Antiprotozoal Aminosteroids from Pachysandra terminalis
Abstract
1. Introduction
2. Results and Discussion
2.1. Antiprotozoal Activity of the Crude Extract and Its Fractions
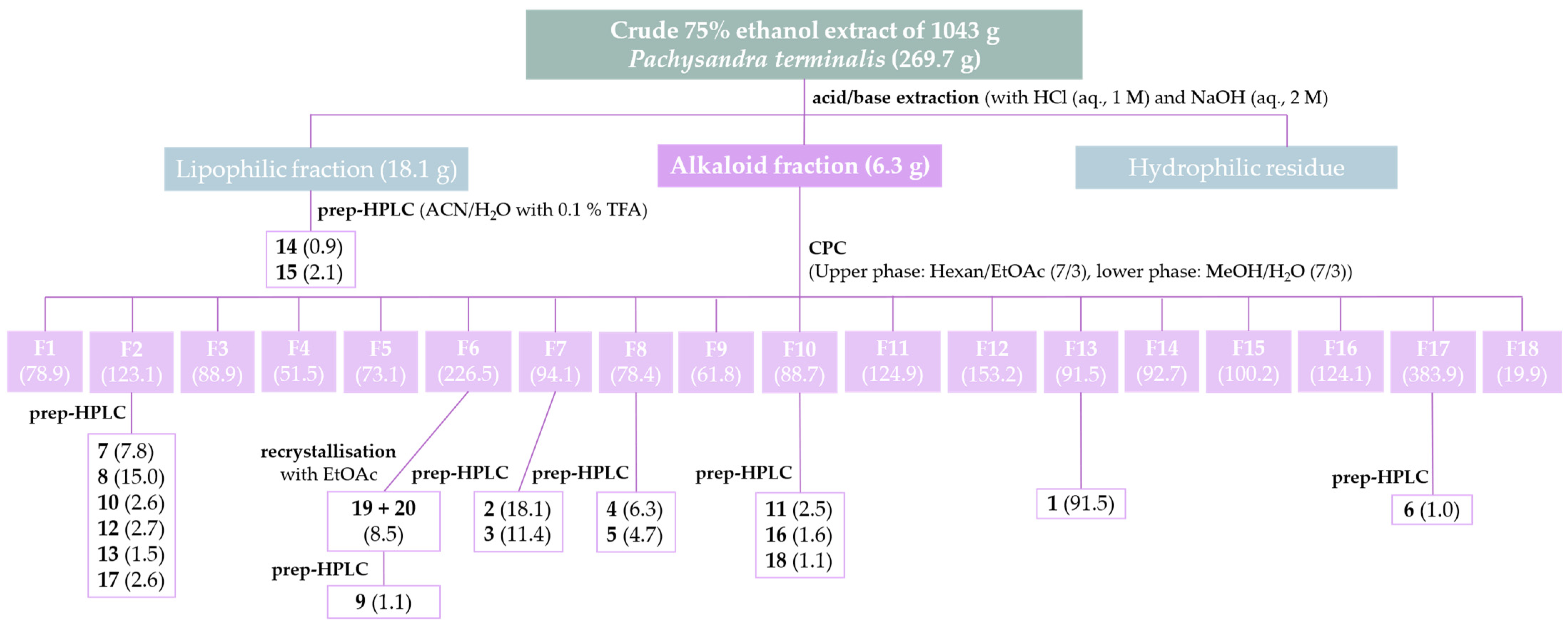
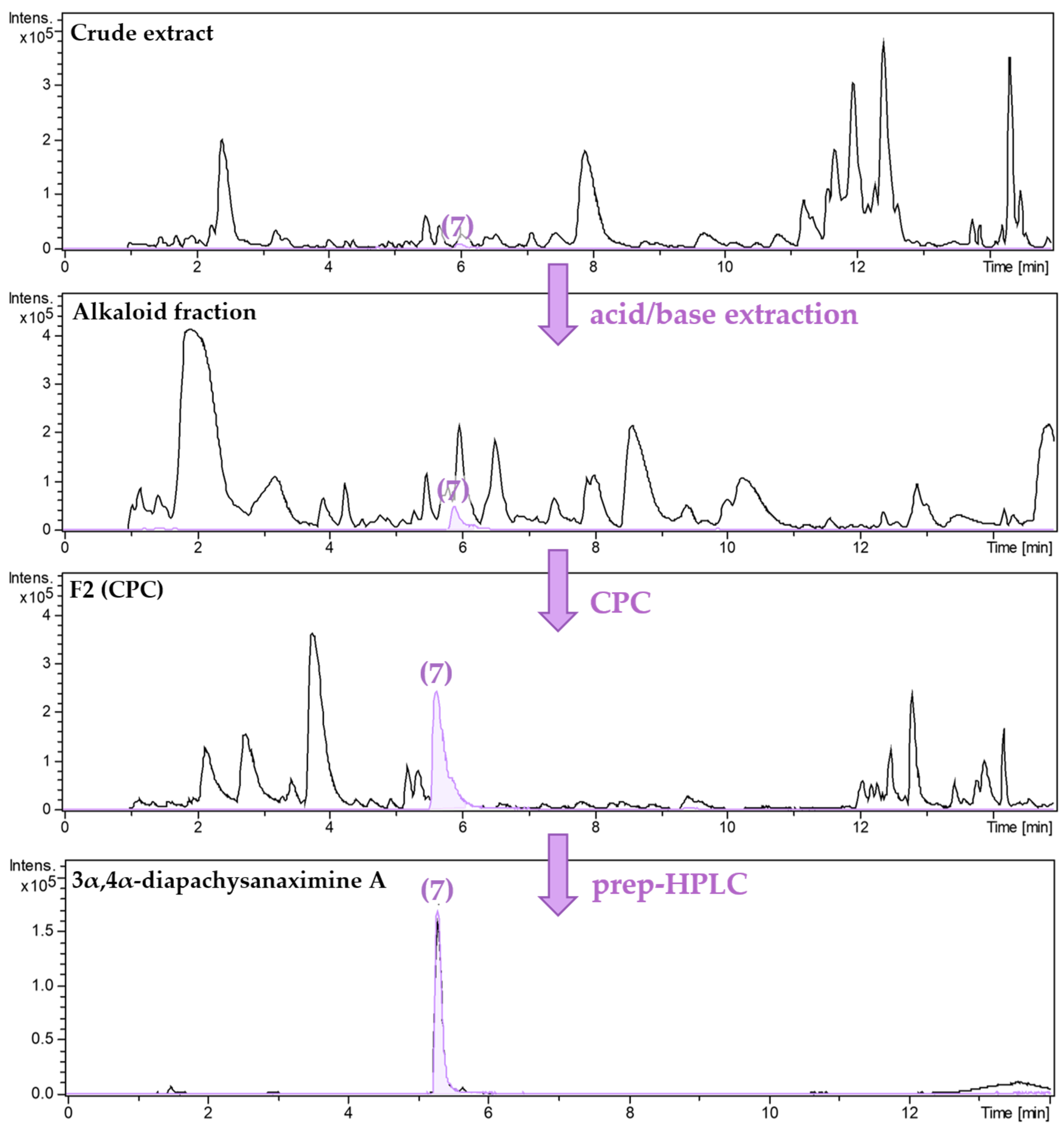
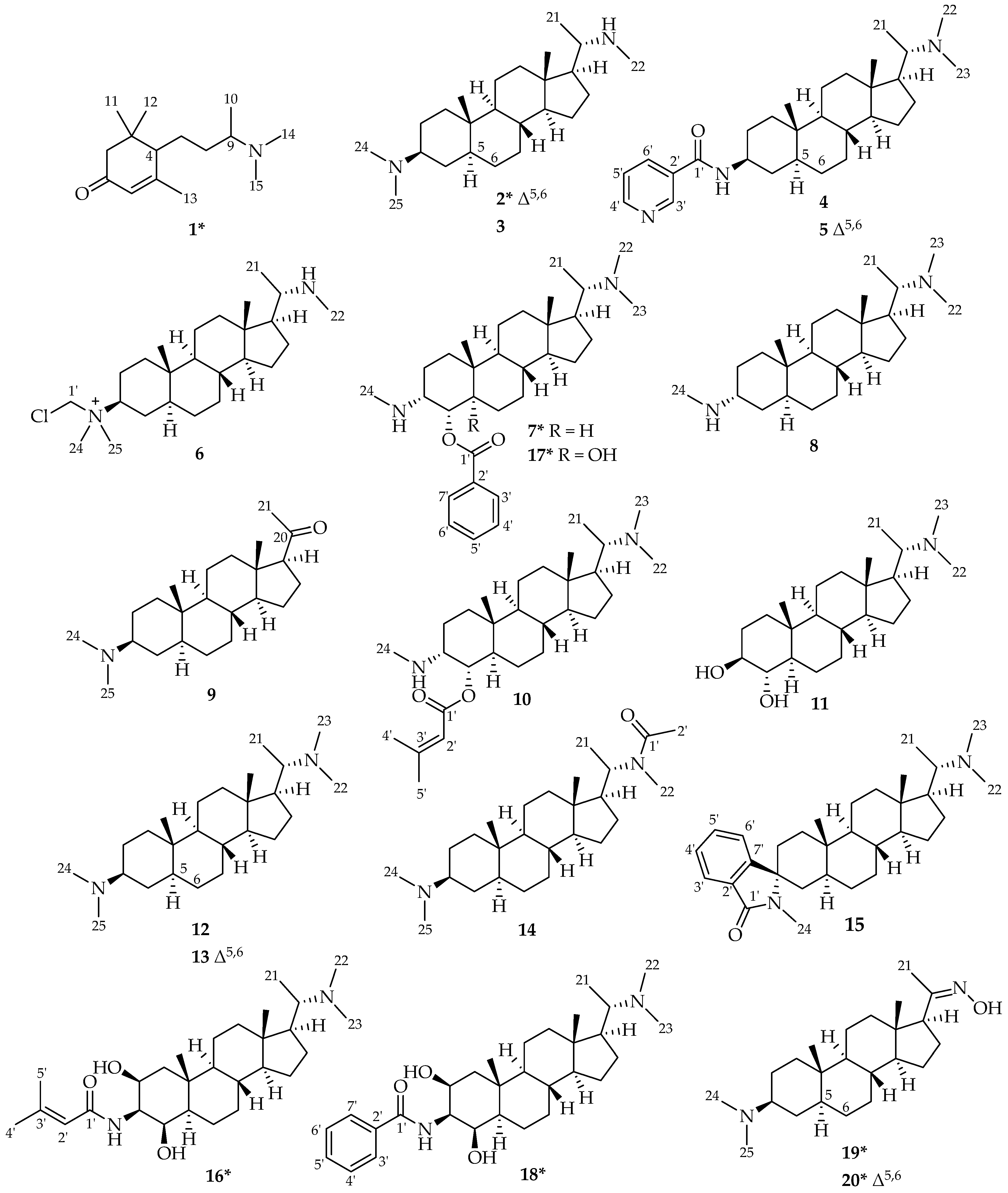
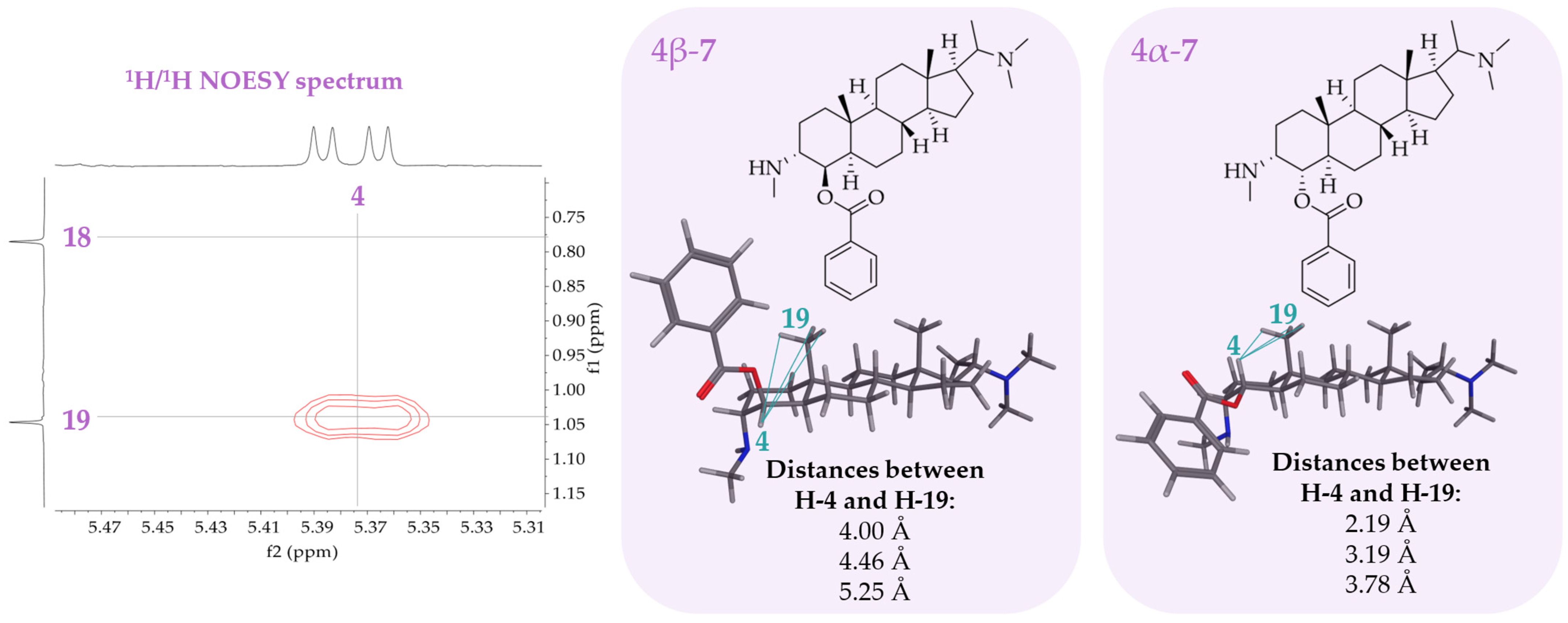
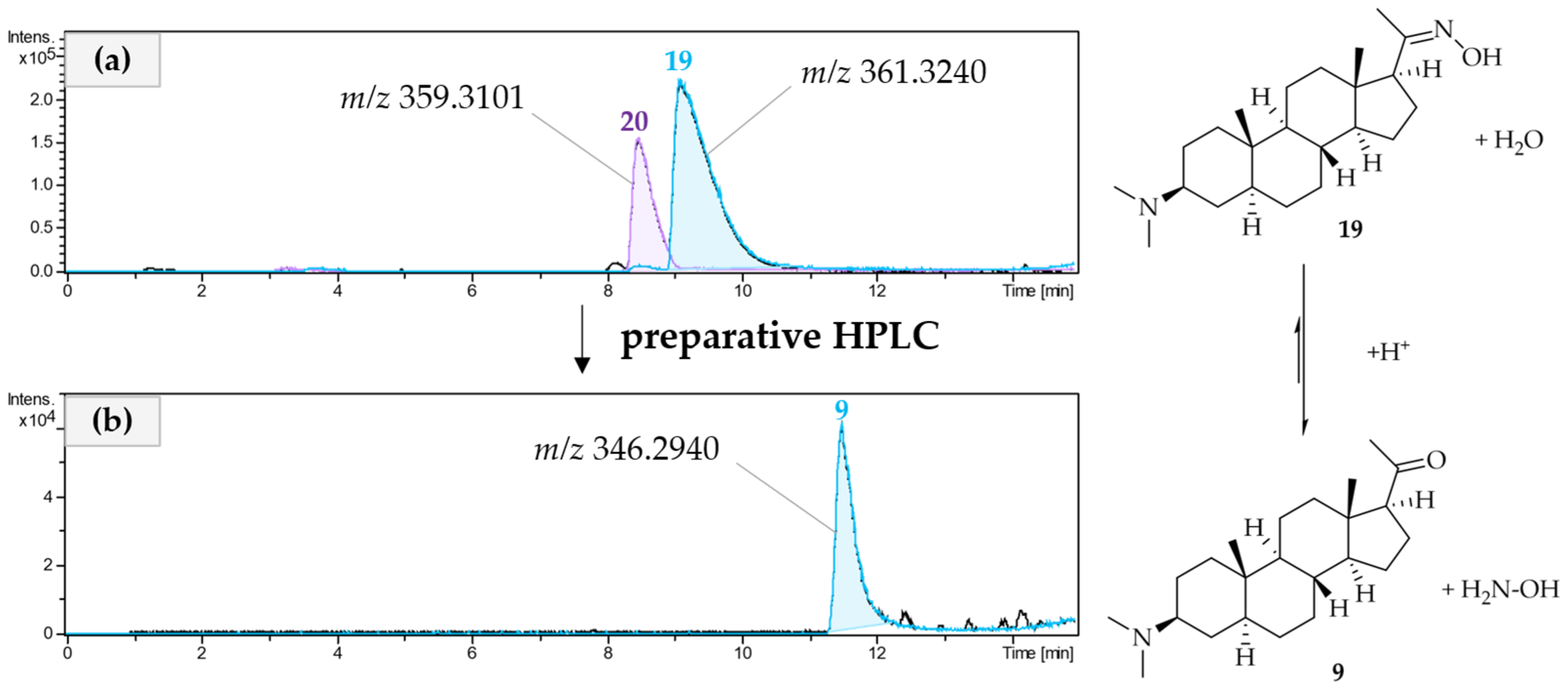
2.2. Isolation and Structural Characterization of Alkaloids from Pachysandra terminals
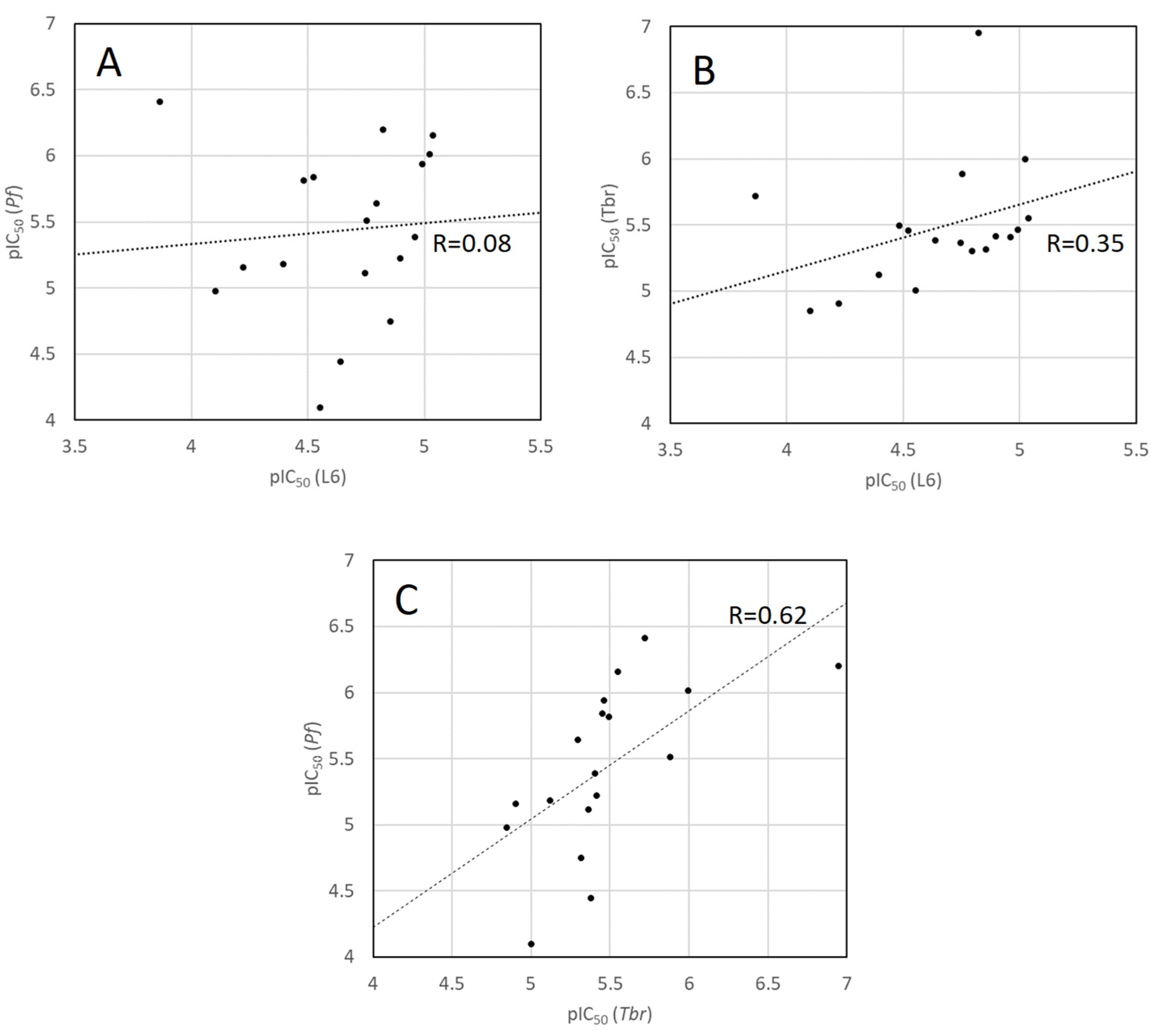
2.3. Antiprotozoal Activity of the Isolated Compounds from Pachysandra terminalis
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of the Plant Material
3.3. Isolation of Alkaloids from Pachysandra terminalis
3.3.1. Isolation of a Megastigmane and Aminosteroids from the Alkaloid Fraction
3.3.2. Isolation of Aminosteroids from the Lipophilic Residue
3.4. Analysis of the Isolated Alkaloids
3.5. Spectral Data of the Isolated Alkaloids
3.6. Biological Activity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Author Statement
References
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Haldar, K.; Bhattacharjee, S.; Safeukui, I. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 2018, 16, 156–170. [Google Scholar] [CrossRef]
- Torreele, E.; Bourdin Trunz, B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazué, G.; Bray, M.A.; Pécoul, B. Fexinidazole—A New Oral Nitroimidazole Drug Candidate Entering Clinical Development for the Treatment of Sleeping Sickness. PLoS Negl. Trop. Dis. 2010, 4, e923. [Google Scholar] [CrossRef] [PubMed]
- Tarral, A.; Blesson, S.; Valverde Mordt, O.; Torreele, E.; Sassella, D.; Bray, M.A.; Hovsepian, L.; Evène, E.; Gualano, V.; Felices, M.; et al. Determination of an Optimal Dosing Regimen of Fexinidazole, a Novel Oral Drug for the Treatment of Human African Trypanosomiasis: First-in-Human Studies. Clin. Pharmocokinet. 2014, 53, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Ma Alves, T.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoen neglected diseases—Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Ma Alves, T.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.G.