Metal Complexes of Bispidine Derivatives: Achievements and Prospects for the Future
Abstract
1. Introduction

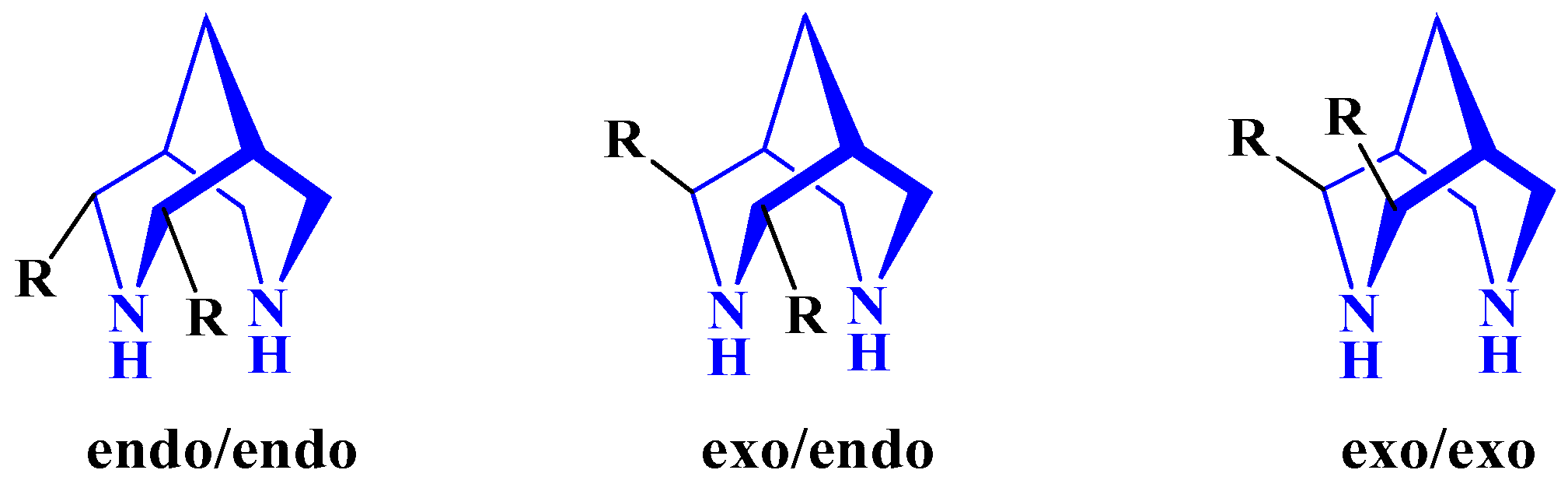
2. The Structure of Bispidine Ligands
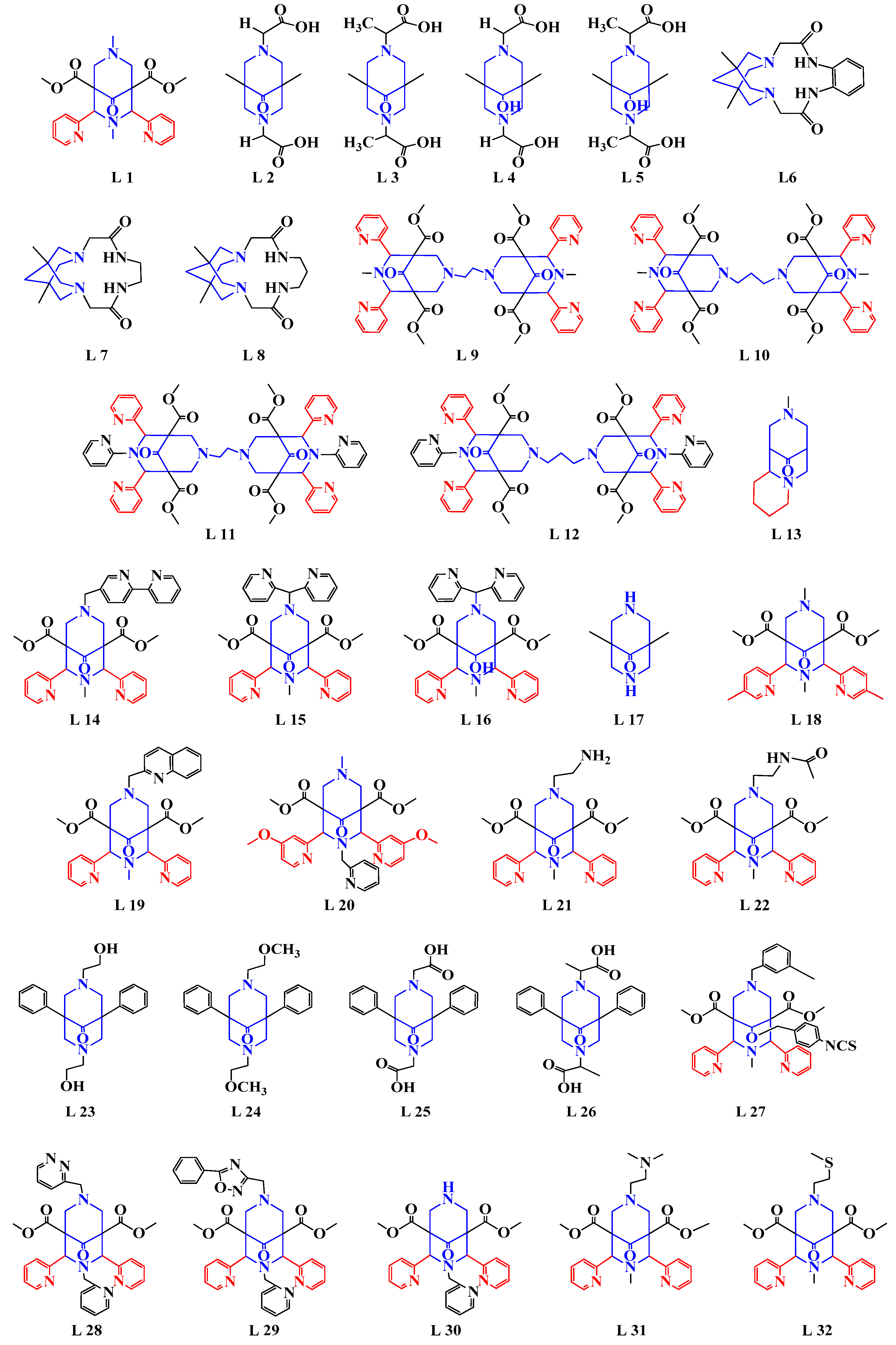
3. Synthesis of Metal Complexes with a Fragment of Bispidine
3.1. Bispidine Complexes of d-Block Transition Metals
3.2. Complexes of p-Block Elements with Bispidines
3.3. Bispidine-Containing Lanthanide Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, H. Degradation of dichloromethane by bispidine. J. Phys. Org. Chem. 2012, 25, 814–827. [Google Scholar] [CrossRef]
- Mukherjee, G.; Sastri, C.V. Eccentricities in spectroscopy and reactivity of non-heme metal intermediates contained in bispidine scaffolds. Isr. J. Chem. 2020, 60, 1032–1048. [Google Scholar] [CrossRef]
- Mukherjee, G.; Velmurugan, G.; Kerscher, M.; Satpathy, J.K.; Sastri, C.V.; Comba, P. Mechanistic insights into amphoteric reactivity of an iron-bispidine complex. Chem. Eur. J. 2024, 30, e202303127. [Google Scholar] [CrossRef]
- Börzel, H.; Comba, P.; Hagen, K.S.; Kerscher, M.; Pritzkow, H.; Schatz, M.; Schindler, S.; Walter, O. Copper-bispidine coordination chemistry: Syntheses, structures, solution properties, and oxygenation reactivity. Inorg. Chem. 2002, 41, 5440–5452. [Google Scholar] [CrossRef]
- Rosetti, A.; Lippi, M.; Marti-Rujas, L.; Sacchetti, A.; Cametti, M. Highly dynamic and tunable behavior of 1D coordination polymers based on the bispidine ligand. Chem. Eur. J. 2018, 24, 19368–19372. [Google Scholar] [CrossRef]
- Walther, M.; Matterna, M.; Juran, S.; Fähnemann, S.; Stephan, H.; Kraus, W.; Emmerling, F. Imidazole-containing bispidine ligands: Synthesis, structure and Cu(II) complexation. Z. Naturforsch. 2011, 66b, 721–728. [Google Scholar] [CrossRef]
- Li, G.; Wang, R.; Ye, D.; Pu, M.; Feng, X.; Lin, L. Bispidine-based S,N-chiral ligands for palladium-catalyzed asymmetric arylation of cyclic N-sulfonyl ketimines. Eur. J. Org. Chem. 2024, 27, e202400008. [Google Scholar] [CrossRef]
- Comba, P.; Starke, M.; Wadepohl, H. Optimization of hexadentate bispidine ligands as chelators for 64CuII PET imaging. ChemPlusChem 2018, 83, 597–604. [Google Scholar] [CrossRef]
- Roux, A.; Gillet, R.; Huclier-Markai, S.; Ehret-Sabatier, L.; Nonat, A.M.; Charbonnière, L. Bifunctional bispidine derivatives for copper-64 labelling and Positron Emission Tomography. Org. Biomol. Chem. 2017, 15, 1475–1483. [Google Scholar] [CrossRef]
- Toàn, N.M.; Vágner, A.; Nagy, G.; Ország, G.; Nagy, T.; Csikos, C.; Váradi, B.; Sajtos, G.Z.; Kapus, I.; Szoboszlai, Z.; et al. [52Mn]Mn-BPPA-Trastuzumab: A promising HER2-Specific PET radiotracer. J. Med. Chem. 2024, 23, 8261–8270. [Google Scholar] [CrossRef]
- Kopp, I.; Cieslik, P.; Anger, K.; Josephy, T.; Neupert, L.; Velmurugan, G.; Gast, M.; Wadepohl, H.; Brühlmann, S.A.; Walther, M.; et al. Bispidine chelators for radiopharmaceutical applications with lanthanide, actinide and main group metal ions. Inorg. Chem. 2023, 62, 20754–20768. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, P.; Kubeil, M.; Zarschler, K.; Ullrich, M.; Brandt, F.; Anger, K.; Wadepohl, H.; Kopka, K.; Bachmann, M.; Pietzsch, J.; et al. Toward personalized medicine: One chelator for imaging and therapy with lutetium-177 and actinium-225. J. Am. Chem. Soc. 2022, 144, 21555–21567. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, A.; Rosetti, A. Synthesis of natural compounds based on the [3,7]-diazabicyclo[3.3.1]nonane (Bispidine) core. Eur. J. Org. Chem. 2021, 10, 1491–1507. [Google Scholar] [CrossRef]
- Erdemoglu, N.; Ozkan, S.; Duran, A.; Tosun, F. GC-MS analysis and antimicrobial activity of alkaloid extract from Genista vuralii. Pharm. Biol. 2009, 47, 81–85. [Google Scholar] [CrossRef]
- Vargas, F.V.; Ceja, L.M. Sparteine as an anticonvulsant drug: Evidence and possible mechanism of action. Seizure 2016, 39, 49–55. [Google Scholar] [CrossRef]
- Plettenberg, M.S.; Hofheim, S.L.; Taunusstein, P.N.; Kandern, M.W. Method for Producing 3,7-Diazabicyclo[3.3.1] Nonane. Compouds Patent US 2011/0152528 A1, 23 June 2011. Available online: https://worldwide.espacenet.com/patent/search/family/041278731/publication/US2011152528A1?q=US2011%2F0152528%20A1 (accessed on 23 June 2011).
