Simultaneous Quantification of Bioactive Triterpene Saponins Calenduloside E and Chikusetsusaponin IVa in Different Plant Parts of Ten Amaranthaceae Species by UPLC-ESI-MS/MS Method
Abstract
1. Introduction
2. Results and Discussion
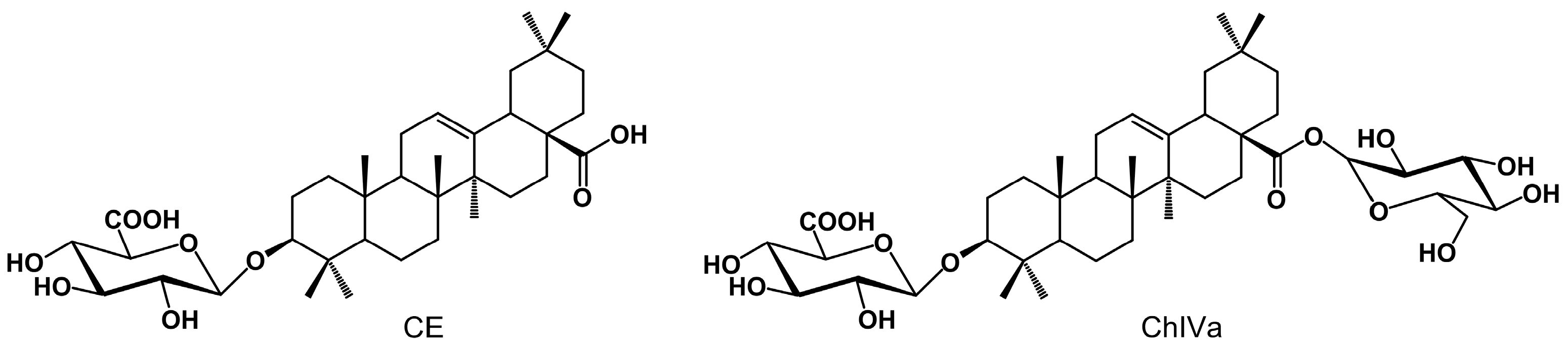
2.1. UPLC-MS/MS Analysis
Method Validation
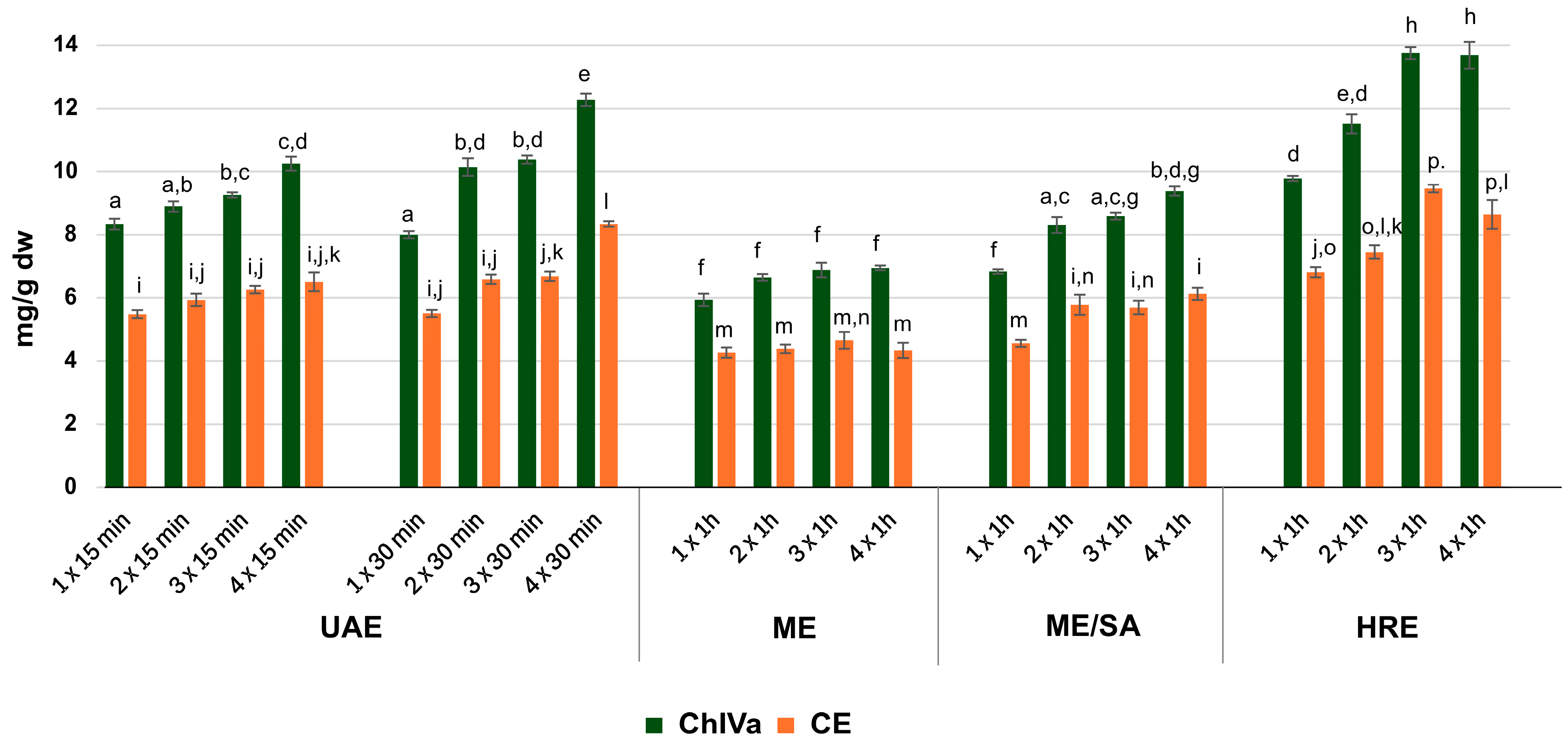
2.2. Optimization of Extraction
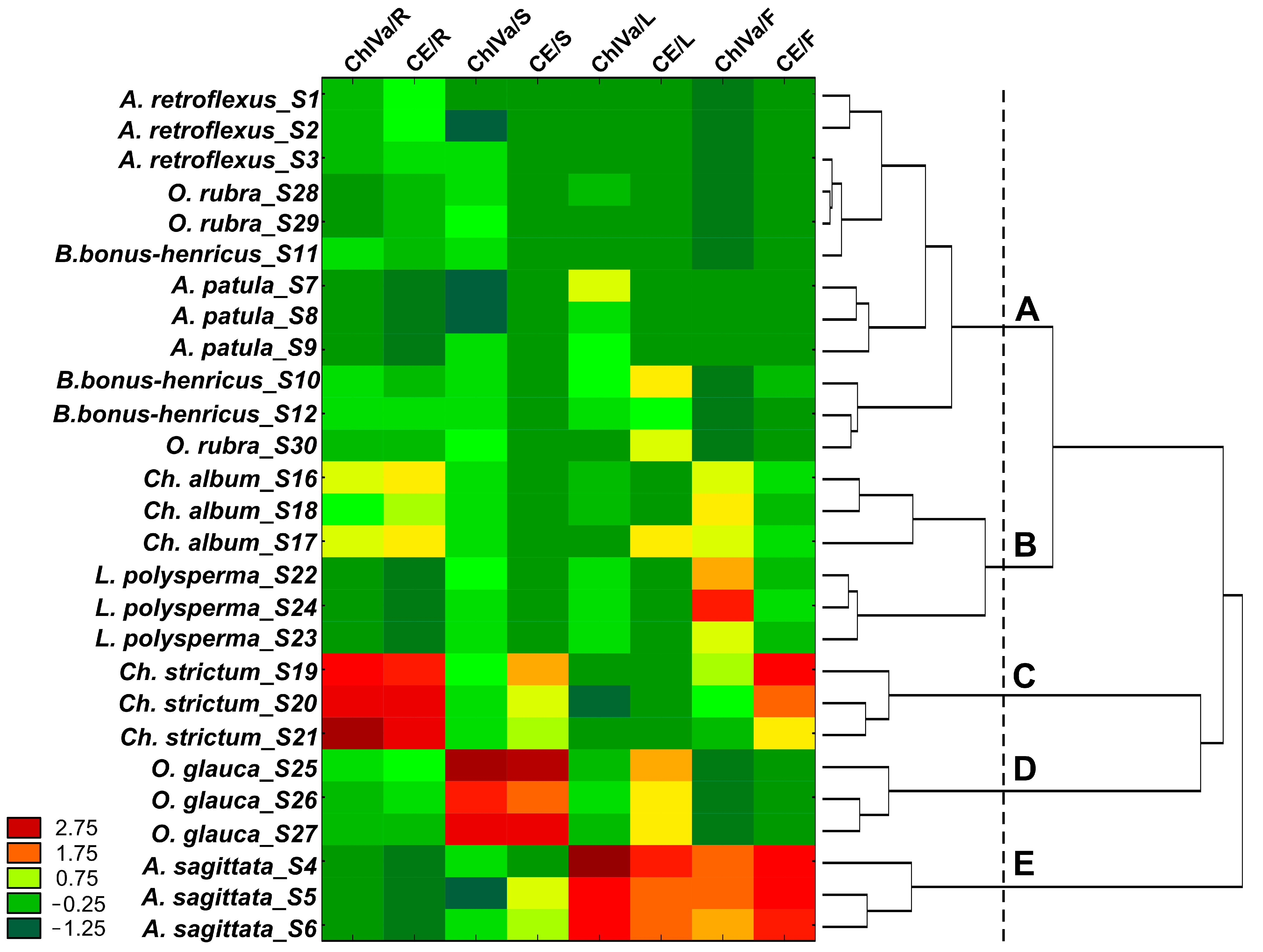
2.3. Quantitation of CE and ChIVa in Plants from the Amaranthaceae Family
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. General Experimental Procedures
3.3. Plant Material
3.4. Selection of the Optimal Extraction Technique
3.5. Solid-Phase Extraction (SPE) Procedure
3.6. Recovery Tests
3.7. Optimized Extraction Procedure
3.8. Preparation of Standards
3.9. Preparation of Calibration Samples
3.10. Preparation of Samples
3.11. Quantitative UPLC-MS/MS Analysis
3.12. Method Validation
3.13. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podolak, I.; Grabowska, K.; Sobolewska, D.; Wróbel-Biedrawa, D.; Makowska-Wąs, J.; Galanty, A. Saponins as cytotoxic agents: An update (2010–2021). Part II—Triterpene saponins. Phytochem. Rev. 2023, 22, 113–167. [Google Scholar] [CrossRef]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-W.; Xiao, S.-Y.; Wang, J.; Hou, W.; Wang, Y.-P. Inhibitory effects of ginsenoside ro on the growth of B16F10 melanoma via its metabolites. Molecules 2019, 24, 2985. [Google Scholar] [CrossRef]
- Grabowska, K.; Galanty, A.; Pecio, Ł.; Stojakowska, A.; Malarz, J.; Żmudzki, P.; Zagrodzki, P.; Podolak, I. Selectivity screening and structure–cytotoxic activity observations of selected oleanolic acid (OA)-type saponins from the Amaranthaceae family on a wide panel of human cancer cell lines. Molecules 2024, 29, 3794. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, K.; Pietrzak, W.; Paśko, P.; Sołtys, A.; Galanty, A.; Żmudzki, P.; Nowak, R.; Podolak, I. Antihyaluronidase and antioxidant potential of Atriplex sagittata Borkh. in relation to phenolic compounds and triterpene saponins. Molecules 2023, 28, 982. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, J.; Li, L.; Wu, T.; Wang, Y.; Wang, X.; Ning, Q. Inhibitory effects of chikusetsusaponin IVa on lipopolysaccharide-induced pro-inflammatory responses in THP-1 cells. Int. J. Immunopathol. Pharmacol. 2015, 28, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Liu, C.; Wang, T.; He, Y.; Zhou, Z.; Dun, Y.; Zhao, H.; Ren, D.; Wang, J.; Zhang, C.; et al. Chikusetsu saponin IVa ameliorates high fat diet-induced inflammation in adipose tissue of mice through inhibition of NLRP3 inflammasome activation and NF-κB signaling. Oncotarget 2017, 8, 31023–31040. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Liu, J.; Guo, J.; Chen, F.; Xu, J.; Chen, H.; Wang, C.; Le, Y.; Lu, D. Calenduloside e ameliorates inflammatory responses in adipose tissue via sirtuin 2-nlrp3 inflammasome axis. J. Agric. Food Chem. 2024, 72, 20959–20973. