A Review of Botany, Phytochemistry, and Biological Activities of Eight Salvia Species Widespread in Kazakhstan
Abstract
:1. Introduction
2. Botany and Distribution of the Genus Salvia in Kazakhstan
3. Extraction Methods
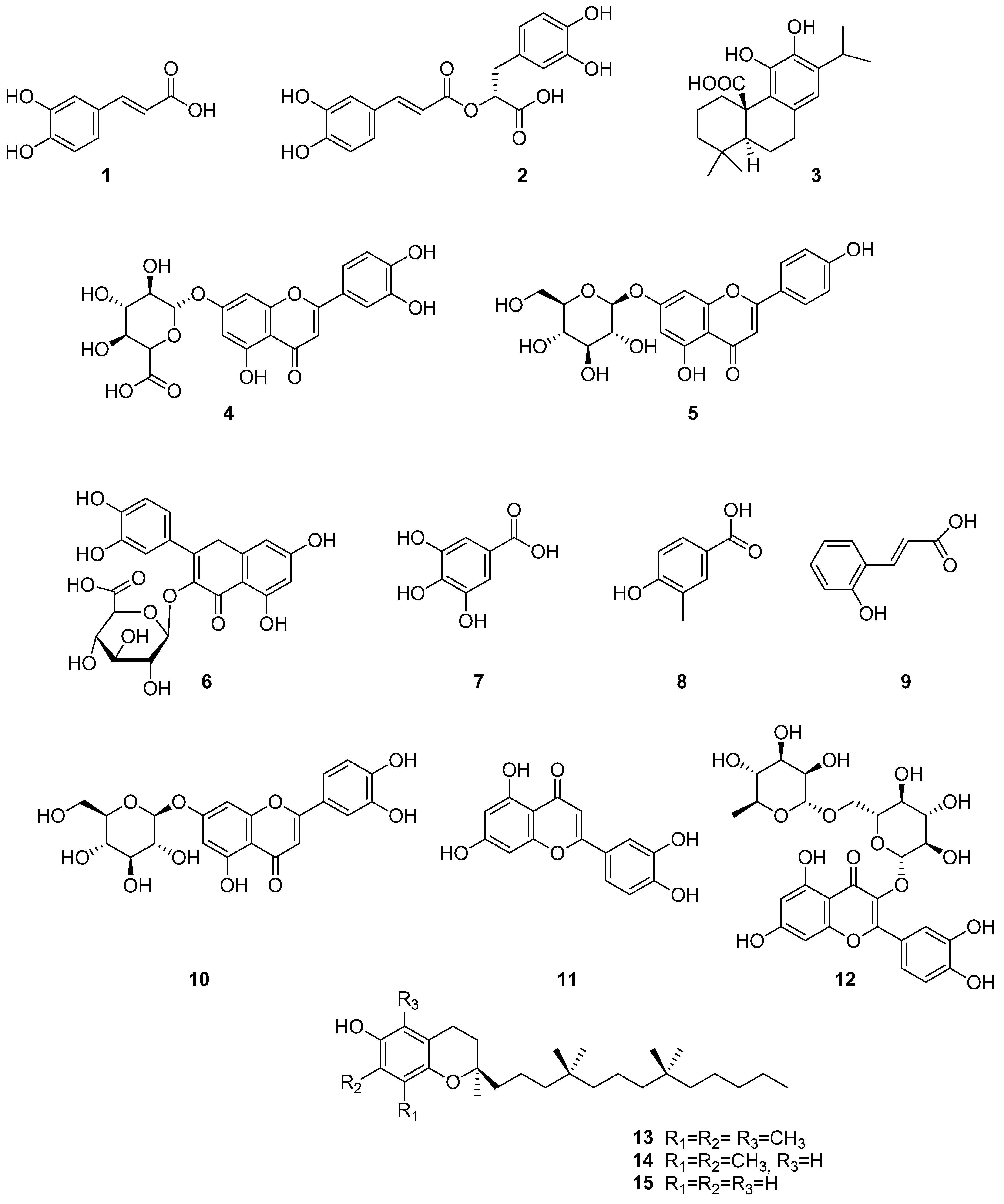
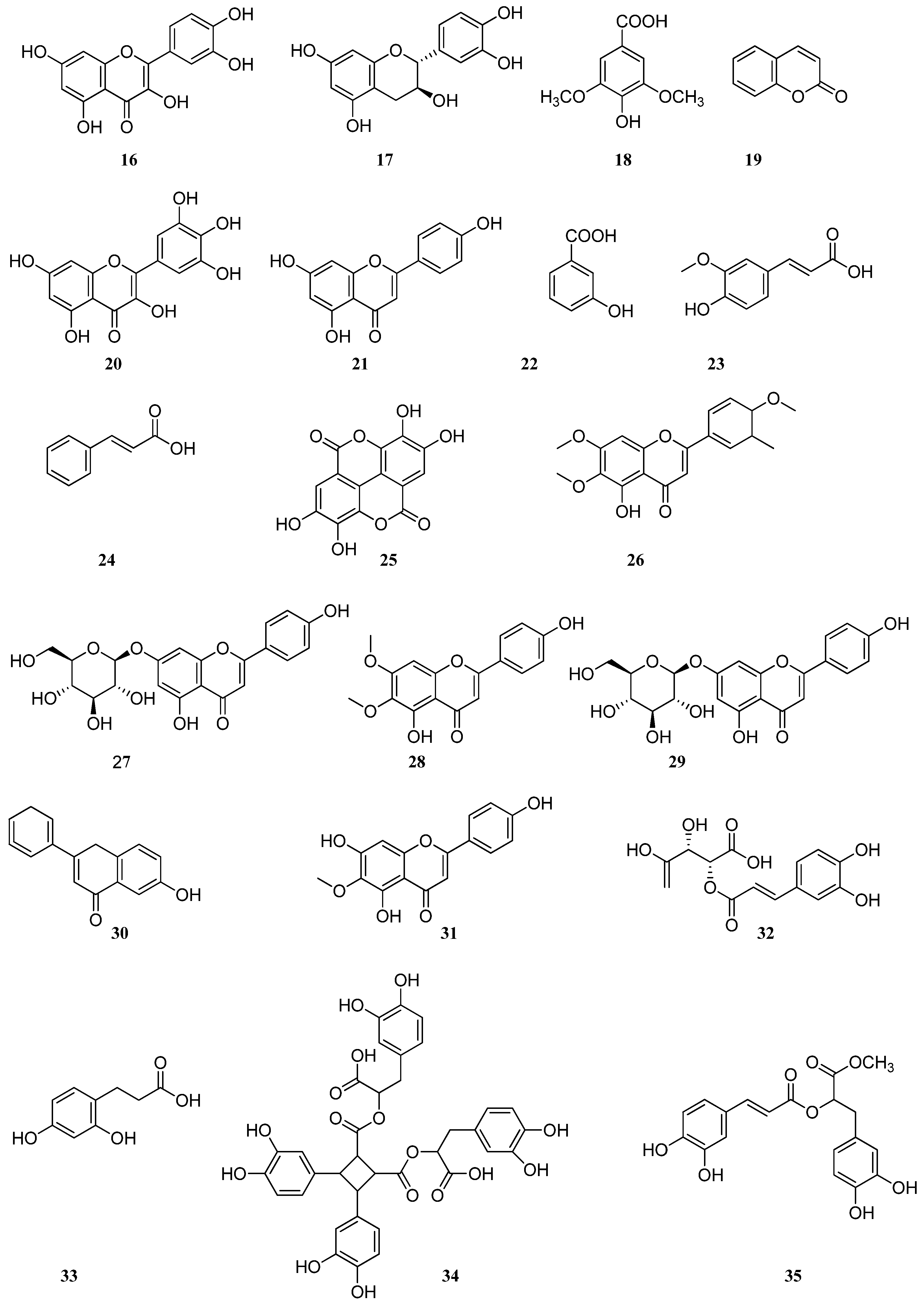
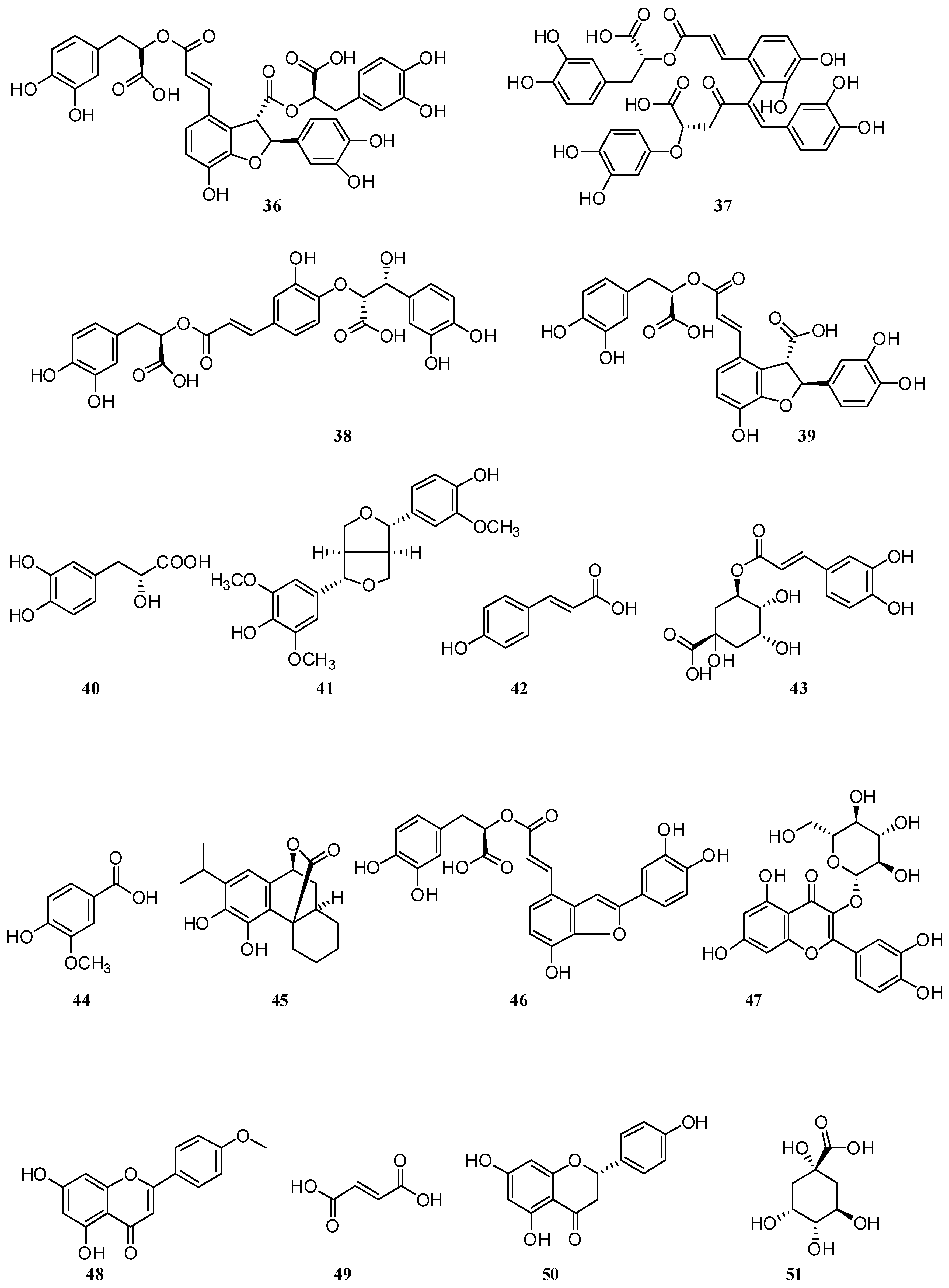
4. Phenolic Compounds and Terpenoids
5. Volatile Organic Compounds (VOCs)
5.1. VOCs from S. virgata Essential Oil
5.2. VOCs from S. macrosiphon Essential Oil
5.3. VOCs from S. sclarea Essential Oil
5.4. VOCs from S. dumetorum Essential Oil
5.5. VOCs from S. verticillata Essential Oil
5.6. VOCs from S. aethiopis Essential Oil
5.7. VOCs from S. deserta Essential Oil
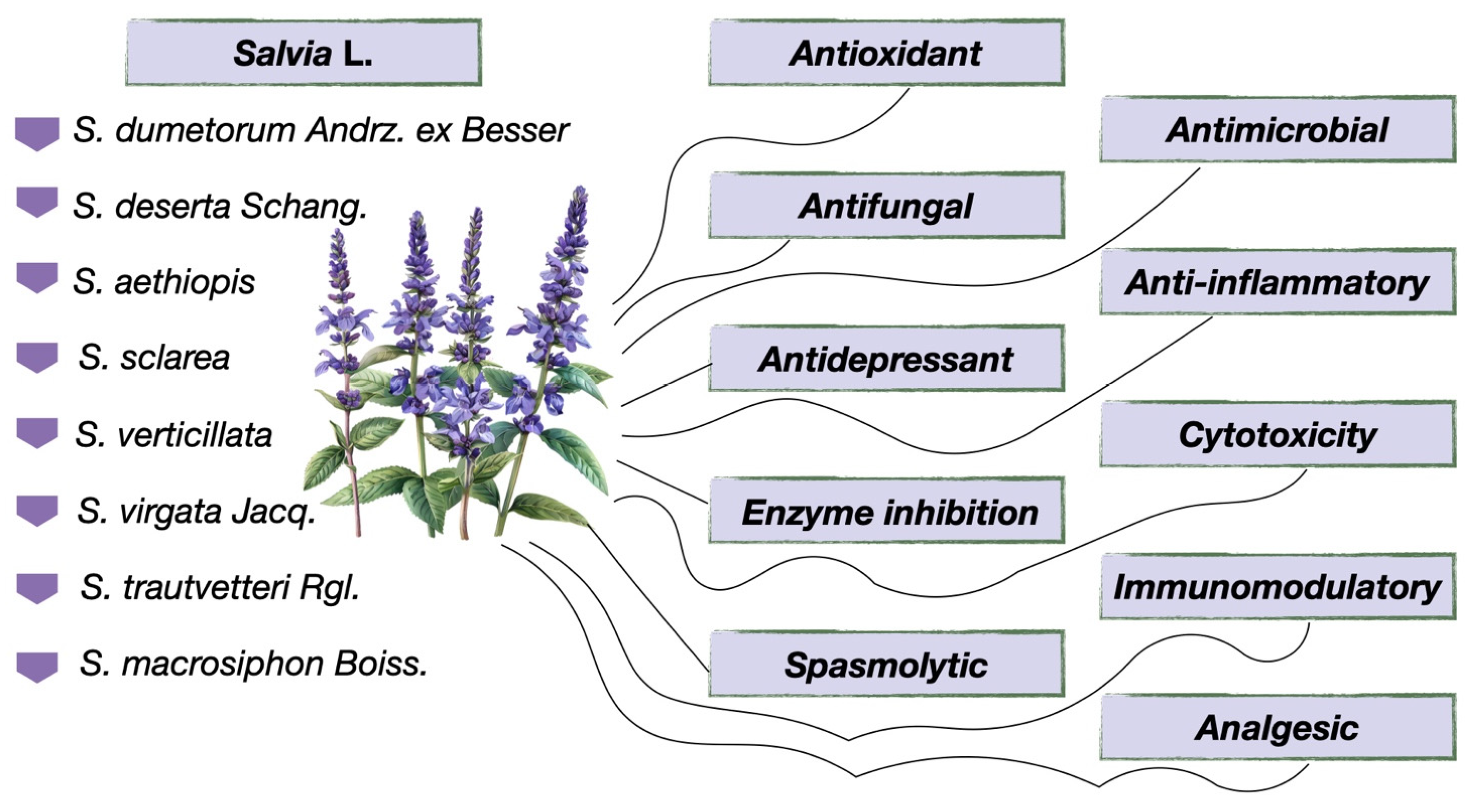
6. Biological Activity
7. Materials and Methods
7.1. Strategy for Literature Search
7.2. Data Mining to Generate the Inventory/Data
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AChE | acetylcholinesterase |
| ADT | arogenate dehydratase |
| BAS | biologically active substances |
| BChE | butyrylcholinesterase |
| BHT | butylated hydroxytoluene |
| CUPRAC | cupric ion reducing antioxidant capacity |
| CBPI | cytokinesis block proliferation index |
| COX | cyclooxygenase |
| DDPH | (1-(2, 6-dimethylphenoxy)-2-(3, 4-dimethoxyphenylethylamino) propane hydrochloride) |
| EO | essential oil |
| FRAP | ferric reducing antioxidant power |
| GA | gallic acid |
| GC–MS | gas chromatography–mass spectrometry |
| HPLC–DAD–ESI–MS | high-performance liquid chromatography coupled with diode array detection and electrospray ionization mass spectrometry |
| HPLC–PDA | high-performance liquid chromatography coupled with photo diode array |
| HPTLC | high-performance thin-layer chromatography |
| QTOF–MS | quadrupole time-of-flight mass spectrometry |
| LPO | lipid peroxidation |
| LPS | lipopolysaccharide |
| LT | luteolin |
| MAE | microwave-assisted extraction |
| MAO-A | monoamine oxidase A |
| MBC | minimum bactericidal concentration |
| MDA | 3,4-methylenedioxyamphetamine |
| MIC | minimum inhibitory concentration |
| MMC | mitomycin C |
| MPO | myeloperoxidase |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) |
| NADES | natural deep eutectic solvent |
| QE | quercetin |
| RA | rosmarinic acid |
| RU | rutin |
| SFE | supercritical fluid extraction |
| TFC | total flavonoid content |
| TNF | tumor necrosis factor |
| TPC | total phenolic content |
| TRP | tryptophan |
| UPLC–Q/TOF | ultra-high performance liquid chromatography with quadrupole time-of-flight |
| VOC | volatile organic compound |
| ZOI | zone of inhibition |
References
- Kew. Plants of the World Online (POWO). Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30000096-2 (accessed on 15 March 2024).