- Kaldybayeva, A.B.; Yu, V.K.; Malmakova, A.E.; Li, T.; Ten, A.Y.; Seilkhanov, T.M.; Praliyev, K.D.; Yu, V.K.; Berlin, K.D. Complexes of 3-[3-(1H-imidazol-1-yl)propyl]-3,7-diazabispidines and β-cyclodextrin as coatings to protect and stimulate sprouting wheat seeds. Molecules 2022, 27, 7406. [Google Scholar] [CrossRef]
- Chigorina, E.A.; Dotsenko, V.V. The first example of 3,7-diazabicyclo[3.3.1]nonane synthesis by aminomethylation of Guareschi imide salts. Chem. Heterocycl. Comp. 2013, 48, 1722–1724. [Google Scholar] [CrossRef]
- Veremeeva, P.N.; Bovina, E.M.; Grishina, I.V.; Lapteva, V.L.; Palyulin, V.A.; Zefirov, N.S. Synthesis of amphiphilic diacyl derivatives of 3,7-diazabicyclo[3.3.1]nonan-9-one. Mendeleev Commun. 2018, 28, 25–26. [Google Scholar] [CrossRef]
- Haridas, V.; Sadanandan, S.; Sharma, Y.K.; Chinthalapalli, S.; Shandilya, A. Bispidine as a secondary structure nucleator in peptides. Tetrahedron Lett. 2012, 53, 623–626. [Google Scholar] [CrossRef]
- Norrehed, S.; Erdélyi, M.; Light, M.E.; Gogoll, A. Protonation-triggered conformational modulation of an N,N′-dialkylbispidine: First observation of the elusive boat–boat conformer. Org. Biomol. Chem. 2013, 11, 6292–6299. [Google Scholar] [CrossRef]
- Galasso, V.; Goto, K.; Miyahara, Y.; Kovač, B.; Klasinc, L. On the structure and spectroscopic properties of bispidine, N,N′-dimethylbispidine and a bis-bispidine macrocycle. Chem. Phys. 2002, 277, 229–240. [Google Scholar] [CrossRef]
- Nonat, A.M.; Roux, A.; Sya, M.; Charbonnière, L.J. 2,4-Substituted bispidines as rigid hosts for versatile applications: From κ-opioid receptor to metal coordination. Dalton Trans. 2019, 48, 16476–16492. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Jakob, M.; Kerscher, M. The tetradentate bispidine ligand dimethyl-(3,7-dimethyl-9-oxo-2,4-bis(2-pyridyl-3,7-diazabicyclo[3.3.1]nonane-1,5-dicarboxylate and its copper(II) complex. In Inorganic Syntheses—Coordination Compounds, 1st ed.; Rauchfuss, T.B., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 35, pp. 70–73. [Google Scholar] [CrossRef]
- Medved’ko, A.V.; Egorova, B.V.; Komarova, A.A.; Rakhimov, R.D.; Krut’ko, D.P.; Kalmykov, S.N.; Vatsadze, S.Z. Copper–bispidine complexes: Synthesis and complex stability study. ACS Omega 2016, 1, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Kubeil, M.; Pietzsch, J.; Rudolf, H.; Stephan, H.; Zarschler, K. Bispidine dioxotetraaza macrocycles: A new class of bispidines for (64) Cu PET imaging. Inorg. Chem. 2014, 53, 6698–6707. [Google Scholar] [CrossRef]
- Born, K.; Comba, P.; Daubinet, A.; Fuchs, A.; Wadepohl, H. Catecholase activity of dicopper(II)-bispidine complexes: Stabilities and structures of intermediates, kinetics and reaction mechanism. J. Biol. Inorg. Chem. 2007, 2, 36–48. [Google Scholar] [CrossRef]
- Breuning, M.; Hein, D.; Steiner, M.; Gessner, V.H.; Strohmann, C. Chiral 2-endo-substituted 9-oxabispidines: Novel ligands for enantioselective copper(II)-catalyzed Henry reactions. Chemistry 2009, 15, 12764–12769. [Google Scholar] [CrossRef]
- Busche, C.; Comba, P.; Mayboroda, A.; Wadepohl, H. Novel RuII complexes with bispidine-based bridging ligands: Luminescence sensing and photocatalytic properties. Eur. J. Inorg. Chem. 2010, 8, 1295–1302. [Google Scholar] [CrossRef]
- Comba, P.; Rudolf, H.; Wadepohl, H. Synthesis and transition metal coordination chemistry of a novel hexadentate bispidine ligand. Dalton Trans. 2015, 44, 2724–2736. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Semashko, V.S.; Manaenkova, M.A.; Krut´ko, D.P.; Nuriev, V.N.; Rakhimov, R.D.; Davlyatshin, D.I.; Churakov, A.V.; Howard, J.A.K.; Maksimov, A.L.; et al. New supramolecular synthons based on 3d transition metal complexes with bidentate bispidines: Synthesis and structural, spectroscopic, and electrochemical studies. Russ. Chem. Bull. 2014, 63, 895–911. [Google Scholar] [CrossRef]
- Comba, P.; Lang, C.; Lopez de Laorden, C.; Muruganantham, A.; Rajaraman, G.; Wadepohl, H.; Zajaczkowski, M. The mechanism of the (bispidine)copper(II)-catalyzed aziridination of styrene: A combined experimental and theoretical study. Chemistry 2008, 14, 5313–5328. [Google Scholar] [CrossRef]
- Comba, P.; Hunoldt, S.; Morgen, M.; Pietzsch, J.; Stephan, H.; Wadepohl, H. Optimization of pentadentate bispidines as bifunctional chelators for 64Cu positron emission tomography (PET). Inorg. Chem. 2013, 52, 8131–8143. [Google Scholar] [CrossRef] [PubMed]