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Guo, J.; Liu, Z.; Liu, J.; Liu, Y.; Chen, H.; Qiu, J.; Wang, C.; Dou, X.; Lu, D. Calenduloside E ameliorates non-alcoholic fatty liver disease via modulating a pyroptosis-dependent pathway. J. Ethnopharmacol. 2024, 319, 117239. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mou, J.; Han, Y.; Wang, M.; Lu, S.; Ma, Q.; Wang, J.; Ye, J.; Sun, G. Calenduloside e modulates macrophage polarization via KLF2-regulated glycolysis, contributing to attenuates atherosclerosis. Int. Immunopharmacol. 2023, 117, 109730. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ji, J.; Zhang, J.; Hou, X.; Fu, X.; Luo, Y.; Mei, Z.; Feng, Z. Anti-inflammatory and osteoprotective effects of Chikusetsusaponin IVa on rheumatoid arthritis via the JAK/STAT signaling pathway. Phytomedicine 2021, 93, 153801. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Du, Y.Y.; Shang, H.; Wang, M.; Sun, Z.H.; Wang, B.Q.; Deng, D.; Wang, S.; Xu, X.D.; Sun, G.B.; et al. Calenduloside E analogues protecting H9c2 cardiomyocytes against H2O2-induced apoptosis: Design, synthesis and biological evaluation. Front. Pharmacol. 2017, 8, 862. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, S.; Shang, H.; Wang, W.Q.; Wang, B.Q.; Zhang, X.; Xu, X.D.; Sun, G.B.; Sun, X.B. The clickable activity-based probe of anti-apoptotic calenduloside E. Pharm. Biol. 2019, 57, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.; Zhou, J.; Dai, Z.; Sun, G.; Sun, X. Calenduloside E suppresses calcium overload by promoting the interaction between L-type calcium channels and Bcl2-associated athanogene 3 to alleviate myocardial ischemia/reperfusion injury. J. Adv. Res. 2020, 34, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.; Liu, B.; Xu, H.; Ye, J.; Sun, X.; Sun, G. Calenduloside E protects against myocardial ischemia-reperfusion injury induced calcium overload by enhancing autophagy and inhibiting L-type Ca2+ channels through BAG3. Biomed. Pharmacother. 2022, 145, 112432. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.Y.; Zhou, J.H.; Xie, X.H.; Sun, G.B.; Sun, X.B. Calenduloside E ameliorates myocardial ischemia-reperfusion injury through regulation of AMPK and mitochondrial OPA1. Oxidative Med. Cell Longev. 2020, 2020, 2415269. [Google Scholar] [CrossRef]
- Li, J.; Bu, Y.; Li, B.; Zhang, H.; Guo, J.; Hu, J.; Zhang, Y. Calenduloside E alleviates cerebral ischemia/reperfusion injury by preserving mitochondrial function. J. Mol. Histol. 2022, 53, 713–727. [Google Scholar] [CrossRef]
- Duan, J.; Yin, Y.; Cui, J.; Yan, J.; Zhu, Y.; Guan, Y.; Wei, G.; Weng, Y.; Wu, X.; Guo, C.; et al. Chikusetsu saponin IVa Ameliorates cerebral ischemia reperfusion injury in diabetic mice via adiponectin-mediated AMPK/GSK-3β pathway in vivo and in vitro. Mol. Neurobiol. 2016, 53, 728–743. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Cui, J.; Jia, N.; Wu, Y.; Xi, M.; Wen, A. Chikusetsu saponin IVa regulates glucose uptake and fatty acid oxidation: Implications in antihyperglycemic and hypolipidemic effects. J. Pharm. Pharmacol. 2015, 67, 997–1007. [Google Scholar] [CrossRef]