- Giannoulis, K.D.; Skoufogianni, E.; Bartzialis, D.; Solomou, A.D.; Danalatos, N.G. Growth and productivity of Salvia officinalis L. under Mediterranean climatic conditions depends on biofertilizer, nitrogen fertilization, and sowing density. Ind. Crops Prod. 2021, 160, 113136. [Google Scholar] [CrossRef]
- Flora of Kazakhstan; Science: Almaty, Kazakhstan, 1964; Volume 7, pp. 440–442. (In Russian)
- Pivovarova, N.S.; Shebitchenko, T.S.; Abrosimova, O.N. Obtaining Callus Culture of Sage Medicinal (Salvia officinalis L.) and its Characteristics. Drug Dev. Regist. 2022, 11, 40–46. [Google Scholar] [CrossRef]
- The State Pharmacopoeia of the Republic of Kazakhstan; Zhybel Zholy: Almaty, Kazakhstan, 2014; Volume 3, pp. 58–59. (In Russian)
- European Pharmacopoeia. Available online: www.pheur.org (accessed on 22 July 2024).
- Russian Pharmacopoeia; M. Medcine: Moscow, Russia, 1990; Volume 11. (In Russian)
- State Pharmacopoeia of Ukraine. The State Pharmacopoeia of Ukraine; Ukrainian Scientific Pharmacopoeial Centre for Quality of Medicines “Pharmacom”: Kharkiv, Ukraine, 2001; Volume 1. (In Ukrainian) [Google Scholar]
- British Pharmacopoeia. Available online: www.pharmacopoeia.co.uk (accessed on 26 July 2018).
- United States Pharmacopeia (USP). Available online: www.usp.org (accessed on 1 November 2022).
- Ilić, Z.S.; Kevrešan, Ž.; Šunić, L.; Stanojević, L.; Milenković, L.; Stanojević, J.; Milenković, A.; Cvetković, D. Chemical Profiling and Antioxidant Activity of Wild and Cultivated Sage (Salvia officinalis L.) Essential Oil. Horticulturae 2023, 9, 624. [Google Scholar] [CrossRef]
- Mocan, A.; Babotă, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical Constituents and Biologic Activities of Sage Species: A Comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (schur ex griseb. & schenk) schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef] [PubMed]
- Levaya, Y.K.; Atazhanova, G.A. Chemical composition and pharmacological activity of some types of sage Modern concepts of scientific research. In Proceedings of the Collection of Scientific Works of the 60th International Scientific Conference of the Eurasian Scientific Association, Moscow, Russia, 7–8 February 2020; ENO: Moscow, Russia, 2020; 420p. (In Russian) [Google Scholar]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Esmaeili, G.; Fatemi, H.; Baghani avval, M.; Azizi, M.; Arouiee, H.; Vaezi, J.; Fujii, Y. Diversity of Chemical Composition and Morphological Traits of Eight Iranian Wild Salvia Species during the First Step of Domestication. Agronomy 2022, 12, 2455. [Google Scholar] [CrossRef]
- Koşar, M.; Göger, F.; Can Başer, K.H. In vitro antioxidant properties and phenolic composition of Salvia virgata Jacq. from Turkey. J. Agric. Food Chem. 2008, 56, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- İnan, Y.; Kurt-Celep, I.; Akyüz, S.; Barak, T.H.; Celep, E.; Yesilada, E. An investigation on the enzyme inhibitory activities, phenolic profile and antioxidant potentials of Salvia virgata Jacq. S. Afr. J. Bot. 2021, 143, 350–358. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Ilgün, S.; Koşar, M. Antioxidant Properties and Phenolic Composition of Salvia. Turk. J. Pharm. Sci. 2016, 13, 201–212. [Google Scholar] [CrossRef]
- Egul, M.; Atas, M.; Bal, H.; Ucar, E.; Eruygur, N.; Senkal, B.C.; Uskutoglu, T. Pharmacological and biological features of ethanol extract of Salvia virgata Jacq. Med. Sci. 2021, 10, 1103–1109. [Google Scholar] [CrossRef]
- Firuzi, O.; Javidnia, K.; Gholami, M.; Soltani, M.; Miri, R. Antioxidant activity and total phenolic content of 24 Lamiaceae speciesgrowing in Iran. Nat. Prod. Commun. 2010, 5, 193–199. [Google Scholar] [CrossRef]
- Mahdieh, E.; Mohammad, R.S.A.; Mohsen, A.; Tahmineh, A.; Maliheh, S.; Elahe, K.R.; Khanavi, M. Biological Activities of the Essential Oil and Total Extract of Salvia macrosiphon Boiss. J. Basic Clin. Pharm. 2017, 8, 82–86. [Google Scholar]
- Kharazian, N. Flavonoid Constituents in Some Species of Salvia L. (Lamiaceae) in Iran. J. Sci. Islam. Repub. Iran 2014, 25, 219–227. [Google Scholar]
- Janicsák, G.; Zupkó, I.; Máthé, I.; Hohmann, J. Comparative study of the antioxidant activities of eleven Salvia species. Nat. Prod. Commun. 2010, 5, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Asadollahi, M.; Eslami, S.; Jassbi, A.R. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran. J. Pharm. Res. IJPR 2013, 12, 801–810. [Google Scholar] [PubMed]
- Luca, S.V.; Skalicka-Woźniak, K.; Mihai, C.-T.; Gradinaru, A.C.; Mandici, A.; Ciocarlan, N.; Miron, A.; Aprotosoaie, A.C. Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe. Antioxidants 2023, 12, 1514. [Google Scholar] [CrossRef]
- Quradha, M.M.; Duru, M.E.; Kucukaydin, S.; Tamfu, A.N.; Iqbal, M.; Bibi, H.; Khan, R.; Ceylan, O. Comparative assessment of phenolic composition profile and biological activities of green extract and conventional extracts of Salvia sclarea. Sci. Rep. 2024, 14, 1885. [Google Scholar] [CrossRef] [PubMed]
- Svydenko, L.; Vergun, O.; Ivanišová, E.; Korablova, O.; Šramková, K.F. Polyphenol Compounds and Antioxidant Activity of Salvia officinalis L. and Salvia sclarea L. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 6, 139–148. [Google Scholar] [CrossRef]
- Hudz, N.; Yezerska, O.; Shanaida, M.; Horčinová Sedláčková, V.; Wieczorek, P.P. Application of the Folin-Ciocalteu method to the evaluation of Salvia sclarea extracts. Pharmacia 2019, 66, 209–215. [Google Scholar] [CrossRef]
- Randjelović, M.; Branković, S.; Jovanović, M.; Kitić, N.; Živanović, S.; Mihajilov-Krstev, T.; Miladinović, B.; Milutinović, M.; Kitić, D. An In Vitro and In Silico Characterization of Salvia sclarea L. Methanolic Extracts as Spasmolytic Agents. Pharmaceutics 2023, 15, 1376. [Google Scholar] [CrossRef] [PubMed]
- Zhussupova, A.; Zhumaliyeva, G.; Ogay, V.; Issabekova, A.; Ross, S.A.; Zhusupova, G.E. Immunomodulatory Effects of Plant Extracts from Salvia deserta Schang. and Salvia sclarea L. Plants 2022, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.; Raal, A.; Kovaleva, A.; Myha, M.; Ilina, T.; Borodina, N.; Komissarenko, A. The Phytochemical and Chemotaxonomic Study of Salvia spp. Growing in Ukraine. J. Appl. Biol. Biotechnol. 2020, 8, 29–36. [Google Scholar]
- Moshari-Nasirkandi, A.; Alirezalu, A.; Alipour, H.; Amato, J. Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling. Sci. Rep. 2023, 13, 16987. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, N.; Radjabian, T. Integrative effects of phytohormones in the phenolic acids production in Salvia verticillata L. under multi-walled carbon nanotubes and methyl jasmonate elicitation. BMC Plant Biol. 2024, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, L.S.; Efthimiou, I.; Giova, L.; Manoli, C.; Sinou, P.S.; Zografidis, A.; Lamari, F.N.; Vlastos, D.; Dailianis, S.; Antonopoulou, M. Phytochemical Profile and Evaluation of the Antioxidant, Cyto-Genotoxic, and Antigenotoxic Potential of Salvia verticillata Hydromethanolic Extract. Plants 2024, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Yumrutas, O.; Sokmen, A.; Ozturk, N. Determination of In Vitro Antioxidant Activities and Phenolic Compounds of Different Extracts of Salvia verticillata ssp. verticillata and spp. amasiaca from Turkey’s Flora. J. Appl. Pharm. Sci. 2011, 1, 43–46. [Google Scholar]
- Katanić Stanković, J.S.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae)—A plant rich in rosmarinic acid. Ind. Crops Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Caylak, E. Determination of antioxidant and anti-inflammatory properties of Salvia aethiopis/sclarea and synthesized silver nanoparticles. Ann. Med. Res. 2024, 31, 169–176. [Google Scholar] [CrossRef]
- Tohma, H.; Köksal, E.; Kılıç, Ö.; Alan, Y.; Yılmaz, M.A.; Gülçin, İ.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef]
- Kostić, M.; Miladinović, B.; Milutinović, M.; Branković, S.; Živanović, S.; Zlatković, B.; Kitić, D. Rosmarinic and caffeic acid content and antioxidant potential of the Salvia aethiopis L. extracts. Acta Med. Median. 2017, 56, 121–128. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Peng, L.; Liang, Z.; Yan, X.; Zhu, Y.; Liu, Y. Comparison of essential oil composition and phenolic acid content of selected Salvia species measured by GC–MS and HPLC methods. Ind. Crops Prod. 2015, 69, 329–334. [Google Scholar] [CrossRef]
- Levaya, Y.K.; Zholdasbayev, M.E.; Atazhanova, G.A.; Akhmetova, S.B. Evaluation of Antibacterial Activity of Salvia stepposa Extracts Isolated Using Microwave Extraction, Growing Wild in Kazakhstan. Trends Sci. 2022, 19, 3217. [Google Scholar] [CrossRef]
- Moussa, H.; Dahmoune, F.; Mróz, M.; Remini, H.; Kadri, N.; Hamid, S.; Kusznierewicz, B. Efficient optimization approaches for microwave assisted extraction of high-quality antioxidant compounds from Salvia officinalis L.: UHPLC-HRMS differential analysis of phenolic profiles obtained by ultrasound and microwave extraction. Sustain. Chem. Pharm. 2023, 35, 101194. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Elez Garofulić, I.; Jukić, M.; Penić, M.; Dent, M. The Influence of Microwave-Assisted Extraction on theIsolation of Sage (Salvia officinalis L.) Polyphenols. Food Technol. Biotechnol. 2012, 50, 377–383. [Google Scholar]
- López-Salazar, H.; Camacho-Díaz, B.H.; Arenas Ocampo, M.L.; Jiménez-Aparicio, A.R. Microwave-assisted Extraction of Functional Compounds from Plants: A Review. BioResources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Ürgeová, E.; Uváčková, Ľ.; Vaneková, M.; Maliar, T. Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species. Plants 2023, 12, 1325. [Google Scholar] [CrossRef] [PubMed]
- Belokurov, S.S.; Narkevich, I.A.; Flisyuk, E.V.; Kaukhova, I.E.; Aroyan, M.V. Modern extraction methods for medicinal plant rawmaterial. Pharm. Chem. J. 2019, 53, 559–563. [Google Scholar] [CrossRef]
- Francik, S.; Francik, R.; Sadowska, U.; Bystrowska, B.; Zawiślak, A.; Knapczyk, A.; Nzeyimana, A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials 2020, 13, 5811. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, W.; Tian, S. Qualitative and Quantitative Analyses of Four Active Components in Different Organs of Salvia deserta Schang by High-Performance Thin-Layer Chromatography. J. Chromatogr. Sci. 2023, 61, 225–233. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yu, Y.; Li, S.M.; Liu, W.; Li, W.; Morris-Natschke, S.L.; Goto, M.; Lee, K.H.; Huang, X.F. Salvisertin A, a New Hexacyclic Triterpenoid, and Other Bioactive Terpenes from Salvia deserta Root. Chem. Biodivers. 2018, 15, e1800019. [Google Scholar] [CrossRef] [PubMed]