- Vatsadze, S.Z.; Tyurin, V.S.; Zyk, N.V.; Churakov, A.V.; Kuz´mina, L.G.; Avtomonov, E.V.; Rakhimov, R.D.; Butina, K.P. Synthesis, crystal structures, and electrochemistry of CuII complexes with tetradentate N2O2 ligands derived from 3,7-diazabicyclo[3.3.1]nonan-9-one. Russ. Chem. Bull. 2005, 54, 1825–1835. [Google Scholar] [CrossRef]
- Kubeil, M.; Neuber, C.; Starke, M.; Arndt, C.; Loureiro, L.R.; Hoffmann, L.; Feldmann, A.; Bachmann, M.; Pietzsch, J.; Comba, P.; et al. 64Cu tumor labeling with hexadentate picolinic acid-based bispidine immunoconjugates. Chem. Eur. J. 2024, 30, e202400366. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, J.L.; Jeanneau, E.; Steinhoff, R.; Hasserodt, J. bispidine platform grants full control over magnetic state of ferrous chelates in water. Chem. Eur. J. 2013, 19, 8839–8849. [Google Scholar] [CrossRef]
- Sahoo, L.; Panwar, P.; Sastri, C.V.; Visser, S.P. Unraveling chlorite oxidation pathways in equatorially heteroatom-substituted nonheme iron complexes. ACS Org. Inorg. Au 2024, 4, 673–680. [Google Scholar] [CrossRef]
- Křižana, M.; Vinklárekb, J.; Erbenb, M.; Růžičkováb, Z.; Honzíčeka, J. Iron (II) complex with modified bispidine ligand: Synthesis and catalytic alkyd drying. Inorganica Chim. Acta 2019, 486, 636–641. [Google Scholar] [CrossRef]
- Börzel, H.; Comba, P.; Hagen, K.S.; Lampeka, Y.D.; Lienke, A.; Linti, G.; Merz, M.; Pritzkow, H.; Tsymbal, L.V. Iron coordination chemistry with tetra-, penta- and hexadentate bispidine-type ligands. Inorganica Chim. Acta 2002, 337, 407–419. [Google Scholar] [CrossRef]
- Comba, P.; Wadepohl, H.; Wiesner, S. Optimization of the efficiency of oxidation catalysts based on iron bispidine complexes. Eur. J. Inorg. Chem. 2011, 16, 2610–2615. [Google Scholar] [CrossRef]
- Cui, H.; Goddard, R.; Pörschke, K.R.; Hamacher, A.; Kassack, M.U. Bispidine analogues of cisplatin, carboplatin, and oxaliplatin. synthesis, structures, and cytotoxicity. Inorg. Chem. 2014, 53, 3371–3384. [Google Scholar] [CrossRef]
- Cui, H.; Goddard, R.; Pörschke, K.R.; Hamacher, A.; Kassack, M.U. Bispidin-9,9-diol analogues of cisplatin, carboplatin, and oxaliplatin: Synthesis, structures, and cytotoxicity. Inorg. Chem. 2016, 55, 2986–2997. [Google Scholar] [CrossRef]
- Rossetti, A.; Sacchetti, A.; Meneghetti, F.; Dugoni, G.C.; Mori, M.; Castellano, C. Synthesis and characterization of new triazole-bispidinone scaffolds and their metal complexes for catalytic applications. Molecules 2023, 28, 6351. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Goddard, R.; Pörschke, K. Synthesis and coordination chemistry of N,N-diallylbispidine. Organometallics 2011, 30, 6241–6252. [Google Scholar] [CrossRef]
- Haberberger, M.; Someya, C.I.; Company, A.; Irran, E.; Enthaler, S. Application of a nickel-bispidine complex as pre-catalyst for C(sp2)–C(sp3) bond formations. Catal. Lett. 2012, 142, 557–565. [Google Scholar] [CrossRef]
- Comba, P.; Grimm, L.; Orvig, C.; Rück, K.; Wadepohl, H. Synthesis and coordination chemistry of hexadentate picolinic acid based bispidine ligands. Inorg. Chem. 2016, 55, 12531–12543. [Google Scholar] [CrossRef]
- Roux, A.; Nonat, A.M.; Brandel, J.; Hubscher-Bruder, V.; Charbonnière, L.J. Kinetically inert bispidol-based Cu(II) chelate for potential application to 64/67Cu Nuclear medicine and diagnosis. Inorg. Chem. 2015, 54, 4431–4444. [Google Scholar] [CrossRef]
- Price, T.W.; Greenmana, J.; Stasiuk, G.J. Current advances in ligand design for inorganic positron emission tomography tracers 68Ga, 64Cu, 89Zr and 44Sc. Dalton Trans. 2016, 45, 15702–15724. [Google Scholar] [CrossRef]
- Gilpin, I.M.F.; Ullrich, M.; Wünsche, T.; Zarschler, K.; Lebeda, O.; Pietzsch, J.; Pietzsch, H.J.; Walther, M. Radiolabelled cyclic bisarylmercury: High chemical and in vivo stability for theranostics. Chem. Med. Chem. 2021, 16, 2645–2649. [Google Scholar] [CrossRef]
- Bruchertseifer, F.; Comba, P.; Martin, B.; Morgenstern, A.; Notni, J.; Starke, M.; Wadepohl, H. First-generation bispidine chelators for 213BiIII radiopharmaceutical applications. Chem. Med. Chem. 2020, 15, 1591–1600. [Google Scholar] [CrossRef]
