1. Introduction
Hypericum perforatum L., known as St John’s wort, is a flowering plant belonging to the family Hypericaceae. This perennial herb can grow up to 1 m tall and is characterized by its many yellow flowers, which have distinct black dots around their edges. The plant features long stamens and three pistils, contributing to its unique floral structure. It is a perennial herbaceous plant that can be found in various temperate regions across the globe and is native to western Asia, Europe, and northern Africa. Over time, St. John’s wort was introduced to North America, South America, southern Africa, Australia, New Zealand, and Japan. This plant thrives in sunny locations, making it commonly found in dry soils and abandoned fields [
1].
St. John’s wort has a long history of use as a medicinal plant. It is widely recognized as a natural antidepressant and is often recommended for managing mild to moderate mood disorders [
2]. In folk medicine, St. John’s wort is used to address digestive issues, including bloating, abdominal pain, and inflammation of the stomach lining. Externally, it is applied to treat wounds, burns (including first- and second-degree burns), eczema, frostbite, and slow-healing wounds [
3]. Its anti-inflammatory properties make it effective for treating abscesses and ulcers. St. John’s wort is also valued for supporting liver function and promoting bile secretion, which can be beneficial in managing liver disorders [
4]. Historically, it was used to relieve neuralgia, neuroses, and other nervous system disorders. In ancient times, it was believed to offer protection against “devilish influences” and was even used to treat snake bites [
5]. Additionally, St. John’s wort has been applied to skin conditions such as vitiligo and employed as a gargle for oral infections. In traditional medicine, it was also used to treat hemorrhoids and as a diuretic [
6]. Furthermore, the anticancer effect of this plant has also been demonstrated [
7].
The different medical properties of St. John’s wort result from its diverse composition of bioactive compounds. It contains, among other compounds, hypericin, hyperforin, hyperoside, flavonoids (e.g., quercetin and rutin), phenolic acids (e.g., caffeic acid and chlorogenic acid), tannins, and essential oils [
8]. Hypericin is the main active component of St. John’s wort, and together with hyperforin, it is responsible for the antidepressant properties of this medical plant. Its flowering parts have been utilized in traditional and phytomedicines due to the presence of specific bioactive phytochemicals such as phloroglucinol derivatives, naphthodianthrones, and xanthone compounds unique to the
Hypericum genus [
2].
While the biological potential of hydrophilic molecules has been extensively studied, research on lipophilic bioactive compounds in St. John’s wort remains limited and requires further exploration [
9]. The available research focuses solely on St. John’s wort leaves or the above-ground parts of the plant without distinguishing between specific sections [
10]. Furthermore, studies have primarily analyzed tocopherol (T) content, with a lack of attention to tocotrienols (T3) [
7]. These compounds (tocotrienols) are rare in nature, particularly in photosynthetic tissues, and their content has not been previously reported in different aerial parts of
H. perforatum except leaves (lack of quantitative data) [
11] and inflorescences [
12]. Recent reports indicate a notable uniqueness of the
Hypericum genus due to the relatively high tocotrienol content found in the leaves of seven [
13] and eleven [
14] species of the Hypericaceae family. This characteristic sets the
Hypericum genus apart from other plants [
13,
14], including both monocots and dicots, which predominantly contain α-T in photosynthetic tissue, with tocotrienols being practically absent [
15]. It is essential to explore new sources of tocotrienols since these lipophilic bioactive phytochemicals exhibit a lower abundance in nature than tocopherols. Novel discovery about the significant content of tocotrienols in St. John’s wort inflorescences [
12] holds particular importance due to the extensive historical use of
H. perforatum in traditional medicine over many centuries. For the above reasons, exploring various parts of St. John’s wort is crucial for a better understanding of the accumulation of these rare secondary metabolites. This research can also facilitate the more effective utilization of this medicinal plant. Simultaneously, it contributes to the development of sustainable use of plant materials, fundamental plant biology knowledge, and advancements in economic, pharmaceutical, and medical fields.