- Golparvar, A.R.; Hadipanah, A.; Gheisari, M.M.; Naderi, D.; Rahmaniyan, S.; Khorrami, M. Chemical composition and antimicrobial activity of essential oil of Salvia officinalis L. and Salvia virgata Jacq. J. Herb. Drugs 2017, 08, 71–78. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Changizi, S.; Vosoughi, M. Essential Oil Composition of Salvia virgata Jacq. from Iran. J. Essent. Oil Bear. Plants 2005, 8, 330–333. [Google Scholar] [CrossRef]
- Cosge Senkal, B.; Uskutoglu, T.; Cesur, C.; Özavci, V.; Dogan, H. Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. grown in culture conditions. Turk. J. Agric. For. 2019, 43, 395–404. [Google Scholar] [CrossRef]
- Yilar, M.; Kadioglu, I.; Telci, I. Essential Oils’ Compositions of Salvia virgata Jacq. and Salvia candidissima subsp. candidissima Vahl. Growing in Natural Habitats of Tokat Province. Turk. J. Weed. Sci. 2017, 20, 70–77, (In Turkish with English Abstract). [Google Scholar]
- Karayel, H.B. Examination of the Change in the Components of Volatile Oil of Salvia virgata Jacq. Plant Grown in Different Locations. J. Multidiscip. Eng. Sci. Technol. JMEST 2018, 5, 9248–9251. [Google Scholar]
- Tomou, E.-M.; Fraskou, P.; Dimakopoulou, K.; Dariotis, E.; Krigas, N.; Skaltsa, H. Chemometric Analysis Evidencing the Variability in the Composition of Essential Oils in 10 Salvia Species from Different Taxonomic Sections or Phylogenetic Clades. Molecules 2024, 29, 1547. [Google Scholar] [CrossRef]
- Dolya, V.S.; Trzhtsynskyi, S.D.; Mozul, V.I.; Tretiak, N.I. Peculiarities of chemical composition of species genus Salvia L. Curr. Issues Pharm. Med. Sci. Pract. 2013, 83–85. (In Ukrainian) [Google Scholar]
- Gad, H.A.; Mamadalieva, R.Z.; Khalil, N.; Zengin, G.; Najar, B.; Khojimatov, O.K.; Al Musayeib, N.M.; Ashour, M.L.; Mamadalieva, N.Z. GC-MS Chemical Profiling, Biological Investigation of Three Salvia Species Growing in Uzbekistan. Molecules 2022, 27, 5365. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, R.; Ramezani, S.; Martignetti, A.; Mari, A.; Piacente, S.; De Giulio, B. Determination of volatile organic compounds in the dried leaves of Salvia species by solid-phase microextraction coupled to gas chromatography mass spectrometry. Nat. Prod. Res. 2015, 30, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Salimpour, F.; Mazooji, A.; Darzikolaei, S.A. Chemotaxonomy of six Salvia species using essential oil composition markers. J. Med. Plants Res. 2011, 5, 1795–1805. [Google Scholar]
- Kariminik, A.; Moradalizadeh, M.; Foroughi, M.M.; Tebyanian, H.; Motaghi, M.M. Chemical composition and antibacterial activity of the essential oils extracted from 4 medicinal plants (Labiatae) of Kerman, Iran. J. Appl. Biotechnol. Rep. 2019, 6, 172–179. [Google Scholar] [CrossRef]
- Nouripour-Sisakht, S.; Diba, A.; Razmjoue, D.; Sadeghi Mansourkhani, H.; Zanganeh, P.; Salahi, M.; Gharaghani, M. Comparing the Anticandidal Activities of Salvia macrosiphon essential oil and Fluconazole. J. Clin. Care Ski. 2024, 5, 5–9. [Google Scholar] [CrossRef]
- Šulniūtė, V.; Baranauskienė, R.; Ragažinskienė, O.; Venskutonis, P.R. Comparison of composition of volatile compounds in ten Salvia species isolated by different methods. Flavour Fragr. J. 2017, 32, 254–264. [Google Scholar] [CrossRef]
- Rzepa, J.; Wojtal, L.; Staszek, D.; Grygierczyk, G.; Labe, K.; Hajnos, M.; Kowalska, T.; Waksmundzka-Hajnos, M. Fingerprint of selected Salvia species by HS-GC-MS analysis of their volatile fraction. J. Chromatogr. Sci. 2009, 47, 575–580. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.S.; Setzer, W.N. The essential oil of Salvia sclarea L. from Tajikistan. Rec. Nat. Prod. 2012, 6, 75–79. [Google Scholar]
- Ben Akacha, B.; Ben Hsouna, A.; Generalić Mekinić, I.; Ben Belgacem, A.; Ben Saad, R.; Mnif, W.; Kačániová, M.; Garzoli, S. Salvia officinalis L. and Salvia sclarea Essential Oils: Chemical Composition, Biological Activities and Preservative Effects against Listeria monocytogenes Inoculated into Minced Beef Meat. Plants 2023, 12, 3385. [Google Scholar] [CrossRef]
- Tasheva, S.; Gandova, V.; Prodanova-Stefanova, V.; Marinova, K.; Dimov, M.; Dobreva, K.; Stoyanova, A. Investigation of the thermodynamic and thermal properties of clary sage (Salvia sclarea L.) essential oil and its main components. E3S Web Conf. 2021, 286, 02003. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films. Gels 2023, 9, 233. [Google Scholar] [CrossRef]
- Korkotadze, T.; Berashvili, D.; Getia, M.; Moshiashvili, G.; Jokhadze, M.; Legault, J.; Mshvildadze, V. Chemical and Biological Characterization of Essential Oil from the Aerial Parts of Salvia sclarea L. Growing in Georgia. Bull. Georg. Natl. Acad. Sci. 2023, 17, 113–117. [Google Scholar]
- Levaya, Y.K.; Atazhanova, G.A.; Kacergius, T.; Ivasenko, S.A.; Marchenko, A.B.; Ishmuratova, M.Y.; Smagulov, M.K. Salvia dumetorum essential oil: GC-MS analysis, antibacterial activity and effect on the formation of Streptococcus mutans biofilms. Nat. Prod. Res. 2023, 38, 3555–3561. [Google Scholar] [CrossRef]
- Giuliani, C.; Ascrizzi, R.; Lupi, D.; Tassera, G.; Santagostini, L.; Giovanetti, M.; Flamini, G.; Fico, G. Salvia verticillata: Linking glandular trichomes, volatiles and pollinators. Phytochemistry 2018, 155, 53–60. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Aytaç, Z.; Can, K.H. The chemical composition of Salvia verticillata L. subsp. verticillata from Turkey. Nat. Volatiles Essent. Oils 2017, 4, 18–28. [Google Scholar]
- Semenchenko, O.M.; Tsurkan, O.O.; Korableva, O.A.; Burmaka, O.V. Determination of volatile compounds of essential oils of different species of genus of Salvia by chromatography-mass spectrometric method. Farm. Zhurnal 2019, 1, 62–65. [Google Scholar]
- Kostić, E.; Kitić, D.; Vujović, M.; Marković, M.; Pavlović, A.; Stojanović, G. A chemometric approach to the headspace sampled volatiles of selected Salvia species from Southeastern Serbia. Bot. Serb. 2022, 46, 285–294. [Google Scholar] [CrossRef]
- Khosravi Dehaghi, N.; Ostad, S.N.; Maafi, N.; Pedram, S.; Ajani, Y.; Hadjiakhoondi, A.; Khanavi, M. Cytotoxic Activity of the Essential Oil of Salvia verticillata L. Res. J. Pharmacogn. 2014, 1, 27–33. [Google Scholar]
- Karayel, H.B.; Akçura, M. Examination of the changes in components of the volatile oil from Abyssinian sage, Musk sage and Medical sage [Salvia aethiopis L., Salvia sclarea L. and Salvia officinalis L. (hybrid)] growing in different locations. Grasas Aceites 2019, 70, e319. [Google Scholar] [CrossRef]
- Damyanova, S.; Stoyanova, A.; Bozov, P. Chemical composition of essential oil from Salvia aethiopis L. from Bulgaria. Sci. Work. NUFT 2016, 5, 242–247. [Google Scholar]
- White, M.; Srivedavyasasri, R.; Cantrell, C.; Ross, S. Isolation and Biological Analysis of Aerial Parts of Salvia aethiopis. Univ. Miss. Undergrad. Res. J. 2017, 2, 11. [Google Scholar]
- Alizadeh, A. Essential Oil Constituents, Antioxidant and Antimicrobial Activities of Salvia virgata Jacq. from Iran. J. Essent. Oil Bear. Plants 2013, 16, 172–182. [Google Scholar] [CrossRef]
- Gohari, A.R.; Hajimehdipoor, H.; Saeidnia, S.; Ajani, Y.; Hadjiakhoondi, A. Antioxidant Activity of some Medicinal Species using FRAP Assay. J. Med. Plants 2011, 10, 54–60. [Google Scholar]
- Balaei-Kahnamoei, M.; Eftekhari, M.; Reza Shams Ardekani, M.; Akbarzadeh, T.; Saeedi, M.; Jamalifar, H.; Safavi, M.; Sam, S.; Zhalehjoo, N.; Khanavi, M. Phytochemical constituents and biological activities of Salvia macrosiphon Boiss. BMC Chem. 2021, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, A.; Jamshidzadeh, A.; Ahmadi, S.; Sohrabpour, M.; Zarshenas, M.M. Salvia macrosiphon seeds and seed oil: Pharmacognostic, anti-inflammatory and analgesic properties. Res. J. Pharmacogn. 2016, 3, 27–37. [Google Scholar]
- Sarkoohi, P.; Fathalipour, M.; Ghasemi, F.; Javidnia, K.; Emamghoreishi, M. Antidepressant Effects of the Aqueous and Hydroalcoholic Extracts of Salvia mirzayanii and Salvia macrosiphon in Male Mice. Shiraz E-Med. J. 2020, 21, e91276. [Google Scholar] [CrossRef]
- Najafi, S.; Mir, N.; Shafeghat, M. Antioxidant and Antibacterial Activities of Six Medicinally Important Species of the Genus Salvia from North East of Iran. J. Genet. Resour. 2016, 2, 41–47. [Google Scholar] [CrossRef]
- Abbas, A.; Raza Naqvi, S.A.; Anjum, F.; Noureen, A.; Rasool, N. Antioxidant, antibacterial and antifungal potential study of Salvia macrosiphon Boiss. stem extracts. Pak. J. Pharm. Sci. 2021, 34, 1903–1907. [Google Scholar]
- Llurba-Montesino, N.; Schmidt, T.J. Salvia Species as Sources of Natural Products with Antiprotozoal Activity. Int. J. Mol. Sci. 2018, 19, 264. [Google Scholar] [CrossRef]
- Yücel, Y.Y.; Nath, E.O. Determination of Antioxidant Activity of Salvia sclarea L. and Its Inhibitory Effects on Acetylcholinesterase and Monoamine Oxidase A. Fabad J. Pharm. Sci. 2023, 48, 255–264. [Google Scholar] [CrossRef]
- Janicsák, G.; Zupkó, I.; Nikolova, M.T.; Forgo, P.; Vasas, A.; Máthé, I.; Blunden, G.; Hohmann, J. Bioactivity-guided Study of Antiproliferative Activities of Salvia Extracts. Nat. Prod. Commun. 2011, 6, 575–579. [Google Scholar] [PubMed]
- Levaya, Y.K.; Zholdasbaev, M.E.; Atazhanova, G.A.; Akhmetova, S.B.; Ishmuratova, M.Y. Antibacterial activity of ultrasonic extracts of Salvia stepposa growing in Kazakhstan. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2021, 1, 45–49. [Google Scholar] [CrossRef]
- Barjaktarevic, A.; Cirovic, T.; Arsenijevic, N.; Volarevic, V.; Markovic, B.S.; Mitic, V.; Jovanovic, V.S.; Cupara, S. Antioxidant, Antimicrobial and Cytotoxic Activities of Salvia verticillata L. Extracts. Indian J. Pharm. Sci. 2021, 83, 1280–1287. [Google Scholar] [CrossRef]
- Mervić, M.; Bival Štefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants 2022, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi-Ghehi, H.; Sonboli, A.; Ebrahimi, S.N.; Esmaeili, M.A.; Mirjalili, M.H. Triterpenic Acid Content and Cytotoxicity of Some Salvia Species from Iran. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Dulger, G.; Dulger, B. Antifungal activity of Salvia verticillata subsp. verticillata against fungal pathogens. Düzce Üniv. Sağlık Bilim. Enst. Derg. 2021, 11, 305–307. [Google Scholar] [CrossRef]
- Coisin, M.; Burzo, I.; Ştefan, M.; Rosenhech, E.; Zamfirache, M.M. Chemical composition and antibacterial activity of essential oils of three Salvia species, widespread in eastern romania. Analele Stiint. Univ. Alexandru Ioan Cuza Iasi 2012, 58, 51–58. [Google Scholar]
- Yashar, A.; Barbaros, S.; Yücel, Y.Y.; Alkan, M.; Polatoğlu, K. AChE-inhibitory properties and the chemical composition of Salvia aethiopis L. essential oil. Facta Univ. Ser. Phys. Chem. Technol. 2018, 16, 171. [Google Scholar]
- Kasimu, R.; Wang, X.; Wang, X.; Hu, J.; Wang, X.; Mu, Y. Antithrombotic effects and related mechanisms of Salvia deserta Schang root EtOAc extracts. Sci. Rep. 2018, 8, 17753. [Google Scholar] [CrossRef] [PubMed]