- Price, T.W.; Yap, S.Y.; Gillet, R.; Savoie, H.; Charbonnière, L.J.; Boyle, R.W.; Nonat, A.M.; Stasiuk, G.J. Evaluation of a bispidine-based chelator for gallium-68 and of the porphyrin conjugate as PET/PDT theranostic agent. Chemistry 2020, 26, 7602–7608. [Google Scholar] [CrossRef]
- Choudhary, N.; Dimmling, A.; Wang, X.; Southcott, L.; Radchenko, V.; Patrick, B.O.; Comba, P.; Orvig, C. Octadentate oxine-armed bispidine ligand for radiopharmaceutical chemistry. Inorg. Chem. 2019, 58, 8685–8693. [Google Scholar] [CrossRef]
- Comba, P.; Jermilova, U.; Orvig, C.; Patrick, B.O.; Ramogida, C.F.; Rück, K.; Schneider, C.; Starke, M. Octadentate picolinic acid-based bispidine ligand for radiometal ions. Chemistry 2017, 23, 15945–15956. [Google Scholar] [CrossRef] [PubMed]
- Braun, F.; Comba, P.; Grimm, L.; Herten, D.; Pokrandt, B.; Wadepohl, H. Ligand-sensitized lanthanide(III) luminescence with octadentate bispidines. Inorganica Chim. Acta 2019, 484, 464–468. [Google Scholar] [CrossRef]
- Abad-Galán, L.; Cieslik, P.; Comba, P.; Gast, M.; Maury, O.; Neupert, L.; Roux, A.; Wadepohl, H. Excited state properties of lanthanide (III) complexes with a nonadentate bispidine ligand. Chemistry 2021, 27, 10303–10312. [Google Scholar] [CrossRef]
- Green, M.A.; Mathias, C.J.; Willis, L.R.; Handa, R.K.; Lacy, J.L.; Miller, M.A.; Hutchins, G.D. Assessment of Cu-ETS as a PET radiopharmaceutical for evaluation of regional renal perfusion. Nucl. Med. Biol. 2007, 34, 247–255. [Google Scholar] [CrossRef]
- Boros, E.; Packard, A.B. Radioactive transition metals for imaging and therapy. Chem. Rev. 2019, 119, 870–901. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Xu, X.; Zhao, M.; Zhang, B.; Deng, S.; Wu, Y. 64Cu-based radiopharmaceuticals in molecular imaging. Technol. Cancer Res. Treat. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Laforest, R.; Ghai, A.; Fraum, T.J.; Oyama, R.; Frye, J.; Kaemmerer, H.; Gaehle, G.; Voller, T.; Mpoy, C.; Rogers, B.E.; et al. First-in-humans evaluation of safety and dosimetry of 64Cu-LLP2A for PET imaging. J. Nucl. Med. 2023, 64, 320–328. [Google Scholar] [CrossRef]
- Comba, P.; Emmerling, F.; Jakob, M.; Kraus, W.; Kubeil, M.; Morgen, M.; Pietzsch, J.; Stephan, H. Copper(II) chemistry of the functionalized macrocycle cyclam tetrapropionic acid. Dalton Trans. 2013, 42, 6142–6148. [Google Scholar] [CrossRef]
- Häring, M.; Pettignano, A.; Quignard, F.; Tanchoux, N.; Día, D.D. Keratin Protein-Catalyzed Nitroaldol (Henry)Reaction and Comparison with Other Biopolymers. Molecules 2016, 21, 1122. [Google Scholar] [CrossRef]
- Comba, P.; Morgen, M.; Wadepohl, H. Tuning of the properties of transition-metal bispidine complexes by variation of the basicity of the aromatic donor groups. Inorg. Chem. 2013, 2, 6481–6501. [Google Scholar] [CrossRef] [PubMed]
- Brox, D.; Comba, P.; Herten, D.P.; Kimmle, E.; Morgen, M.; Rühl, C.L.; Rybina, A.; Stephan, H.; Storch, G.; Wadepohl, H. CuII-selective bispidine-dye conjugates. J. Inorg. Biochem. 2015, 148, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Kerscher, M.; Schiek, W. Bispidine coordination chemistry. Prog. Inorg. Chem. 2007, 55, 613–704. [Google Scholar] [CrossRef]
- Bleiholder, C.; Börzel, H.; Comba, P.; Ferrari, R.; Heydt, M.; Kerscher, M.; Kuwata, S.; Laurenczy, G.; Lawrance, G.A.; Lienke, A.; et al. Coordination chemistry of a new rigid, hexadentate bispidine-based bis(amine)tetrakis(pyridine) ligand. Inorg. Chem. 2005, 44, 8145–8155. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Morgen, M.; Wadepohl, H. First row transition metal complexes of a hexadentate pyrazole-based bispidine ligand. Polyhedron 2013, 52, 1239–1245. [Google Scholar] [CrossRef]
- Keinänen, O.; Fung, K.; Brennan, J.M.; Zia, N.; Harris, M.; Dam, E.; Biggin, C.; Hedt, A.; Stoner, J.; Donnelly, P.S.; et al. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 28316–28327. [Google Scholar] [CrossRef]
- Krasnovskaya, O.O.; Abramchuck, D.; Erofeev, A.; Gorelkin, P.; Kuznetsov, A.; Shemukhin, A.; Beloglazkina, E.K. Recent advances in 64Cu/67Cu-based radiopharmaceuticals. Int. J. Mol. Sci. 2023, 24, 9154. [Google Scholar] [CrossRef]
- Morgan, K.A.; Rudd, S.E.; Noor, A.; Donnelly, P.S. Theranostic nuclear medicine with gallium-68, lutetium-177, copper-64/67, actinium-225, and lead-212/203 radionuclides. Chem. Rev. 2023, 123, 12004–12035. [Google Scholar] [CrossRef]