- Cui, J.; Xi, M.M.; Li, Y.W.; Duan, J.L.; Wang, L.; Weng, Y.; Jia, N.; Cao, S.S.; Li, R.L.; Wang, C.; et al. Insulinotropic effect of Chikusetsu saponin IVa in diabetic rats and pancreatic β-cells. J. Ethnopharmacol. 2015, 164, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Seo, C.S.; Hwang, I.H.; Lee, M.W.; Song, K.H. Anti-obesity activities of Chikusetsusaponin IVa and Dolichos lablab L. seeds. Nutrients 2018, 10, 1221. [Google Scholar] [CrossRef]
- Cui, J.; Duan, J.; Chu, J.; Guo, C.; Xi, M.; Li, Y.; Weng, Y.; Wei, G.; Yin, Y.; Wen, A.; et al. Chikusetsu saponin IVa protects pancreatic β cell against intermittent high glucose-induced injury by activating Wnt/β-catenin/TCF7L2 pathway. Aging 2020, 12, 1591–1609. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duan, J.; Jia, N.; Liu, M.; Cao, S.; Weng, Y.; Zhang, W.; Cao, J.; Li, R.; Cui, J.; et al. IRS-2/Akt/GSK-3β/Nrf2 pathway contributes to the protective effects of chikusetsu saponin IVa against lipotoxicity. Oxidative Med. Cell Longev. 2021, 2021, 8832318. [Google Scholar] [CrossRef] [PubMed]
- Rattanathongkom, A.; Lee, J.B.; Hayashi, K.; Sripanidkulchai, B.O.; Kanchanapoom, T.; Hayashi, T. Evaluation of chikusetsusaponin IV a isolated from Alternanthera philoxeroides for its potency against viral replication. Planta Med. 2009, 75, 829–835. [Google Scholar] [CrossRef]
- Dai, Q.; Wu, S.T.; Zheng, X.; You, P.T.; Liu, Y.W.; Zhao, Y.; Zhang, X.Q. Chikusetsusaponin IVa targets Nrf2 to inhibit H9N2 avian influenza virus infection. Pharmacogn. Mag. 2024. [Google Scholar] [CrossRef]
- Kim, K.M.; Im, A.R.; Shim, K.S.; Seo, C.S.; Lee, Y.; Lee, J.; Yoo, J.S.; Choi, S.; Chae, S. Chikusetsusaponin IVa from Dolichos lablab Linne attenuates UVB-induced skin photoaging in mice by suppressing MAPK/AP-1 signaling. Appl. Biol. Chem. 2024, 67, 79. [Google Scholar] [CrossRef]
- Liu, Y.W.; Luo, R.Y.; Liu, A.Q.; Wang, J.W.; Hu, N.P.; Li, W.T.; Li, J.K.; Wang, J.W.; Duan, J.L. Identification of chikusetsusaponin IVa as a novel lysine-specific demethylase 1 inhibitor that ameliorates high fat diet-induced MASLD in mice. Acta Pharmacol. Sin. 2024, 46, 632–652. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Sun, Z.; Wang, W.; Shang, H.; Wang, B.; Deng, D.; Ma, G.; Wu, H.; Zhu, N.; Xu, X.; et al. Semisynthesis and biological evaluation of oleanolic acid 3-O-β-d-glucuronopyranoside derivatives for protecting H9c2 cardiomyoblasts against H2O2-induced injury. Molecules 2018, 23, 44. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Sun, Y.; Cheng, L.; Zhao, S.; Xu, H.; Fawcett, J.P.; Sun, X.; Gu, J. Pharmacokinetic study of calenduloside E and its active metabolite oleanolic acid in beagle dog using liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2014, 951–952, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Liu, C. Biopharmaceutics classification and intestinal absorption of chikusetsusaponin IVa. Biopharm. Drug Dispos. 