- Búfalo, J.; Cantrell, C.L.; Jacob, M.R.; Schrader, K.K.; Tekwani, B.L.; Kustova, T.S.; Ali, A.; Boaro, C.S. Antimicrobial and Antileishmanial Activities of Diterpenoids Isolated from the Roots of Salvia deserta. Planta Med. 2016, 82, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yuan, X.; Jiao, S.S.; He, X.P.; Hu, H.; Kang, J.J.; Luo, S.H.; Liu, Y.; Guo, K.; Li, S.H. Clerodane diterpenoids from the Uygur medicine Salvia deserta with immunosuppressive activity. Phytochemistry 2023, 214, 113823. [Google Scholar] [CrossRef] [PubMed]
- Marčetić, M.; Arsenijević, J. Antioxidant activity of plant secondary metabolites. Arh. Farm. 2023, 73, 264–277. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T. Fast Antioxidant Reaction of Polyphenols and Their Metabolites. Antioxidants 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Mittova, V.; Tsetskhladze, Z.R.; Palumbo, R.; Pastore, R.; Roviello, G.N. Georgian Medicinal Plants as Rich Natural Sources of Antioxidant Derivatives: A Review on the Current Knowledge and Future Perspectives. Curr. Med. Chem. 2024, 31, 4407–4424. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.; Mittova, V.; Motsonelidze, C.; Vakhania, M.; Vicidomini, C.; Roviello, G.N. Antimicrobial Metabolites of Caucasian Medicinal Plants as Alternatives to Antibiotics. Antibiotics 2024, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019, 7010467. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, L.D.S.; Dias, A.D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Steward, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
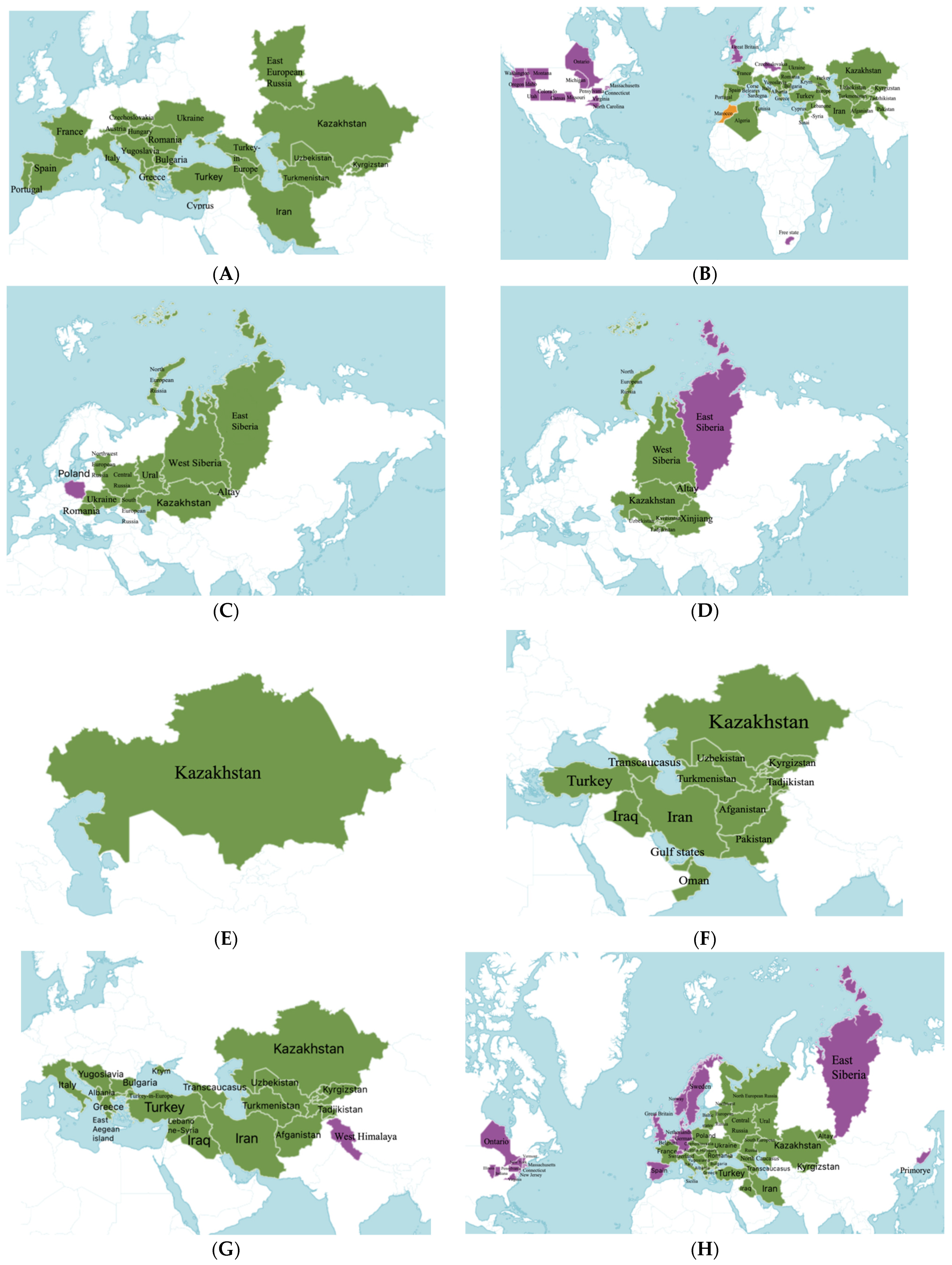


| Morphological Characteristics | S. aethiopis | S. sclarea | S. dumetorum | S. deserta | S. trautvetteri | S. macrosiphon | S. virgata | S. verticillata |
|---|---|---|---|---|---|---|---|---|
| Stem | Short, pyramidal branched, ribbed, densely pubescent. | Longer than inflorescence, pubescent with curly hairs. | Short and rarely pubescent at the base with a thick rosette of basal leaves. | In the upper part, longer than the inflorescence, densely pubescent. | Covered with glandular and simple spaced hairs. | Densely hairy, emerges from woody root. | Shorter than inflorescences, leafy, pubescent below with multicellular hairs. | Numerous, densely pubescent multicellular hairs. |
| Leaves | Basal ovate, oblong heart-shaped, sharp or blunt, town-dentate at the edges, sometimes lobed. Stem sessile, oblong-ovate, sharp- or blunt-toothed. | Basal ones are smaller, curl early and dry out. Stem ovate or ovate- oblong, sharp or blunt, wrinkled, long-leaved. | Basal numerous, oblong or barely ovate, slightly heart-shaped at the base, blunt. Stem often larger than basal, short tiled or sessile. | Basal leaves are small, drying early. Stem significantly smaller, ovate or at the apex long thinly drawn, sessile or very short-leaved. | Basal pinnately dissected, oblong or oblong-elliptical in outline, 5–7 cm long, 3–4 cm wide. Stem reduced, short-leaved, upper sessile. | Basal oblong-ovate, twofold and sharply gnawed-serrated along the edge, softly pubescent along the veins on both sides. | Basal elliptical, oblong or ovate-oblong, blunt or rounded along the edge of the town, twofold town. Stem lanceolate, sessile, almost lobed along the edge, pubescent. | Basal on petioles of equal plates, stem heart-ovate, 4–13 cm long, 3–10 cm wide, sharp, the edges of the leaf blade are town-like. |
| Inflorescence | Pyramidal panicle, branches with 4–6 close, 6–10-flowered false whorls. | Paniculate branched, with 2–6 floral false whorls. | Simple or with one or two pairs of lower branches, with 5–10 false 4–6-flowered whorls. | With one or two pairs of simple branches, with 20–25 false 4–6-flowered whorls. | A simple or branched brush, glandularly pubescent pedicels, 1–1.5 cm long, 2–3 in whorls. | Spreading panicle, with 3–6 color whorls spaced. | Long, with 2–3 pairs of protruding, long rod-shaped branches, with 6-flowered false whorls. | Simple or with one or two pairs of long branches, of 20–40-flowered false whorls. |
| Calyx | 10–16 mm long, tubular-bell-shaped, two-lipped, with five well-developed acutely pointed teeth. | 10–12 mm long. | 7 mm long, upper lip shorter than lower, semicircular, lower— two-toothed. | 7 mm long, upper lip shorter than lower, rounded, lower—two-toothed. | Two-lipped, bell-shaped, green, sometimes painted in purple tones, three-dentate. | 15–20 mm long, narrow-tube with protruding veins. | 8–9 mm long, upper lip shorter than lower, with three short close teeth, lower lip three-dentate. | Tubular, often lilac. |
| Corolla | White, 12–22 mm long. | Pinkish, white or lilac. | Dark blue, 15–18 mm long. | Dark purple, 10–16 mm long. | White, occasionally bluish, 30–40 mm long. | White, 20–25 mm long. | Pale purple, 18–25 mm long. | Purple, sometimes white, 10–13 mm long. |
| Fruits | Ellipsoidal trihedral, 2–2.5 mm long, greenish-brown. | Ellipsoidal, 2–3 mm long, reticular-wrinkled, brown. | Trihedral spherical, 2 mm in diameter, dark brown. | Trihedral spherical, 1.5–2 mm in diameter, dark brown. | Trihedral spherical, brown, with stripes. | Naked, round-ovoid, brownish. | Spherical, 2 mm diameter, smooth trihedral, dark brown. | Round elliptical, smooth, 1.5–5 mm long, from light brown to dark. |
| Habitat | In the steppes and on the meadow slopes of the steppe mountains. | On gravelly and rocky mountain slopes, in gorges and valleys. | In the steppes and on dry steppe meadows. | In the steppe zone, along the mountain slopes, forest edges, river banks. | On the steppe, gravelly and rocky mountain slopes. | In the foothills, along gravelly and loess slopes, rocky valleys. | On meadow mountain slopes, lawns and edges of walnut and deciduous forests. | On rocky scree, in pine forests, on dry elevated places on rocky and clay soil. |
| Distribution in Kazakhstan | Chu-Ili Mountains, Karatau. | Chu-Ili Mountains, Karatau, Western Tien Shan. | Tobolsk-Ishim, Irtysh, Semipalatinsk, Kokchetav, Caspian, Aktobe, Western and Eastern small hills, Altai. | Tobolsk-Ishim, Irtysh, Semipalatinsk, Kokchetav, Caspian, Aktobe, Altai, Turkestan, Dzungarian Alatau, Zaili Alatau, Chu-Ili, Karatau, Western Tien Shan. | Karatau. | Western Tien Shan. | Western Tien Shan. | Tobolsk-Ishim. |
| Species | Plant Part | Location | Extraction Method | Solvent | TPC (mg eq-QE/g) | TFC (mg eq-QE/g) | RA (mg/g) | LT (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| S. virgata | L R S F | Iran | M | 70% MeOH | 35.6 ± 7.6 15.8 ± 3.3 18.7 ± 2.6 36.7 ± 5.7 | 83.9 ± 9.4 94.9 ± 4.7 82.1 ± 7.3 100.4 ± 8.7 | ND | ND | [16] |
| AP | Turkey | S | H EA MeOH 50% MeOH W | 28.31 ± 0.58 64.47 ± 1.04 133.79 ± 0.79 212.30 ± 0.43 116.22 ± 0.84 | 0.81 ± 0.09 0.10 ± 0.02 6.53 ± 0.11 3.57 ± 0.05 3.87 ± 0.06 | ND 4.48 ± 0.13 59.75 ± 1.66 48.49 ± 2.84 23.23 ± 0.43 | ND 0.06 ± 0.00 0.14 ± 0.01 0.36 ± 0.02 0.58 ± 0.02 | [17] | |
| AP | Turkey | M | 80% MeOH | 125.11 ± 5.44 | 28.03 ± 0.44 | 37.93 ± 1.42 | ND | [18] | |
| AP | Turkey | M | 70% MeOH W | 195.22 ± 0.25 120.14 ± 2.27 | 62.20 ± 0.57 14.17 ± 0.83 | 66.94 ± 0.471 26.81 ± 0.047 | 0.97 0.22 | [19] | |
| AP | Turkey | M | 80% EtOH | 283.35 ± 10.4 | 13.37 ± 1.6 | ND | ND | [20] | |
| AP | Iran | M | MeOH | 7.5 ± 0.7 | ND | ND | ND | [21] | |
| S. macrosiphon | L R | Iran | M | 70% MeOH | 14.7 ± 2.0 5.8 ± 0.8 | 51.9 ± 5.5 15.9 ± 1.9 | ND | ND | [16] |
| AP | Iran | M | 80% MeOH | 111.9 ± 0.06 | ND | ND | ND | [22] | |
| AP | Iran | M | MeOH | 7.1 ± 1.0 | ND | ND | ND | [21] | |
| L | Iran | P | 85% MeOH | ND | ND | ND | 6.25% | [23] | |
| S. sclarea | L R | Iran | M | 70% MeOH | 19.3 ± 2.3 1.5 ± 0.2 | 69.9 ± 3.2 14.9 ± 2.1 | ND | ND | [16] |
| L | Hungary | U | 50% MeOH | 3.07% | ND | 0.87% | ND | [24] | |
| ND | Lithuania | SFE-CO2 | EtOH | ND | ND | 7.323 ± 0.084 | ND | [25] | |
| AP | Moldova | M | EtOH | 110.90 ± 0.26 | ND | ND | ND | [26] | |
| AP | Iran | M | 80% MeOH | 14.13 ± 0.90 | ND | ND | ND | [27] | |
| AP | Turkey | M U P | MeOH MeOH NADES | ND | ND | 0.179 ± 0.00 0.191 ± 0.00 0.090 ± 0.00 | 0.009 ± 0.00 0.008 ± 0.00 0.002 ± 0.00 | [28] | |
| WP | Ukraine | M | 80% EtOH | 29.39–91.02 | 25.91–53.82 | – | – | [29] | |
| AP | Ukraine | U+M M P+M M | 70% EtOH 65% EtOH 70% EtOH 70% EtOH | 832.8 567.9 703.0 1045.9 | 1675.6 1142.01 1414.09 2103.12 | ND | ND | [30] | |
| AP | Serbia | M U M U | MeOH MeOH 80% MeOH 80% MeOH | ND | ND | 175.66 ± 2.02 177.77 ± 1.89 197.48 ± 2.00 171.99 ± 1.88 | 1.45 ± 0.01 1.13 ± 0.01 0.96 ± 0.02 0.80 ± 0.02 | [31] | |
| AP | Kazakhstan | U | 50% EtOH | ND | 7.71% | ND | ND | [32] | |
| S. dumetorum | ND | Lithuania | SFE-CO2 | EtOH | ND | ND | 5.024 ± 0.109 | 0.193 ± 0.094 | [25] |
| L | Hungary | U | 50% MeOH | 6.54% | ND | 2.09% | ND | [24] | |
| L | Ukraine | U | MeOH | ND | ND | 1.223 | ND | [33] | |
| S. verticillata | ND | Lithuania | SFE-CO2 | EtOH W | ND | ND | 15.436 ± 0.112 1.383 ± 0.032 | 0.294 ± 0.0075 0.402 ± 0.0057 | [25] |
| AP | Moldova | M | EtOH | 107.62 ± 0.08 | ND | ND | ND | [26] | |
| AP | Iran | U | 80% MeOH | 32.61 ± 0.39 | 11.32 ± 0.23 | ND | ND | [34] | |
| L | Iran | M | MeOH | ND | ND | 4.78 | ND | [35] | |
| L | Greece | U | 70% MeOH | ND | ND | 223.12 ± 1.63 | 23.92 ± 5.7 | [36] | |
| L | Turkey | S | MeOH | ND | ND | 37.1 | ND | [37] | |
| AP | Serbia | M | MeOH | 175.6 ± 16.3 | 244.4 ± 4.7 | 23.458 ± 0.52 | – | [38] | |
| L | Ukraine | U | MeOH | ND | ND | 12.310 | ND | [33] | |
| S. aethiopis | AP | Moldova | M | EtOH | 81.43 ± 0.25 | ND | ND | ND | [26] |
| AP | Iran | M | 80% MeOH | 14.13 ± 0.90 | ND | ND | ND | [27] | |
| ND | Cyprus | M | MeOH | 90.75 | 121.76 | 4.18 ± 0.9 | 5.56 ± 0.2 | [39] | |
| ND | Turkey | M | EtOH | ND | ND | 1.904 | 0.1715 | [40] | |
| AP | Serbia | U | MeOH 80% MeOH EtOH 60% EtOH 80% EtOH EA | ND | ND | 0.222 ± 0.030 0.223 ± 0.022 0.140 ± 3.21 0.0725 ± 0.00 0.231 ± 0.041 0.0058 ± 0.00 | ND | [41] | |
| L | Ukraine | U | MeOH | ND | ND | 0.552 | ND | [33] | |
| S. deserta | AP | Kazakhstan | U | 50% EtOH | ND | 10.14% | ND | ND | [32] |
| L F | China | M | 70% MeOH | ND | ND | 30 8 | ND | [42] | |
| S. trautvetteri | No data available | ||||||||
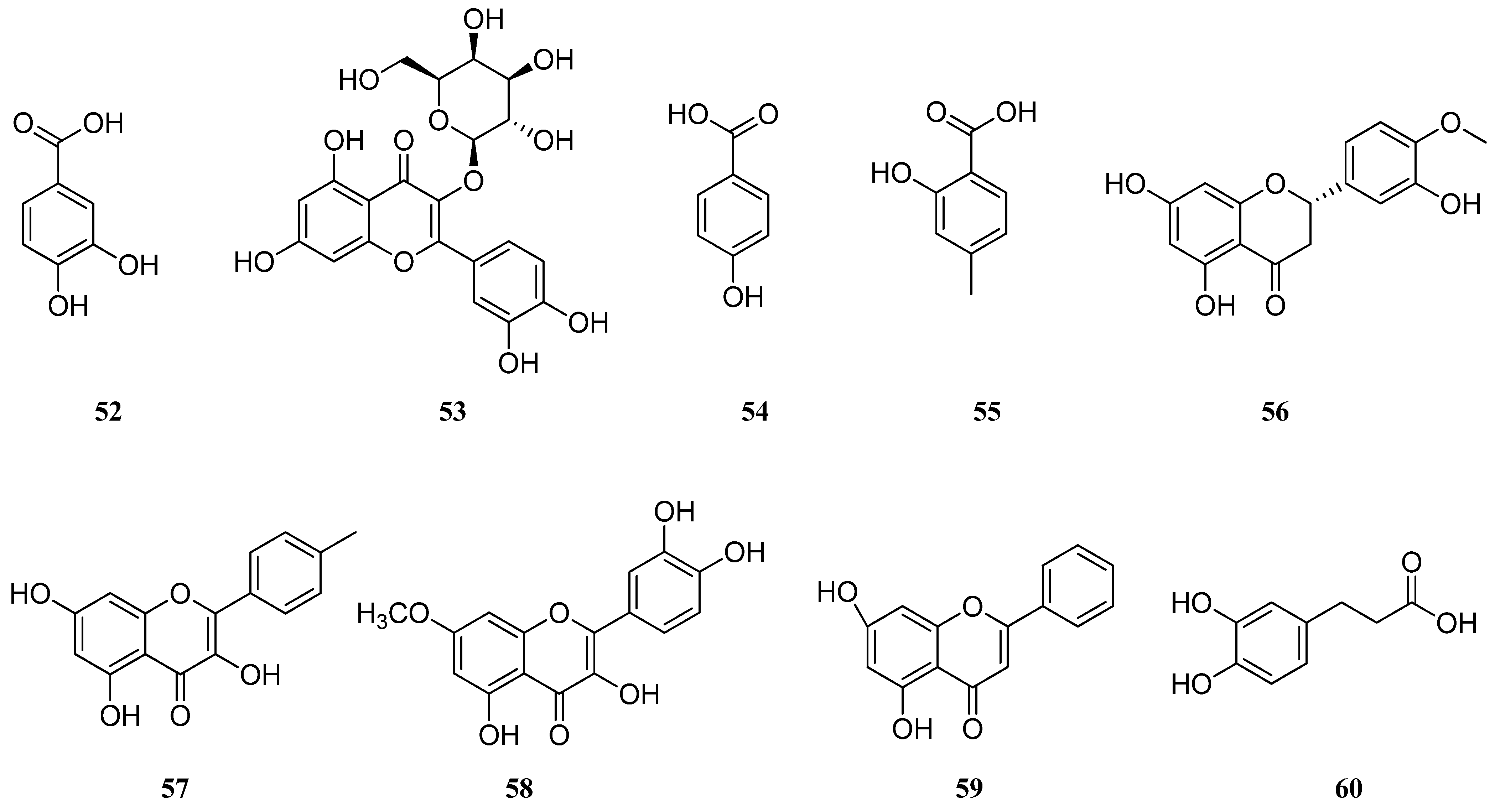
| Number | Compound Name | Type | Reference |
|---|---|---|---|
| (1) | Caffeic acid | Hydroxycinnamic acid | [17,24,25,31,32,33,39,42,50] |
| (2) | Rosmarinic acid | Phenolic acids | [17,18,24,25,28,31,32,33,36,42,50] |
| (3) | Carnosic acid | Phenolic diterpene | [25,38] |
| (4) | Luteolin-7-glucuronide | Flavonoid | [25] |
| (5) | Apigenin-7-glucuronide | Flavonoid | [25] |
| (6) | Quercetin-3-glucuronide | Flavonoid | [25] |
| (7) | Gallic acid | Phenolic acid | [17,28,37] |
| (8) | p-OH-benzoic acid | Phenolic acid | [17] |
| (9) | o-coumaric acid | Hydroxycinnamic acid | [17,36,37] |
| (10) | Cynaroside | Flavonoid | [17,31,33] |
| (11) | Luteolin | Flavonoid | [17,28,31,32,36,39,40] |