- Comba, P.; Laorden, C.L.; Pritzkov, H. Tuning the properties of copper(II) complexes with tetra- and pentadentate bispidine (=3,7-diazabicyclo[3.3.1]nonane) ligands. Helv. Chim. Acta 2005, 88, 647–664. [Google Scholar] [CrossRef]
- Josephy, T.; Kumar, R.; Bleher, K.; Röhs, F.; Glaser, T.; Rajaraman, G.; Comba, P. Synthesis, characterization, and reactivity of bispidine-iron(IV)-tosylimido species. Inorg. Chem. 2024, 63, 12109–12119. [Google Scholar] [CrossRef]
- Bukowski, M.R.; Comba, P.; Lienke, A.; Limberg, C.; Laorden, C.L.; Mas-Balleste, R.; Merz, M.; Que, L. Katalytische epoxidierung und 1,2-dihydroxylierung von olefinen mit bispidin-eisen(II)/H2O2-Systemen. Angew. Chem. 2006, 118, 3524–3528. [Google Scholar] [CrossRef]
- Bautz, J.; Comba, P.; Laorden, C.L.; Menzel, M.; Rajaraman, G. Biomimetic high-valent non-heme iron oxidants for the cis-dihydroxylation and epoxidation of olefins. Angew. Chem. 2007, 119, 8213–8216. [Google Scholar] [CrossRef]
- Comba, P.; Maurer, M.; Vadivelu, P. Oxidation of cyclohexane by high-valent iron bispidine complexes: Tetradentate versus pentadentate ligands. Inorg. Chem. 2009, 48, 10389–10396. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Wunderlich, S. Iron-catalyzed halogenation of alkanes: Modeling of nonheme halogenases by experiment and DFT calculations. Chem. Eur. J. 2010, 16, 7293–7299. [Google Scholar] [CrossRef] [PubMed]
- Jaccob, M.; Comba, P.; Maurer, M.; Vadivelu, P.; Venuvanalingam, P. A combined experimental and computational study on the sulfoxidation by high-valent iron bispidine complexes. Dalton Trans. 2011, 40, 11276–11281. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Koten, G.K.; Gebbink, R.J.M. Mononuclear non-heme iron enzymes with the 2-His-1-carboxylate facial triad: Recent developments in enzymology and modeling studies. Chem. Soc. Rev. 2008, 37, 2716–2744. [Google Scholar] [CrossRef]
- Comba, P.; Fukuzumi, S.; Kotani, H.; Wunderlich, S. Elektronentransfereigenschaften eines effizienten Nichthäm-Eisenkatalysators mit einem vierzähnigen Bispidinligand. Angew. Chem. 2010, 122, 2679–2682. [Google Scholar] [CrossRef]
- Hong, S.; Lee, Y.-M.; Shin, W.; Fukuzumi, S.; Nam, W. Dioxygen activation by mononuclear nonheme iron(II) complexes generates iron–oxygen intermediates in the presence of an NADH analogue and proton. J. Am. Chem. Soc. 2009, 131, 13910–13911. [Google Scholar] [CrossRef]
- Touti, F.; Singh, A.K.; Maurin, P.; Canaple, L.; Beuf, O.; Samarut, J.; Hasserodt, J. An electroneutral macrocyclic iron(II) complex that enhances MRI contrast in vivo. J. Med. Chem. 2011, 54, 4274–4278. [Google Scholar] [CrossRef]
- Dorazio, S.J.; Tsitovich, P.B.; Siters, K.E.; Spernyak, J.A.; Morrow, J.R. Iron(II) PARACEST MRI contrast agents. J. Am. Chem. Soc. 2011, 133, 14154–14156. [Google Scholar] [CrossRef]
- Tinzl, M.; Diedrich, J.V.; Mittl, P.R.E.; Clémancey, M.; Reiher, M.; Proppe, J.; Latour, J.-M.; Hilvert, D. Myoglobin-catalyzed azide reduction proceeds via an anionic metal amide intermediate. J. Am. Chem. Soc. 2024, 146, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Kraut, D.A.; Carroll, K.S.; Herschlag, D. Challenges in enzyme mechanism and energetics. Annu. Rev. Biochem. 2003, 72, 517–571. [Google Scholar] [CrossRef] [PubMed]
- Dunham, N.P.; Arnold, F.H. Nature’s machinery, repurposed: Expanding the repertoire of iron-dependent oxygenases. ACS Catal. 2020, 10, 12239–12255. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, M.R.; Comba, P.; Limberg, C.; Merz, M.; Que, L.; Wistuba, T. Bispidin-ligandeneffekte in der eisen-wasserstoffperoxid-chemie. Angew. Chem. 2004, 116, 1303–1307. [Google Scholar] [CrossRef]
- Comba, P.; Fukuzumi, S.; Kotani, H.; Wunderlich, S. Electron-transfer properties of an efficient nonheme iron oxidation catalyst with a tetradentate bispidine ligand. Angew. Chem. Int. Ed. 2010, 49, 2622–2625. [Google Scholar] [CrossRef]
- Semenov, V.A.; Larina, L.I. Stereochemical and computational NMR survey of 1,2,3-triazoles: In search of the original tauto-conformers. J. Phys. Chem. A 2024, 128, 3231–3240. [Google Scholar] [CrossRef]
- Gillet, R.; Roux, A.; Brandel, J.; Huclier-Markai, S.; Camerel, F.; Jeannin, O.; Nonat, A.M.; Charbonnière, L.J. A Bispidol chelator with a phosphonate pendant arm: Synthesis, Cu(II) complexation, and 64Cu Labeling. Inorg. Chem. 2017, 56, 11738–11752. [Google Scholar] [CrossRef]