Tocopherols, particularly α and γ homologues, are widely distributed in nature in leaves, seeds, and plant oils. They are classified as members of the vitamin E family. However, as highlighted by Azzi [
16], the term “vitamin E” should not be used interchangeably for all tocochromanol compounds. This distinction arises because only α-T fulfills the specific criteria for preventing vitamin E deficiency-related ataxia, a neurological disorder caused by insufficient vitamin E levels in humans. Tocochromanols exist in multiple forms, each varying in its antioxidant and health effectiveness [
17]. α-T protects cells from oxidative stress, supports the cardiovascular system by lowering LDL levels and preventing atherosclerosis, and enhances neurological functions by slowing neurodegenerative processes. It also exhibits anti-cancer properties by stabilizing biological membranes and inhibiting the growth of certain cancers [
18]. In contrast, tocotrienols and other tocochromanol-related compounds are less prevalent and have been less extensively studied and identified in natural sources [
19,
20,
21]. As a result, there is a limited number of studies available regarding the detection, screening, taxonomic distribution, biosynthesis, metabolism, and physiological roles of these tocochromanols, despite their promising potential in promoting human health [
21]. Tocotrienols lower LDL cholesterol levels, reduce inflammation, and have strong chemo—preventive effects by inhibiting the proliferation of cancer cells [
18]. Tocotrienols are recognized for their safety and beneficial effects on health, as they do not pose toxicity risks to healthy cells. These compounds demonstrate anti-metastatic and anti-angiogenic properties, with a unique capacity to target and eradicate cancer stem cells selectively. Consequently, there is a growing consensus on the potential benefits of incorporating tocotrienols into mono-targeted or combination therapies in conjunction with other chemotherapeutic agents [
22]. While α-T has not shown clear cancer-preventive effects, the potential impact of γ-T, δ-T, and tocotrienols on cancer risk is still unclear and requires further exploration through additional research studies [
23]. Due to the positive effects of tocotrienols on human health and the occurrence of δ-T3 in nature limited to latex
Hevea brasiliensis Muli. Arg., fruits of
Elaeis guineensis Jacq., and seeds of
Bixa orellana L. [
24], the presence of tocotrienols in the
H. perforatum can make this plant uniquely suitable as raw material for tocotrienol recovery in temperate climate zones.
Hence, the objective of this study is to show that various aerial parts of H. perforatum (stems, leaves, flower buds, flowers, dead petals, and unripe seed pods) contain relatively high concentrations of tocotrienols, especially δ-T3, tocotrienol which occur very rarely in nature, particularly in photosynthetic tissue. Knowledge demonstrated in this study can expand the use of H. perforatum in the food, pharmaceutical, and medical industries, as well as improve the understanding of the positive health potential of St. John’s wort.
2. Results and Discussion
The harvested
H. perforatum plant and its separated parts can be seen in
Figure S1 (Supplementary Materials). The obtained chromatograms of the profile of tocochromanols in St. John’s wort show a noticeably different composition and/or concentration in each of the analyzed parts of this plant, with some similarities (
Figure 1).