2019, 40, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.P.; Guo, M.H.; Wang, Z. Determination and validation of chikusetsusaponin IVa in rat plasma by UPLC-MS/MS and its application to pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Du, Y.; Li, W.; Cai, B. Development and validation of an UHPLC-MS/MS approach for simultaneous quantification of five bioactive saponins in rat plasma: Application to a comparative pharmacokinetic study of aqueous extracts of raw and salt-processed Achyranthes bidentata. J. Pharm. Biomed. Anal. 2018, 151, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H.; Wu, H.; Deng, R.; Li, F. Chemical and metabolic analysis of Achyranthes bidentate saponins with intestinal microflora-mediated biotransformation by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry coupled with metabolism platform. J. Pharm. Biomed. Anal. 2019, 170, 305–320. [Google Scholar] [CrossRef]
- Liang, Y.; Fan, T.; Bai, M.; Cui, N.; Li, W.; Wang, J.; Guan, Y. Chikusetsu Saponin IVa liposomes modified with a retro-enantio peptide penetrating the blood-brain barrier to suppress pyroptosis in acute ischemic stroke rats. J. Nanobiotechnol. 2024, 22, 393. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cui, D.; Xu, H.; Wei, F.; Wen, F.; Wang, S.; Zhou, J.; Ding, T. Process for Extracting Oleanolic Acid-3-O-Beta-d-Pyraneglucuronide from General Aralia chinensis saponin and Its Usage. Chinese Patent Specification 00123317.3, 26 November 2000. Available online: https://patents.google.com/patent/CN1355172A/en (accessed on 24 February 2025).
- Ye, C.; Tang, H.; Huang, L.; Yuan, C.; Zhang, J.; Yuan, D.; He, Y. Quality analysis on roots and rhizomes of wild and cultivated Panax japonicus. Chin. Pharm. J. 2023, 58, 925–932. [Google Scholar] [CrossRef]
- Mroczek, A. Phytochemistry and bioactivity of triterpene saponins from Amaranthaceae family. Phytochem. Rev. 2015, 14, 577–605. [Google Scholar] [CrossRef]
- Guan, F.; Wang, Q.; Wang, M.; Shan, Y.; Chen, Y.; Yin, M.; Zhao, Y.; Feng, X.; Liu, F.; Zhang, J. Isolation, identification and cytotoxicity of a new noroleanane-type triterpene saponin from Salicornia bigelovii Torr. Molecules 2015, 20, 6419–6431. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.; Dawid, C.; Ralla, T.; Stark, T.D.; Salminen, H.; Weiss, J.; Hofmann, T. Fast and sensitive LC-MS/MS method for the quantitation of saponins in various sugar beet materials. J. Agric. Food Chem. 2020, 68, 15027–15035. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, X.; Zhang, Y.; Wang, R.; Wang, J.; Yu, S.; Jia, L. Method for Extracting Marigold Saponin E from Radix Achyranthis bidentatae and Application of Marigold Saponin E in Preparation of Medicine for Treating Acute Liver Injury. Chinese Patent Specification 201510730999, 25 February 2011. Available online: https://patents.google.com/patent/CN102648925A/en (accessed on 24 February 2025).
- Su Hong Zhang, P.; Wang, L.; Bu, X.; Lu, W.; Liu, Z. Preparation Method of Calendula Officinalis Glycoside E. Chinese Patent Specification 201911279307.9, 13 December 2019. Available online: https://patents.google.com/patent/CN110950922B/en (accessed on 24 February 2025).