- Ndiaye, D.; Sy, M.; Pallier, A.; Même, S.; Silva, I.; Lacerda, S.; Nonat, A.M.; Charbonnière, L.J.; Tóth, É. Unprecedented kinetic inertness for a Mn2+−bispidine chelate: A novel structural entry for Mn2+−based imaging agents. Angew. Chem. Int. Ed. 2020, 59, 11958–11963. [Google Scholar] [CrossRef]
- Juran, S.; Walther, M.; Stephan, H.; Bergmann, R.; Steinbach, J.; Kraus, W.; Emmerling, F.; Comba, P. Hexadentate bispidine derivatives as versatile bifunctional chelate agents for copper(II) radioisotopes. Bioconjug. Chem. 2009, 20, 347–359. [Google Scholar] [CrossRef]
- Gao, F.; Sihver, W.; Bergmann, R.; Walther, M.; Stephan, H.; Belter, B.; Neuber, C.; Haase-Kohn, C.; Bolzati, C.; Pietzsch, J.; et al. Radiochemical and radiopharmacological characterization of a 64Cu-labeled α-MSH analog conjugated with different chelators. J. Label. Compd. Radiopharm. 2019, 62, 495–509. [Google Scholar] [CrossRef]
- Comba, P.; Haaf, C.; Lienke, A.; Muruganantham, A.; Wadepohl, H. Synthesis, structure, and highly efficient copper-catalyzed aziridination with a tetraaza-bispidine ligand. Chem. Eur. J. 2009, 15, 10880–10887. [Google Scholar] [CrossRef] [PubMed]
- Bleher, K.; Comba, P.; Gast, M.; Kronenberger, S.; Josephy, T. Copper-bispidine-catalyzed aziridination—A new twist in small molecule activation. Inorganica Chim. Acta 2022, 532, 120752. [Google Scholar] [CrossRef]
- Suslov, E.V.; Ponomarev, K.Y.; Patrusheva, O.S.; Kuranov, S.O.; Okhina, A.A.; Rogachev, A.D.; Munkuev, A.A.; Ottenbacher, R.V.; Dalinger, A.I.; Kalinin, M.A.; et al. Novel bispidine-monoterpene conjugates—Synthesis and application as ligands for the catalytic ethylation of chalcones. Molecules 2021, 26, 7539. [Google Scholar] [CrossRef] [PubMed]
- Scharnagel, D.; Müller, A.; Prause, F.; Eck, M.; Goller, J.; Milius, W.; Breuning, M. The first modular route to core-chiral bispidine ligands and their application in enantioselective copper(II)-catalyzed Henry reactions. Chem. Eur. J. 2015, 21, 12488–12500. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Lienke, A. Bispidine copper (II) compounds: Effects of the rigid ligand backbone. Inorg. Chem. 2001, 40, 5206–5209. [Google Scholar] [CrossRef]
- Comba, P.; Martin, B.; Muruganantham, A.; Straub, J. Structure, bonding, and catecholase mechanism of copper bispidine complexes. Inorg. Chem. 2012, 51, 9214–9225. [Google Scholar] [CrossRef]
- Comba, P.; Kuwata, S.; Linti, G.; Pritzkow, H.; Tarnai, M.; Wadepohl, H. Oxidative N-dealkylation in cobalt-bispidine-H2O2 systems. Chem. Commun. 2006, 19, 2074–2076. [Google Scholar] [CrossRef]
- Comba, P.; Pokrandt, B.; Wadepohl, H. Cobalt bispidine complexes: Oxidation with dioxygen. Aust. J. Chem. 2017, 70, 576–580. [Google Scholar] [CrossRef]
- Comba, P.; Kerscher, M.; Krause, T.; Schöler, H.F. Iron-catalysed oxidation and halogenation of organic matter in nature. Environ. Chem. 2015, 12, 381–395. [Google Scholar] [CrossRef]
- Barman, P.; Vardhaman, A.K.; Martin, B.; Wörner, S.J.; Sastri, C.V.; Comba, P. Influence of ligand architecture on oxidation reactions by high-valent nonheme manganese oxo complexes using water as a source of oxygen. Angew. Chem. Int. Ed. 2015, 54, 2095–2099. [Google Scholar] [CrossRef]
- Pollak, D.; Goddard, R.; Pörschke, K.-R. Synthesis and structures of 9-oxabispidine analogues of cisplatin, carboplatin, and oxaliplatin. Inorg. Chem. 2016, 55, 9424–9435. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Martin, B.; Sanyal, A.; Stephan, H. The computation of lipophilicities of 64Cu PET systems based on a novel approach for fluctuating charges. Dalton Trans. 2013, 42, 11066–11073. [Google Scholar] [CrossRef] [PubMed]
- Kubeil, M.; Martínez, I.I.S.; Bachmann, M.; Kopka, K.; Tuck, K.L.; Stephan, H. Dual-Labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives. Pharmaceuticals 2022, 15, 432. [Google Scholar] [CrossRef] [PubMed]



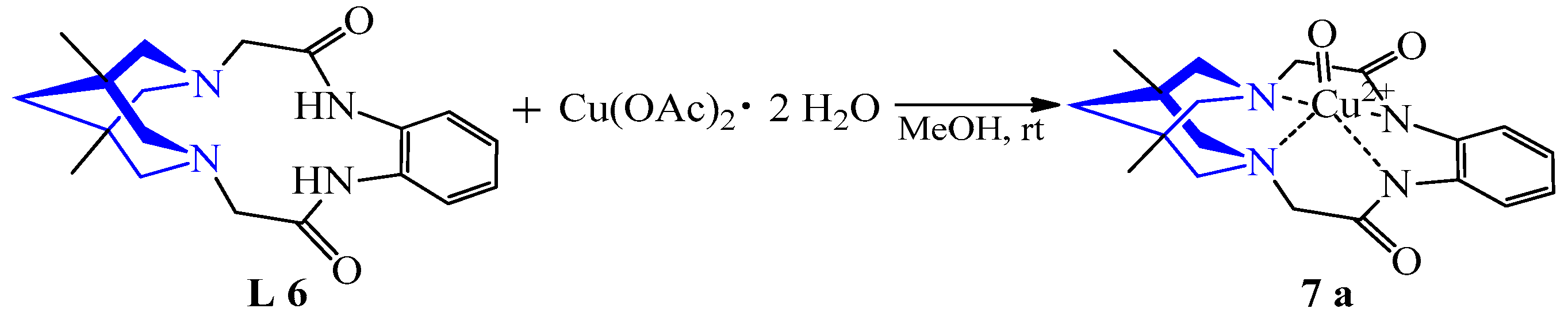
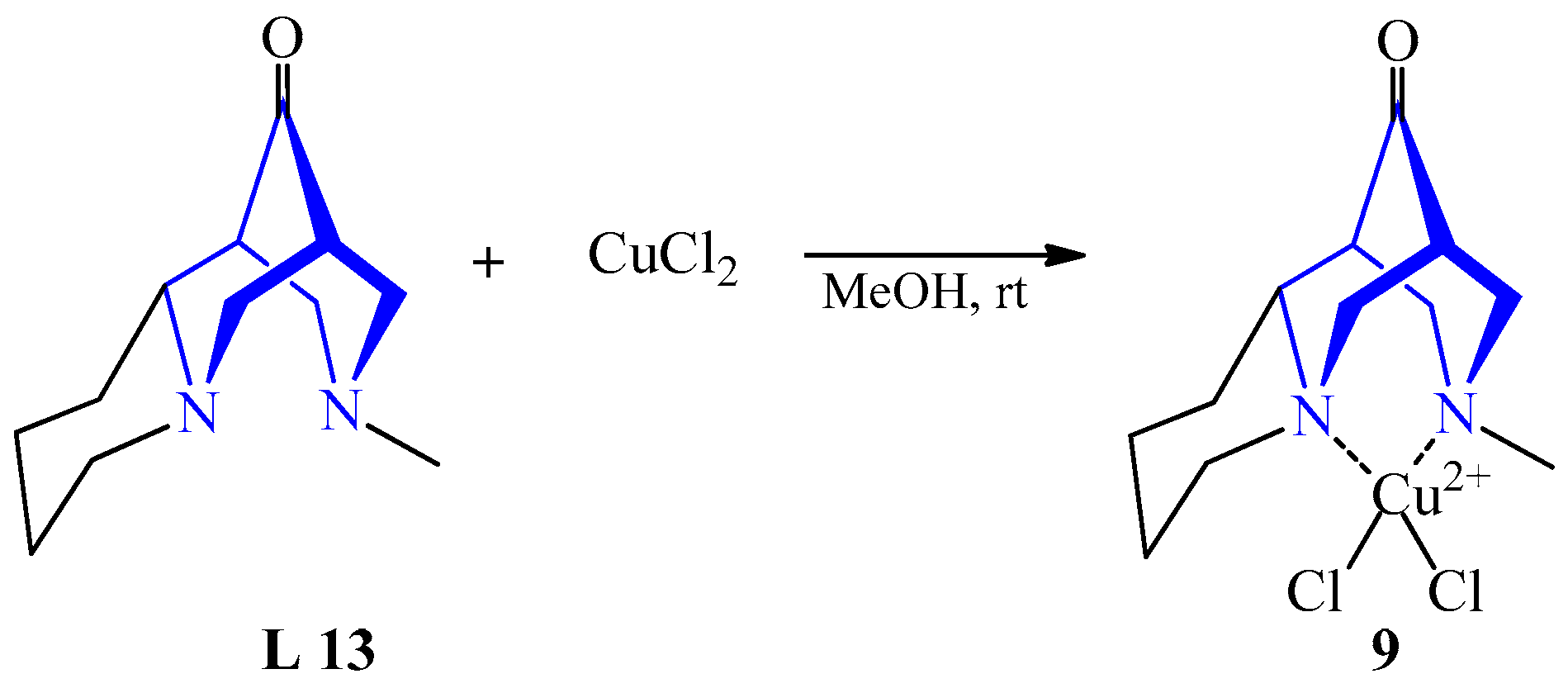
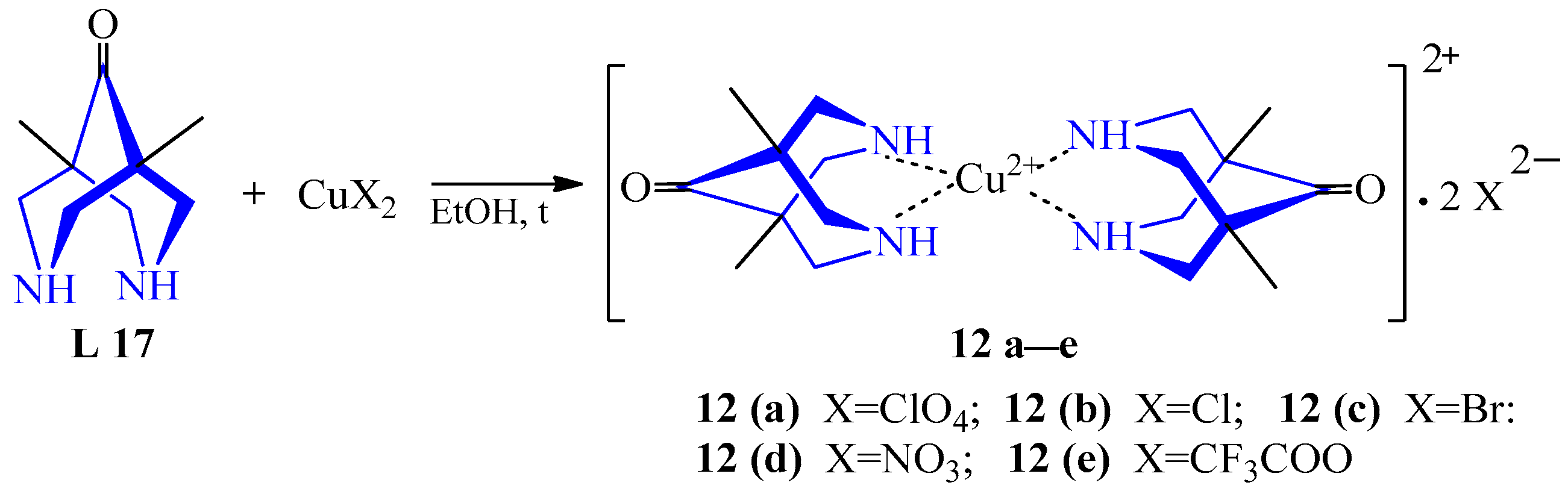





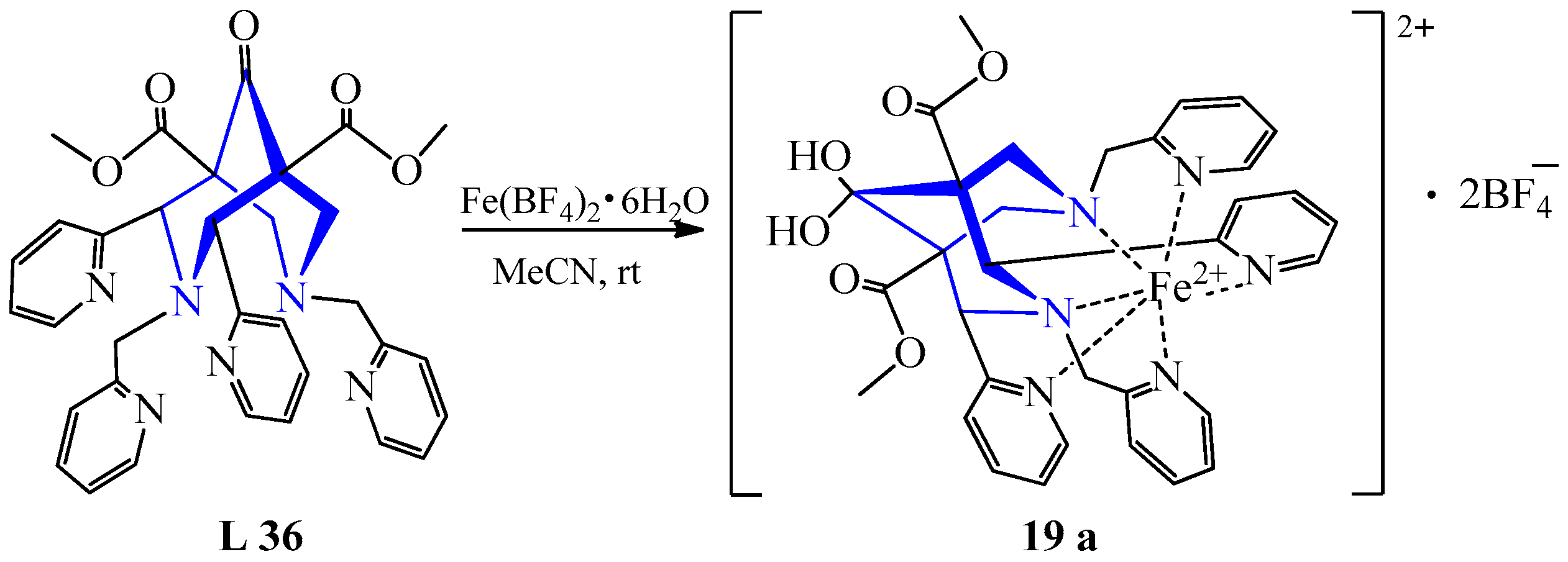




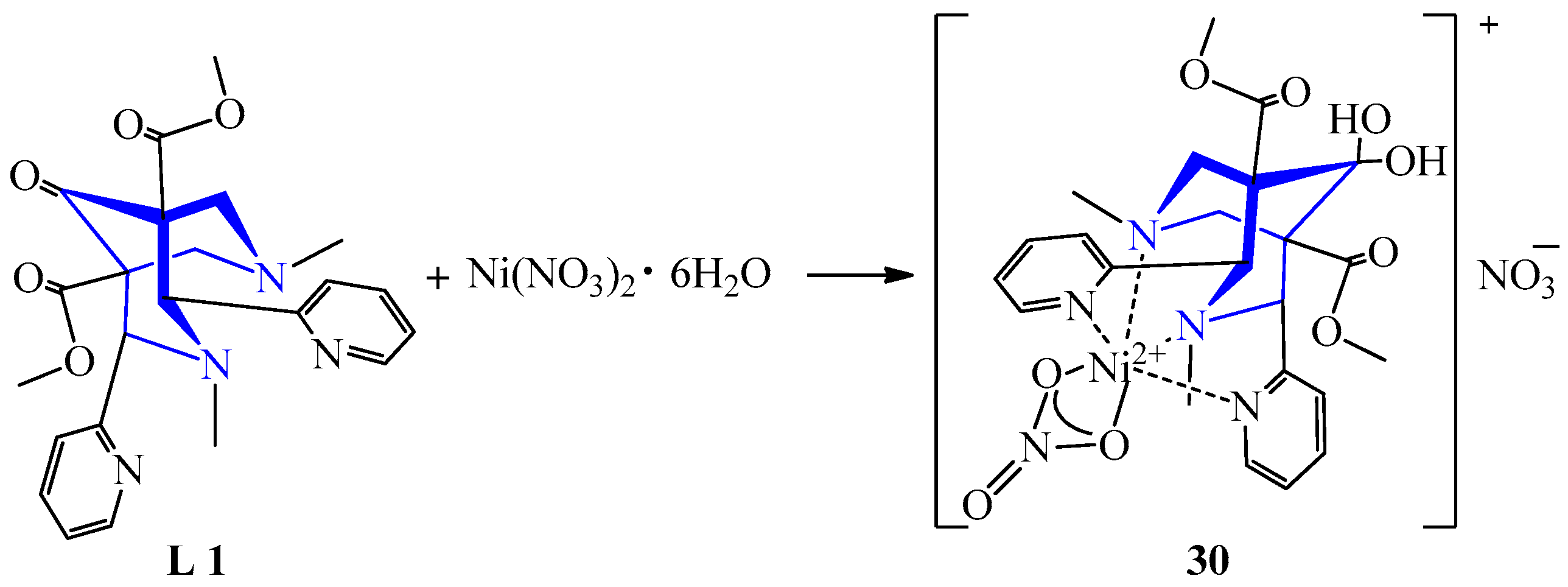



| No | Salts | Lig. (L) | Complex, Solvent | λmax {ε(M−1 cm−1)} | Yield, % | The Color of the Solid | Synthesis Method | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
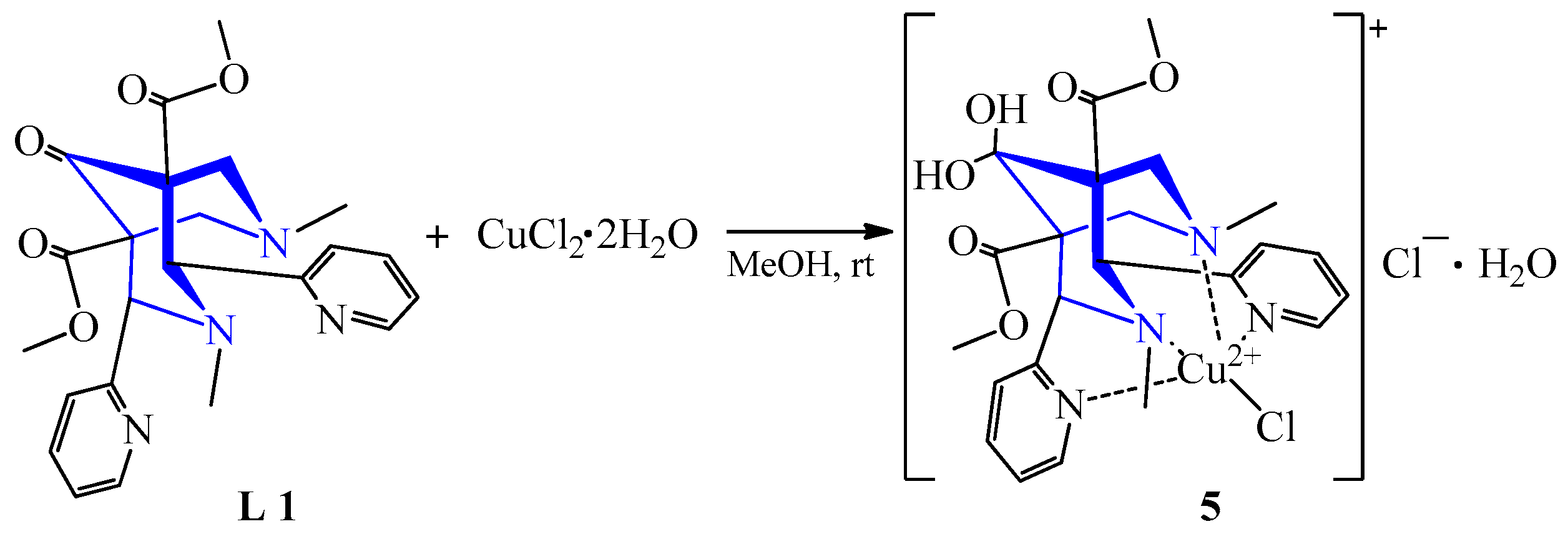