| (12) | Rutin | Flavonoid | [18,28,33] |
| (13) | α-tocopherol | Tocopherol | [25] |
| (14) | γ-tocopherol | Tocopherol | [25] |
| (15) | δ-tocopherol | Tocopherol | [25] |
| (16) | Quercetin | Flavonoid | [28,33] |
| (17) | Catechin | Flavonoid | [21,28] |
| (18) | Syringic acid | Phenolic acid | [28] |
| (19) | Coumarin | Flavonoid | [28] |
| (20) | Myricetin | Flavonoid | [28] |
| (21) | Apigenin | Flavonoid | [25,28,31,32,33,36,40] |
| (22) | 3-hydroxy benzoic acid | Phenolic acid | [28] |
| (23) | Ferulic acid | Hydroxycinnamic acid | [28,36] |
| (24) | Trans-cinnamic acid | Phenolic acid | [28] |
| (25) | Ellagic acid | Phenolic acid | [28] |
| (26) | Salvigenin | Flavonoid | [31,32,36] |
| (27) | Cosmosiin | Flavonoid | [31,33] |
| (28) | Cirsimaritin | Flavonoid | [32,33,36] |
| (29) | Kaempferol-3-O-glucoside | Flavonoid | [33] |
| (30) | 6-hydroxyluteolin-5-glucoside | Flavonoid | [33] |
| (31) | Hispidulin | Flavonoid | [33,36] |
| (32) | Caftaric acid | Hydroxycinnamic acid | [36] |
| (33) | Dimer-β-(3,4-dihydroxyphenyl) lactic acid | Phenolic acid | [36] |
| (34) | Sagerinic acid | Cyclobutane lignans | [36] |
| (35) | Methyl-rosmarinic acid | Hydroxycinnamic acid | [36] |
| (36) | Salvianolic acid B | Stilbenoid | [32,36] |
| (37) | Salvianolic acid E | Stilbenoid | [36] |
| (38) | Salvianolic acid K | Stilbenoid | [36] |
| (39) | Lithospermic acid | Phenolic acid | [36] |
| (40) | Danshensu | Phenolic acid | [32,36] |
| (41) | Medioresinol | Furanoid ligans | [36] |
| (42) | p-coumaric acid | Hydroxycinnamic acid | [37,40] |
| (43) | Chlorogenic acid | Phenolic acid | [37,40] |
| (44) | Vanillic acid | Flavonoid | [32,37] |
| (45) | Carnosol | Phenolic diterpenoid | [38] |
| (46) | Salvianolic acid C | Stilbenoid | [38] |
| (47) | Isoquercetin | Flavonoid | [39] |
| (48) | Acecetin | Flavonoid | [39] |
| (49) | Fumaric acid | Dicarboxylic acid | [39] |
| (50) | Naringenin | Flavonoid | [39,40] |
| (51) | Quinic acid | Cyclohexanecarboxylic acid | [40] |
| (52) | Protocatechuic acid | Hydroxybenzoic acid | [40] |
| (53) | Hyperoside | Flavonoid | [40] |
| (54) | 4-hydroxybenzoic acid | Hydroxybenzoic acid | [40] |
| (55) | Salicylic acid | Phenolic acid | [40] |
| (56) | Hesperetin | Flavonoid | [40] |
| (57) | Kaempferol | Flavonoid | [40] |
| (58) | Rhamnetin | Flavonoid | [40] |
| (59) | Chrystin | Flavonoid | [40] |
| (60) | Dihydrocaffeic acid | Hydroxycinnamic acid | [32] |
| (61) | Protocatechuicaldehyde | Hydroxybenzaldehydes | [32] |
| (62) | Luteolin 7-O-glucoside | Flavonoid | [32] |
| (63) | Apigenin 7-O-glucoside | Flavonoid | [32] |
| (64) | Tetramethoxeflavone | Flavonoid | [32] |
| (65) | Methoxycoumarin | Coumarin | [32] |
| (66) | Jaceosidin | Flavonoid | [32] |
| (67) | Viscosine | Flavonoid | [32] |
| (68) | Eupatorin | Flavonoid | [32] |
| (69) | Genkwanine | Flavonoid | [32] |
| (70) | Oleanolic acid | Triterpenoid | [50] |
| (71) | Ursolic acid | Triterpenoid | [50] |
| (72) | Salvisertin A | Triterpenoid | [51] |
| (73) | Dichroanone | Diterpenoid | [51] |
| (74) | Sugiol | Diterpenoid | [51] |
| Component | Area, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Iran | Turkey | Greece | Krym | Uzbekistan | |||||
| [16] | [52] | [53] | [54] | [55] | [56] | [57] | [58] | [59] | |
| 3-Ethyl-3-hydroxyandrostan-17-one | 10.22 | – | – | – | – | – | – | – | – |
| Aromadendrene | 0.48 | – | – | – | – | – | 15.2 | – | 0.3 |
| Bicyclogermacrene | – | – | – | 1.85 | – | – | 0.6 | – | – |
| Borneol | – | – | – | 0.81 | 1.5 | – | 0.3 | 0.22 | 0.3 |
| Camphene | – | – | – | – | – | – | 2.2 | 3.19 | 2.6 |
| Camphor | – | – | – | – | 0.74 | – | – | 0.63 | – |
| Carvacrol | – | – | – | – | – | – | – | – | 0.5 |
| Caryophyllene oxide | – | 30.23 | 34.4 | 6.9 | 7.43 | 13.22 | 6.6 | 6.03 | – |
| cis-Z-α-Bisabolene epoxide | 2.73 | – | – | – | – | – | – | – | – |
| cis-β-Faesene | 1.48 | – | – | 0.78 | – | – | 0.5 | – | – |
| Doconexent | 3.97 | – | – | – | – | – | – | – | – |
| Elemen | 0.96 | 0.24 | – | – | 0.67 | – | – | – | – |
| Eucalyptol | – | – | – | – | 0.21 | – | – | – | – |
| Falcarinol | 2.07 | – | – | – | – | – | – | – | – |
| Germacrene B | – | – | – | 0.49 | – | – | – | – | – |
| Germacrene D | – | – | – | 3.23 | 6.01 | 9.75 | 6.1 | 0.34 | – |
| Hexadecane | – | – | 0.1 | – | – | – | – | – | – |
| Humulene epoxide II | – | – | 0.2 | 1.37 | – | 0.74 | 0.3 | – | – |
| Limonene | – | 1.45 | – | – | – | – | 0.8 | – | 6.2 |
| Linalool | – | – | 0.1 | – | – | 0.73 | – | 30.50 | 0.2 |
| Linalyl acetate | – | – | – | – | – | 0.64 | – | 22.05 | 0.2 |
| Myrcene | – | – | – | – | – | - | 0.8 | 0.50 | – |
| Phytol | – | – | – | 6.83 | – | – | – | – | – |
| Sabinene | – | 11.82 | – | - | 2.02 | 21.2 | – | – | |
| Selinene | 0.48 | – | – | 0.51 | – | – | – | – | – |
| Seychellene | 1.20 | – | – | – | – | – | – | – | |
| Spathulenol | – | 0.17 | 25.6 | 6.09 | 0.71 | 0.84 | 0.6 | 2.83 | 1.0 |
| Squalene | – | – | – | 0.86 | – | – | – | – | – |
| Terpinolene | – | – | – | – | – | – | 0.3 | – | – |
| Thymol | 0.34 | 0.75 | 0.2 | - | – | – | – | – | 0.3 |
| trans-β-Ocimene | – | – | – | – | – | – | – | 0.29 | – |
| Valeranone | 26.09 | – | – | 1.19 | – | – | – | – | – |
| α-Pinene | – | 1.14 | – | – | – | – | 1.9 | – | – |
| α-Copaene | – | – | – | – | – | 2.03 | 0.4 | 0.71 | 0.8 |
| α-Gurjunene | 5.62 | - | – | – | – | – | – | – | – |
| α-Humulene | 0.50 | 0.95 | 0.1 | 1.03 | 10.88 | 2.83 | 1.1 | 0.55 | 1.2 |
| α-Terpineol | – | – | – | – | – | – | – | – | 1.0 |
| α-Thujone | – | – | – | – | 0.2 | 0.82 | – | 0.12 | – |
| β-Caryophyllene | 7.08 | 22.6 | 3.1 | 1.96 | 36.26 | 48.12 | – | – | – |
| β-Eudesmol | – | – | – | – | – | – | – | – | 1.2 |
| β-Bourbonene | – | – | – | – | 1.82 | – | – | 0.19 | – |
| β-Ionone | – | – | – | – | – | – | 0.2 | – | – |
| β-Pinene | – | – | – | 0.28 | 1.05 | – | 1.3 | – | – |
| γ-Terpinene | – | 1.12 | 0.3 | – | 0.93 | 1.03 | 1.9 | – | 5.2 |
| δ-Cadinene | 23.3 | – | – | 0.44 | 0.15 | 3.6 | 0.3 | – | – |
| Component | Reference | |||||
|---|---|---|---|---|---|---|
| [16] | [22] | [60] | [61] | [62] | [63] | |
| 1,8-Cineole | – | – | 5.82 | – | 1.1 | – |
| 4-Terpineol | – | – | 0.16 | – | 1.0 | – |
| Aromadendrene | 2.88 | – | – | 1.1 | – | 0.39 |
| Benzyl benzoate | – | – | – | – | – | 0.68 |
| Bicyclogermacrene | – | – | 0.70 | 1.8 | – | 0.71 |
| Borneol | 0.77 | – | – | – | – | – |
| Butyl benzoate | – | – | – | – | – | 49.16 |
| Camphene | – | – | 0.18 | – | – | – |
| Camphor | 3.10 | – | 0.48 | – | – | – |
| Carvacrol | – | 9.96 | – | – | – | – |
| Caryophyllene oxide | 14.63 | – | 0.20 | – | – | 1.19 |
| Cyperene | – | – | – | – | – | 4.10 |
| Ethyl benzoate | – | – | – | – | – | 0.31 |
| Fenchone | – | – | – | – | 1.1 | – |
| Germacrene A | – | 0.91 | – | – | – | – |
| Germacrene B | 1.32 | 0.73 | – | 0.2 | – | – |
| Germacrene D | 7.59 | 0.93 | 2.14 | 4.3 | – | 1.62 |
| Heptacosane | 0.30 | – | – | – | – | – |
| Hexanal | – | – | 0.25 | – | – | – |
| Hexanol | – | – | – | – | 1.4 | – |
| n-Hexyl benzoate | – | – | – | – | – | 7.0 |
| Hexyl isobutyrate | – | 0.96 | – | – | – | 0.82 |
| Hexyl isovaleriate | – | 5.06 | – | – | – | – |
| Hexyl n-valerate | – | – | – | 4.8 | – | – |
| Humulene epoxide II | 0.22 | – | – | – | – | – |
| Isocomene | – | 1.15 | – | – | – | – |
| Limonene | 0.22 | – | 2.73 | – | – | – |
| Linalool | 27.20 | 19.00 | 0.34 | – | 54.8 | 3.31 |
| Linalyl acetate | 1.55 | – | – | – | – | – |
| Manool | – | 0.67 | – | – | 27.3 | – |
| Manoyl oxide | – | – | – | – | 1.3 | – |
| Methyl benzoate | – | – | – | – | – | 0.27 |
| Metyl chavicol | – | – | – | – | 1.1 | – |
| Myrcene | – | – | 1.01 | – | – | – |
| o-Cymene | – | – | 1.03 | – | – | – |
| Octyl benzoate | – | – | – | – | – | 2.75 |
| p-Cymene | – | – | 7.25 | – | – | – |
| Sabinene | – | – | 0.85 | – | – | – |
| Sclareol | – | 0.6 | – | 8.6 | – | – |
| Spathulenol | 5.86 | – | – | 5.80 | 1.7 | 4.83 |
| Thymol | – | 8.73 | 0.44 | – | – | – |
| Valencene | – | – | – | 1.4 | – | – |
| α-Cadinene | – | 1.04 | – | 1.8 | – | – |
| α-Cubebene | – | – | 0.38 | – | – | – |
| α-Pinene | – | – | 0.39 | – | – | – |
| α-Caryophyllene | – | – | 0.45 | 0.6 | – | 1.34 |
| α-Copaene | – | – | 1.26 | 0.9 | – | – |
| α-Eudesmol | 1.25 | 0.82 | – | – | – | – |
| α-Gurjunene | – | – | – | 0.6 | – | – |
| α-Terpinene | – | – | 1.11 | 2.6 | – | – |
| α-Terpineol | 0.21 | – | – | – | 1.7 | – |
| α-Murolol | – | – | – | – | 1.4 | – |
| α-Thujene | – | – | 4.28 | – | – | – |
| α-Thujone | – | – | 17.84 | – | – | – |
| β-Caryophyllene | 13.50 | – | 9.92 | – | – | 3.54 |
| β-Cedrene | – | 14.64 | – | – | – | – |
| β-Cubebene | – | – | 0.42 | 0.3 | – | - |
| β-Elemene | – | 13.33 | – | 5.4 | – | 3.02 |
| β-Eudesmol | – | – | – | 3.9 | – | – |
| β-Fanesene | – | – | – | 0.5 | – | – |
| β-Selinene | – | 3.18 | 0.40 | 2.2 | – | – |
| β-Pinene | – | – | 1.52 | – | – | – |
| β-Thujone | – | – | 1.83 | – | – | – |
| γ-Elemene | – | 0.6 | 0.39 | 0.2 | – | – |
| γ-Terpinene | 0.85 | – | 14.75 | – | – | – |
| δ-Cadinene | – | – | 1.32 | 0.7 | – | – |
| δ-Elemene | – | 4.02 | – | – | – | – |
| δ-Selinene | – | – | – | – | – | 2.86 |
| Component | Area, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Iran [16] | Uzbekistan [59] | Lithuania [64] | Poland [65] | Slovakia [66] | Tadjikistan [67] | Italy [68] | Bulgaria [69] | India [70] | Georgia [71] | |
| 1,8-Cineole | – | 1.2 | – | – | – | 0.1 | – | – | – | – |
| 2-Tridecanone | – | 0.8 | 1.2 | – | – | – | – | – | – | – |
| 9-octadecenoic acid | – | 6.9 | – | – | – | – | – | – | – | – |
| Aromadendrene | – | – | – | – | – | – | 0.4 | 0.27 | – | – |
| Bicyclogermacrene | – | – | – | – | 0.2 | 1.2 | 0.8 | – | – | – |
| Borneol | – | 0.2 | – | – | – | 0.1 | – | – | – | – |
| Camphene | – | – | – | 23.36 | – | – | – | – | – | – |
| Camphor | – | 0.4 | – | 2.74 | – | – | 0.1 | – | – | – |
| Carvacrol | – | – | 4.0 | – | – | 1.3 | – | – | – | – |
| Caryophyllene oxide | 0.55 | – | 14.0 | – | 0.3 | 0.2 | 0.2 | – | – | 1.56 |
| Elemen | – | – | – | – | – | 0.2 | – | – | – | – |
| Eugenol | – | 1.1 | – | – | – | – | – | – | – | – |
| Germacrene D | 16.4 | – | 9.6 | – | 0.2 | 11.4 | 10.5 | – | – | 0.35 |
| Hexacosane | – | – | 6.6 | – | – | – | – | – | – | – |
| Hexadecane | – | – | 1.7 | – | – | – | – | – | – | – |
| Humulene epoxide II | – | – | – | – | – | – | 0.3 | – | – | – |
| Limonene | – | 0.4 | – | – | 2.20 | 0.2 | 0.1 | 1.05 | – | – |
| Linalool | 26.2 | – | – | – | 20.6 | 12.5 | 11.3 | 17.67 | 13.78 | 14.9 |
| Linalyl acetate | 20.5 | 4.7 | – | – | 49.1 | 39.2 | 59.3 | 34.62 | 20.58 | 18.8 |
| Methyl eugenol | – | 0.3 | – | – | – | – | – | – | – | – |
| Myrcene | 1.85 | – | – | – | 0.6 | 0.7 | 0.5 | 1.82 | 8.54 | 1.21 |
| n-Butyloctadecenoate | – | 5.7 | – | – | – | – | – | – | – | – |
| Nerol | 2 | – | – | – | 1.1 | 1.1 | – | 0.15 | – | 1.66 |