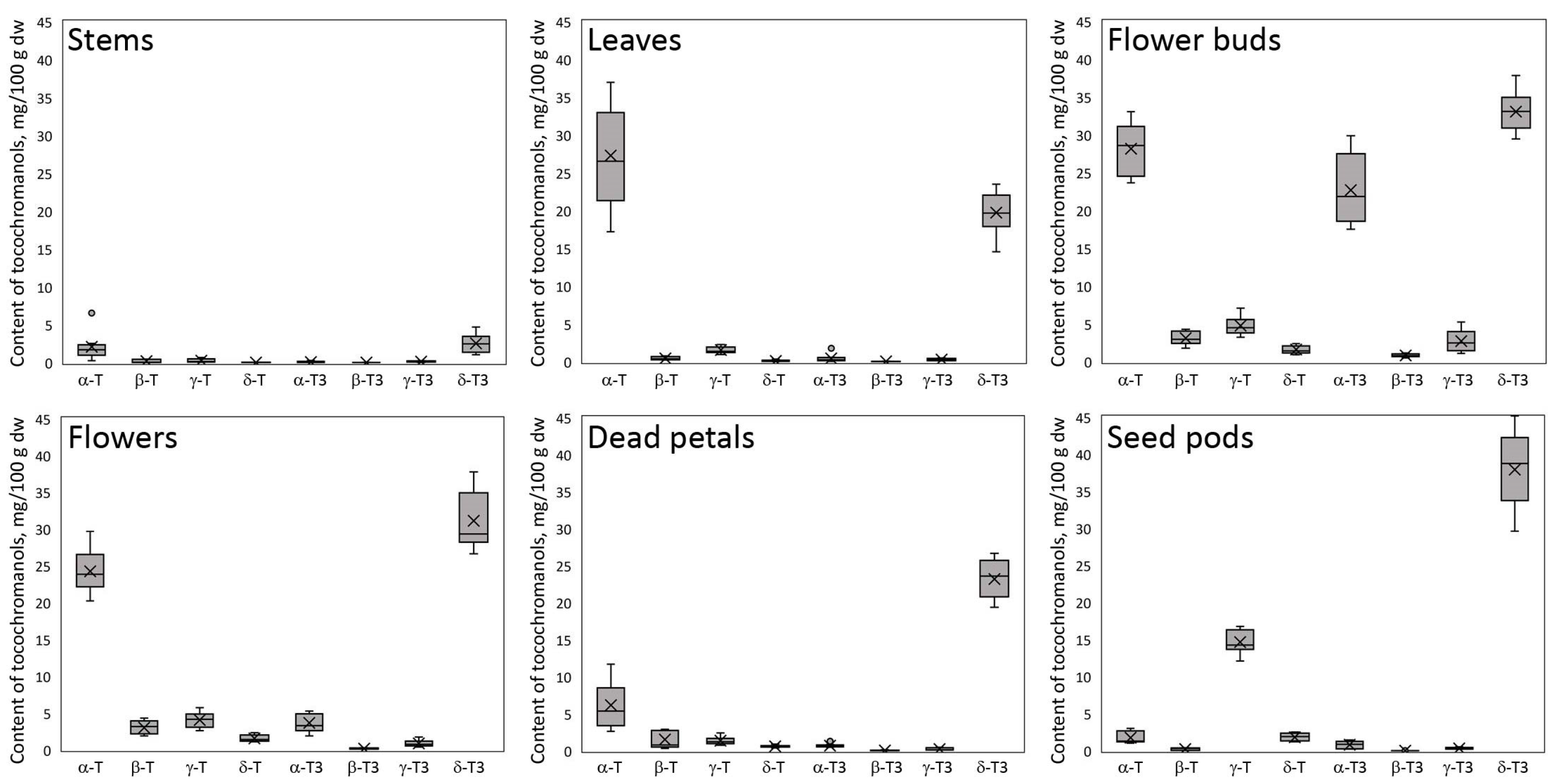
The flower buds have the most diverse tocochromanol profile, while the stems have the least. Despite some differences, each part was characterized by the distinct presence of δ-T3 and α-T, constituting a substantial part of the identified tocochromanols. The exception was seed pods, where, instead of α-T, the γ-T was the main tocopherol.
Figure 1 gives an illusion of domination of δ-T3 in each plant organ (the highest peak); however, due to the physicochemical properties of various tocochromanols, as well as elution order in isocratic separation by RP-HPLC-FLD, tocopherols and tocotrienols peak areas in chromatograms, especially between the homologues α and δ, are not representative of their content in the analyzed material [
20]. The real content and ratio of tocopherols and tocotrienols in stems, leaves, flower buds, flowers, dead petals, and seed pods of wild
H. perforatum can be seen in
Table S1 (Supplementary Materials). In four of the six investigated aerial parts of St. John’s wort—stems, leaves, flowers, and dead petals—the tocochromanol profile was similar, but their content greatly varied between the different parts. The flower buds and unripe seed pods were more diverse in terms of the tocochromanol composition than other parts of