| 1 | CuCl2·2H2O | L 1 | [Cu(L)Cl]Cl·2H2O, MeOH | 656 {90} | 72 | Blue | Diffusion of ether | [24] | |
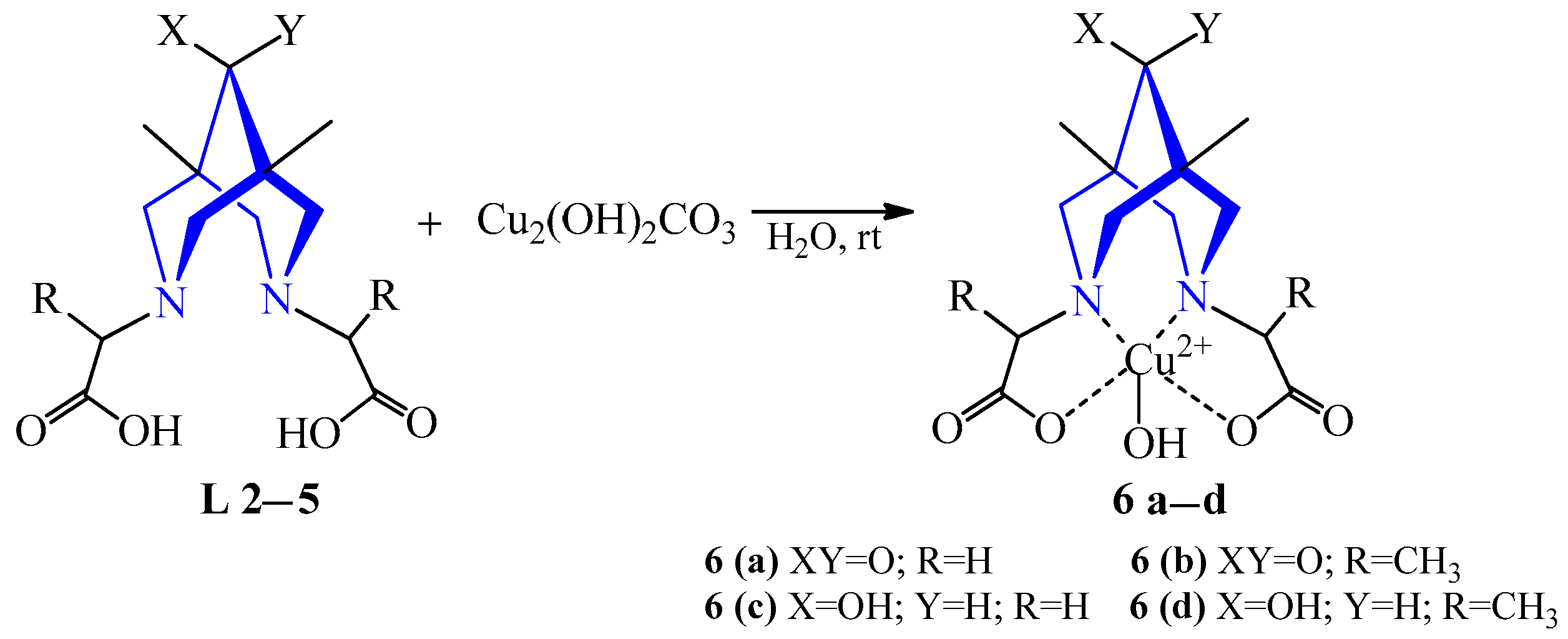
| 2 | Cu2(OH)2CO3 | L 2–5 | [L2Cu(OH)2], H2O | 602–612 {247–252} | Blue | Neutralization with acidic chelate N2O2 | Radiopharmaceuticals, positron emission tomography (PET) | [25] | |
| 3 | Cu(OAc)2·2H2O | L 6 L 7–8 | [Cu(L)(O)], MeOH [Cu(L)(OH2)], MeOH | 483 {149} 476 {117} 524 {189} | 56 | Red and purple | Recrystallization | 64Cu PET visualization | [26] |
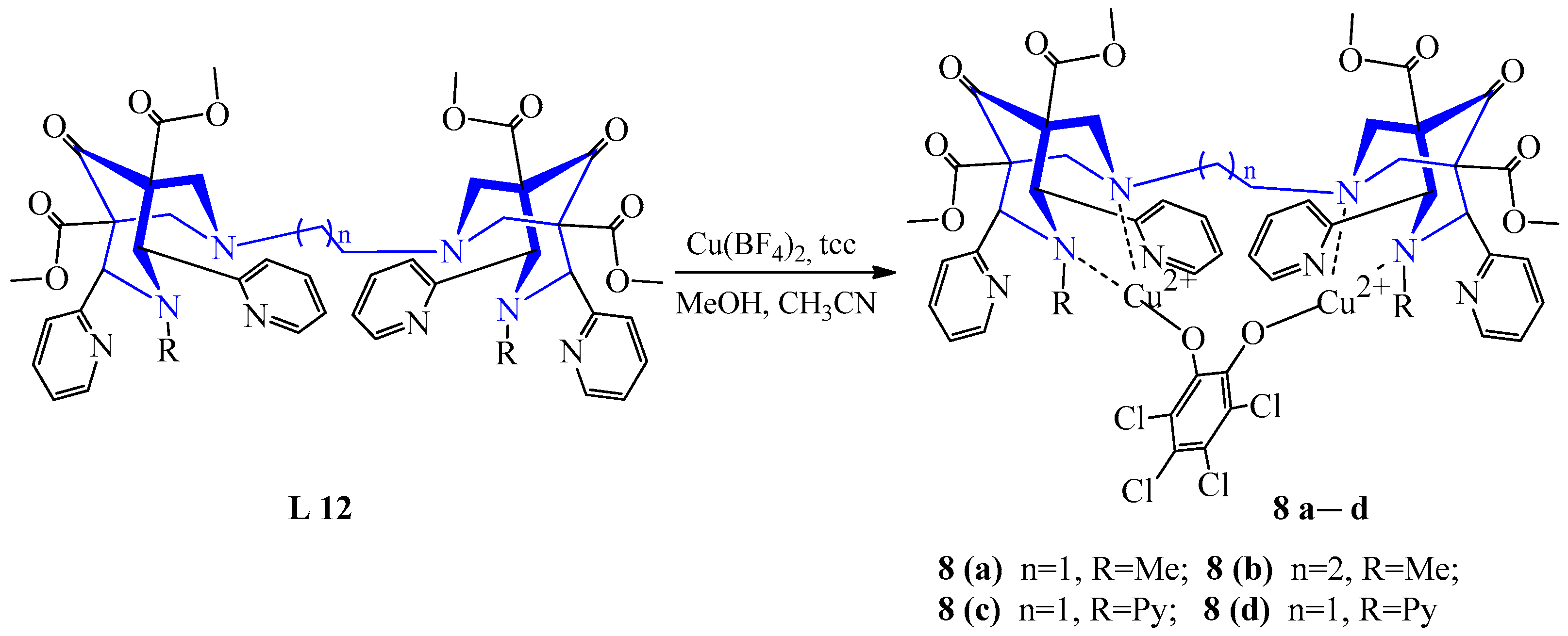
| 4 | Cu(BF4)2 | L 1, L 9–12 | [Cu(L)NCMe)]2+, MeCN [Cu2(L)NCMe)]4+, MeCN, MeOH | 450 {510} 453 {577} 456 {685} 468 {651} 532 {1640} | 58 | Catalytic enzymatic oxidation of catecholamines | [27] | ||
| 5 | CuCl2 | L 13 | [Cu(L)Cl]Cl, MeOH | 55 | Green | Recrystallization | The catalyst for Henry’s enantioselective reaction | [28] | |
| 6 | Cu(ClO4)2·6H2O | L 14 | [Cu(L)](ClO4)2, MeOH:H2O | 412 | 70 | Green | Heating, concentration at low pressure | The aziridination reaction | [29] |
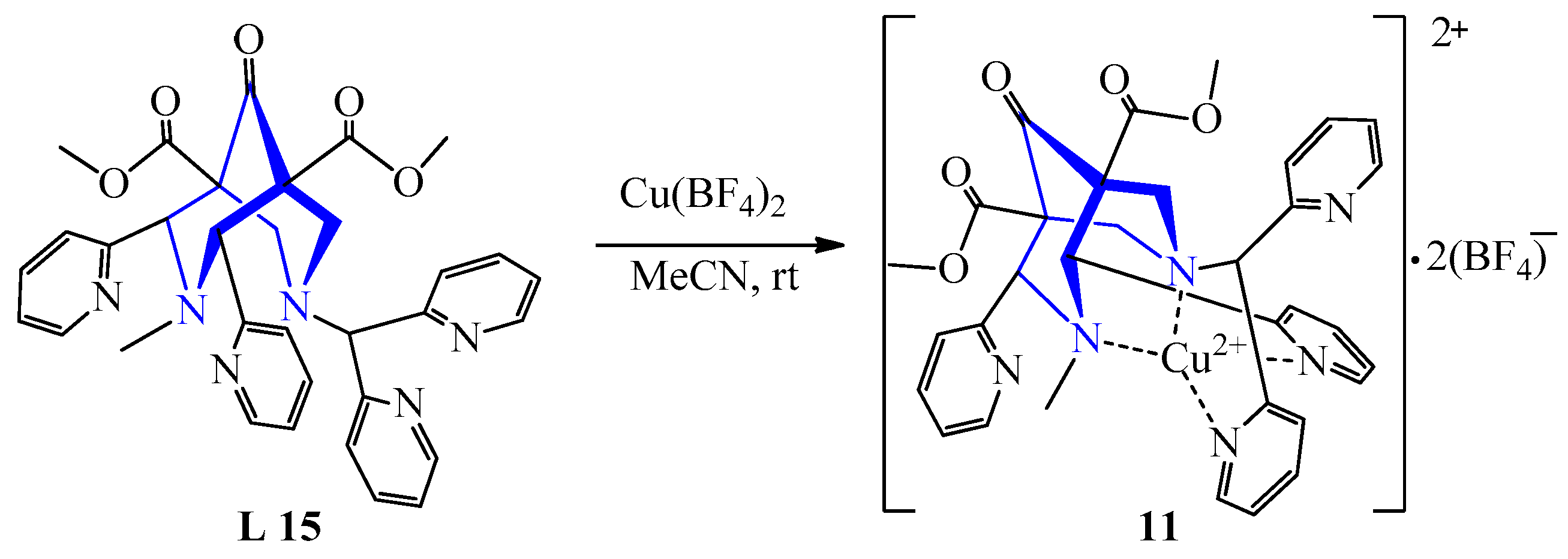
| 7 | Cu(BF4)2 | L 15 L 16 | [Cu(L)](BF4)2·3H2O, MeCN [Cu(L)](BF4)2·2H2O, MeCN | 626 {119} 630 {112} | 74 54 | Blue | Evaporation, diffusion of ether heating, diffusion of ether | The aziridination reaction | [30] |
| 8 | Cu(ClO4)2·6H2O CuCl2·2H2O CuBr2·2H2O Cu(NO3)2·3H2O Cu(CF3COO)2·4H2O | L 17 | [Cu(L)2](ClO4)2, EtOH [Cu(L)2Cl]Cl, EtOH [Cu(L)2Br]Br, EtOH [Cu(L)2](NO3)2, EtOH [Cu(L)2](CF3COO)2, EtOH | 284 {450} 273 {462} 272 {482} 288 {450} 284 {485} | 95 - 68 51 - | Pink Blue Purple Yellow | Concentration at low pressure, recrystallization | Development of supramolecular coordination polymers | [31] |
| 9 | Cu(CF3SO3)2 Cu(BF4)2·6H2O | L 1 L 18 L 19 | [Cu(L)](CF3SO3)2, MeCN [Cu(L)](BF4)2·3H2O, MeCN [Cu(L)](BF4)2·4H2O, MeCN | - 624 {25} 881 {60} | 83 88 89 | Diffusion of ether | The aziridination reaction | [32] | |
| 10 | Cu(ClO4)2·6H2O | L 20–21 L 22 | [Cu(L)](ClO4)2, MeCN [Cu(L)](ClO4), MeCN | 629 627 564 | 84 69 92 | Blue Blue Purple | Diffusion of ether | 64Cu PET visualization | [33] |
| 11 | CuCl2 Cu(ClO4)2 Cu(OH)2 | L 23 L 24 L 25 L 26 | [Cu(L)]Cl2, EtOH [Cu(L)](ClO4)2, CHCl3 [Cu(L)](OH)2, DMF | Blue Purple Dark- blue | Heating, recrystallization | Catalysts for enantioselective reactions | [34] | ||
| 12 | CuX2 | L 27 | [Cu(L)X]X | For the synthesis of radiopharmaceuticals based on Cu(II) ions | [35] | ||||
| 13 | Fe(BF4)2·6H2O Fe(ClO4)2·H2O Fe(BF4)2·6H2O | L 36 L 28 L 29 L 30 | [Fe(L)] 2BF4, MeCN [Fe(L)]·2ClO4, MeCN [Fe(L)(MeCN)]·2BF4, MeCN | 458 {5560} 455 {10,870} 443 {12,570} 444 {7144} | 71 36 25 17 | Red-black Dark-brown - Dark-brown | Diethyl ether diffusion, heating, recrystallization | For the design of responsive off–on probes | [36] |
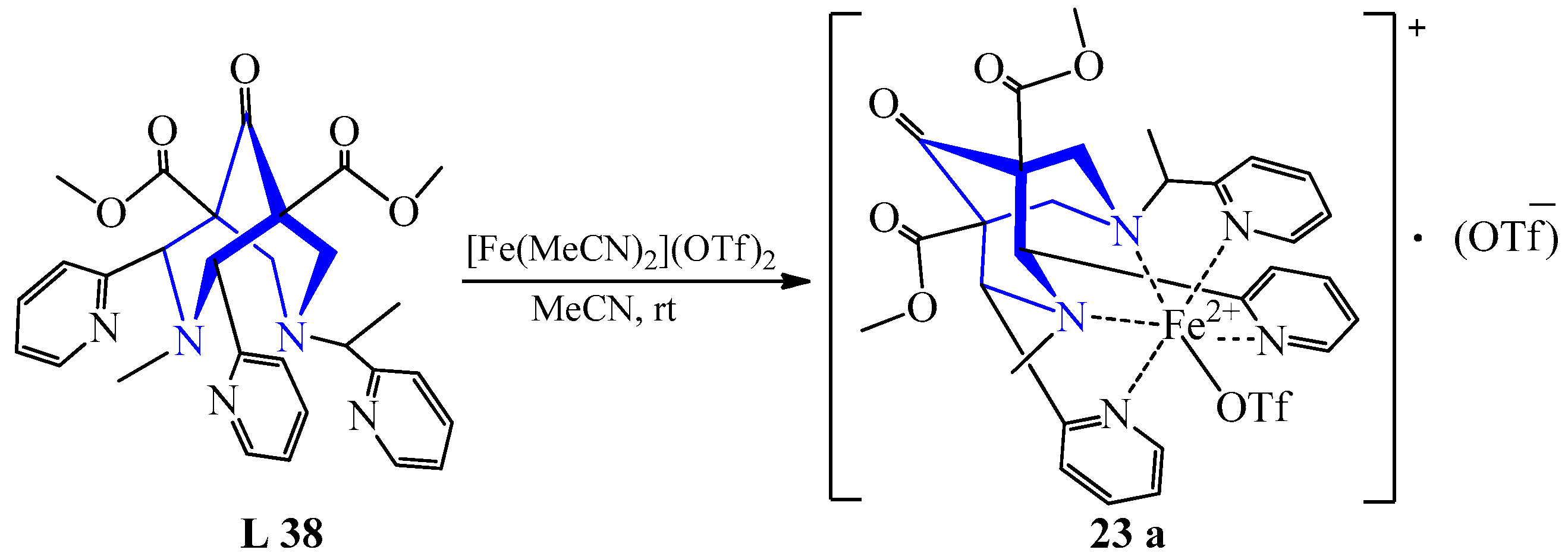
| 14 | Fe(MeCN)2(OTf)2 | L 31 L 32 L 33 | [Fe(L) MeCN)]2+, MeCN | 37 {1061] 380 {1057} 373 {1112} | 88 84 88 | Yellow Brown Light-yellow | Slow vapor diffusion with diethyl ether | For oxidizing ClO2− to ClO2 | [37] |
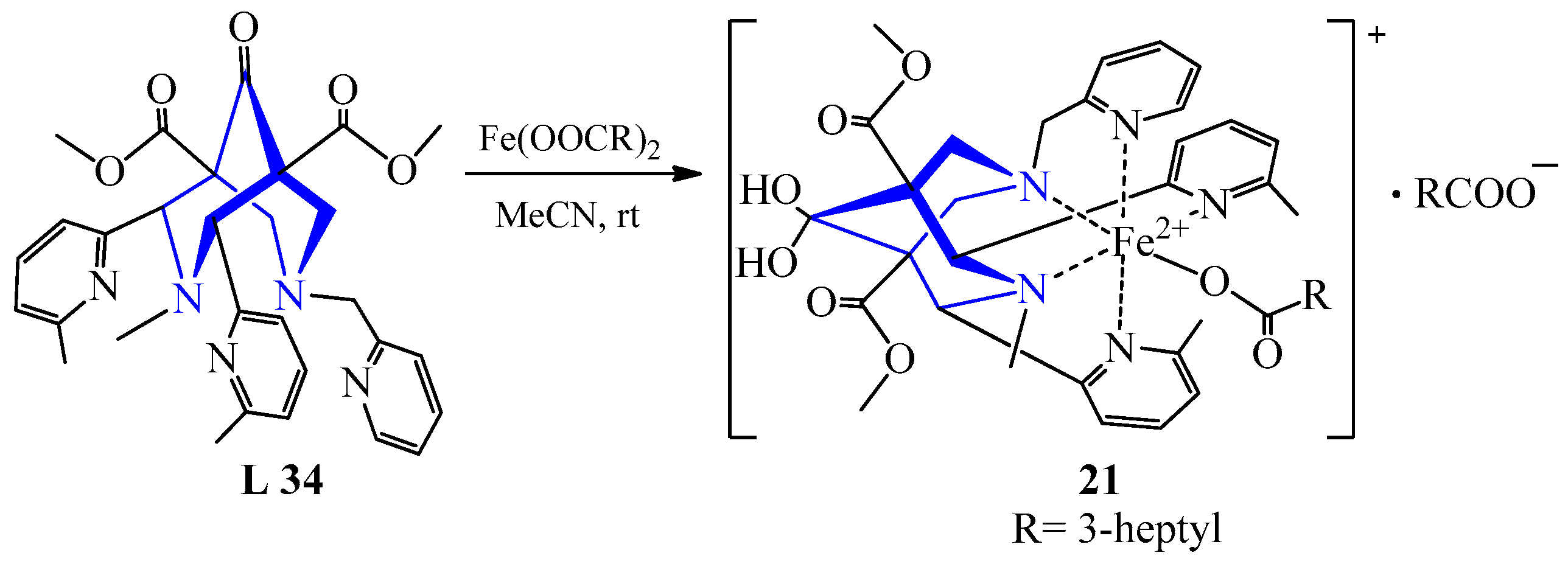
| 15 | Fe(OOCC7H15)2 | L 34 | [Fe(L)OOCC7H15] (OOCC7H15) | 400 | 57 | Canary | Evaporation, recrystallization | Catalyst for the auto-oxidation process | [38] |
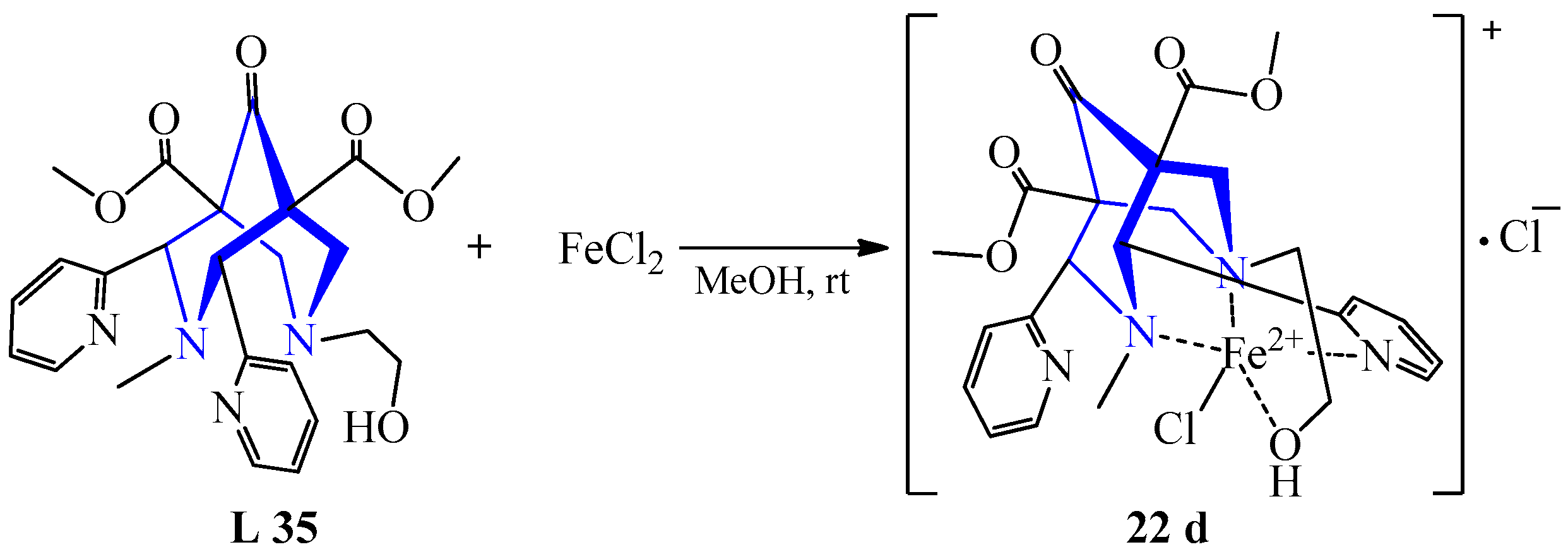
| 16 | [Fe(NCCH3)2·(CF3SO3)2] Fe(SCN)2 FeSO4 FeCl2 Fe(ClO4)2 FeCl2 | L 1 L 1 L 34 L 35 L 36 L 37 | [Fe(L)(CF3SO3)2], CD3CN [Fe(L)(SCN)2], CD3CN [Fe(L)SO4], MeOH [Fe(L)Cl]Cl, MeOH [Fe(L)](ClO4)2, MeOH [Fe(L)Cl]Cl, MeOH | 392 375 404 412 {1819} 564 {215} 579 {948} | 40–70 | Ether diffusion, recrystallization | Catalysts | [39] | |
| 17 | [Fe(NCCH3)2]·(CF3SO3)2 | L 38 L 39 | [Fe(L)(CF3SO3)] (CF3SO3), MeCN [Fe(L)(CF3SO3)] (CF3SO3)·3H2O, MeCN | 73 33 | Yellow | Recrystallization | A catalyst for bioinspired catalysis | [40] | |
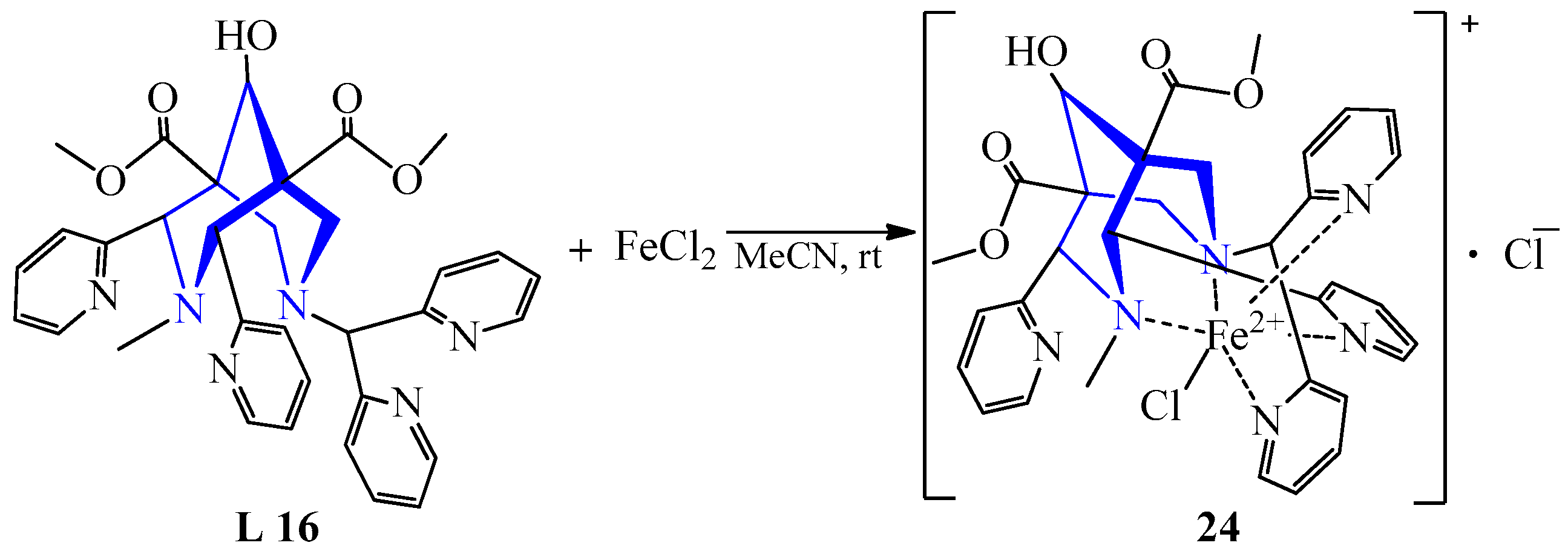
| 18 | FeCl2 Fe(ClO4)2 Fe(BF4)2·6H2O | L 16 L 15 L 15 | [Fe(L)Cl]Cl, MeCN [Fe(L)(OCH3)](ClO4)2, MeOH [Fe(L)(OCH3)](BF4)2, MeOH | 412 {1381} - 364 {1245} | 52 49 54 | Yellow | Diffusion of ether | The activation process | [30] |
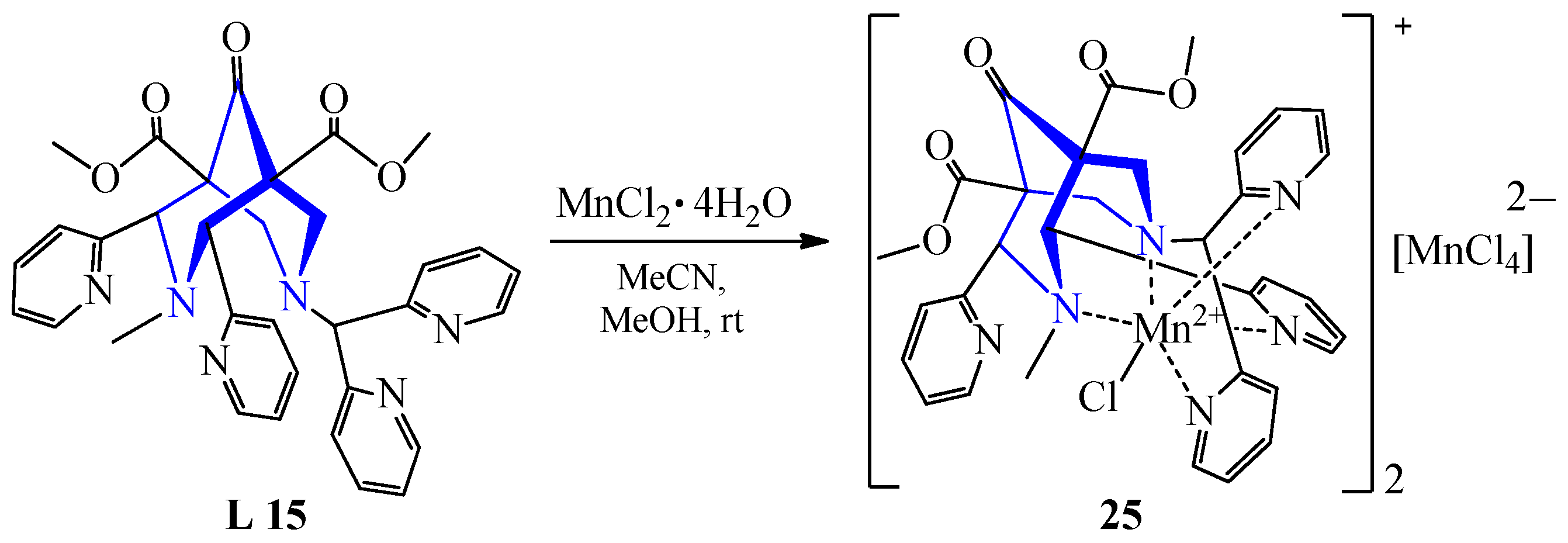
| 19 | MnCl2·4H2O | L 15 | [Mn(L)Cl]2[MnCl4], MeCN: MeOH | 56 | Colorless | Evaporation, diffusion of ether | PET visualization | [30] | |
