3/g for UPC-96 at 273 and 298 K, whereas those for UPC-97 were only 28.8 and 25.7 cm3/g at 273 and 298 K, respectively. For CO2, the uptake capacities were 40.5 and 29.6 cm3/g for UPC-96 at 273 and 298 K, respectively, which were higher than those obtained for UPC-97 (22.5 and 18.4 cm3/g). Notably, the CO2 uptake capacity of UPC-96 exceeds many reported MOF adsorbents, such as ZNU-11 (22.5 cm3/g) [40], BSF-10 (25.8 cm3/g) [41], and SNNU-27-Cd (29.5 cm3/g) [42]. In contrast, both MOFs exhibited negligible CH4 uptake, with the maximum amounts of 16.2 and 7.9 cm3/g for UPC-96 and 9.4 and 5.7 cm3/g for UPC-97 at 273 and 298 K, respectively. In spite of its low specific surface and porosity values, UPC-97 exhibited a considerable adsorption capacity for C2H2 and CO2 and a negligible adsorption capacity for CH4, which may be due to the fact that the kinetic diameter of C2H2 and CO2 is smaller than that of N2 (3.64 Å) and CH4 (3.8 Å). In addition to the molecular sieving effect, the differential interaction between different gases and MOFs can also explain the different adsorption capacities. The highest C2H2 capacity could be attributed to the framework and C2H2 molecules having stronger host–guest interactions than CO2 and CH4.
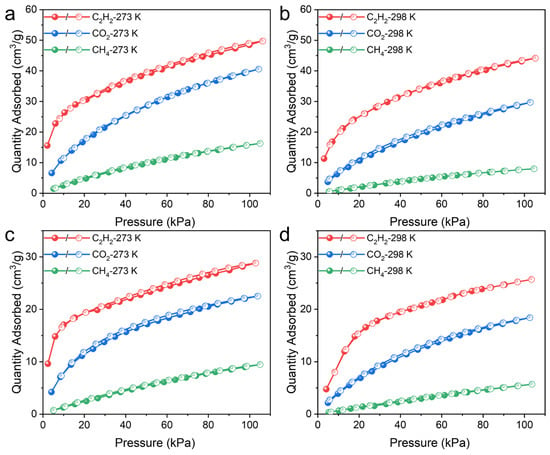
Figure 8.
Single-component adsorption/desorption isotherms of C2H2, CO2, and CH4: UPC-96 at (a) 273 and (b) 298 K; UPC-97 at (c) 273 and (d) 298 K.
Cycling stability is an important evaluation indicator for adsorbents in practical applications. The repeatability of UPC-96 and UPC-97 was tested for five cycles without a reactivation process at 298 K (Figure 9). For UPC-96, there are only 3.8%, 5.3%, and 6.0% decreases in the adsorption capacity of C2H2, CO2, and CH4, respectively; for UPC-97, there are only 4.2%, 5.7%, and 6.5% decreases in the adsorption capacity of C2H2, CO2, and CH4, respectively, indicating their good reproducibility. Meanwhile, the PXRD patterns of UPC-96 and UPC-97 after adsorption match well with the original patterns (Figure 3), suggesting their good stability.
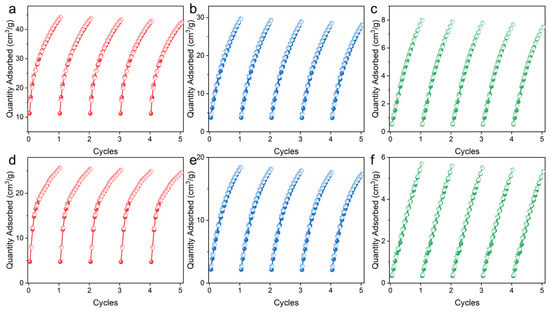
Figure 9.
Cycling adsorption/desorption isotherms at 298 K: UPC-96 for (a) C2H2, (b) CO2, and (c) CH4; UPC-97 for (d) C2H2, (e) CO2, and (f) CH4.