- Li, J.; Li, P.; Li, H.J.; Song, Y.; Bi, Z.M.; Li, Y.J. Simultaneous qualification and quantification of eight triterpenoids in Radix Achyranthis bidentatae by high-performance liquid chromatography with evaporative light scattering detection and mass spectrometric detection. J. Sep. Sci. 2007, 30, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qi, H.; Qi, L.W.; Yi, L.; Li, P. Simultaneous determination of main phytoecdysones and triterpenoids in Radix Achyranthis bidentatae by high-performance liquid chromatography with diode array-evaporative light scattering detectors and mass spectrometry. Anal. Chim. Acta. 2007, 596, 264–272. [Google Scholar] [CrossRef]
- Mroczek, A.; Kapusta, I.; Stochmal, A.; Janiszowska, W. MS/MS and UPLC-MS profiling of triterpenoid saponins from leaves and roots of four red beet (Beta vulgaris L.) cultivars. Phytochem. Lett. 2019, 30, 333–337. [Google Scholar] [CrossRef]
- Mroczek, A.; Kapusta, I.; Janda, B.; Janiszowska, W. Triterpene saponin content in the roots of red beet (Beta vulgaris L.) cultivars. J. Agric. Food Chem. 2012, 60, 12397–12402. [Google Scholar] [CrossRef]
- Ossipov, V.; Khazieva, F.; Baleev, D.; Salminen, J.-P.; Sidelnikov, N. Comparative metabolomics of ligulate and tubular flowers of two cultivars of Calendula officinalis L. Metabolites 2024, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, W.; Bialy, Z. Chromatographic determination of plant saponins—An update (2002–2005). J. Chromatogr. A 2006, 1112, 78–91. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Tao, L.; Zhang, X.; Hao, F.; Zhao, S.; Han, L.; Bai, C. Recent advances in separation and analysis of saponins in natural products. Separations 2022, 9, 163. [Google Scholar] [CrossRef]
- Savarino, P.; Demeyer, M.; Decroo, C.; Colson, E.; Gerbaux, P. Mass spectrometry analysis of saponins. Mass Spectrom. Rev. 2023, 42, 954–983. [Google Scholar] [CrossRef] [PubMed]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Majinda, R.R.T. Extraction and isolation of saponins. Methods Mol. Biol. 2012, 864, 415–426. [Google Scholar] [CrossRef]
- Yin, M.; Wang, X.; Wang, M.; Chem, Y.; Dong, Y.; Zhao, Y.; Feng, X. A new triterpenoid saponin and other saponins from Salicornia europea. Chem. Nat. Compd. 2012, 48, 258–261. [Google Scholar] [CrossRef]
- Kamel, M.S.; Mohamed, K.M.; Hassanean, H.A.; Ohtani, K.; Kasai, R.; Yamasaki, K. Acylated flavonoid glycosides from Bassia muricata. Phytochemistry 2001, 57, 1259–1262. [Google Scholar] [CrossRef]
- Li, Y.J.; Wei, H.L.; Qi, L.W.; Chen, J.; Ren, M.T.; Li, P. Characterization and identification of saponins in Achyranthes bidentata by rapid-resolution liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2010, 24, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Lavaud, C.; Voutquenne, L.; Bal, P.; Pouny, I. Saponins from Chenopodium album. Fitoterapia 2000, 71, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zu, Y.G.; Fu, Y.J.; Yang, Y.C.; Li, S.M.; Li, Z.N.; Wink, M. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Jegal, J.; Jeong, E.J.; Yang, M.H. A review of the different methods applied in ginsenoside extraction from Panax ginseng and Panax quinquefolius roots. Nat. Prod. Commun. 2019, 14, 1934578X19868393. [Google Scholar] [CrossRef]
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Ultrasound-assisted extraction of total saponins from Aralia taibaiensis: Process optimization, phytochemical characterization, and mechanism of α-glucosidase inhibition. Drug Des. Dev. Ther. 2022, 16, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Wei, Y.; Sun, J.; Zhao, X.; Xie, J. Ultrasound-assisted extraction and antioxidant activity of triterpenoid saponins from Platycodon grandiflorum roots: Optimization, purification, and molecular insights. Ind. Crops Prod. 2025, 223, 119877. [Google Scholar] [CrossRef]
- Mroczek, A.; Klimczak, U.; Kowalczyk, M. Determination of saponins in leaves of four Swiss chard (Beta vulgaris L.) cultivars by UHPLC-CAD/QTOF-MS/MS. Pol. J. Food Nutr. Sci. 2021, 71, 147–159. [Google Scholar] [CrossRef]
- Islam, M.; Malakar, S.; Rao, M.V.; Kumar, N.; Sahu, J.K. Recent advancement in ultrasound-assisted novel technologies for the extraction of bioactive compounds from herbal plants: A review. Food Sci. Biotechnol. 2023, 32, 1763–1782. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Q.; Zhao, H.T.; Zhang, X.L.; Zhang, W.T.; Liu, X.C.; Gao, S.H. Comparison of different extraction techniques and optimization of the microwave-assisted extraction of saponins from Aralia elata (Miq.) Seem fruits and rachises. Chem. Pap. 2020, 74, 3077–3087. [Google Scholar] [CrossRef]
- Podolak, I.; Żuromska-Witek, B.; Grabowska, K.; Żebrowska, S.; Galanty, A.; Hubicka, U. Comparative quantitative study of ardisiacrispin a in extracts from Ardisia crenata Sims varieties and their cytotoxic activities. Chem. Biodivers. 2021, 18, e2100335. [Google Scholar] [CrossRef]
- Christensen, S.B.; Omar, A.A. Atriplex nummularia, a source for the two molluscicide saponins: Hederagenin-3-O-β-D-glucuronopyranoside and calenduloside E. J. Nat. Prod. 1985, 48, 161. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Thongphasuk, P.; Chamulitrat, W.; Wink, M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry 2008, 69, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Pecetti, L.; Biazzi, E.; Tava, A. Variation in saponin content during the growing season of spotted medic [Medicago arabica (L.) Huds.]. J. Sci. Food Agric. 2010, 90, 2405–2410. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- De Santis, G.; Maddaluno, C.; D’Ambrosio, T.; Rascio, A.; Rinaldi, M.; Troisi, J. Characterisation of quinoa (Chenopodium quinoa Willd.) accessions for the saponin content in Mediterranean environment. Ital. J. Agron. 2016, 11, 774. [Google Scholar] [CrossRef]
- Grabowska, K.; Pecio, Ł.; Galanty, A.; Żmudzki, P.; Oleszek, W.; Podolak, I. Serjanic acid glycosides from Chenopodium hybridum L. with good cytotoxicity and selectivity profile against several panels of human cancer cell lines. Molecules 2021, 26, 4915. [Google Scholar] [CrossRef]
- Fuentes-Bazan, S.; Uotila, P.; Borsch, T. A novel phylogeny-based generic classification for Chenopodium sensu lato, and a tribal rearrangement of Chenopodioideae (Chenopodiaceae). Willdenowia 2012, 42, 5–24. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, M.; Li, B.; Kan, Y.; Zhang, B.; Zheng, X.; Feng, W. Raw and salt-processed Achyranthes bidentata attenuate LPS-induced acute kidney injury by inhibiting ROS and apoptosis via an estrogen-like pathway. Biomed. Pharmacother. 2020, 129, 110403. [Google Scholar] [CrossRef]
- Lim, J.G.; Park, H.M.; Yoon, K.S. Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd.). Food Sci. Nutr. 2019, 8, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Podolak, I.; Żmudzki, P.; Koczurkiewicz, P.; Michalik, M.; Zajdel, P.; Galanty, A. Minor triterpene saponins from underground parts of Lysimachia thyrsiflora: Structure elucidation, LC-ESI-MS/MS quantification, and biological activity. Nat. Prod. Commun. 2013, 8, 1934578X1300801208. [Google Scholar] [CrossRef]
- International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). ICH Harmonised Tripartite Guideline: Validation and Analytical Procedures: Text and Methodology ICH-Q2 (R1); ICH: Geneva, Switzerland, 2005; Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline.pdf (accessed on 24 February 2025).