| Selinene | – | – | – | – | – | 0.2 | – | – | – | – |
| Spathulenol | – | 2.5 | 1.5 | – | – | 0.2 | 0.3 | 0.72 | – | – |
| Squalene | – | – | 10.9 | – | – | – | – | – | – | 9.07 |
| Tetradecane | – | – | 2.0 | – | – | – | – | – | – | – |
| Thujol | – | – | – | 12.31 | – | – | – | – | – | – |
| Thymol | 0.74 | 0.4 | – | – | – | 1.5 | – | – | – | – |
| trans-β-Ocimene | 1.82 | 0.5 | 0.7 | – | 0.5 | 0.2 | 0.1 | 1.59 | – | 0.5 |
| Triacontane | – | – | 1.3 | – | – | – | – | – | – | – |
| α-Pinene | – | – | – | – | 2.4 | 0.1 | – | – | – | – |
| α-Caryophyllene | – | 0.3 | – | – | – | 0.1 | 1.7 | 0.30 | – | – |
| α-Copaene | – | 1.3 | 4.9 | – | 0.2 | 1.0 | 1.2 | – | – | – |
| α-Eudesmol | – | – | 1.9 | – | – | – | – | – | – | – |
| α-Terpineol | – | 2.5 | 0.8 | – | 4.9 | 5.5 | – | 4.84 | 17.82 | – |
| α-Thujone | 3.21 | – | – | – | – | 0.4 | – | – | – | – |
| β-Caryophyllene | – | – | – | – | 5.1 | 2.4 | 3.7 | 5.6 | 27.00 | 1.23 |
| β-Cubebene | 6.18 | – | 12.3 | – | – | – | – | – | – | – |
| β-Eudesmol | 0.65 | 1.3 | – | – | – | 0.5 | 0.1 | – | – | – |
| β-Bourbonene | 0.71 | – | – | – | – | – | – | 0.38 | – | – |
| β-Pinene | – | – | 1.4 | – | 0.2 | – | – | – | – | – |
| γ-Terpinene | – | 0.3 | 2.2 | – | – | 0.3 | – | – | – | – |
| δ-Cadinene | 0.68 | 1.4 | – | – | – | 0.4 | 0.7 | 0.97 | – | – |
| Component | Area, % | |||
|---|---|---|---|---|
| Ukraine [33] | Lithuania [64] | Poland [65] | Kazakhstan [72] | |
| 1,8-Cineole | 0.1 | – | – | 0.13 |
| Aromadendrene | – | – | – | 0.52 |
| Bicyclogermacrene | – | – | – | 5.59 |
| Camphene | – | – | 17.26 | - |
| Camphor | – | – | 2.66 | 0.89 |
| Caryophyllene oxide | – | 24.7 | – | 11.16 |
| Docosane | 0.9 | – | – | – |
| Dotriacontane | 3.6 | – | – | – |
| Elemen | – | – | – | 0.45 |
| Germacrene D | – | – | – | 1.13 |
| Heneicosane | 1.4 | – | – | – |
| Hentriacosane | 15.3 | – | – | – |
| Heptacosane | 2.4 | – | – | – |
| Hexacosane | – | 12.9 | – | – |
| Hexadecane | – | – | – | 0.22 |
| Humulene epoxide II | – | 1.0 | – | 1.07 |
| Limonene | 0.1 | – | – | – |
| Linalool | – | – | – | 0.14 |
| Linalyl acetate | – | – | – | 0.08 |
| Nonacosane | 5.3 | – | – | – |
| Pentacosane | 1.0 | – | – | – |
| Phytol | – | 6.7 | – | – |
| Sabinene | – | 0.6 | – | – |
| Selinene | – | - | – | 2.6 |
| Spathulenol | – | 4.8 | – | 10.75 |
| Squalene | – | 20.1 | – | – |
| Tetradecane | 3.0 | 1.0 | – | 0.16 |
| Thujol | – | – | 8.42 | – |
| Thymol | – | – | – | 0.46 |
| Triacontane | – | 9.0 | – | – |
| Tritiacontane | 12.9 | – | – | – |
| α-Pinene | 0.1 | – | 2.88 | – |
| α-Humulene | – | – | – | 1.82 |
| α-Gurjunene | – | – | – | 0.33 |
| α-Ionol | – | 0.9 | – | – |
| α-Terpineol | – | – | – | 0.20 |
| α-Thujone | – | – | – | 0.59 |
| β-Caryophyllene | – | 0.7 | – | 13.21 |
| β-Cubebene | – | 0.8 | – | – |
| β-Famesene | – | – | – | 0.18 |
| β-Sitosterol | – | – | 2.77 | – |
| γ-Sitosterol | 4.0 | – | – | – |
| γ-Terpinene | – | – | – | 0.11 |
| δ-Cadinene | – | – | 6.77 | – |
| Component | Area, % | |||||||
|---|---|---|---|---|---|---|---|---|
| Greece * [57] | Lithuania [64] | Poland [65] | Italy [73] | Turkey [74] | Ukraine [75] | Serbia [76] | Iran [77] | |
| 1,8-Cineole | ND–18.4 | – | – | – | 2.5 | 6.757 | – | – |
| 4-Terpineol | ND–0.2 | – | – | – | 0.6 | 2.226 | – | 0.28 |
| 4aα,7α,7aα-Nepetalactone | ND–51.4 | – | – | – | – | – | – | – |
| Aromadendrene | – | 2.2 | – | 0.1–0.4 | – | – | – | 0.05 |
| Bicyclogermacrene | ND–−0.1 | – | – | 11.5–14.8 | – | 6.631 | – | 6.32 |
| Borneol | – | – | 0.33 | – | 0.4 | – | – | 0.20 |
| Camphene | – | – | 2.39 | – | – | 0.799 | – | 0.18 |
| Camphor | ND–0.1 | 2.8 | 5.23 | – | 0.6 | 2.757 | – | – |
| Carvacrol | – | 2.5 | – | – | 2.1 | – | – | – |
| Caryophyllene oxide | 2.6–6.0 | – | – | 1.0 | 1.9 | 4.355 | – | 0.89 |
| cis-Z-α-Bisabolene epoxide | – | – | – | – | – | – | – | 0.58 |
| Eucaliptol | – | – | 0.80 | – | – | – | – | – |
| Germacrene B | – | 35.7 | – | – | – | – | – | – |
| Germacrene D | 11.5–2.8 | 14.3 | - | 39.5–40.7 | – | 11.881 | – | 0.21 |
| Hecadecanoic acid | – | – | – | – | 2.3 | - | – | - |
| Hexacosane | – | 0.8 | – | – | – | – | – | – |
| Hexadecane | – | 2.0 | – | – | – | – | – | – |
| Hexanal | – | – | – | – | 0.5 | – | – | – |
| Humulene epoxide II | 0.4–1.0 | – | 0.2–0.4 | 0.9 | – | – | – | |
| Limonene | 1.8–ND | – | 5.85 | 3.9 | 4.1 | – | – | 3.80 |
| Linalool | 0.2–0.1 | – | – | 0.4–0.8 | 2.2 | 0.310 | – | 0.05 |
| Myrcene | 0.3–0.2 | – | – | 0.8–1.1 | 1.4 | 0.142 | 6.0–6.6 | 1.92 |
| Myrtenol | ND–0.1 | – | – | – | – | – | – | – |
| Nerol | – | 28.1 | – | – | – | – | – | – |
| Nerolidol | 35.0–ND | – | – | – | – | – | – | – |
| o-Cymene | – | – | – | – | – | – | 0.5–0.6 | – |
| p-Cymene | – | – | – | 0.2–0.3 | 1.0 | – | – | 0.22 |
| Phytol | – | – | – | – | – | – | – | 0.84 |
| Sabinene | 0.2–0.6 | – | – | 0.8 | – | – | 1.7–5.5 | 4.44 |
| Sabinene hydrate | ND–0.1 | – | – | – | – | – | – | – |
| Seychellene | – | 13.1 | – | – | – | – | – | – |
| Spathulenol | 1.1–0.1 | 44.2 | – | 3.1–6.6 | 31.0 | – | - | 5.89 |
| Squalene | – | 0.7 | – | – | – | – | – | – |
| Tetradecane | 0.3–ND | – | – | 0.4–−0.7 | – | – | – | – |
| Thymol | – | – | – | – | 0.4 | – | – | – |
| Thujol | – | 2.18 | – | – | – | – | – | |
| Cis-ocimene | – | 1.54 | – | – | – | – | – | |
| trans-β-Ocimene | – | 7.0 | – | 0.6–2.9 | – | – | – | 1.65 |
| Tridecsne | – | – | – | 0.4–0.3 | – | – | – | – |
| Valeranone | – | – | – | – | – | 3.298 | – | 0.38 |
| Verbenone | – | – | – | – | 0.8 | – | – | – |
| Virodoflorol | ND–0.2 | – | – | – | – | 15.502 | – | 0.12 |
| α-Pinene | 1.0–0.6 | – | 10.72 | 0.5–1.3 | 8.2 | 0.145 | 1.9–21.1 | 3.03 |
| α-Copaene | 2.3–0.2 | – | – | 0.3–0.5 | – | – | – | 0.25 |
| α-Cubebene | 0.4–ND | – | – | 0.7–0.1 | – | – | – | 0.23 |
| α-Gurjunene | 0.4–ND | 2.5 | – | – | – | – | – | 3.22 |
| α-Humulene | 0.7–0.4 | – | – | 2.7–5.9 | – | 5.864 | 0.2 | 8.61 |
| α-Terpineol | ND–1.2 | – | – | – | 1.0 | – | – | – |
| α-Thujone | – | – | – | 0.3 | – | 11.013 | 1.6–2.5 | – |
| β-Phellandrene | – | – | – | 4.5–4.9 | – | – | 43.9–55.5 | 9.08 |
| β-Caryophyllene | 3.8–1.3 | 5.7 | – | 7.3–11.9 | 0.7 | – | 0.3–0.9 | 24.40 |
| β-Copaene | ND–0.1 | – | – | 0.6–0.7 | – | – | – | – |
| β-Cubebene | 0.8–ND | – | – | 0.3 | – | – | – | 0.23 |
| β-Elemene | 0.7–0.2 | – | – | 0.3–0.4 | – | – | – | – |
| β-Farnesene | – | – | – | 1.5–2.4 | – | – | – | – |
| β-Bourbonene | 3.0–1.3 | – | – | 1.8–3.1 | – | 0.368 | – | 0.17 |
| β-Pinene | 6.1–1.6 | – | 2.49 | 2.6–3.7 | 2.0 | – | 3.0–3.6 | 5.00 |
| β-Thujone | – | – | – | – | – | 0.932 | – | – |
| γ-Cadinene | 0.8–ND | – | – | 1.5–0.3 | – | – | – | – |
| γ-Muurolene | 1.2–ND | – | – | 0.2–0.4 | – | – | 7.9 | – |
| γ-Terpinene | ND–0.7 | – | – | – | 1.1 | – | – | 0.21 |
| δ-Cadinene | 1.2–0.2 | – | – | 0.7–1.2 | – | 0.302 | – | – |
| δ-Elemene | – | – | – | 1.4–7.4 | – | – | – | – |
| Component | Area, % | |||
|---|---|---|---|---|
| Greece [57] | Serbia [76] | Turkey [78] | Bulgaria * [79] | |
| Bicycloelemene | – | – | 1.27 | – |
| Bicyclogermacrene | 0.6 | – | 2.55 | – |
| Camphene | 0.1 | – | – | – |
| Caryophyllene oxide | 8.4 | – | 1.82 | 0.09–0.35 |
| Germacrene A | 0.5 | – | – | – |
| Germacrene D | 20.0 | – | 15.20 | 29.37–21.19 |
| Heptacosane | – | – | – | 0.69–0.20 |
| Hexacosane | – | – | – | 1.73–0.51 |
| Humulene epoxide II | 1.3 | – | – | – |
| Phytol | 0.6 | – | – | – |
| Sabinene | 0.3 | 0.8 | – | – |
| Spathulenol | – | – | – | 0.10–0.24 |
| Valeranone | 0.5 | – | – | – |
| Viridiflorol | – | – | 0.80 | – |
| α-Pinene | 0.3 | 1.3 | – | 0.17–0.19 |
| α-Copaene | 13.7 | 33.4 | 16.46 | 13.55–17.24 |
| α-Cubebene | 0.4 | – | 0.81 | 0.97–0.64 |
| α-Humulene | 6.4 | 1.5 | 7.4 | 5.46–6.79 |
| α-Muurolene | – | 7.2 | – | – |
| β-Caryophyllene | 30.6 | 36.8 | 30.46 | 23.55–21.91 |
| β-Cubebene | 4.4 | – | 7.04 | 7.02–9.71 |
| β-Elemene | 1.1 | 7.3 | 3.01 | – |
| β-Bourbonene | 0.7 | 0.2 | – | – |
| β-Pinene | 0.2 | 0.7 | – | 0.20–0.23 |
| γ-Cadinene | – | – | – | 0.98–0.63 |
| γ-Elemene | – | – | – | 0.62–1.28 |
| γ-Muurolene | – | 10.3 | – | – |
| δ-Cadinene | 3.6 | 0.2 | 5.73 | 5.56–6.69 |
| Component | Area, % | |
|---|---|---|
| China * [42] | Poland [65] | |
| 1-Octen-3-ol | 4.98–ND | – |
| 4-Terpineol | ND–10.91 | – |
| Camphene | – | 4.01 |
| Caryophyllene oxide | 5.99–4.95 | – |
| Eucalyptol | ND–0.73 | – |
| Germacrene D | ND–0.94 | – |
| Humulene epoxide II | 0.94–1.26 | – |
| Ledol | 8.36–6.98 | – |
| Limonene | ND–0.96 | 7.45 |
| Manool | 1.39–4.83 | – |
| Myrcene | ND–0.71 | – |
| o-Cymene | ND–2.15 | – |
| Thujol | – | 2.79 |
| α-Pinene | ND–0.68 | 35.35 |
| α-Copaene | ND–0.84 | – |
| α-Humulene | ND–0.99 | – |
| α-Terpineol | ND–0.72 | – |
| β-Caryophyllene | 0.25–4.6 | – |
| β-Phellandrene | ND–29.74 | – |
| β-Pinene | – | 13.02 |
| β-Terpineol | ND–2.62 | – |
| γ-Muurolene | 0.21–0.16 | – |
| γ-Terpinene | ND–3.02 | – |
| δ-Cadinene | ND–0.33 | – |
| Species | Biological Activity | Assay Results | Ref. |
|---|---|---|---|
| S. virgata | Lipid peroxidation | Methanol, water, ethyl acetate, and hexane extracts inhibited β-carotene/linoleic acid co-oxidation in the range of 40%. | [17] |
| Antioxidant | The IC50 for DPPH for the fractions: aqueous methanol 0.2 mg/mL > water 0.3 mg/mL > methanol 0.4 mg/mL > ethyl acetate 1.65 mg/mL. | [17] | |
| Non-digested methanolic extract IC50 for DPPH 0.57 ± 0.57 μg/mL, FRAP 1.43 ± 0.18 mM FeSO4 eq.in 1 g sample. | [18] | ||
| FRAP value was 27.5 ± 1.2 μM eq-QE/g dry weight and IC50 DDPH scavenging 644.8 ± 65.3 μg dry weight/mL. | [21] | ||
| The IC50 of EO for DPPH: 1.98 ± 0.23 mg/mL, in ABTS: 0.75 ± 0.02 mg/mL, in CUPRAC: 0.39 ± 0.02 mg/mL, in FRAP: 0.28 ± 0.01 mg/mL. | [59] | ||
| Ethanolic extract IC50 for DPPH 291.58 ± 0.004 μg/mL, IC50 values for ABTS 16.74 ± 0.007 μg/mL. | [20] | ||
| The IC50 of EO for DPPH: 22.12–24.45 mg/mL, for FRAP 26.84–28.46 μM eq-QE/g dw. | [81] | ||
| Enzyme inhibitory | Non-digested methanolic extract showed α-glucosidase, α-amylase, AChE, and BChE inhibition activity in a concentration of 1 mg/mL was 70.2, 81.2, 16.45, and 25.98%; in a concentration of 0.5 mg/mL it was 55.7, 64.4, 11.87, and 17.85%, respectively. AGEs inhibitory potential for 1 mg/mL was 86.2%, and for 0.5 mg/mL, it was 55.5%. | [18] | |
| EO showed high BChE activity IC50 of 0.60 ± 0.01 mg/mL. While EO did not exhibit any α-glucosidase inhibition and AChE, it also exhibited weak activity as an α-amylase inhibitor. | [59] | ||
| Ethanolic extract α-glucosidase and α-amylase inhibition activity were 75.73 and 62.72%, respectively. | [20] | ||
| Cytotoxic | Ethanolic extract decreased MDA-MB-231 cell viability (p < 0.05) in a dose-dependent manner (IC50 = 0.118 mg/mL), and displayed no considerable cytotoxicity on the L929 cell line (0.0625–1 mg/mL). | [20] | |
| Antimicrobial | EO has the high activity against Staphylococcus epidermidis, Penicillium funiculosum, and Escherihia coli. | [52] | |