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoen neglected diseases—Part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, C.O.; Nwodo, N.J.; Kaiser, M.; Brun, R.; Schmidt, T.J. Steroid Alkaloids from Holarrhena africana with strong activity against Trypanosoma brucei rhodesiense. Molecules 2017, 22, 1129. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, C.O.; Ebiloma, G.U.; Black, J.A.; Nwodo, N.J.; Lemgruber, L.; Schmidt, T.J.; de Koning, H.P. Potent Antitrypanosomal Activities of 3-Aminosteroids against African Trypanosomes: Investigation of Cellular Effects and of Cross-Resistance with Existing Drugs. Molecules 2019, 24, 268. [Google Scholar] [CrossRef]
- Althaus, J.B.; Jerz, G.; Winterhalter, P.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal Activity of Buxus sempervirens and Activity-Guided Isolation of O-tigloylcyclovirobuxeine-B as the Main Constituent Active against Plasmodium falciparum. Molecules 2014, 19, 6184–6201. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.U.; Kaiser, M.; Mäser, P.; Schmidt, T.J. Antiprotozoal Nor-Triterpene Alkaloids from Buxus sempervirens L. Antibiotics 2021, 10, 696. [Google Scholar] [CrossRef]
- Müller, F.; Ritz, C.M.; Welk, E.; Wesche, K. Rothmaler Exkursionsflora von Deutschland, 22nd ed.; Springer Spektrum: Berlin/Heidelberg, Germany, 2021; p. 338. [Google Scholar] [CrossRef]
- Knaack, W.; Geissman, T. Steroidal alkaloids of Pachysandra terminalis (Buxaceae). Tetrahedron Lett. 1964, 5, 1381–1385. [Google Scholar] [CrossRef]
- Tomita, M.; Uyeo, S.; Kikuchi, T. Studies on the alkaloids of Pachysandra terminalis Sieb. et Zucc.: Structure of Pachysandrine A and B. Tetrahedron Lett. 1964, 5, 1053–1061. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uyeo, S.; Ando, M.; Yamamoto, A. Alkaloids of Pachysandra terminalis. III. Sytematic separation and characterization of alkaloids. Tetrahedron Lett. 1964, 27–28, 1817–1823. [Google Scholar] [CrossRef]
- Xiang, M.; Hu, B.; Qi, Z.; Wang, X.; Xie, T.; Wang, Z.; Ma, D.; Zeng, Q.; Luo, X. Chemistry and bioactivities of natural steroidal alkaloids. Nat. Prod. Bioprospecting 2022, 12, 23. [Google Scholar] [CrossRef]