To evaluate the affinity between the framework and gas molecules, the Virial equation was employed to calculate the adsorption enthalpy (Qst). In both MOFs, the Qst value follows the same order as the adsorption capacity, indicating stronger interactions with C2H2 molecules. At near-zero coverage, the Qst values of UPC-96 reached 51.7, 38.3, and 32.4 kJ/mol for C2H2, CO2, and CH4, respectively, which are higher than those for UPC-97 (38.9, 30.3, and 21.6 kJ/mol) (Figure 10). It is worth noting that the Qst values of CO2 are comparable to many MOFs, such as ZNU-11 (33.9 kJ/mol) [40], BSF-10 (27.4 kJ/mol) [41], and SNNU-27-Cd (24.6 kJ/mol) [42], suggesting a much stronger host–guest interaction between the framework and CO2. The high Qst values of UPC-96 and UPC-97 could be attributed to the abundant open metal sites after the removal of the coordinated solvent molecules [43].
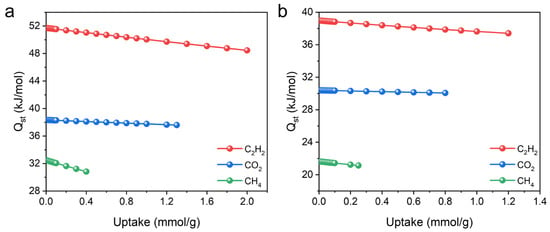
Figure 10.
Adsorption enthalpy of (a) UPC-96 and (b) UPC-97 for C2H2, CO2, and CH4.
To predict the separation performance, the ideal adsorbed solution theory (IAST) was used to calculate the equimolar C2H2/CO2 and CO2/CH4 selectivity. In agreement with previous experimental results, UPC-96 exhibited a better separation performance than UPC-97. At 298 K, UPC-96 showed good C2H2/CO2 and CO2/CH4 selectivity values of 5.7 and 10.9, respectively, whereas C2H2/CO2 and CO2/CH4 selectivity values only reached 3.2 and 6.2 for UPC-97 (Figure 11). The C2H2/CO2 selectivity is on par with that of some reported adsorbents, such as SNNU-27-Fe (2.0) [42], SIFSIX-Cu-TPA (5.3) [44], and BSF-10 (5.86) [41], while the CO2/CH4 selectivity surpasses that of MOF-303(Al) (4) [45], PCN-222 (4.3) [46], and Fe-dbai (7.5) [47]. The significant difference between UPC-96 and UPC-97 in adsorption capacity, Qst value, and IAST selectivity suggests the important role of solvent regulation in the optimization of gas adsorption and separation performance.
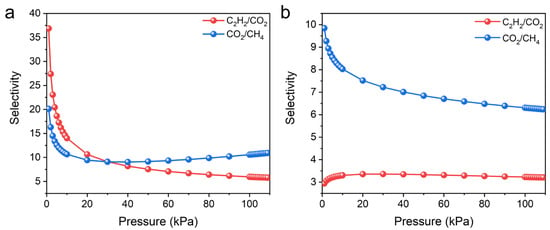
Figure 11.
IAST selectivity of (a) UPC-96 and (b) UPC-97 for equimolar C2H2/CO2 and CO2/CH4 mixtures at 298 K.
3. Materials and Methods
3.1. Materials and Instruments
All reagents were commercially available and used without further purification.
1H nuclear magnetic resonance (NMR) spectrum was obtained using an Inova 500 MHz spectrometer (Inova, Falls Church, VA, USA). Powder X-ray diffraction was carried out on a Bruker D8-Focus Bragg-Brentano X-ray powder diffractometer (Bruker, Billerica, MA, USA) equipped with a Cu sealed tube at 40 kV and 15 mA. Elemental analysis was conducted on a Vario EL III elemental analyzer (Vario, Bohmte, Germany). Scanning electron microscopy images were taken with Hitachi JSM-7500F (Hitachi, Tokyo, Japan) scanning electron microscope. Thermogravimetric analysis was performed using a Mettler Toledo TGA/DSC1 instrument (Mettler Toledo, Columbus, OH, USA) under a static N2 atmosphere with a heating rate of 10 °C/min in the range of 40–900 °C. Infrared spectroscopy spectrum was determined using a Nicolet 330 FTIR spectrometer (Nicolet, Mountain, WI, USA) within 4000–400 cm−1 region. Gas adsorption measurements were conducted using a Micrometritics ASAP 2020 surface area analyzer (Micrometritics, Norcross, GA, USA).