| Ethanolic extract has moderate activity towards E. coli (0.312 mg/mL) and Staphylococcus aureus (0.312 mg/mL), and weak antimicrobial activity towards Bacillus cereus (0.625 mg/mL). | [20] | ||
| EO showed moderate antimicrobial activity against S. aureus and Candida albicans. | [81] | ||
| S. macrosiphon | Antioxidant | The highest radical scavenging activity was in roots, IC50 = 10.9 μg/mL, followed by leaves, IC50 = 36.7 μg/mL. | [16] |
| FRAP value was 17.4 ± 2.3 μM eq-QE/g dry weight and IC50 DDPH scavenging was 415.3 ± 17.9 μg dry weight/mL. | [21] | ||
| The IC50 determined using DDPH of EO and the total extract was 1.83 μg/mL and 55.07 μg/mL, respectively. | [22] | ||
| Methanolic extract possesses activity FRAP 404.12 mmol of FeSO4/100 g dried plant. | [82] | ||
| The IC50 DDPH of methanolic extract of aerial part showed higher activity than ethanolic and n-hexane extracts quantified as 230.29 ± 0.64 mg/mL. | [83] | ||
| DPPH scavenging potential (78.0 ± 2.0%) of stem methanolic extract. | [84] | ||
| Cytotoxic | The EO revealed a potent cytotoxic activity on MCF-7, MDA-MB-231, and T47D cell lines (IC50 < 0.15 μg/mL). Total extract exhibited moderate cytotoxic activity (IC50 < 100 μg/mL). | [22] | |
| Significant cytotoxicity of n-hexane fraction from hydromethanolic extract against cancerous cell lines A549, MCF-7, and MDA-MB-231 with IC50 20.89 ± 0.35, 10.24 ± 0.15, and 20.98 ± 0.25 μg/mL, respectively. | [85] | ||
| Enzyme inhibitory | AChE IC50 = 0.169 ± 0.045, while BChE activity was >0.5 μg/mL. Total extract did not exhibit AChE and BChE inhibitory effects at concentrations up to 500 μg/mL. | [22] | |
| Antimicrobial | MIC and MBC against S. aureus were 2.5 and 5 mg/mL, Listeria monocetogenes 2 and 10 mg/mL, Salmonella enterica 5 and 10 mg/mL, and there was no activity against B. cereus. | [62] | |
| n-hexane fractions from hydromethanolic extract MIC against S. aureus and E. coli were 1.25 and 2.50 mg/mL. | [85] | ||
| The MIC value of methanolic extract of aerial part against S. aureus was 1.25 mg/mL, S. epidermidis 2.5 mg/mL, Bacillus subtilis 2.5 mg/mL, K. pneumonia 5 mg/mL, and Pseudomonas aeruginosa 10 mg/mL. The MIC value of ethanolic extract of aerial part against S. aureus was 2.5 mg/mL, S. epidermis 2.5 mg/mL, B. subtilis 2.5 mg/mL, K. pneumonia 10 mg/mL, and P. aeruginosa 10 mg/mL. The MIC of n-hexane extract of aerial part against S. aureus was 5 mg/mL, S. epidermidis 5 mg/mL, and B. subtilis 5 mg/mL. | [83] | ||
| Stem butanol extract has activity against P. aeruginosa with a ZOI = 23 ± 2.00 mm. | [84] | ||
| Antifungal | The MICs of EO against C. albicans, Candida parapsilosis, and Candida glabrata were 0.44, 0.056, and 0.088 μL/mL. | [63] | |
| Stem methanol extract showed the ZOI (11 ± 0.67 mm) against Fusarium brachygibbosum. | [84] | ||
| Anti-inflammatory | It was revealed that only the highest examined dose of the seed EO was somehow effective in the early phase of the formalin test in rats. | [86] | |
| Analgesic | Seed EO could not significantly inhibit the neutrophil-induced damage by reducing MPO activity in the paws of the rat. | [86] | |
| Antidepressant | The results did not show any significant effect of hydroethanolic extract on immobility, swimming, and climbing behaviors. However, aqueous extract decreased immobility at doses of 300, 600, and 900 mg/kg (11.1, 13.3, and 14.2%, respectively, p < 0.05), and increased swimming at doses of 600 and 900 mg/kg (53.3% and 61.8%, p < 0.05) as compared with the control group. | [87] | |
| Locomotors | Significant decreases in locomotor activity were found in the hydroethanolic extract at doses of 1800 mg/kg. | [87] | |
| S. sclarea | Antioxidant | The IC50 for root extract in DPPH assay was 14.9 μg/mL. | [16] |
| The IC50 DPPH of methanolic extract 190.74 ± 5.7 μg plant extracted or μg eq-QE/1 mL 10−4 M DPPH. | [26] | ||
| The IC50 for extract in the DPPH test was 32.33 ± 0.35, ABTS 17.20 ± 0.10, 29.67 ± 0.02 μg/mL. | [27] | ||
| The green and methanolic extracts exhibited potent LPO activity with IC50 5.61 ± 0.47 and 5.37 ± 0.27; CUPRAC A0.5 = 12.28 ± 0.12 and A0.5 = 17.22 ± 0.36; DDPH with IC50 = 6.31 ± 0.23 and IC50 = 19.20 ± 0.70; and ABTS+ with IC50 = 6.50 ± 0.45 and 8.64 ± 0.63 μg/mL, respectively. | [28] | ||
| Extract had significantly high antioxidant activity in MDA tests 521.5 ± 16.2 pmol/mg, while in DPPH and FRAP tests it had lower activity than vitamin C and Trolox 29.52 ± 4.7%, FRAP 26.23 ± 3.8 mg-trolox/g, respectively. | [39] | ||
| The antioxidant activity of EO is lower compared to the Trolox. DPPH radical scavenging ability was determined at 11.76 ± 1.34% inhibition, and the ABTS radical cation at 29.70 ± 1.45%. | [66] | ||
| The IC50 for EO in the DPPH assay was 123 ± 0.99 μg/mL. | [68] | ||
| The DDPH radical scavenging activity of methanolic extract in a concentration of 10 mg/mL was higher than ethanolic extract measured as 88.49 ± 2.63 and 82.21 ± 1.79%. | [88] | ||
| Antimicrobial | The methanolic extract MIC against E. coli was 5 mg/mL, Klebsiella pneumoniae 2.5 mg/mL, Salmonella Typhi 0.31 mg/mL, B. subtilis 5 mg/mL, S. epidermidis 0.31 mg/mL, and S. aureus 1.25 mg/mL. | [26] | |
| The extract indicated moderate activity against S. aureus and Streptococcus pneumoniae at 1.25 mg/mL. | [27] | ||
| The methanolic extract MIC = 0.5 mg/mL showed high activity against E. coli. The ultrasonic extract and green extract MICs against E. faecalis were 0.625 and 1.25 mg/mL, and against P. aeruginosa (strain PA01) were 2.5 and 1.25 g/mL, respectively. The green extract showed the best effect against S. aureus, MIC = 0.625 mg/mL. The MIC values of methanolic, ultrasonic, and green extracts against Chromobacterium violaceum (strain CV026) were recorded as 0.5, 0.25, and 0.25 mg/mL, respectively. | [28] | ||
| Hydromethanolic extract had strong effect on P. aeruginosa with an MIC and MBC of 50 mg/mL. Methanolic extract prepared by ultrasound showed the best antimicrobial activity toward S. aureus, B. cereus, Listeria monocytogenes, P. aeruginosa, and Enterobacter aerogenes with the MIC values of 6.25, 12.5, 25, 50, and 50 mg/mL, respectively. | [31] | ||
| The EO indicated high antimicrobial activity against S. aureus, MIC50 = 1.48 μL/mL, and biofilm-forming Pseudomonas fluorescens, MIC50 = 2.93 μL/mL. | [66] | ||
| EO has activity against S. aureus, P. aeruginosa, L. monocytogenes, and S. enterica with MIC values of 5.6 ± 0.68, 11.2 ± 0.31, 4.6 ± 0.31, and 7.5 ± 0.0 mg/mL. In addition, EO showed the same MIC value of 7.5 mg/mL for B. cereus, Enterococcus faecalis, and Micrococcus luteus. | [68] | ||
| Cytotoxic | A slight increase was found in the number of viable cells at tested doses for the extract on MCF-7 and MDA-MB-231 cell lines. | [27] | |
| The cytotoxic effects of conventional and ultrasonic extracts on HDFn using the MTT assay do not cause a significant toxic effect on human fibroblasts. | [32] | ||
| Enzyme inhibitory | The green extract showed a maximum AChE inhibition of 47.00 ± 1.50% with IC50 200 μg/mL, and BChE inhibition of 61.79 ± 0.63% with IC50 131.6 ± 0.98 μg/mL. The methanolic extract inhibited 50.70 ± 0.94% BChE with IC50 192.4 ± 1.25 μg/mL. The ultrasonic and methanolic extracts have moderated urease inhibitory activity of 55.12 ± 0.88% and 52.31 ± 0.74% with an IC50 of 171.6 ± 0.95 μg/mL and 187.5 ± 1.32 μg/mL, respectively. | [28] | |
| The ethanolic extract inhibitory effect on AChE was nearly four times higher with an IC50 of 0.27 ± 0.005 mg/mL, while the methanolic extract effect on MAO-A was found to be higher, with an IC50 of 3.03 ± 0.05 mg/mL. | [88] | ||
| Spasmolytic | The hydromethanolic extract prepared by maceration was reported as the strongest bronchodilator agent, which inhibited spontaneous ileal contractions, and carbachol- and KCl-induced tracheal smooth muscle contractions. Methanolic extract by maceration was indicated as the causing most powerful relaxation of KCl-induced ileal contractions. Hydromethanolic extract by ultrasound generated the best spasmolytic effects in the acetylcholine-induced ileal contractions. | [31] | |
| Immunomodulatory | Immunomodulatory effects of the conventional and ultrasonic extracts had either a stimulating effect on inactivated macrophages or suppressed cytokine-producing activity in LPS-activated macrophages. Conventional extraction significantly increased the secretion of TNF-α by intact and Con A-activated splenic T lymphocytes. | [32] | |
| Anti-inflammatory | The IC50 of extracts was significantly lower compared to the anti-inflammatory IC50 of acetylsalicylic acid. IC50 for heat-induced hemolysis was 3.96 ± 0.86 mg/mL, for proteinase was 3.75 ± 0.71 mg/mL, and for albumin denaturation was 4.69 ± 0.96 mg/mL. | [39] | |
| The EO at concentration 80 μg/mL exhibited a 74% inhibition of nitric oxide with an IC50 of 37 ± 3 μg/mL. | [71] | ||
| Antifungal | The best effect of EO against C. tropicalis was an MIC50 of 2.93 μL/mL. The ZOI against Aspergillus flavus at a concentration of 500 μL/mL was (8.00 ± 3.00 mm), Botrytis cinerea at a concentration of 250 μL/mL was (9.67 ± 1.53 mm), and Penicillium citrinum at a concentration of 250 μL/mL was (7.33 ± 0.58). | [66] | |
| Insecticidal | EO showed insecticidal activity on Oxycarenus lavaterae. The best activity was found for a 100% concentration of EO. | [66] | |
| Anti-trypanosomal | Water, methanolic, chlorophorm, and n-hexane extracts have activity against protozoan Trypanosoma brucei rhodesiense with an IC50 of 10.31, 6.44, 4.4, and 2.4 μg/mL, and for Trypanosoma cruzi, >90, 56.82, 52.51, and 18.17 μg/mL, respectively. | [89] | |
| Anti-leishmanial | Water, methanolic, chlorophorm, and n-hexane extracts have activity against protozoan Leishmania spp. with an IC50 of 47.88, 12.95, 8.31, and 5.25 μg/mL, respectively. | [89] | |
| Anti-plasmodial | Water, methanolic, chlorophorm, and n-hexane extracts have activity against protozoan Plasmodium falciparum with an IC50 of >20, 6.6, 2.54, and 3.78 μg/mL, respectively. | [89] | |
| S. dumetorum | Cytotoxic | Hexane extract at a concentration of 10 μg/mL showed cell proliferation on the MCF-7 cancer cell line of 27.8 ± 1.0%, while chloroform extract has activity on the A431 cell line of 41.1 ± 0.9%. | [90] |
| Antimicrobial | A 30% flower ultrasonic extract has the most pronounced activity towards S. aureus with a ZOI of 35 ± 1 mm, 40% leaf ultrasonic extract towards Bacillus subtilis with a ZOI of 49 ± 1 mm, and 70% leaf ultrasonic extract towards E. coli with a ZOI of 24 ± 1 mm. | [91] | |
| S. verticillata | Antioxidant | The IC50 for extract in the DPPH test was 27.36 ± 0.32 μg/mL, ABTS 13.40 ± 0.10 μg/mL, 19.75 ± 0.02 μg/mL. | [27] |