- Zhai, H.Y.; Zhao, C.; Zhang, N.; Jin, M.N.; Tang, S.A.; Qin, N.; Kong, D.X.; Duan, H.Q. Alkaloids from Pachysandra terminalis Inhibit Breast Cancer Invasion and Have Potential for Development As Antimetastasis Therapeutic Agents. J. Nat. Prod. 2012, 75, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, C.; Wang, F.; Zhang, Y.; Huang, W.; Zhang, H.; Li, Y.; Zhang, D.; Song, X. Pregnane alkaloids with BRD4 inhibitory and cytotoxic activities from Pachysandra terminalis. Phytochem. Lett. 2021, 45, 63–67. [Google Scholar] [CrossRef]
- Li, X.-Y.; Yu, Y.; Jia, M.; Jin, M.-N.; Qin, N.; Zhao, C.; Duan, H.-Q.; Terminamines, K.-S. Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis. Molecules 2016, 21, 1283. [Google Scholar] [CrossRef] [PubMed]
- Flittner, D.; Kaiser, M.; Mäser, P.; Lopes, N.P.; Schmidt, T.J. The Alkaloid-Enriched Fraction of Pachysandra terminalis (Buxaceae) Shows Prominent Activity against Trypanosoma brucei rhodesiense. Molecules 2021, 26, 591. [Google Scholar] [CrossRef]
- Pollitt, L.C.; MacGregor, P.; Matthews, K.; Reece, S.E. Malaria and trypanosomes transmission: Different parasites, same rules? Trends Parasitol. 2011, 27, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Das, B.; Dutta, C.P.; Mukherjee, K.S. Steroid alkaloids of Saracococa pruniformis lindl. Tetrahedron Lett. 1965, 6, 67–72. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Zaheer-ul-Haq; Feroz, Z.; Khalid, A.; Nawaz, S.A.; Khan, M.R.; Choudhary, M.I. New Cholinesterase-Inhibiting Steroidal Alkaloids from Sarcococca saligna. Helv. Chim. Acta 2004, 87, 439–448. [Google Scholar] [CrossRef]
- Tomita, M.; Kikuchi, T.; Uyeo, S.; Nishinaga, T.; Yasunishi, M.; Yamamoto, A. Pachysandra Alkaloids. I. Systematic isolation and characterization of alkaloids. Yakugaku Zasshi 1967, 87, 215–227. [Google Scholar] [CrossRef][Green Version]
- Kikuchi, T.; Uyeo, S.; Nishinaga, T. Alkaloids of Pachysandra terminalis. IV. Structure of epipachysamine B, epipachysamine C and terminaline. Tetrahedron Lett. 1965, 24, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xu, Y.; Fan, H.; Huang, W.; Zhang, H.; Wang, W.; Song, X. A new C21 steroidal compound from the whole herb of Pachysandra terminalis Sieb. et Zucc. Biochem. Syst. Ecol. 2020, 91, 104056. [Google Scholar] [CrossRef]