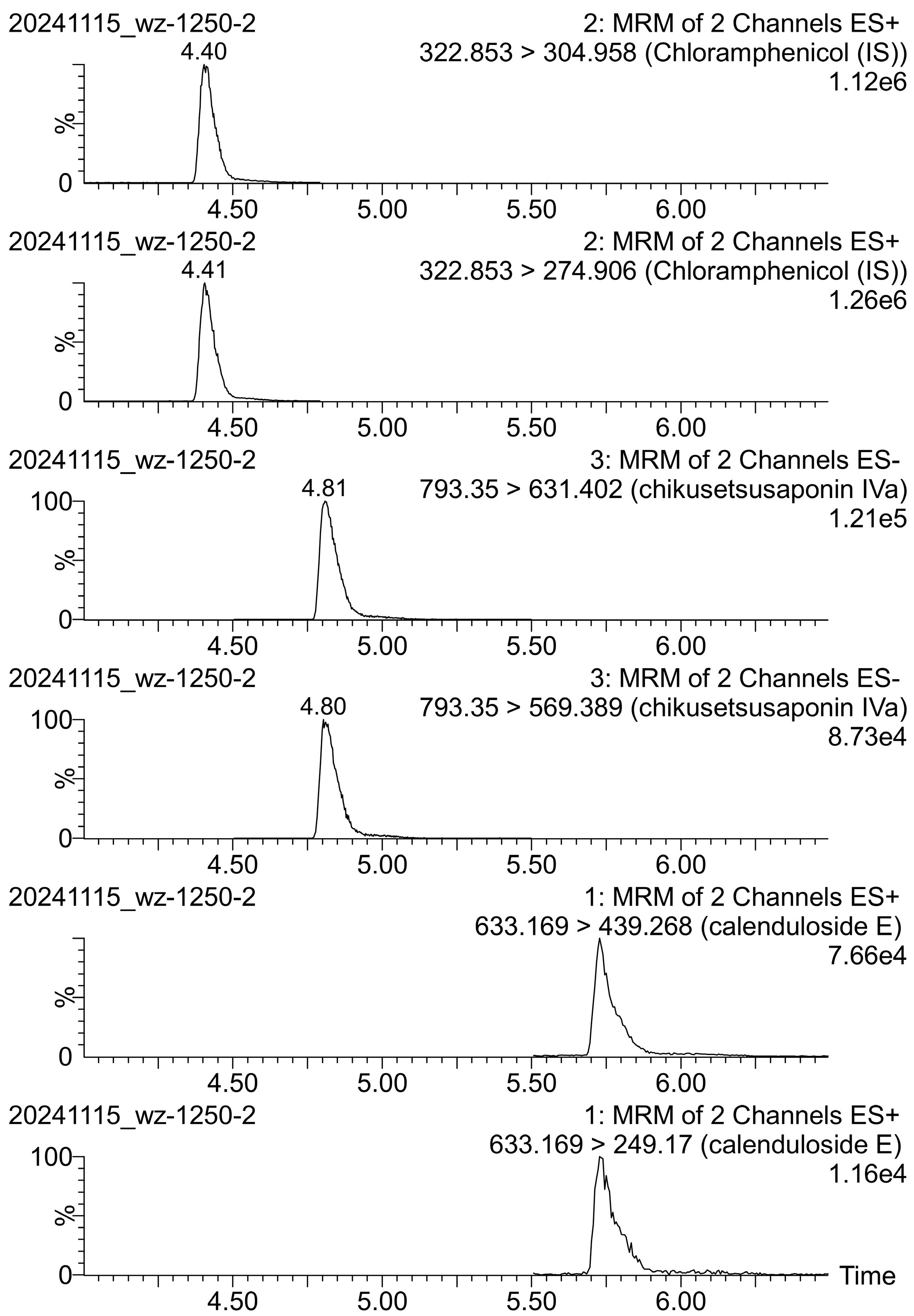
| Compound | Mode | Rt [min] | MRM Transition Q1→Q2 [m/z] | Cone Potential [V] | Collision Energy [eV] |
|---|---|---|---|---|---|
| Chikusetsusaponin IVa (ChIVa) | ES− | 4.80 | 793.3→569.4 | 96 | 28 |
| 793.3→631.4 | 20 | ||||
| Calenduloside E (CE) | ES− | 5.73 | 633.2→249.2 | 16 | 24 |
| 633.2→439.3 | 14 | ||||
| Chloramphenicol (IS) | ES+ | 4.40 | 323.1→275.0 | 16 | 10 |
| 323.1→305.0 | 4 |
| Parameters | Chikusetsusaponin IVa | Calenduloside E |
|---|---|---|
| a0 | −13.65 ± 0.97 (p < 10−4) | −9.27 ± 0.29 (p < 0.01) |
| a1 | 0.0987 ± 0.0049 (p < 10−6) | 0.0432 ± 0.0047 (p < 10−6) |
| a2 | −6.71 × 10−6 ± 0.99 × 10−6 (p < 10−6) | 7.04 × 10−6 ± 0.96 × 10−6 (p < 10−6) |
| r | 0.9947 | 0.9963 |
| r2 | 0.9887 | 0.9920 |
| LOD [ng/mL] | 143 | 236 |
| LOQ [ng/mL] | 151 | 304 |
| Calibration range [ng/mL] | 151–5000 | 304–5000 |
| Shapiro–Wilk test for residuals | p > 0.32 | p > 0.21 |
| Mandel’s fitting test | p < 10−6 | p < 10−6 |
| Asymmetry factor | 2.3 | 3.1 |
| Tailing factor | 1.4 | 1.7 |
| Concentration: 156 ng/mL: | ||
| Accuracy [%] | 105.1 | nd |
| Intra-day RSD [%] | 11.01 | nd |
| Inter-day RSD [%] | 12.60 (p > 0.21) | nd |
| Concentration: 312 ng/mL: | ||
| Accuracy [%] | nd | 92.8 |
| Intra-day RSD [%] | nd | 4.34 |
| Inter-day RSD [%] | nd | 6.24 (p > 0.67) |
| Concentration: 625 ng/mL: | ||
| Accuracy [%] | 94.2 | 91.8 |
| Intra-day RSD [%] | 7.88 | 8.32 |
| Inter-day RSD [%] | 9.02 (p > 0.42) | 8.78 (p > 0.61) |
| Concentration: 5000 ng/mL: | ||
| Accuracy [%] | 98.3 | 99.4 |
| Intra-day RSD [%] | 1.38 | 0.13 |
| Inter-day RSD [%] | 5.21 (p > 0.52) | 1.31 (p > 0.52) |
| Method of Extraction | Extraction Yield [%] | ChIVa Content [%] | CE Content [%] | |
|---|---|---|---|---|
| UAE | 1 × 15 min | 12.11 ± 0.85 | 6.91 ± 0.50 a,b | 4.57 ± 0.31 a |
| 2 × 15 min | 14.76 ± 0.58 | 6.03 ± 0.29 | 3.97 ± 0.13 | |
| 3 × 15 min | 16.05 ± 1.36 | 5.79 ± 0.43 | 3.94 ± 0.32 | |
| 4 × 15 min | 15.98 ± 0.58 | 6.43 ± 0.36 | 4.29 ± 0.31 | |
| 1 × 30 min | 12.96 ± 0.64 | 6.18 ± 0.34 | 4.33 ± 0.13 b | |
| 2 × 30 min | 15.14 ± 0.40 | 6.71 ± 0.36 c | 4.25 ± 0.20 | |
| 3 × 30 min | 15.37 ± 0.60 | 6.76 ± 0.26 d | 4.24 ± 0.18 | |
| 4 × 30 min | 16.25 ± 0.76 | 7.56 ± 0.43 e,f,g,h | 5.13 ± 0.29 c,d,e,f,g | |
| ME | 1 × 1 h | 9.87 ± 2.03 | 6.22 ± 1.46 | 4.37 ± 1.12 h |
| 2 × 1 h | 12.51 ± 1.52 | 5.36 ± 0.61 e,i | 3.54 ± 0.43 c,i | |
| 3 × 1 h | 13.51 ± 0.90 | 5.10 ± 0.17 a,f,j,k,l | 3.41 ± 0.05 d,j,k,l | |