3.2. Synthesis of Ligand
TCPB-Me was synthesized according to the previous literature [48]. 1H NMR spectrum proved the good purity of ligand (Figure 12). 1H NMR (500 MHz, DMSO-d6): δ = 7.82 (d, 8H), 7.28 (d, 8H), 1.74 (s, 6H).
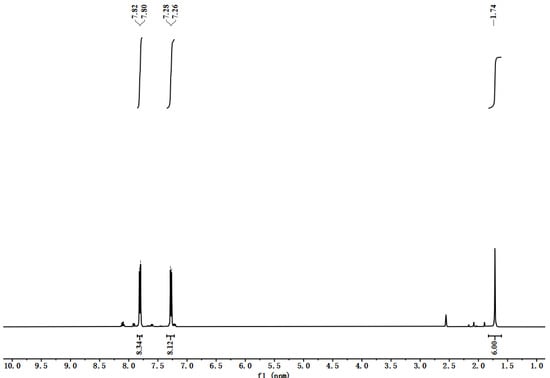
Figure 12.
1H NMR spectrum of TCPB-Me.
3.3. Synthesis of MOFs
UPC-96: A mixture of TCPB-Me (5.0 mg) and Zn(NO3)2·6H2O (10.0 mg) in DMA/H2O (5 mL, v/v = 1/1) was placed into a glass vial (10 mL) and heated at 100 °C for 40 h. The vial was then cooled to room temperature at a rate of 5 °C/h. The obtained colorless rod-shaped crystals were filtered, washed with DMA, and dried in air.
UPC-97 was synthesized with the same method as that for UPC-96 except for the addition of HNO3/H2O (80 μL, v/v = 1/10), and colorless lamellar crystals were obtained.
3.4. Single-Crystal X-Ray Diffraction
The as-synthesized crystals were taken from the mother liquid without further treatment, transferred to oil, and mounted onto a loop. The data were collected using an Agilent Technologies SuperNova diffractometer equipped with graphite monochromatic Cu Kα radiation (λ = 1.54184 Å). With the help of Olex2 (Version: Olex2-1.5), the structure was solved with the Superflip structure solution program using charge flipping and refined with the ShelXL (Version: ShelXL-2019/3) refinement package using least squares minimization. The structure was treated anisotropically, whereas the hydrogen atoms were placed in calculated ideal positions and refined depending on their respective nonhydrogen atoms. Table 1 shows the crystallographic data and refinement details.

Table 1.
Crystal data of UPC-96 and UPC-97.
3.5. Gas Adsorption Measurements
The activated samples were prepared by immersing the as-synthesized MOFs in chromatography-grade methanol and dichloromethane for solvent exchange followed by activation under vacuum for 8 h at 373 K. Gas adsorption experiments containing C2H2, CO2, and CH4 at 273 and 298 K and N2 at 77 K were performed by using ASAP-2020 surface area analyzer (Micrometritics, Norcross, GA, USA). Liquid nitrogen bath was used to stabilize the temperature at 77 K, whereas other test temperatures were maintained via a circulating water bath.
3.6. Isosteric Heat of Adsorption
A Virial equation comprising the temperature-independent parameters ai and bj was employed to calculate the enthalpies of adsorption for C2H2, CO2, and CH4 in UPC-96 and UPC-97, which were measured at 273 and 298 K.
Here, P is the pressure expressed in mmHg, N is the amount absorbed in mmol/g, T is the temperature in K, ai and bj are virial coefficients, and m, n represent the number of coefficients required to adequately describe the isotherms (herein, m = 5 and n = 2). Qst is the coverage-dependent isosteric heat of adsorption and R is the universal gas constant.