- Tomita, M.; Uyeo, S.; Kikuchi, T. Studies on the alkaloids of Pachysandra terminalis Sieb. et Zucc. (2).: Structure of pachysamine -A and -B. Tetrahedron Lett. 1964, 25, 1641–1644. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uyeo, S. Pachysandra alkaloids. III. Structures of pachysandrine-B, -C and -D. Chem. Pharm. Bull. 1967, 15, 207–213. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Devkota, K.P.; Nawaz, S.A.; Ranjit, R.; Rahman, A. Cholinesterase inhibitory pregnane-type steroidal alkaloids from Sarcococca hookeriana. Steroids 2005, 4, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Uyeo, S.; Nishinaga, T. Pachysandra Alkaloids. V. Structure of Epipachysamine-A, -B, -C, -D, -E and -F. Chem. Pharm. Bull. 1967, 15, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Nishinaga, T.; Inagaki, M.; Niwa, M.; Kuriyama, K. Pachysandra Alkaloids. XIII. Structure and stereochemistry of spiropachysine, a novel spiro-lactam alkaloid. Chem. Pharm. Bull. 1975, 23, 416–429. [Google Scholar] [CrossRef][Green Version]
- Musharraf, S.; Goher, M.; Ali, A.; Adhikari, A.; Choudhary, M.; Atta-Ur-Rahman. Rapid characterization and identification of steroidal alkaloids in Sarcococca coriacea using liquid chromatography coupled with electrospray ionization quadropole time-of-flight mass spectrometry. Steroids 2012, 77, 138–148. [Google Scholar] [CrossRef]
- Jin, M.N.; Ma, S.N.; Zhai, H.Y.; Duan, H.; Kong, D. A new megastigmane alkaloid from Pachysandra terminalis with antitumor metastasis effect. Chem. Nat. Compd. 2015, 51, 311–315. [Google Scholar] [CrossRef]
- Qui, M.; Nie, R.; Li, Z.; Zhou, J. Three new steroidal alkaloids from Pachysandra axillaris. Zhiwu Xuebao 1990, 32, 626–630. [Google Scholar]
- Iqbal, N.; Adhikari, A.; Kanwal, N.; Abdalla, O.M.; Mesaik, M.A.; Musharraf, A.G. New immunomodulatory steroidal alkaloids from Sarcococa saligna. Phytochem. Lett. 2015, 14, 203–208. [Google Scholar] [CrossRef]
- Devkota, K.; Wansi, J.; Lenta, B.; Khan, S.; Choudhary, M.; Sewald, N. Bioactive Steroidal Alkaloids from Sarcococca hookeriana. Planta Med. 2010, 76, 1022–1025. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, Y.X.; Chen, J.C.; Lu, L.; Zhang, X.M.; Li, Y.; Qiu, M.H. Pregnane alkaloids from Pachysandra axillaris. Steroids 2010, 75, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden in der organischen Chemie; Thieme: Stuttgart, Germany, 2005. [Google Scholar]