H. perforatum, due to the presence of considerable amounts of α-T3 (23%) and γ-T (25%), respectively.
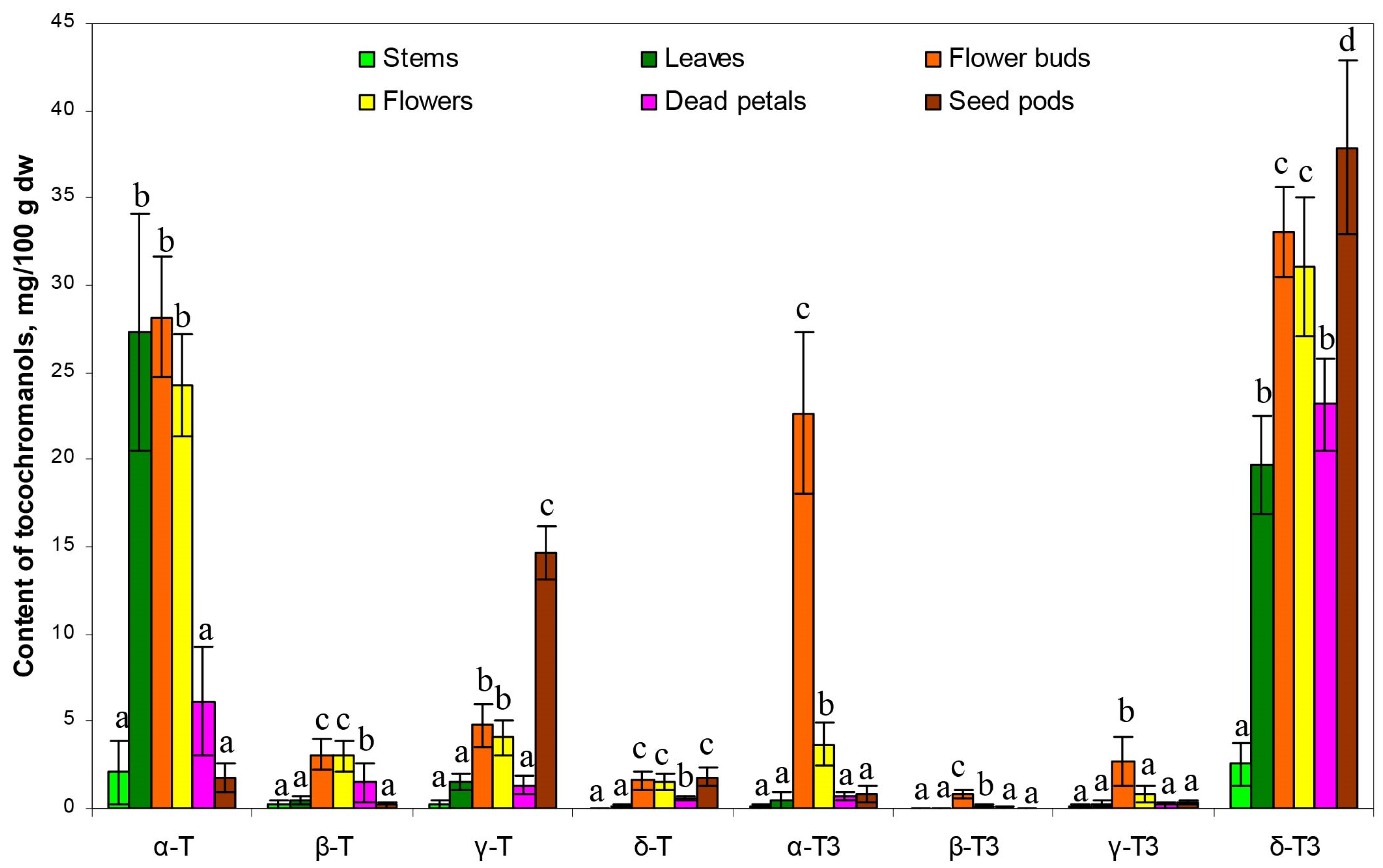
Figure 2 presents the median value, upper and lower quartile, and upper and lower extreme measurements of value distribution, while
Figure 3 presents the average content proportion (%) of tocochromanols in the aerial parts of wild