3.7. IAST Selectivity
Before estimating the selectivity for binary gas mixture, the single-component gas adsorption isotherms were first fitted to a dual-site Langmuir–Freundlich (DSLF) model:
where q is the amount of adsorbed gas (mmol/g), p is the bulk gas-phase pressure (kPa), qsat is the saturation amount (mmol/g), b is the Langmuir–Freundlich parameter (kPa−1), and n is the Langmuir–Freundlich exponent (dimensionless) for two adsorption sites A and B, indicating the presence of weak and strong adsorption sites. bA and bB are both temperature-dependent.
The adsorption selectivity Sads was calculated by ideal adsorbed solution theory as follows:
where q1 and q2 are the molar loadings in the adsorbed phase at equilibrium with the bulk gas phase; p1 and p2 are partial pressure.
4. Conclusions
In conclusion, we reported the solvent regulation of pH using the solvothermal method to synthesize two layered Zn-MOFs, UPC-96 and UPC-97. UPC-96 with a completely deprotonated ligand was obtained without the addition of acid, whereas UPC-97 with a partially deprotonated ligand was obtained by adjusting the pH value of solvents to be more acidic. The solvent regulation brought about quite different structures, in which UPC-96 consisted of 2D layers of sql topology, and UPC-97 was composed of infinite S-shaped chains. The significant differences in structure thus resulted in differences in their gas adsorption and separation performance. UPC-96 performed better than UPC-97 in BET surface area, pore volume, adsorption capacity, enthalpy, and selectivity. This work provides guidance for the regulation of MOFs’ structure to effectively optimize their specific gas separation performance.
Supplementary Materials
CCDC 2413283-2413284 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, accessed on 4 March 2025, by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
Conceptualization, X.Z., X.C., C.Q. and X.W.; methodology, X.Z. and M.X.; software, X.W.; validation, X.Z., X.W. and W.F.; formal analysis, X.W.; investigation, X.Z.; resources, D.S.; data curation, W.F.; writing—original draft preparation, X.Z. and X.W.; writing—review and editing, W.F., Q.M. and D.S.; visualization, W.F.; supervision, D.S.; project administration, D.S.; funding acquisition, W.F. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Basic Research Projects of Natural Science Foundation of Shandong Province (ZR2023ZD40), the Key Research and Development Projects of Shandong Province (2023CXGC010315), the National Natural Science Foundation of China (22275210, 22201305), the Outstanding Youth Science Fund Projects of Shandong Province (2022HWYQ-070), and the Taishan Scholar Foundation of Shandong Province (tsqnz20221123), and sponsored by CNPC Innovation Found (2024DQ02-0202).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The original contributions presented in this study are included in the article/Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sokolov, S.; Seeburg, D.; Wohlrab, S.; Friedel, M.; Nitzsche, J.; Kondratenko, E.V. An Approach Using Oxidative Coupling of Methane for Converting Biogas and Acid Natural Gas into High-Calorific Fuels. Ind. Eng. Chem. Res. 2018, 58, 2454–2459. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sust. Energ. Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhang, H.D.; Zhou, Z.; Ge, Z.L.; Chen, C.J.; Hou, Y.D.; Ye, M.L. Current Status and Effective Suggestions for Efficient Exploitation of Coalbed Methane in China: A Review. Energy Fuels 2021, 35, 9102–9123. [Google Scholar] [CrossRef]