- Rodríguez, J.; Nuñez, L.; Peixinho, S.; Jiménez, C. Isolation of the first natural 6-hydroximino-4-en-3-one-steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997, 38, 1833–1836. [Google Scholar] [CrossRef]
- Latscha, H.P.; Kazmaier, U.; Klein, H. Organische Chemie—Chemie-Basiswissen II; Springer Spektrum: Berlin/Heidelberg, Germany, 2023; pp. 250–251. [Google Scholar] [CrossRef]
- Rudine, A.B.; Walter, M.G.; Wamser, C.C. Reaction of Dichlormethane with Pyridine Derivatives under Ambient Conditions. J. Org. Chem. 2010, 75, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, C.O.; Althaus, J.B.; Nwodo, N.J.; Schmidt, T.J. 3D-QSAR Study on the Antitrypanosomal and Cytotoxic Activities of Steroid Alkaloids by Comparative Molecular Field Analysis. Molecules 2018, 23, 1113. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Schmidt, T.J. Target-Guided Isolation of O-tigloylcyclovirobuxeine-B from Buxus sempervirens L. by Centrifugal Partition Chromatography. Molecules 2020, 25, 4804. [Google Scholar] [CrossRef]
- Brun, R.; Schumacher, R.; Schmid, C.; Kunz, C.; Burri, C. The phenomenon of treatment failures in human African trypanosomiasis. Trop. Med. Int. Health 2001, 6, 906–914. [Google Scholar] [CrossRef]
- Huber, W.; Koella, J.C. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993, 55, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ponnudurai, T.; Leeuwenberg, A.D.; Meuwissen, J.H. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geogr. Med. 1981, 33, 50–54. [Google Scholar] [PubMed]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef]
- Matile, H.; Pink, J.R.L. Plasmodium falciparum Malaria Parasite Cultures and Their Use in Immunology; Lefkovits, I., Pernis, B., Eds.; Immunological Methods; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Dorn, A.; Stoffel, R.; Matile, H.; Bubendorf, A.; Ridley, R.G. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 1995, 374, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- American Type Culture Collection. Available online: https://www.atcc.org/products/crl-1458?matchtype=&network=x&device=c&adposition=&keyword=&gad_source=1&gclid=Cj0KCQiAqL28BhCrARIsACYJvkc4HaNakUs4XdDO-iagFhY7g-WUWkR3dcXPQQJmUXVK2-SkbQY31t4aAogjEALw_wcB (accessed on 11 February 2025).