| 4 × 1 h | 14.26 ± 0.42 | 4.87 ± 0.05 b,c,d,g,m,n,o,p | 3.08 ± 0.03 a,b,e,h,m,n,o,p | |
| ME/SA | 1 × 1 h | 13.03 ± 1.69 | 5.30 ± 0.67 h,r | 3.60 ± 0.46 f,r |
| 2 × 1 h | 14.14 ± 1.53 | 5.94 ± 0.85 | 3.88 ± 0.67 g | |
| 3 × 1 h | 14.26 ± 0.61 | 6.02 ± 0.08 | 4.02 ± 0.15 | |
| 4 × 1 h | 15.07 ± 0.37 | 6.22 ± 0.10 | 4.15 ± 0.08 | |
| HRE | 1 × 1 h | 14.41 ± 1.10 | 6.81 ± 0.49 m | 4.70 ± 0.35 j,m |
| 2 × 1 h | 16.78 ± 1.80 | 6.92 ± 0.84 j,n | 4.55 ± 0.61 n | |
| 3 × 1 h | 18.51 ± 1.02 | 7.44 ± 0.44 i,k,o,r | 5.10 ± 0.34 i,k,o,r | |
| 4 × 1 h | 19.28 ± 0.81 | 7.11 ± 0.51 l,p | 4.72 ± 0.41 l,p | |
| 1 × 3 h | 16.27 ± 0.42 | 6.55 ± 0.02 | 4.14 ± 0.06 | |
| Species | ChIVa Content [mg/g dw] | CE Content [mg/g dw] | ||||||
|---|---|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | Fruits | Roots | Stems | Leaves | Fruits | |
| A. retroflexus_S1 | 0.57 ± 0.01 | 0.11 ± 0.08 | 0.16 ± 0.03 | <LOQ | 1.11 ± 0.04 | <LOD | <LOQ | <LOD |
| A. retroflexus_S2 | 0.79 ± 0.03 | <LOQ | 0.16 ± 0.02 | <LOQ | 1.22 ± 0.03 | <LOD | <LOQ | <LOD |
| A. retroflexus_S3 | 0.74 ± 0.02 | 0.17 ± 0.02 | 0.17 ± 0.04 | <LOQ | 0.80 ± 0.03 | <LOQ | <LOD | <LOD |
| A. sagittata_S4 | 0.16 ± 0.02 | 0.17 ± 0.01 | 0.75 ± 0.05 | 13.59 ± 0.49 | <LOQ | <LOQ | 0.57 ± 0.12 | 8.17 ± 0.49 |
| A. sagittata_S5 | 0.16 ± 0.01 | <LOQ | 0.56 ± 0.03 | 13.41 ± 0.86 | <LOQ | 0.35 ± 0.04 | 0.51 ± 0.02 | 8.08 ± 0.45 |
| A. sagittata_S6 | 0.17 ± 0.02 | 0.17 ± 0.03 | 0.55 ± 0.09 | 12.46 ± 0.59 | <LOQ | 0.31 ± 0.08 | 0.48 ± 0.16 | 7.26 ± 0.64 |
| A. patula_S7 | 0.19 ± 0.04 | <LOQ | 0.36 ± 0.08 | 1.11 ± 0.27 | <LOQ | <LOD | <LOQ | 0.41 ± 0.04 |
| A. patula_S8 | 0.18 ± 0.03 | <LOQ | 0.24 ± 0.04 | 1.34 ± 0.09 | <LOQ | <LOD | <LOQ | 0.43 ± 0.02 |
| A. patula_S9 | 0.19 ± 0.02 | 0.16 ± 0.03 | 0.3 ± 0.01 | 1.11 ± 0.32 | <LOQ | <LOD | <LOQ | 0.37 ± 0.05 |
| B. bonus-henricus_S10 | 1.31 ± 0.06 | 0.18 ± 0.01 | 0.29 ± 0.01 | 0.21 ± 0.02 | 0.56 ± 0.04 | <LOD | 0.36 ± 0.05 | 0.57 ± 0.10 |
| B. bonus-henricus_S11 | 1.33 ± 0.02 | 0.17 ± 0.05 | 0.17 ± 0.02 | 0.16 ± 0.01 | 0.57 ± 0.06 | <LOD | <LOQ | 0.41 ± 0.04 |
| B. bonus-henricus_S12 | 1.35 ± 0.02 | 0.16 ± 0.03 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.83 ± 0.13 | <LOQ | 0.23 ± 0.11 | 0.48 ± 0.14 |
| Ch. hybridum_S13 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Ch. hybridum_S14 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Ch. hybridum_S15 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Ch. album_S16 | 3.68 ± 0.07 | 0.16 ± 0.01 | 0.19 ± 0.03 | 9.46 ± 0.29 | 2.41 ± 0.07 | <LOQ | <LOQ | 1.42 ± 0.15 |