- Babar, M.; Bustam, M.A.; Ali, A.; Shah Maulud, A.; Shafiq, U.; Mukhtar, A.; Shah, S.N.; Maqsood, K.; Mellon, N.; Shariff, A.M. Thermodynamic data for cryogenic carbon dioxide capture from natural gas: A review. Cryogenics 2019, 102, 85–104. [Google Scholar] [CrossRef]
- Yusuf, M.; Kumar, R.; Ali Khan, M.; Ahmed, M.J.; Otero, M.; Muthu Prabhu, S.; Son, M.; Hwang, J.-H.; Hyoung Lee, W.; Jeon, B.-H. Metal-organic framework-based composites for biogas and natural gas uptake: An overview of adsorption and storage mechanisms of gaseous fuels. Chem. Eng. J. 2023, 478, 147302. [Google Scholar] [CrossRef]
- Yang, H.; Xue, L.; Yang, X.; Xu, H.; Gao, J. Advances in metal-organic frameworks for efficient separation and purification of natural gas. Chin. J. Struct. Chem. 2023, 42, 100034. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal–organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Zhu, X.; Ke, T.; Zhou, J.; Song, Y.; Xu, Q.; Zhang, Z.; Bao, Z.; Yang, Y.; Ren, Q.; Yang, Q. Vertex Strategy in Layered 2D MOFs: Simultaneous Improvement of Thermodynamics and Kinetics for Record C2H2/CO2 Separation Performance. J. Am. Chem. Soc. 2023, 145, 9254–9263. [Google Scholar] [CrossRef]
- Wang, J.W.; Fan, S.C.; Li, H.P.; Bu, X.; Xue, Y.Y.; Zhai, Q.G. De-Linker-Enabled Exceptional Volumetric Acetylene Storage Capacity and Benchmark C2H2/C2H4 and C2H2/CO2 Separations in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202217839. [Google Scholar] [CrossRef]
- Liu, S.; Han, X.; Chai, Y.; Wu, G.; Li, W.; Li, J.; da Silva, I.; Manuel, P.; Cheng, Y.; Daemen, L.L.; et al. Efficient Separation of Acetylene and Carbon Dioxide in a Decorated Zeolite. Angew. Chem. Int. Ed. 2021, 60, 6526–6532. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, C.; An, F.; Wang, H.; Wang, Q.; Xiao, A.; Wang, L.; Zhu, L. Effective separation of acetylene from carbon dioxide via commensurate adsorption in a microporous metal-organic framework. Sep. Purif. Technol. 2023, 324, 124557. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, W.; Xue, W.; Zhu, H.; Zheng, M.; Huang, H.; Zhong, C. Construction of π back donation active site in metal-organic framework for dense packing of C2H2 and efficient C2H2/CO2 separation. Sep. Purif. Technol. 2023, 322, 124345. [Google Scholar] [CrossRef]
- State of the Art: CCS Technologies 2023. Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/state-of-the-art-ccs-technologies-2023/ (accessed on 28 August 2023).
- Xu, S.; Liu, R.-S.; Zhang, M.-Y.; Lu, A.-H. Designed synthesis of porous carbons for the separation of light hydrocarbons. Chin. J. Chem. Eng. 2022, 42, 130–150. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, S.; Wang, W.; Feng, L.; Liu, X.; Zhang, X.; Wang, X.; Kang, Z.; Dai, F.; Yuan, D.; et al. Optimizing Multivariate Metal–Organic Frameworks for Efficient C2H2/CO2 Separation. J. Am. Chem. Soc. 2020, 142, 8728–8737. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Zhang, X.; Li, M.-Z.; Li, J.-R. Non-CO2 greenhouse gas separation using advanced porous materials. Chem. Soc. Rev. 2024, 53, 2056–2098. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Sanaeepur, H.; Ghomi, M.; Luque, R.; Garcia, H.; Chen, B. Flexible–robust MOFs/HOFs for challenging gas separations. Coordin. Chem. Rev. 2024, 505, 215660. [Google Scholar] [CrossRef]
- Khan, N.A.; Hassan, M.; Lee, H.J.; Jhung, S.H. Highly porous polyaniline- or polypyrrole-derived carbons: Preparation, characterization, and applications in adsorption. Chem. Eng. J. 2023, 474, 145472. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Developing High-Capacity Solid “Molecular Basket” Sorbents for Selective CO2 Capture and Separation. Acc. Chem. Res. 2023, 56, 3358–3368. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Elhenawy, S.E.M.; Khraisheh, M.; AlMomani, F.; Walker, G. Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2. Catalysts 2020, 10, 1293. [Google Scholar] [CrossRef]