- Schäfer, L.; Kaiser, M.; Mäser, P.; Schmidt, T.J. Antiprotozoal activity of alkaloid fractions and isolation of a new megastigmane alkaloid from leaves of Pachysandra terminalis. In Proceedings of the 71st International Congress of the Society for Medicinal Plant and Natural Product Research (GA), Dublin, Ireland, 2–5 July 2023. Planta Med. 2023, 89, 1317–1318. [Google Scholar] [CrossRef]
- Schäfer, L.; Kaiser, M.; Mäser, P.; Schmidt, T.J. Isolation, structural characterization and antiprotozoal activity of steroidal alkaloids from Pachysandra terminalis. In Proceedings of the International Congress on Natural Products Research (ICNPR), Krakow, Poland, 13–17 July 2024; Abstract Book. Available online: https://www.icnpr2024.org/AbstractBook (accessed on 17 January 2024).
| Pf | Tbr | Cytotox | SI (Pf) | SI (Tbr) | |
|---|---|---|---|---|---|
| Small scale extracts | |||||
| DCM extract | 8.5 ± 0.1 | 5.6 ± 0.6 | 31 ± 8 | 3.6 | 5.5 |
| Alkaloid fraction (DCM) | 0.96 ± 0.04 | 6.07 ± 0.02 | 17 ± 1 | 17.3 | 2.7 |
| 75% EtOH extract | 9.3 ± 0.5 | 13.15 ± 0.05 | 45 ± 2 | 4.8 | 3.4 |
| Alkaloid fraction (EtOH) | 0.33 ± 0.01 | 0.85 ± 0.37 | 14 ± 1 | 41.2 | 16.0 |
| Large-scale extracts and fractions | |||||
| 75% EtOH extract | 14 ± 1 | 52 ± 11 | 76 ± 3 | 5.6 | 1.5 |
| Alkaloid fraction | 0.31 ± 0.03 | 1.8 ± 0.4 | 13 ± 3 | 41.0 | 6.9 |
| Lipophilic residue | 2.5 ± 0.2 | 2.0 ± 0.2 | 15 ± 1 | 6.0 | 7.4 |
| Hydrophilic residue | >50 | >100 | >100 | - | - |
| Positive controls | |||||
| Chloroquine | 0.002 ± 0.000 | ||||
| Melarsoprol | 0.007 ± 0.002 | ||||
| Podophyllotoxin | 0.009 ± 0.001 | ||||
| δH [ppm], mult., J [Hz] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pos. | 1 a | 2 b | 6 b | 7 b | 9 b | 16 b | 17 b | 18 b | 19 a | 20 a |
| 1 | - | 2.05, m 1.20, m | 1.95, m 1.17, m | 1.73, m 1.27, m | 1.92, m 1.12, ddd (dt), 13.5, 4.1 | 2.12, m 1.31, m | 1.73, m 1.43, m | 2.17, dd, 14.4, 3.1 1.36, m | 1.79, m 0.97, m | 1.89, m 1.07, m |
| 2 | 2.40, d, 17.3 2.05, d, 17.3 | 1.99, m 1.74, m | 2.03, m 1.78, m | 2.12, m 2.11, m | 1.91, m 1.63, m | 4.06, dd, 3.3, 1.4 | 2.12, m 2.16, m | 4.19, d, 3.4 | 1.82, m 1.50, m | 1.83, m 1.49, m |
| 3 | - | 3.08, dddd (tt), 12.2, 4.1 | 3.69, dddd (tt), 12.3, 3.9 | 3.68, m | 3.19, dddd (tt), 13.2, 4.3 | 3.84, dd (t), 3.4 | 3.63, m | 4.05, dd (t), 3.5 | 2.53, m | 2.25, m |
| 4 | 1.87, m | 2.49, dt 12.6, 12.5, 2.6 2.45, ddd, 12.8, 4.6, 2.6 | 1.76, m 1.67, m | 5.37, dd, 12.5/4.3 | 1.67, m 1.47, m | 3.74, m | 5.46, d, 4.4 | 3.87, br s | 1.57, m 1.35, m | 2.25, m |
| 5 | - | - | 1.31, m | 1.73, m | 1.25, m | 1.25, m | - | 1.31, m | 1.11, m | - |
| 6 | 5.82, s | 5.53, dd, 4.9, 2.4 | 1.37, m, 2H | 1.79, m 1.32, m | 1.38, m, 2H | 1.86, m 1.41, m, | 1.61, m 1.36, m | 1.89, m 1.41, m | 1.29, m 1.25, m | 5.34, m |
| 7 | 1.71, m 1.48, m | 2.06, m 1.63, m | 1.76, m 1.02, m | 1.81, m 1.00, m | 1.74, dd 13.3/3.3 1.00, m | 1.80, m 1.05, m | 1.68, m 1.61, m | 1.82, m 1.05, m | 1.68, m 0.89, m | 1.99, m 1.55, m |
| 8 | 1.78, m 1.41, m | 1.56, m | 1.42, m | 1.53, m | 1.44, m | 1.51, m | 1.53, m | 1.52, m | 1.33, m | 1.45, m |
| 9 | 2.68, br s | 1.05, m | 0.78, m | 0.98, m | 0.79, ddd, 12.2, 10.5, 4.1 | 0.73, m | 1.17, m | 0.76, m | 0.67, m | 0.97, m |
| 10 | 1.05, d, 3H | - | - | - | - | - | - | - | - | - |
| 11 | 1.02, s, 3H | 1.62, m 1.57, m | 1.59, m 1.36, m | 1.62, m 1.40, m | 1.64, m 1.36, m | 1.55, m 1.38, m | 1.51, m 1.35, m | 1.57, m 1.40, m | 1.26, m 1.54, m | 1.43, m 1.29, m |
| 12 | 1.07, s, 3H | 2.03, m 1.31, m | 1.98, m 1.27, m | 1.99, m 1.34, m | 2.04, m 1.46, m | 1.96, m 1.29, m | 1.98, m 1.34, m | 1.98, m 1.32, m | 1.85, m 1.26, m | 1.85, m 1.26, m |
| 13 | 1.99, s, 3H | - | - | - | - | - | - | - | - | - |