| Methanolic extract has antioxidant capabilities in the DPPH (58.05 mg AAE/g DW) and FRAP (41.38 μmol Fe++/g DW) assays. | [34] | ||
| The scavenging potential of the hydromethanolic extract towards the DPPH free radical (IC50 = 15.8 μg/mL) was higher than the BHT (IC50 = 94.6 μg/mL). | [36] | ||
| The antioxidant potential of methanolic and dichlormethane extracts in the DPPH assay was 16.0 ± 2.12 and 21.3 ± 0.86 mg/mL, and in the β-carotene/linoleic acid assay was 61.3 ± 5.38 and 30.4 ± 1.21 mg/mL. | [37] | ||
| The scavenging potential of methanolic extract for the DPPH assay was IC50 33.04 ± 5.83 μg/mL, ABTS+ assay was IC50 67.01 ± 13.62 μg/mL, NO radical scavenging assay was IC50 73.12 ± 19.04 μg/mL, and MDA assay was 58.07 ± 9.72 μg/mL. | [38] | ||
| Five assays were used to determine the antioxidant capacity of the chloroform and petroleum ether extracts. Chloroform extract exhibited higher values in the FRAP and CUPRAC assays, measured as 50.22 ± 0.65 μgTE/mg and 69.90 ± 0.36 μgTE/mg, than petroleum ether extract, 30.12 ± 0.45 μgFE/mg and 38.58 ± 0.59 μgFE/mg. Results in ABTS, DPPH, and TRP fluorometric assays correlated with each other. | [92] | ||
| Leaf ethanolic extract showed a high potency of free radical scavenging in DPPH assay with an IC50 value of 2.49 µg/mL and demonstrated the strongest antioxidative properties equal to Trolox (IC50 = 2.50 µg/mL). The ethanolic extract of leaves possesses NO radical scavenging activity at concentrations of 100, 200, and 400 µg/mL with IC50 65.04 + 3.13, 80.17 + 3.46, and 81.55 + 1.72 µg/mL, respectively. | [93] | ||
| Antimicrobial | The MIC values of the extract were 1.25 mg/mL against S. aureus and 2.5 mg/mL against S. pneumoniae, E. coli, P. aeruginosa, and C. albicans. | [27] | |
| Methanolic extract was very active on B. cereus with growth inhibition at a concentration of 1.25 mg/mL. | [38] | ||
| Petroleum ether had activity against Salmonella Enteritidis, E. coli, E. aerogenes, Enterococcus faecalis, and S. aureus with an MIC value of 6.25 mg/mL, while chloroform extract had the same MIC value against B. cereus. | [92] | ||
| Cytotoxic | A slight increase was found in the number of viable cells at tested doses for the extract on MCF-7 and MDA-MB-231 cell lines. | [27] | |
| The S. verticillata extract was tested at concentrations of 10, 25, and 50 μg/mL. High concentrations of the extract at 50 μg/mL exhibit significant cytotoxicity in a dose-dependent manner on the CBPI compared to the control. MMC cells treated with extract exhibit cytotoxicity in all concentrations. | [36] | ||
| EO has demonstrated cytotoxic activity in HT-29, T-47D, Caco-2, and NIH-3T3 cell lines with IC50 90.90 ± 14.88, 80.20 ± 8.91, 125.12 ± 27.59, and 81.81 ± 3.47 μg/mL, respectively. | [77] | ||
| Chloroform extract at a concentration of 10 μg/mL showed cell proliferation on MCF7 and A431 cancer cell lines of 30.9 ± 0.6 and 48.3 ± 1.5%, while hexane extract has activity only on the A431 cell line at 32.1 ± 0.6%. | [90] | ||
| The IC50 value for chloroform extracts was on MDA-MB-231 77.16 μg/mL and HCT 116 105.08 μg/mL cell lines; for petroleum ether extract, the IC50 value was 30.90 μg/mL for the MDA-MB-231 cell line and 44.28 μg/mL for the HCT 116 cell line. | [92] | ||
| The methanolic extract of leaves was studied in an MTT assay on human cancer cell lines (MCF-7, SH-SY5Y, and HL-60) with an IC50 of 166.3 ± 2.4, 72.8 ± 1.9, and 127.8 ± 2.7 μg/mL, respectively. | [94] | ||
| Genotoxic and antigenotoxic | Cells treated with hydromethanolic extract at concentrations of 10, 25, and 50 μg/mL showed low MN frequencies compared to control, thus indicating the absence of genotoxicity. | [36] | |
| Antifungal | The lowest MIC value of methanolic extract was found on Penicillium canescens (5 mg/mL), C. albicans, and Fusarium oxysporum (10 and 20 mg/mL, respectively). | [38] | |
| The MIC of the leaf, rootstock, and the combined ethanol extracts ranged from 3.12 to 25, 6.25 to 25, and 1.56 to 12.5 mg/mL, respectively. The combined extracts have shown strong antifungal effect against Cryptococcus laurentii, Cryptococcus neoformans, and Geotrichum capitatum with MIC values of 1.56 mg/mL, followed by C. glabrata with an MIC value of 3.12 mg/mL, and C. albicans and Candida guillermondii with MIC values of 6.25 mg/mL. Candida tropicalis, C. parapsilosis, Debaryomyces hansenii, and Kluyveromyces fragilis have shown a moderate activity with an MIC value of 12.5 mg/mL. | [95] | ||
| Enzyme inhibitory | The inhibition of the AChE of ethanolic leaf extract with an IC50 of 1600 μg/mL was 51.30%, while less concentrations were inactive. | [93] | |
| S. aethiopis | Antioxidant | The IC50 DPPH of methanolic extract was 123.37 ± 8.05 μg plant extracted or μg eq-QE/1 mL 10−4 M DPPH. | [26] |
| The IC50 for extract in the DPPH test was 146.6 ± 1.1 μg/mL, ABTS 59.16 ± 0.05 μg/mL, 80.02 ± 0.05 μg/mL. | [27] | ||
| MDA values of 521.5 ± 16.2 pmol/mg of the extract were found to be significantly higher compared to reference vitamin C 105.2 ± 15.8 pmol/mg. The DPPH scavenger and FRAP activities had significantly lower levels for the extract, quantified as 29.52 ± 4.7% and 26.23 ± 3.8 mg-trolox/g, respectively. | [39] | ||
| In the CUPRAC assay, the water extract showed an activity similar to ascorbic acid. In the FRAP assay, the ethanol extract showed higher reducing power at a concentration of 30 μg/mL, the same as BHT. In the DPPH assay, it showed low antioxidant activity. | [40] | ||
| The 80% methanolic extract was the most efficient in the DPPH antioxidant assay with IC50 23.79 ± 0.42 μg/mL, while 60% ethanolic extract expressed the best anti-lipoperoxidant activity in the β-carotene/linoleic acid assay at 37.82 ± 1.45 μg/mL. | [41] | ||
| Antimicrobial | The methanolic extract has an MIC against Salmonella Typhi of 5 mg/mL and against K. pneumoniae of 10 mg/mL. | [26] | |
| The extract indicated activity against S. aureus, P. aeruginosa, and S. pneumoniae with MIC values of 2.5 mg/mL and against E. coli and C. albicans with MIC values of 5 mg/mL. | [27] | ||
| ZOIs of the extract for S. aureus, E. aerogenes, E. coli, and P. aeruginosa were 12 ± 0.00, 10 ± 0.47, 10 ± 0.00, and 9 ± 0.47 mm, respectively. | [40] | ||
| The EO MIC assessed for the Staphylococcus strain was at 0.25% oil concentration. | [96] | ||
| Cytotoxic | It was found that there was a slight increase in the number of viable cells at tested doses for the extract on MCF-7 and MDA-MB-231 cell lines. | [27] | |
| Hexane extract at a concentration of 10 μg/mL showed cell proliferation on MCF7 and A431 cancer cell lines of 33.5 ± 1.5 and 20.3 ± 1.3%. | [90] | ||
| Anti-inflammatory | In heat-induced hemolysis, proteinase inhibitory activity, and albumin denaturation in vitro assays, low anti-inflammatory activity was found compared to the IC50 value of a standard drug. | [39] | |
| Inhibition of cannabinoid and opioid receptors | The ethanolic extract of aerial parts showed a moderate level of inhibition in cannabinoid (CB1—37.0% displacement and CB2—31.0% displacement) and opioid (Delta—46.3%, Kappa—45.3%, and Mu—32.9% displacement) receptors. | [80] | |
| Enzyme inhibitory | The EO inhibited 46.4 ± 0.8% (n = 3) of AChE activity, at 1 mg/mL. | [97] | |
| S. deserta | Cytotoxic | The cytotoxic effects of conventional and ultrasonic extracts on HDFn using the MTT assay do not cause a significant toxic effect on human fibroblasts. | [32] |
| Antithrombotic | Ethanolic root extract had significant inhibitory effects on ADP-induced maximum platelet aggregation rate (10.2 ± 2.6 vs. control 35.7 ± 5.2), reduced the FeCl3-induced rat common carotid artery thrombus weight and thrombus area ratio, significantly decreased plasma TXB2, vWF, and PAI-1 levels and increased 6-keto-PGF1α and t-PA levels in a dose-dependent manner. Thus, the ratio of TXB2/6-keto-PGF1α was significantly decreased, while the ratio of t-PA/PAI-1 was significantly increased. Enhanced AT-III and PC activities indicated coagulation inactivation effects of ethanolic root extracts. | [98] | |
| Anti-plasmodial | Diterpenoids from the root extract possessed weak activity with values of 1.6 mg/L. | [99] | |
| Immunomodulatory | No significant difference was found between conventional and ultrasonic extracts, both significantly suppressed the level of TNF-α production and LPS-activated macrophages. The measurement of IL-1β levels in intact macrophages showed that the ultrasonic extract caused a significant increase in IL-1β level compared to the control and conventional extract. | [32] | |
| Cleropane diterpenoids isolated from aerial part possessed a terminal α,β-unsaturated-γ-lactone moiety, and were assayed for their immunosuppressive activity via inhibiting the secretion of cytokines TNF-α and IL-6 in macrophages RAW264.7. Among them, (5R,8R,9S,10R)-18-nor-cleroda-2,13-dien-16,15-olide-4-one obviously suppressed the secretion of TNF-α and IL-6 with IC50 values of 8.55 and 13.65 μM, respectively. | [100] | ||
| Anti-leishmanial | Taxodione isolated from root extract had significant anti-leishmanial activity with an IC50 value of 1.46 μM (0.46 mg/L) against Leishmania donovani. | [99] | |
| Antibacterial | Phenolic abietane diterpene isolated from root extract called ferruginol showed the strongest activity against Streptococcus iniae. | [99] | |
| Antifungal | Taxodione isolated from the root extract had IC50 values of 3.0 μM (0.93 mg/L) and 8.5 μM (2.67 mg/L) against C. neoformans and Candida glabrata, respectively. | [99] | |
| Antimicrobial | Taxodinone and ferruginol isolated from the root extract showed antibacterial activity against S. aureus and MRSA with IC50 values from 8.8 to 14.0 μM (2.78 to 4.00 mg/L) and from 8.4 to 9.5 μM (2.63 to 2.71 mg/L), respectively. | [99] | |
| S. trautvetteri | No data available. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levaya, Y.; Atazhanova, G.; Gabe, V.; Badekova, K. A Review of Botany, Phytochemistry, and Biological Activities of Eight Salvia Species Widespread in Kazakhstan. Molecules 2025, 30, 1142. https://doi.org/10.3390/molecules30051142
Levaya Y, Atazhanova G, Gabe V, Badekova K. A Review of Botany, Phytochemistry, and Biological Activities of Eight Salvia Species Widespread in Kazakhstan. Molecules. 2025; 30(5):1142. https://doi.org/10.3390/molecules30051142
Chicago/Turabian StyleLevaya, Yana, Gayane Atazhanova, Vika Gabe, and Karakoz Badekova. 2025. "A Review of Botany, Phytochemistry, and Biological Activities of Eight Salvia Species Widespread in Kazakhstan" Molecules 30, no. 5: 1142. https://doi.org/10.3390/molecules30051142
APA StyleLevaya, Y., Atazhanova, G., Gabe, V., & Badekova, K. (2025). A Review of Botany, Phytochemistry, and Biological Activities of Eight Salvia Species Widespread in Kazakhstan. Molecules, 30(5), 1142. https://doi.org/10.3390/molecules30051142