H. perforatum.
A diverse array of concentrations for both tocopherol and tocotrienol homologues, along with significant variability, were noted among the sampled aerial components of St. John’s wort (
Figure 2). Both figures (
Figure 2 and
Figure 3) clearly demonstrate the domination of two tocochromanols, α-T and δ-T3, and concentration considerably differed between the different parts of St. John’s wort (2.1–28.2 and 2.5–37.9 mg/100 g dw, on average, respectively). Exceptions are flower buds and seed pods, which, apart from α-T and δ-T3, had significant amounts of α-T3 and γ-T (17.5–29.9 and 12.1–16.7 mg/100 g dw), respectively. In stems, leaves, flowers, and dead petals, the α-T and δ-T3 comprise 81–94% of total tocochromanols. Except for leaves dominated by tocopherols (55% of α-T), other investigated parts of St. John’s wort were predominated by tocotrienols (52–72%), where δ-T3 was a main tocochromanol (34–69%) (
Figure 3). A high content of γ-T in unripe seed pods of
H. perforatum is not surprising, since γ-T in seeds and their oil across various taxa is a predominant tocochromanol [
19]. In contrast to high levels of δ-T3 and relatively notable amounts of α-T3, tocotrienol isomers (β-T3 and γ-T3) were recorded in low quantities (0.0–1.1 and 0.0–5.3 mg/100 g dw, respectively). β-T3 was present only in two parts, mainly in flower buds and sequentially in flowers. γ-T3 was detected in all
H. perforatum parts, with the highest content in flower buds (2.7 mg/100 g dw on average). In contrast, in
Borago officinalis L. flower buds and flowers, β-T3 and γ-T3 were the only detected tocotrienols; however, their quantities (sum of tocotrienols) were lower than recorded in the present study in
H. perforatum flower buds and flowers [
25]. From four tocopherols, three of them–β-T, γ-T, and δ-T–constituted a minor proportion of the tocochromanols, with the exception of seed pods, which contained a notable amount of γ-T (14.6 mg/100 g dw on average). The accumulation of γ-T in unripe seed pods results from seed formation. γ-T is the dominant tocochromanol in ripe
H. perforatum seeds. Total tocochromanols content differs depending on the part of the plant. The richest source of tocochromanols were flower buds, 96.9 mg/100 g dw. On the other hand, the lowest amount was obtained for stems, 5.3 mg/100 g dw. The ratio between tocopherols and tocotrienols also differed. The biggest participation of tocopherols was for St. John wort stems, leaves, and flowers (ratio Ts/T3s–0.9)
(Table S1, Supplementary Material). In most parts of a plant, α-T predominated among tocopherols, besides seed pods, where γ-T was the primary form and δ-T3 among tocotrienols (
Figure 3). Tocopherols can be found in both the subterranean and aerial parts of higher plants, including roots, tubers, stems, leaves, flower buds, flowers, fruits, and seeds [
24]. The predominance of α-T in St. John’s wort photosynthesis tissues (leaves and stems) is unsurprising, as α-T is well-established as the primary tocochromanol in the foliage of numerous plant species, with some exceptions where γ-T was detected as a major tocochromanol, e.g.,
Kalanchoe daigremontiana and lettuce [
26]. The age of the leaf and its location play an important role in the profile of tocochromanols, as well as their concentration [
26,
27]. Conversely, tocotrienols are less commonly found in green plant tissues [
15]. Exceptions include the species
Hypericum and
Clusia, where substantial quantities of tocotrienols have been identified in the leaves of five
Clusia species and seven
Hypericum species in one study [
13], and eleven in another [
14]. The composition and concentration of tocopherols and tocotrienols in the leaves of
H. perforatum observed in this study are consistent with previous findings [
13]. Among the various
Hypericum species analyzed,
H. perforatum uniquely demonstrates a predominance of the δ homologue among the four tocotrienols, while other
Hypericum species primarily contain α-T3 or γ-T3 as their main tocotrienols. Interestingly, four out of the five
Clusia species also exhibited a dominance of δ-T3 as the principal tocotrienol, similar to
H. perforatum. It is important to note that in all
Clusia and
Hypericum species examined, α-T was the tocochromanol present in the highest concentrations [
13,
14].