| 20 | (C6H10)PtCl2 | L 40 | [Pt(L)Cl]Cl, DMF [Pt(L)Cl]Cl·C3H7NO, DMF [Pt(L)Cl]Cl·3H2O, H2O | 87 87 60 | Yellow | Recrystallization, cooling heating, recrystallization | Cytotoxicity against human cancer cell lines K562 (chronic myeloid leukemia), A2780 (ovarian cancer) | [41] | |
| 21 | (C6H10)PtCl2 | L 41 | [Pt(L)Cl]Cl, DMF | 97 | Yellow | Heating, recrystallization | Preparation of compounds with cytotoxic activity | [42] | |
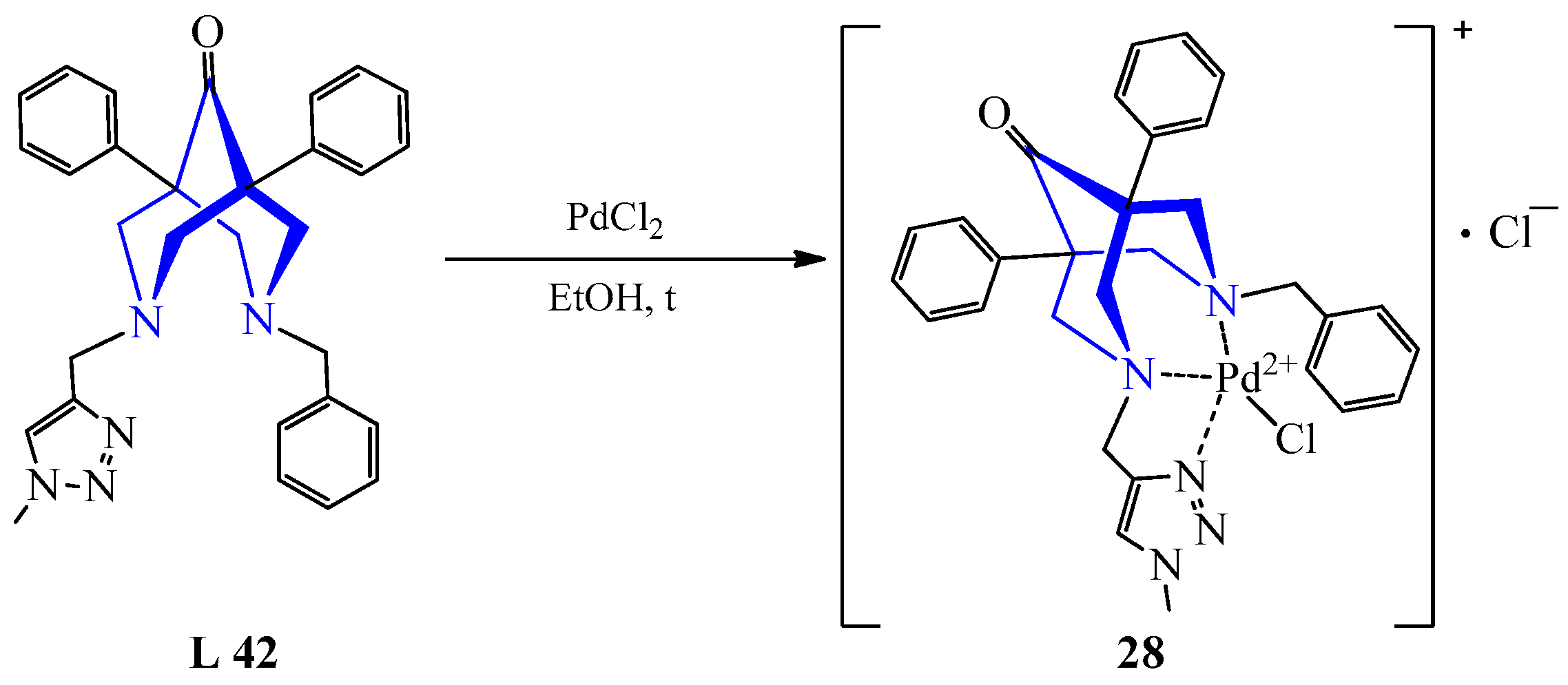
| 22 | PdCl2 | L 42 | [Pd(L)Cl]Cl, MeCN | Might be used as catalysts under microwave irradiation | [43] | ||||
| 23 | Ni(acac)2 | L 43 | [Ni(L)](acac)2, C5H12 | 99 | Blue | Cooling | Immobilization of metal heterogeneous surfaces | [44] | |
| 24 | Ni(NO3)2·6H2O | L 1 | [Ni(L)(NO3)](NO3), MeCN: MeOH | 98 | Pink | Evaporation, recrystallization | A catalyst for bond formation C(sp2)-C(sp3) | [45] | |
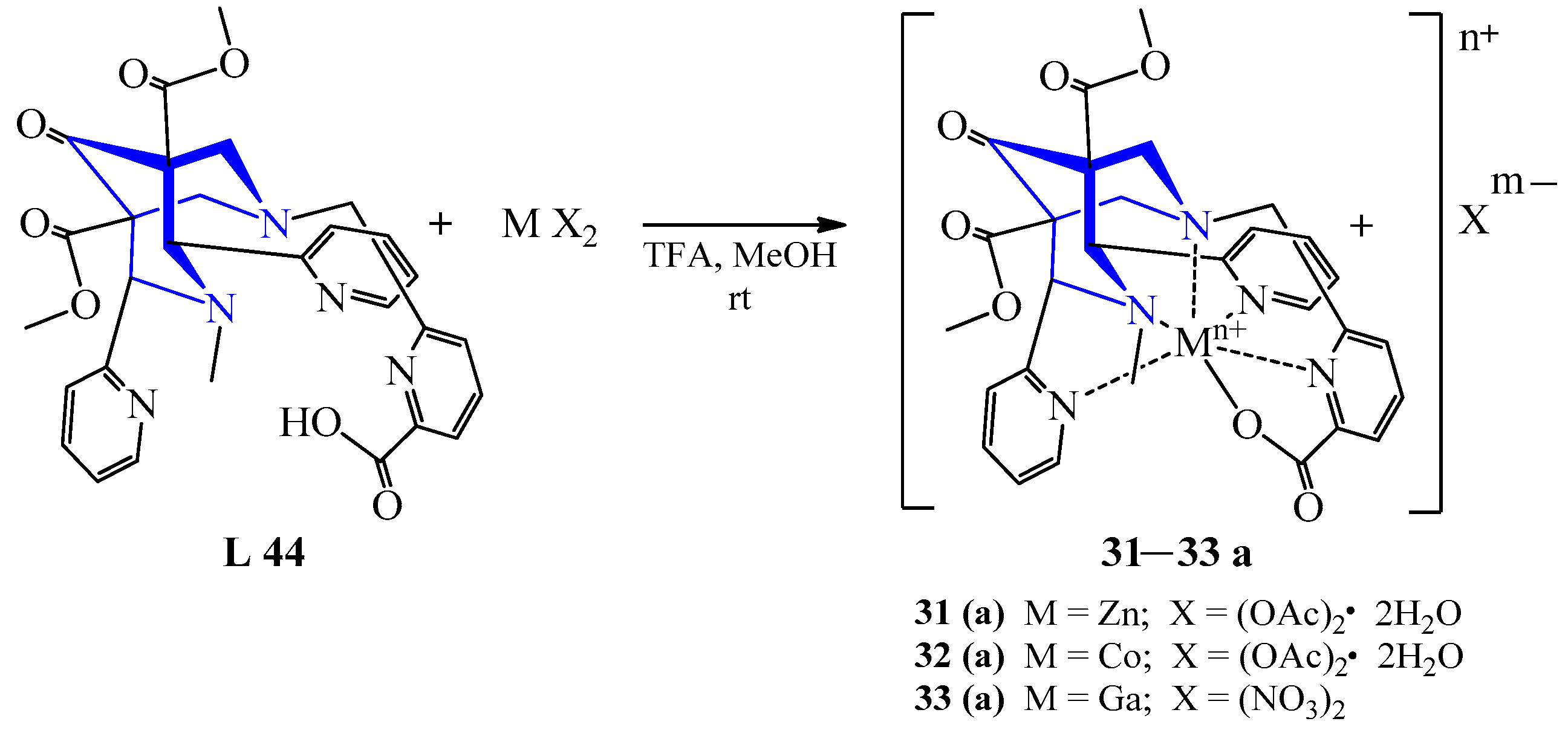
| 25 | Zn(OAc)2·2H2O | L 44 L 45 | [Zn(L)](TFA), TFA, MeOH | 98 61 | Colorless | Evaporation, diffusion of ether | Bifunctional Chelators for 64Cu PET | [46] | |
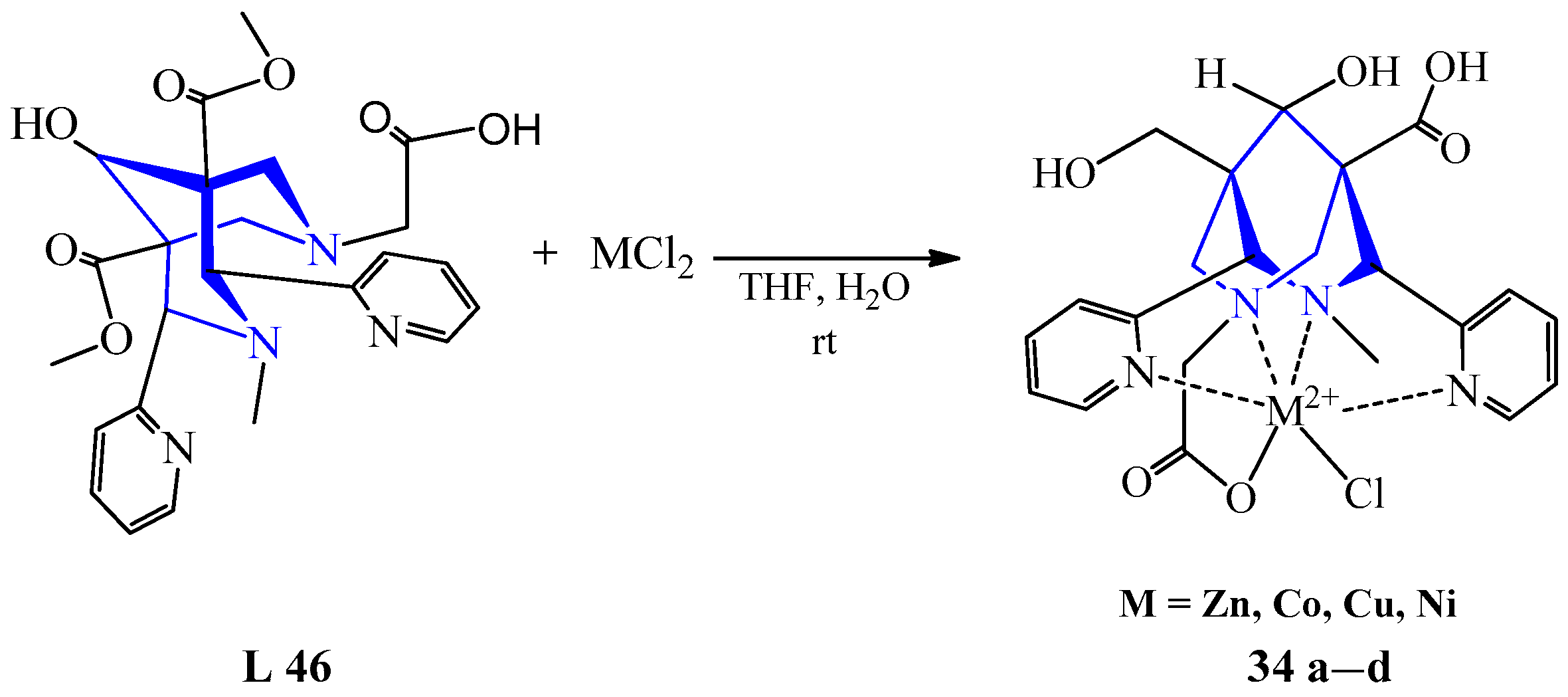
| 26 | ZnCl2 | L 46 | [Zn(L)Cl], THF, H2O | Evaporation, centrifugation | Chelator for radiolabeling studies with 64Cu | [47,48] | |||
| 27 | Co(OAc)2·2H2O | L 44 L 45 | [Co(L)](TFA), TFA, MeOH | 470 {123} 475 {117} | 32 67 | Red Brown | Evaporation, diffusion of ether | Bifunctional Chelators for 64Cu PET | [46] |
| 28 | 197HgCl2 NatHgCl2 | L 47 | [Hg(L)], EtOH [Hg(L)], THF | Heating, evaporation | Theranostic applications in radiopharmaceuticals | [49] | |||
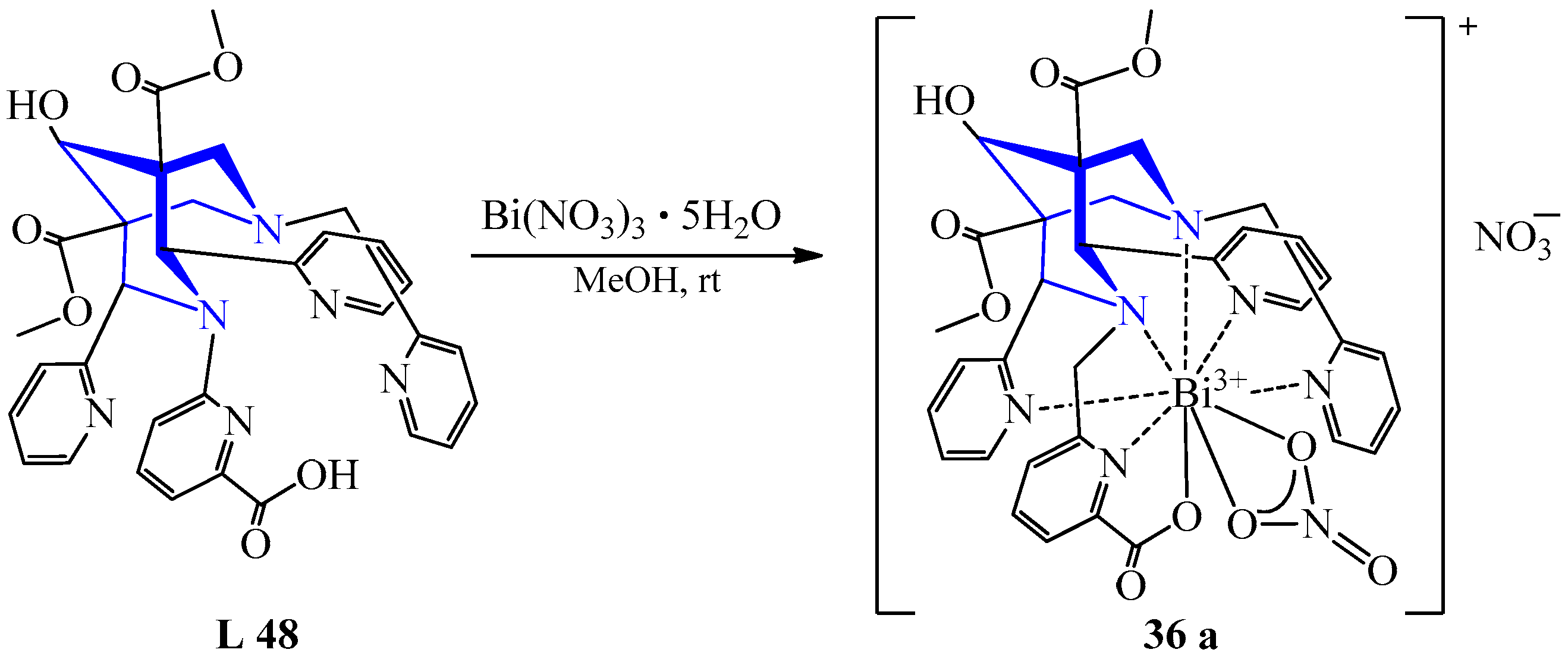
| 29 | Bi(NO3)3·5H2O | L 48 L 49 L 50 L 51 | [Bi(L)](NO3)2, MeOH [Bi(L)(NO3)](NO3), MeOH [Bi(L)(Br)0,5(NO3)0,5], MeOH [Bi(L)(NO3)], MeOH [Bi(L)(NO3)](NO3), MeOH | 93 99 60 95 67 | Recrystallization Evaporation, recrystallization | Targeted 213Bi alpha therapy | [50] | ||
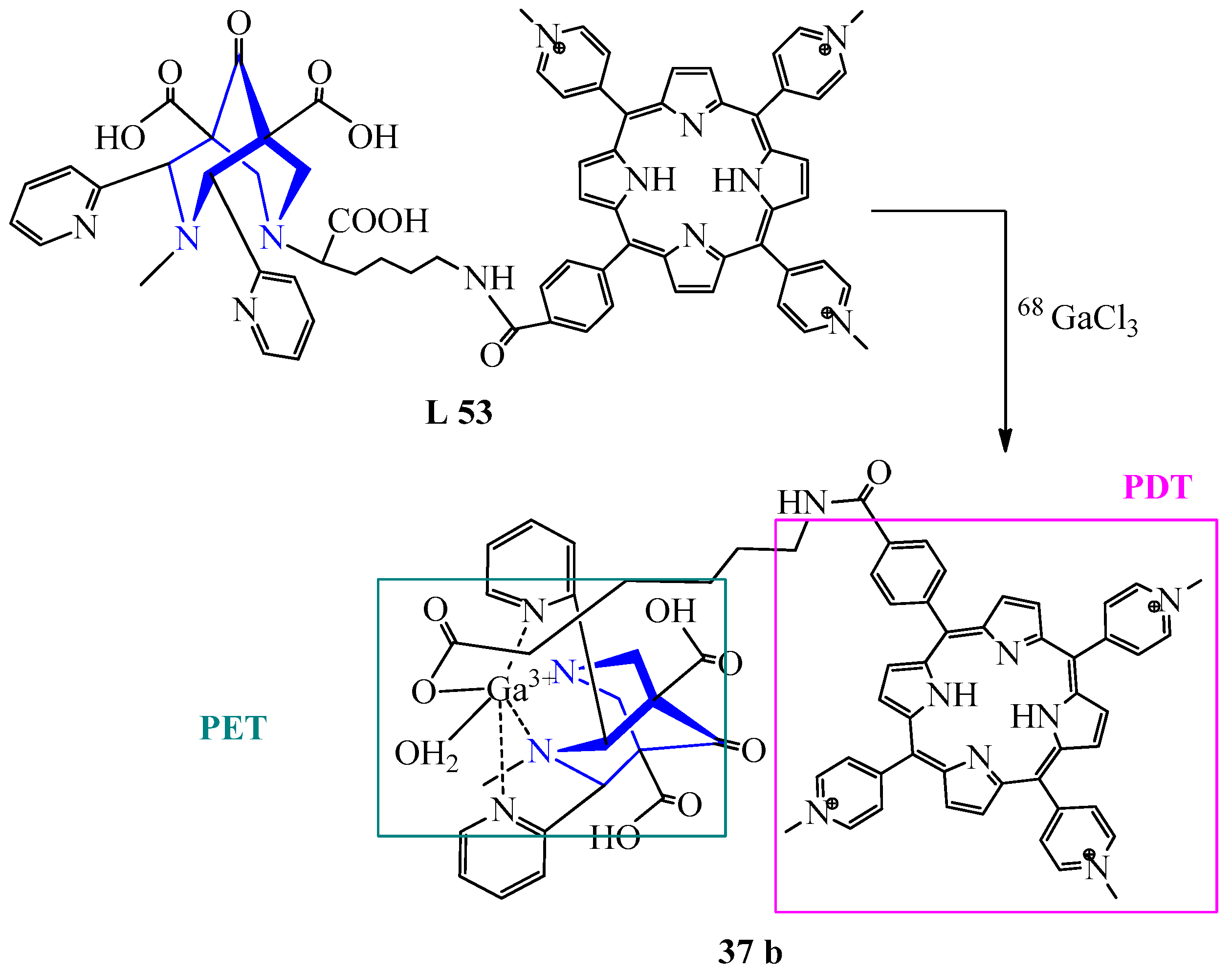
| 30 | GaCl3 | L 52 L 53 | [Ga(L)]Cl, H2O | - 422 {240} | 90 | White Purple | Heating, recrystallization | PET visualization, creating stable theranostic probes for PET/PDT | [51] |
| 31 | Ga(NO3)3 | L 48 | [Ga(L)](TFA)(NO3), TFA, MeOH:H2O | 65 | Colorless | Heating, evaporation | Bifunctional Chelators for 64Cu PET | [46] | |
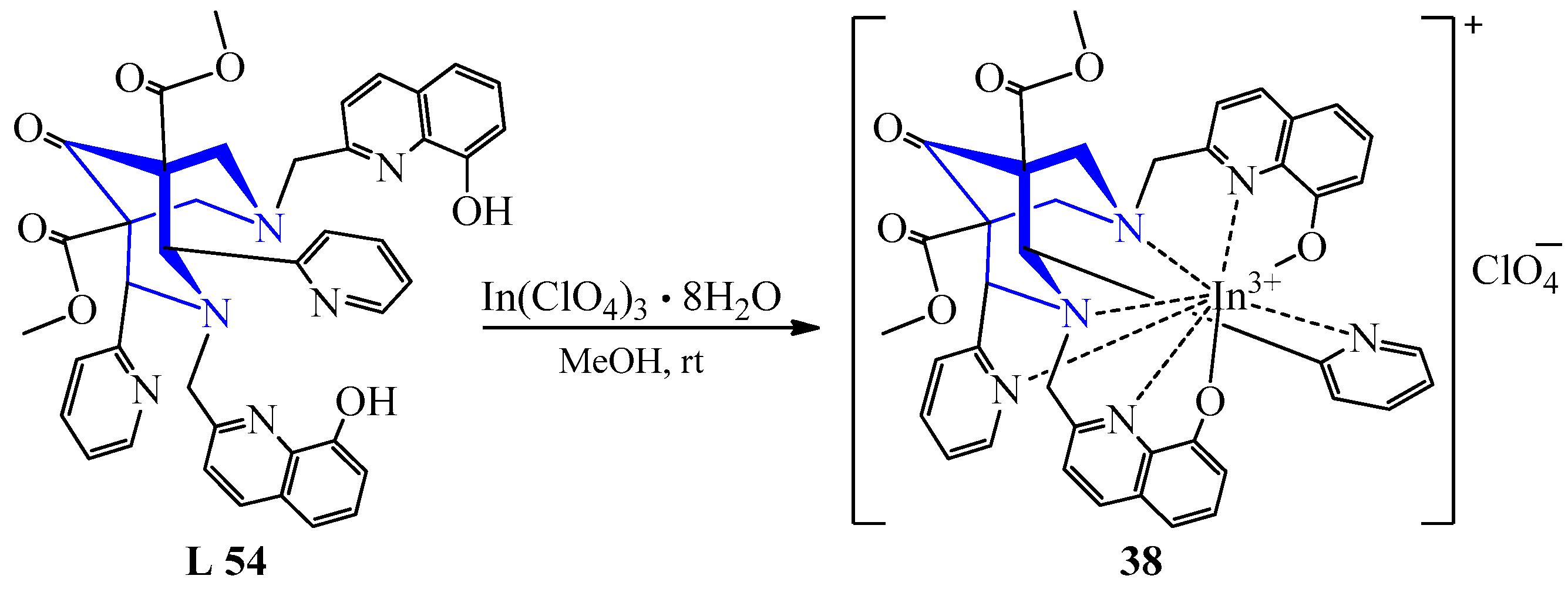
| 32 | In(ClO4)3·8H2O | L 54 | [In(L)](ClO4), MeOH | 39 | Orange | Concentration in a vacuum | Creation of a chelating component in radiopharmaceuticals based on In | [52] | |
| 33 | In(OAc)3·5H2O Lu(OAc)3·4H2O LaCl3·7H2O | L 51 | [In(L)](TFA), TFA, MeOH, H2O [Lu(L)](TFA), TFA, MeOH [La(L)](TFA), TFA, MeOH | 88 84 81 | Colorless | Evaporation Evaporation, diffusion of ether Evaporation, concentration in vacuum | Chelators in nuclear medicine used in single-photon emission computed tomography (SPECT) | [53] | |
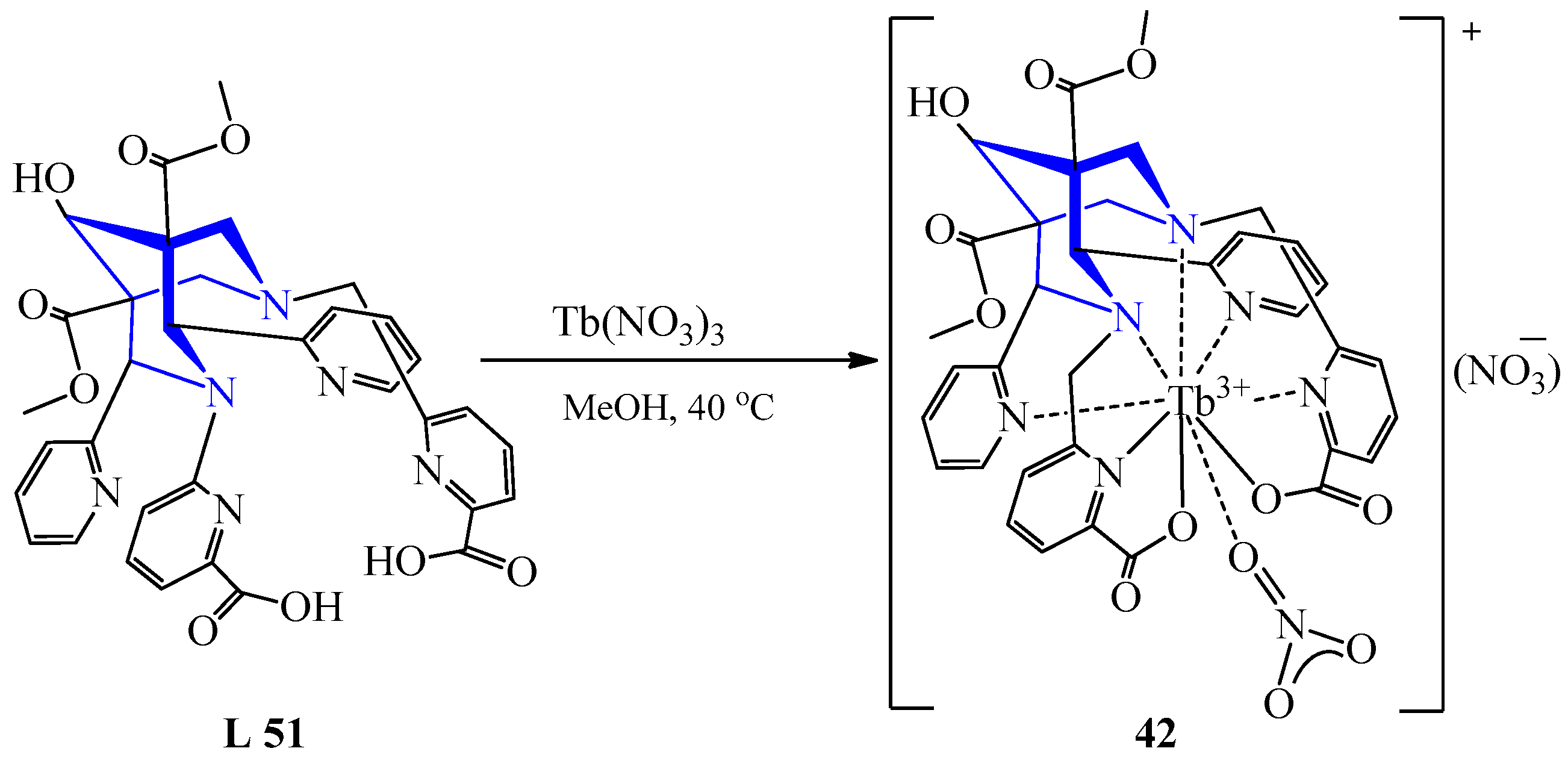
| 34 | Nd(OAc)3 Tb(NO3)3 Dy(OAc)3 | L 51 | [Nd(L)(OH)2](TFA)·(MeOH), TFA, MeOH [Tb(L)(OH)2](TFA)·2H2O·MeCN, TFA, H2O, MeCN [Dy(L)(OH)2](TFA)·1.5H2O·MeCN, TFA, H2O, MeCN | - 491 - | Colorless | Evaporation, diffusion of ether | Acting as an antenna for energy transmission | [54] | |
| 35 | Tb(NO3)3 Eu(OAc)3 Yb(NO3)3 | L 55 | [Tb(L)](NO3) MeOH·H2O, MeOH:H2O [Eu(L)](OAc), EtOH:MeOH [Yb(L)] (NO3) MeOH, H2O, MeOH, | 261 261 262 | 40–70 | Heating, evaporation | Biological fluorescent probes with red-shifted absorption | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaldybayeva, A.B.; Yu, V.K.; Durap, F.; Aydemir, M.; Tassibekov, K.S. Metal Complexes of Bispidine Derivatives: Achievements and Prospects for the Future. Molecules 2025, 30, 1138. https://doi.org/10.3390/molecules30051138
Kaldybayeva AB, Yu VK, Durap F, Aydemir M, Tassibekov KS. Metal Complexes of Bispidine Derivatives: Achievements and Prospects for the Future. Molecules. 2025; 30(5):1138. https://doi.org/10.3390/molecules30051138
Chicago/Turabian StyleKaldybayeva, Altynay B., Valentina K. Yu, Feyyaz Durap, Murat Aydemir, and Khaidar S. Tassibekov. 2025. "Metal Complexes of Bispidine Derivatives: Achievements and Prospects for the Future" Molecules 30, no. 5: 1138. https://doi.org/10.3390/molecules30051138
APA StyleKaldybayeva, A. B., Yu, V. K., Durap, F., Aydemir, M., & Tassibekov, K. S. (2025). Metal Complexes of Bispidine Derivatives: Achievements and Prospects for the Future. Molecules, 30(5), 1138. https://doi.org/10.3390/molecules30051138