- Goncalves, R.B.; Collados, C.C.; Malliakas, C.D.; Wang, Z.; Thommes, M.; Snurr, R.Q.; Hupp, J.T. Chemically Reversible CO2 Uptake by Dendrimer-Impregnated Metal–Organic Frameworks. Langmuir 2024, 40, 9299–9309. [Google Scholar] [CrossRef] [PubMed]
- El Yazeed, W.S.A.; Mansour, B.N.H.; Ibrahim, A.A.; Ahmed, A.I.; Salama, R.S.; El-Shinawi, H. Activated carbon encapsulated Aluminum metal–organic frameworks as an active and recyclable adsorbent for removal of different dyes and lead from aqueous solution. Inorg. Chem. Commun. 2025, 171, 113558. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Li, S.; Guo, S.; Shen, L.; Zhou, T.; Zhong, H.; Wu, L.; Meng, Q.; Zhang, Y. Oxygen-Vacancy-Enhanced Peroxidase-like Activity of Reduced Co3O4 Nanocomposites for the Colorimetric Detection of H2O2 and Glucose. Inorg. Chem. 2020, 59, 3152–3159. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, S.; Wu, C.; Li, T. Surface-Seal Encapsulation of a Homogeneous Catalyst in a Mesoporous Metal–Organic Framework. J. Am. Chem. Soc. 2022, 144, 685–689. [Google Scholar] [CrossRef]
- Lu, J.; Wang, S.; Ding, C.; Lv, W.; Zeng, Y.; Liu, N.; Wang, H.; Meng, Q.; Liu, Q. Metal organic frameworks derived CoSe2@N-Doped-carbon-nanorods as highly efficient electrocatalysts for oxygen evolution reaction. J. Alloys Compd. 2019, 778, 134–140. [Google Scholar] [CrossRef]
- Bonneau, M.; Lavenn, C.; Zheng, J.-J.; Legrand, A.; Ogawa, T.; Sugimoto, K.; Coudert, F.-X.; Reau, R.; Sakaki, S.; Otake, K.-I.; et al. Tunable acetylene sorption by flexible catenated metal–organic frameworks. Nat. Chem. 2022, 14, 816–822. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Fan, W.; Sun, D. Fe-Based Metal-Organic Frameworks: From Various Synthesis, Diverse Structures to Multifunctional Applications. Chin. J. Chem. 2023, 41, 3772–3791. [Google Scholar] [CrossRef]
- Meng, Q.; Xin, X.; Zhang, L.; Dai, F.; Wang, R.; Sun, D. A multifunctional Eu MOF as a fluorescent pH sensor and exhibiting highly solvent-dependent adsorption and degradation of rhodamine B. J. Mater. Chem. A 2015, 3, 24016–24021. [Google Scholar] [CrossRef]
- Gao, X.; Yan, W.-H.; Hu, B.-Y.; Huang, Y.-X.; Zheng, S.-M. Porous Metal–Organic Frameworks for Light Hydrocarbon Separation. Molecules 2023, 28, 6337. [Google Scholar] [CrossRef]
- Wang, G.D.; Krishna, R.; Li, Y.Z.; Shi, W.J.; Hou, L.; Wang, Y.Y.; Zhu, Z. Boosting Ethane/Ethylene Separation by MOFs through the Amino-Functionalization of Pores. Angew. Chem. Int. Ed. 2022, 61, e202213015. [Google Scholar] [CrossRef]
- Knebel, A.; Caro, J. Metal–organic frameworks and covalent organic frameworks as disruptive membrane materials for energy-efficient gas separation. Nat. Nanotechnol. 2022, 17, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Li, Y.; Yang, X.; Gao, F.; Wang, X.; Kang, Z.; Fan, W.; Sun, D. Metal-organic frameworks for C2H2/CO2 separation: Recent development. Coordin. Chem. Rev. 2023, 482, 215093. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, G.-N.; Lian, X.; Yang, S.-Q.; Hu, T.-L. Customizing Pore System in a Microporous Metal–Organic Framework for Efficient C2H2 Separation from CO2 and C2H4. Molecules 2022, 27, 5929. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Wang, H.; Zhou, M.; Ke, G.; Zhang, L.; Wu, J.; Gao, Z.; Lu, D. A comprehensive transformer-based approach for high-accuracy gas adsorption predictions in metal-organic frameworks. Nat. Commun. 