The concentrations of α-T and δ-T3, two tocochromanols identified in all examined aerial parts of
H. perforatum, exhibited significant variability in stems (coefficient of variation 0.871 and 0.471, respectively). In contrast, the lowest variability was observed in flower buds (0.122 and 0.079, respectively)
(Table S1, Supplementary Materials). The lowest proportion for α-T was recorded in seed pods (3%) and the highest in leaves (55%), while δ-T3 was most predominant in dead petals and seed pods (69% and 66%, respectively) and the lowest percentage was found in flower buds (34%). The highest content of tocotrienols was noted in flower buds (59.3 mg/100 g dw on average) and then subsequently in seed pods, flowers, dead petals, leaves, and stems (39.0 > 35.8 > 24.1 > 14.7 > 2.8 mg/100 g dw, respectively). Relative to other aerial parts, flower buds were characterized by a high content of α-T3 (22.7 mg/100 g dw on average), while in the other parts, α-T3 constituted much less (<5.3 mg/100 g dw). β-T3 and γ-T3 constituted the smallest portion of tocotrienols in
H. perforatum parts and only reached notable concentration in flower buds (1.1 and 5.3 mg/100 g dw, respectively, on average). This makes δ-T3 the most dominant tocotrienol in St. John’s wort
(Table S1, Supplementary Materials). The prevalence of δ-T3 dominance is rare in plants, with few exceptions, such as annatto
(Bixa orellana L.) [
28] and lychee (
Litchi chinensis Sonn.) [
29] seeds and their oils, which are known for being abundant sources of δ-T3. Due to the profile and concentration of tocochromanols, St. John’s wort is more similar to lychee than to annatto seeds (tocotrienol pair domination δ-T3 and α-T3 vs. δ-T3 andγ-T3, respectively).
The composition of tocotrienols in flower buds varied the most and changed during plant development (flower buds > flowers > unripe seed pods). For three tocotrienol homologues, α-, β-, and γ-T3 content decreased in subsequent stages of development. However, δ-T3 content slightly decreased and became the lowest in flowers, and then increased to be the highest in unripe seed pods. Flowers, compared to flower buds, recorded notable losses of both α homologues (α-T and α-T3), with a particular emphasis on the α-T3. The significant decrease in α-T3 content implies the protective function of the reproductive/pollen system of the plants in St. John’s wort. This finding cannot be confirmed with literature due to a lack of studies and plant sources with high content of tocotrienols, as similarly discovered in
H. perforatum in the present study. However, this phenomenon can be understood through several key mechanisms and findings from recent studies. One of the explanations for decreasing α-T3 content during plant development is the antioxidant/protective character of α-T3 in
H. perforatum as a plant responds to external stress, e.g., solar radiation, no longer producing and recycling α-T3. Typically, α-T predominates as a tocochromanol in flowers, with tocotrienols present in minor quantities (Fernandes et al., 2020). α-T serves as a crucial antioxidant within chloroplasts, effectively scavenging singlet oxygen and preventing lipid peroxidation propagation, a unique function not shared by other antioxidants. This pivotal role underscores its significance in safeguarding plants against photo-oxidative damage induced by abiotic stress. Recent studies have unveiled novel roles of α-T at the systemic level, including suggesting potential non-antioxidant functions in regulating flower and fruit development as well as leaf senescence [
30]. Our studies suggest that α-T3 may play a similar role in
H. perforatum. However, this phenomenon requires future investigations for a detailed understanding. The concentration of α-T is highly variable, fluctuating in response to environmental stressors and reflecting the species’ ability to withstand such stress [
31]. α-T is the main tocochromanol in photosynthetic tissues, where it is responsible for the neutralization of lipid peroxy radicals and maintaining the fluidity and integrity of membranes. The highest content of this tocochromanol was detected in flower buds, leaves, and flowers (28.2, 27.3 and 24.2 mg/100 g dw, respectively). In the other parts of a plant, the content of α-T was much lower, even 10 times lower in unripe seed pods.