| 14 | 2.35, s, 6H | 1.17, m | 1.18, m | 1.24, m | 1.23, m | 1.22, m | 1.26, m | 1.23, m | 1.10, m | 1.04, m |
| 15 | 1.78, m 1.30, m | 1.77, m 1.30, m | 1.64, m 1.29, m | 1.70, m 1.22, m | 1.82, m 1.31, m | 1.76, m 1.27, m | 1.80, m 1.30, m | 1.65, m 1.16, m | 1.65, m 1.16, m | |
| 16 | 1.96, m 1.54, m | 1.92, m 1.49, m | 1.90, m 1.55, m | 2.13, m 1.66, m | 1.89, m 1.51, m | 1.90, m 1.55, m | 1.91, m 1.52, m | 2.08, m 1.65, m | 2.08, m 1.65, m | |
| 17 | 1.59, m | 1.58, m | 1.71, m | 2.63, t, 9.1 | 1.67, m | 1.67, m | 1.68, m | 2.20, m | 2.08, m | |
| 18 | 0.79, s, 3H | 0.77, s, 3H | 0.79, s, 3H | 0.61, s, 3H | 0.77, s, 3H | 0.78, s, 3H | 0.78, s, 3H | 0.61, s, 3H | 0.64, s, 3H | |
| 19 | 1.07, s, 3H | 0.90, s, 3H | 1.05, s, 3H | 0.88, s, 3H | 1.27, s, 3H | 1.16, s, 3H | 1.30, s, 3H | 0.79, s, 3H | 0.99, s, 3H | |
| 20 | 3.23, m | 3.21, m | 3.38, m | - | 3.35, m | 3.36, m | 3.36, m | - | - | |
| 21 | 1.36, d, 6.6, 3H | 1.35, d, 6.6 3H | 1.33, d, 6.6, 3H | 2.11, s, 3H | 1.33, d, 6.7, 3H | 1.33, d, 6.6, 3H | 1.33, d, 6.6, 3H | 1.86, s, 6.6, 3H | 1.86, s, 6.6, 3H | |
| 22 | 2.65, s, 3H | 2.65, s, 3H | 2.88, s, 3H | 2.88, s, 3H | 2.88, s, 3H | 2.88, s, 3H | ||||
| 23 | 2.70, s, 3H | 2.70, s, 3H | 2.70, s, 3H | 2.70, s, 3H | ||||||
| 24 | 2.88, s, 6H | 3.16, s, 6H | 2.75, s, 3H | 2.84, s, 3H | 2.69, s, 3H | 2.47, s (broad), 6H | 2.38, s (broad), 6H | |||
| 25 | ||||||||||
| 1′ | 5.30, s | - | - | - | - | |||||
| 2′ | - | 5.83, sep, 1.3 | - | - | ||||||
| 3′/7′ | 7.53, m | - | 8.20, m | 7.87, dd, 7.3, 1.0, 2H | ||||||
| 4′/6′ | 8.09, m | 1.88, d, 1.3, 3H | 7.53, m | 7.49, d, 7.8, 2H | ||||||
| 5′ | 7.66, m | 2.13, d, 1.3, 3H | 7.67, m | 7.56, t, 7.8 | ||||||
| δC [ppm] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pos. | 1 a | 2 b | 6 b | 7 b | 9 b | 16 b | 17 b | 18 b | 19 a | 20 a |
| 1 | 199.4, Cq | 38.4, CH2 | 38.4, CH2 | 32.0, CH2 | 37.9, CH2 | 45.8, CH2 | 26.6, CH2 | 45.7, CH2 | 37.5, CH2 | 38.3, CH2 |
| 2 | 47.4, CH2 | 23.9, CH2 | 22.4, CH2 | 21.8, CH2 | 23.8, CH2 | 72.0, CH | 21.9, CH2 | 71.5, CH | 24.8, CH2 | 23.7, CH2 |
| 3 | 36.4, Cq | 67.4, CH | 73.6, CH | 60.1, CH | 66.9, CH | 54.2, CH | 60.2, CH | 55.1, CH | 64.8, CH | 65.2, CH |
| 4 | 51.4, CH | 33.9, CH2 | 28.7, CH2 | 72.9, CH | 29.9, CH2 | 76.4, CH | 73.5, CH | 75.8, CH | 30.1, CH2 | 34.8, CH2 |
| 5 | 165.1, Cq | 139.5, Cq | 46.8, CH | 45.9, CH | 46.3, CH | 51.6, CH | 77.8, Cq | 51.4, CH | 45.7, CH | 141.3, Cq |
| 6 | 125.3, CH | 124.8, CH | 29.6, CH2 | 25.1, CH2 | 29.6, CH2 | 27.0, CH2 | 21.8, CH2 | 27.0, CH2 | 28.9, CH2 | 121.4, CH |
| 7 | 27.2, CH2 | 32.8, CH2 | 32.8, CH2 | 32.1, CH2 | 33.0, CH2 | 33.2, CH2 | 28.6, CH2 | 33.3, CH2 | 32.0, CH2 | 32.2, CH2 |
| 8 | 33.4, CH2 | 32.9, CH | 36.5, CH | 36.0, CH | 36.6, CH | 36.1, CH | 35.2, CH | 36.1, CH | 35.8, CH | 32.0, CH |
| 9 | 60.3, CH | 51.1, CH | 54.9, CH | 55.0, CH | 55.2, CH | 57.6, CH | 46.0, CH | 57.5, CH | 54.4, CH | 50.4, CH |
| 10 | 12.9, CH3 | 37.7, Cq | 36.3, Cq | 38.9, Cq | 36.7, Cq | 36.1, Cq | 42.3, Cq | 36.1, Cq | 35.9, Cq | 37.0, Cq |
| 11 | 29.0, CH3 | 21.9, CH2 | 22.1, CH2 | 21.8, CH2 | 22.3, CH2 | 21.5, CH2 | 26.1, CH2 | 21.5, CH2 | 21.3, CH2 | 21.1, CH2 |
| 12 | 27.3, CH3 | 40.1, CH2 | 40.4, CH2 | 40.3, CH2 | 40.0, CH2 | 40.4, CH2 | 40.4, CH2 | 40.4, CH2 | 39.0, CH2 | 38.8, CH2 |
| 13 | 24.7, CH3 | 43.8, Cq | 44.0, Cq | 44.2, Cq | 45.3, Cq | 44.3, Cq | 44.2, Cq | 44.3, Cq | 44.1, Cq | 43.9, Cq |
| 14 | 40.4, CH3 | 57.5, CH | 57.2, CH | 57.2, CH | 57.7, CH | 57.3, CH | 57.0, CH | 57.3, CH | 56.0, CH | 56.3, CH |
| 15 | 25.2, CH2 | 25.1, CH2 | 23.6, CH2 | 25.4, CH2 | 25.2, CH2 | 25.0, CH2 | 25.2, CH2 | 24.3, CH2 | 24.4, CH2 | |
| 16 | 27.2, CH2 | 27.2, CH2 | 26.7, CH2 | 23.6, CH2 | 26.8, CH2 | 26.8, CH2 | 26.8, CH2 | 23.2, CH2 | 23.2, CH2 | |