2024, 15, 1904. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Wang, Z.; Gong, Q.; Li, M.; Bo, Y.; Xu, H.; Jiang, G.; Chi, K. Exquisitely Constructing a Robust MOF with Dual Pore Sizes for Efficient CO2 Capture. Molecules 2023, 28, 6276. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, A.; Hong, T.; Jia, X.; Liu, M. Tailored porous structure and CO2 adsorption capacity of Mg-MOF-74 via solvent polarity regulation. Chem. Eng. J. 2023, 476, 146845. [Google Scholar] [CrossRef]
- Leszczyński, M.K.; Justyniak, I.; Gontarczyk, K.; Lewiński, J. Solvent Templating and Structural Dynamics of Fluorinated 2D Cu-Carboxylate MOFs Derived from the Diffusion-Controlled Process. Inorg. Chem. 2020, 59, 4389–4396. [Google Scholar] [CrossRef]
- Heo, C.Y.; Díaz-Ramírez, M.L.; Park, S.H.; Kang, M.; Hong, C.S.; Jeong, N.C. Solvent-Driven Dynamics: Crafting Tailored Transformations of Cu(II)-Based MOFs. ACS Appl. Mater. Interfaces 2024, 16, 9068–9077. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Hu, J.; Wang, L.; Zhang, Y. Efficient C2H2/CO2 and C2H2/C2H2 separations in a novel fluorinated metal–organic framework. Sep. Purif. Technol. 2024, 332, 125777. [Google Scholar] [CrossRef]
- Sun, W.; Jin, Y.; Wu, Y.; Lou, W.; Yuan, Y.; Duttwyler, S.; Wang, L.; Zhang, Y. A new boron cluster anion pillared metal organic framework with ligand inclusion and its selective acetylene capture properties. Inorg. Chem. Front. 2022, 9, 5140–5147. [Google Scholar] [CrossRef]
- Xue, Y.Y.; Bai, X.Y.; Zhang, J.; Wang, Y.; Li, S.N.; Jiang, Y.C.; Hu, M.C.; Zhai, Q.G. Precise Pore Space Partitions Combined with High-Density Hydrogen-Bonding Acceptors within Metal–Organic Frameworks for Highly Efficient Acetylene Storage and Separation. Angew. Chem. Int. Ed. 2021, 60, 10122–10128. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Gu, Y.; Li, F. Progress and potential of metal-organic frameworks (MOFs) for gas storage and separation: A review. J. Environ. Chem. Eng. 2022, 10, 108300. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Chen, C.; Di, Z.; Yuan, D.; Pang, J.; Wei, W.; Wu, M.; Hong, M. An Unprecedented Pillar-Cage Fluorinated Hybrid Porous Framework with Highly Efficient Acetylene Storage and Separation. Angew. Chem. Int. Ed. 2021, 60, 7547–7552. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, K.; Zhai, L.; Wang, Z.; Wang, H.; Zhao, Y.; Wang, J. Constructing multiple sites of metal-organic frameworks for efficient adsorption and selective separation of CO2. Sep. Purif. Technol. 2023, 307, 122725. [Google Scholar] [CrossRef]
- Lv, D.; Shi, R.; Chen, Y.; Chen, Y.; Wu, H.; Zhou, X.; Xi, H.; Li, Z.; Xia, Q. Selective Adsorptive Separation of CO2/CH4 and CO2/N2 by a Water Resistant Zirconium–Porphyrin Metal–Organic Framework. Ind. Eng. Chem. Res. 2018, 57, 12215–12224. [Google Scholar] [CrossRef]
- Tu, S.; Yu, L.; Liu, J.; Lin, D.; Wu, Y.; Li, Z.; Wang, H.; Xia, Q. Efficient CO2 Capture under Humid Conditions on a Novel Amide-Functionalized Fe-soc Metal–Organic Framework. ACS Appl. Mater. Interfaces 2023, 15, 12240–12247. [Google Scholar] [CrossRef]
- Angeli, G.K.; Sartsidou, C.; Vlachaki, S.; Spanopoulos, I.; Tsangarakis, C.; Kourtellaris, A.; Klontzas, E.; Froudakis, G.E.; Tasiopoulos, A.; Trikalitis, P.N. Reticular Chemistry and the Discovery of a New Family of Rare Earth (4, 8)-Connected Metal-Organic Frameworks with csq Topology Based on RE4(μ3-O)2(COO)8 Clusters. ACS Appl. Mater. Interfaces 2017, 9, 44560–44566. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).