Hence, the occurrence of δ-T3 in approximately 40% of the identified tocochromanols in
H. perforatum leaves is distinctive. In the realm of scientific research, a singular instance has been documented in which photosynthetic tissues exhibited significant levels of tocotrienols, albeit at a concentration 2.5 times lower than that of tocopherols. Specifically, β and γ isomers of tocotrienol were observed in
Vellozia gigantea leaves, which is a monocot and not related to
Hypericum (dicot) [
32]. Tocotrienols can accumulate in leaves through genetic modification, leading to a shift from a predominance of tocopherols to tocotrienols. For example, in wild-type
Nicotiana tabacum L., α-T is the primary tocochromanol found in young leaves, but transgenic tobacco plants exhibited a higher abundance of α-T3 and γ-T3 in their leaves [
33]. Nevertheless, to date, significant δ-T3 content has not been reported in the leaves of any species; however, presence of δ-T3 in
H. perforatum leaves was already reported over decade ago (lack of quantitative data) [
11]. Tocotrienols are reported mainly in latex, e.g.,
H. brasiliensis; fruits, e.g.,
E. guineensis; seeds, e.g.,
B. orellana [
24]; grain bran, e.g.,
Secale cereale L. [
34]; some seed oils, e.g., American cranberry (
Vaccinium macrocarpon Aiton) and grapes (
Vitis sp.); and cereal bran oils, e.g., wheat (
Triticum aestivum L.) [
19], but not in the leaves of wild-type plants.
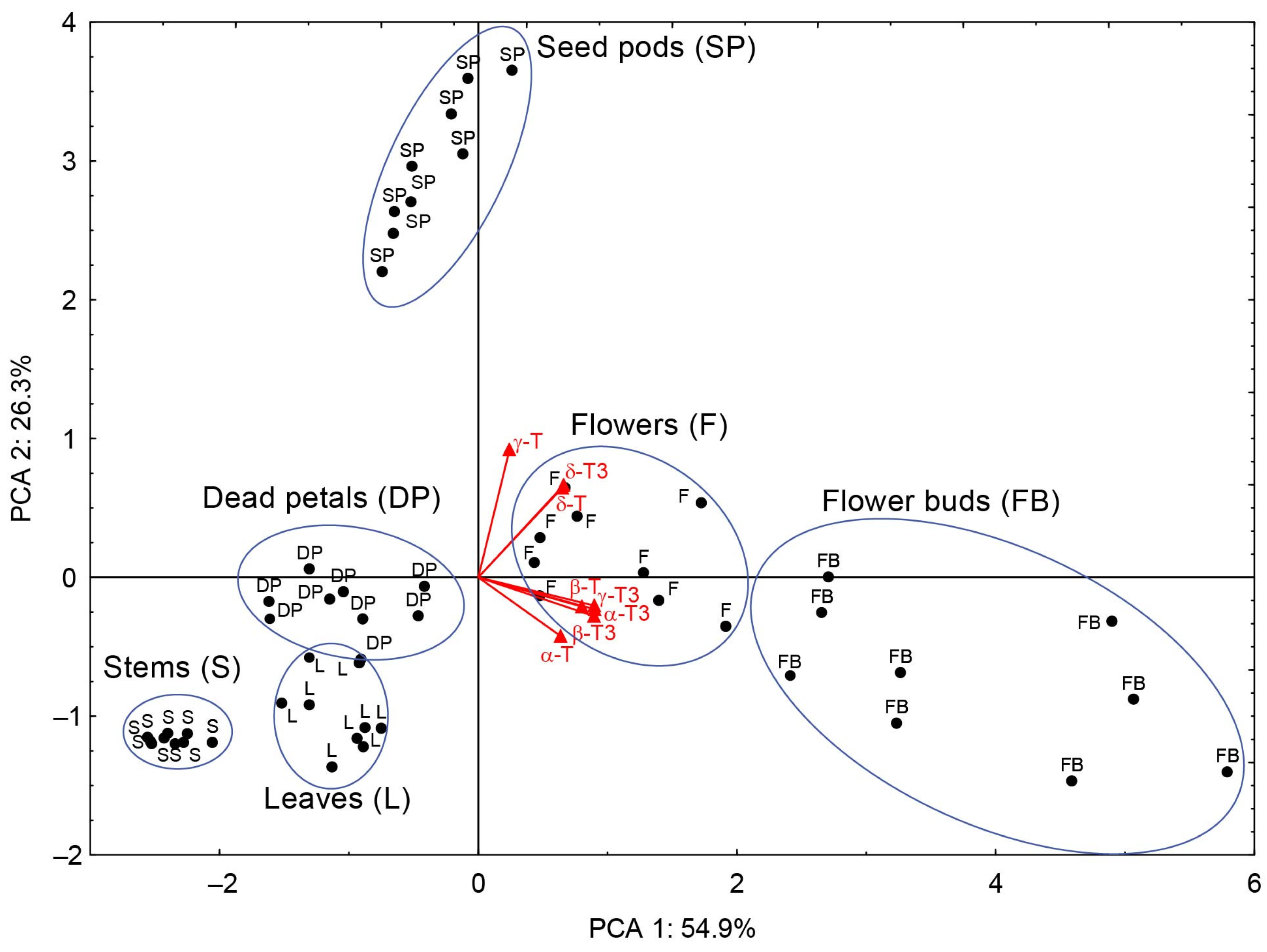
Principal component analysis (PCA) was employed to analyze the data and facilitate the discovery of hidden patterns and relationships between variables. By transforming the data into new, uncorrelated variables (principal components), it becomes easier to understand the structures within the data and their interactions. The obtained results of the principal components (PC), PC1 (54.9%) and PC2 (26.3%) of the PCA explain 81.2% of the variation. PC1 was highly positively correlated (