| 17 | 54.1, CH | 54.2, CH | 53.1, CH | 64.7, CH | 53.1, CH | 53.1, CH | 53.2, CH | 57.0, CH | 56.9, CH | |
| 18 | 12.2, CH3 | 12.4, CH3 | 12.5, CH3 | 13.8, CH3 | 12.5, CH3 | 12.5, CH3 | 12.5, CH3 | 13.5, CH3 | 13.3, CH3 | |
| 19 | 19.5, CH3 | 12.5, CH3 | 13.0, CH3 | 12.5 CH3 | 17.4, CH3 | 15.9, CH3 | 17.3, CH3 | 12.5, CH3 | 19.6, CH3 | |
| 20 | 59.7, CH | 59.7, CH | 67.0, CH | 212.3, Cq | 67.1, CH | 67.0, CH | 67.1, CH | 158.9, Cq | 158.8, Cq | |
| 21 | 15.9, CH3 | 15.9, CH3 | 12.0, CH3 | 31.6, CH3 | 11.9, CH3 | 12.0, CH3 | 12.0, CH3 | 15.2, CH3 | 15.2, CH3 | |
| 22 | 29.5, CH3 | 29.5, CH3 | 43.4, CH3 | 43.4, CH3 | 43.4, CH3 | 43.4, CH3 | ||||
| 23 | 35.8, CH3 | 35.8, CH3 | 35.8, CH3 | 35.8, CH3 | ||||||
| 24 | 40.5, CH3 | 47.6, CH3 | 33.2, CH3 | 40.4, CH3 | 33.5, CH3 | 40.9, CH3 | 40.4, CH3 | |||
| 25 | ||||||||||
| 1′ | 69.3, CH2 | 166.9, Cq | 162.2, Cq | 167.1, Cq | 169.6, Cq | |||||
| 2′ | 130.5, Cq | 119.7, CH | 130.7, Cq | 135.7, Cq | ||||||
| 3′/7′ | 129.8, CH | 139.7, Cq | 131.2, CH | 129.6, CH | ||||||
| 4′/6′ | 130.9, CH | 27.2, CH3 | 129.7, CH | 132.8, CH | ||||||
| 5′ | 134.9, CH | 20.0, CH3 | 134.94, CH | 128.30, CH | ||||||
| Compound | Pf [µg/mL] | Tbr [µg/mL] | Cytotox [µg/mL] | SI (Pf) | SI(Tbr) |
|---|---|---|---|---|---|
| 1 | 0.93 ± 0.07 (3.91 µM) | 6.2 ± 0.9 (26.2 µM) | 47 ± 1 (198 µM) | 51 | 8 |
| 2 b | 0.898 ± 0.002 (1.53 µM) | 1.9 ± 0.2 (3.2 µM) | 19 ± 5 (33 µM) | 21 | 10 |
| 3 b | 0.85 ± 0.03 (1.44 µM) | 2.0 ± 0.2 (3.5 µM) | 18 ± 3 (30 µM) | 21 | 7 |
| 4 b | 2.3 ± 0.2 (3.4 µM) | 1.6 ± 0.5 (2.4 µM) | 5.2 ± 0.3 (7.7 µM) | 2 | 3 |
| 5 b | 0.91 ± 0.02 (1.34 µM) | 1.93 ± 0.06 (2.85 µM) | 5.8 ± 0.4 (8.6 µM) | 6 | 3 |
| 6 b | 0.25 ± 0.03 (0.39 µM) | 1.2 ± 0.4 (1.9 µM) | 87 ± 13 (136 µM) | 346 | 74 |
| 7 b | 0.45 ± 0.09 (0.63 µM) | 0.079 ± 0.001 (0.112 µM) | 10 ± 4 (15 µM) | 23 | 133 |
| 8 b | 1.82 ± 0.06 (3.09 µM) | 0.8 ± 0.1 (1.3 µM) | 10.4 ± 0.8 (17.7 µM) | 6 | 13 |
| 9 a | 8 ± 2 (18 µM) | 2.21 ± 0.06 (4.80 µM) | 6 ± 2 (14 µM) | 0.8 | 3 |
| 10 b | 0.66 ± 0.13 (0.97 µM) | 0.69 ± 0.03 (1.01 µM) | 6.5 ± 0.7 (9.5 µM) | 10 | 9 |
| 11 a | 3 ± 1 (7 µM) | 5.9 ± 0.3 (12.4 µM) | 28 ± 13 (60 µM) | 8 | 5 |
| 12 b | 2.5 ± 0.3 (4.1 µM) | 2.3 ± 0.2 (3.9 µM) | 7 ± 2 (11 µM) | 3 | 3 |
| 13 b | 4.6 ± 0.7 (7.7 µM) | 2.6 ± 0.4 (4.3 µM) | 11 ± 3 (18 µM) | 2 | 4 |
| 14 a | 18 ± 1 (36 µM) | 2.14 ± 0.05 (4.14 µM) | 12 ± 3 (23 µM) | 0.6 | 5 |
| 15 a | 3 ± 1 (6 µM) | 2.21 ± 0.04 (3.83 µM) | 7.3 ± 0.7 (12.7 µM) | 2 | 3 |
| 16 a | 6.1 ± 0.5 (10.6 µM) | 8.1 ± 0.6 (14.1 µM) | 45 ± 1 (79 µM) | 7 | 6 |
| 17 b | 1.6 ± 0.4 (2.3 µM) | 3 ± 2 (5 µM) | 12 ± 1 (16 µM) | 7 | 3 |
| 18 a | 47 ± 7 (80 µM) | 5.9 ± 0.4 (9.9 µM) | 17 ± 2 (28 µM) | 0.4 | 3 |
| 19 c + 20 c | 2.38 ± 0.11 (6.61 µM) | 2.71 ± 0.67 (7.52 µM) | 14.51 ± 0.31 (40.27 µM) | 6 | 5 |
| Trifluoroacetate | >100 | >100 | >100 | - | - |
| Chloroquine | 0.002 ± 0.000 | ||||
| Melarsoprol | 0.007 ± 0.002 | ||||
| Podophyllotoxin | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, L.; Cal, M.; Kaiser, M.; Mäser, P.; Schmidt, T.J. Antiprotozoal Aminosteroids from Pachysandra terminalis. Molecules 2025, 30, 1093. https://doi.org/10.3390/molecules30051093
Schäfer L, Cal M, Kaiser M, Mäser P, Schmidt TJ. Antiprotozoal Aminosteroids from Pachysandra terminalis. Molecules. 2025; 30(5):1093. https://doi.org/10.3390/molecules30051093
Chicago/Turabian StyleSchäfer, Lizanne, Monica Cal, Marcel Kaiser, Pascal Mäser, and Thomas J. Schmidt. 2025. "Antiprotozoal Aminosteroids from Pachysandra terminalis" Molecules 30, no. 5: 1093. https://doi.org/10.3390/molecules30051093
APA StyleSchäfer, L., Cal, M., Kaiser, M., Mäser, P., & Schmidt, T. J. (2025). Antiprotozoal Aminosteroids from Pachysandra terminalis. Molecules, 30(5), 1093. https://doi.org/10.3390/molecules30051093