r ≥ 0.80) with four tocochromanols (β-T, α-T3, β-T3, and γ-T3) and moderately correlated with α-T, δ-T, and δ-T3 (
r ≥ 0.64), whereas PC2 showed high loads only with γ-T (correlations
r = 0.92) and was moderately correlated with δ-T and δ-T3 (
r ≥ 0.64). According to PCA, the investigated
H. perforatum samples were classified into six nearly completely separate groups, each group representing a different aerial part of St. John’s wort (
Figure 4). The aerial parts of St. John’s wort have been separated based on the distinct profile and/or concentration of tocochromanols found in each part of the
H. perforatum plant, as has been described above. Based on
Figure 5, it can be seen that the aerial parts of
H. perforatum differ statistically significantly (
p < 0.05) in their concentrations of all tocochromanols.
Most noteworthy are statistical differences recorded for the content of α-T and δ-T3, the main tocochromanols in all parts of St. John’s wort. For α-T, it is possible to distinguish two groups that differ statistically significantly, while within-groups do not. The first group with low α-T content includes stems, dead petals, and seed pods. The second group with high content includes leaves, flower buds, and flowers. In the case of the δ-T3 content, most of the aerial parts of H. perforatum differed statistically significantly except for only two pairs, that is, leaves and dead petals, and flowers and flower buds, which were not statistically significantly different.