| Ch. album_S17 | 3.81 ± 0.3 | 0.19 ± 0.01 | 0.17 ± 0.01 | 9.73 ± 0.22 | 2.38 ± 0.03 | <LOQ | 0.4± | 1.29 ± 0.19 |
| Ch. album_S18 | 2.17 ± 0.04 | 0.18 ± 0.01 | 0.2 ± 0.01 | 10.82 ± 0.28 | 1.66 ± 0.02 | <LOQ | <LOD | 1.23 ± 0.23 |
| Ch. strictum_S19 | 6.67 ± 0.09 | 0.21 ± 0.07 | 0.16 ± 0.02 | 8.12 ± 0.05 | 3.25 ± 0.45 | 0.47 ± 0.08 | <LOQ | 8.11 ± 0.06 |
| Ch. strictum_S20 | 7.34 ± 0.08 | 0.19 ± 0.03 | 0.1 ± 0.04 | 5.3 ± 0.31 | 3.68 ± 0.04 | 0.34 ± 0.05 | <LOQ | 6.42 ± 0.07 |
| Ch. strictum_S21 | 9.3 ± 0.17 | 0.19 ± 0.02 | 0.17 ± 0.08 | 3.16 ± 0.28 | 3.9 ± 0.53 | 0.32 ± 0.07 | <LOQ | 5.09 ± 0.14 |
| L. polysperma_S22 | 0.17 ± 0.04 | 0.24 ± 0.04 | 0.25 ± 0.02 | 12.57 ± 0.64 | <LOQ | <LOD | <LOQ | 0.89 ± 0.04 |
| L. polysperma_S23 | 0.17 ± 0.03 | 0.17 ± 0.01 | 0.25 ± 0.05 | 9.66 ± 1.09 | <LOD | <LOD | <LOQ | 0.54 ± 0.08 |
| L. polysperma_S24 | 0.05 ± 0.01 | 0.16 ± 0.03 | 0.25 ± 0.07 | 14.38 ± 1.23 | <LOD | <LOD | <LOQ | 1.81 ± 0.24 |
| O. glauca_S25 | 1.12 ± 0.04 | 0.68 ± 0.09 | 0.18 ± 0.03 | <LOQ | 1.03 ± 0.03 | 0.86 ± 0.13 | 0.43 ± 0.05 | <LOD |
| O. glauca_S26 | 0.59 ± 0.03 | 0.48 ± 0.02 | 0.23 ± 0.01 | <LOQ | 0.79 ± 0.15 | 0.53 ± 0.09 | 0.39 ± 0.03 | <LOD |
| O. glauca_S27 | 0.58 ± 0.09 | 0.55 ± 0.02 | 0.21 ± 0.02 | 0.1 ± 0.02 | 0.69 ± 0.09 | 0.73 ± 0.08 | 0.38 ± 0.03 | <LOD |
| O. rubra_S28 | 0.33 ± 0.02 | 0.18 ± 0.03 | 0.19 ± 0.06 | 0.21 ± 0.08 | 0.71 ± 0.02 | <LOQ | <LOQ | <LOQ |
| O. rubra_S29 | 0.4 ± 0.06 | 0.19 ± 0.04 | 0.17 ± 0.03 | 0.17 ± 0.03 | 0.69 ± 0.05 | <LOQ | <LOQ | 0.35 ± 0.02 |
| O. rubra_S30 | 0.44 ± 0.07 | 0.2 ± 0.02 | 0.18 ± 0.07 | 0.18 ± 0.02 | 0.67 ± 0.03 | <LOQ | 0.32 ± 0.05 | 0.34 ± 0.06 |
| Species | Symbol | Collection Site: Geographical Coordinates | Date of Collection | Reference No. of Voucher Specimens |
|---|---|---|---|---|
| Amaranthus retroflexus L. | S1 | 49°55′05.6″ N 20°11′58.8″ E | July 2024 | Fg/A.R/2024/07/1 |
| S2 | 50°02′19.4″ N 19°55′28.4″ E | August 2023 | Fg/A.R/2023/08/2 | |
| S3 | 50°01′50.3″ N 19°47′47.2″ E | August 2019 | Fg/A.R/2019/08/2 | |
| Atriplex patula L. | S4 | 49°55′02.1″ N 20°12′02.1″ E | October 2024 | Fg/A.P/2024/10/1 |
| S5 | 50°02′18.1″ N 19°55′26.2″ E | October 2024 | Fg/A.P/2024/10/2 | |
| S6 | 50°05′56.0″ N 19°54′24.1″ E | September 2020 | Fg/A.P/2020/09/1 | |
| Atriplex sagittata Borkh. (syn. Atriplex nitens Schkuhr) | S7 | 50°21′29.1″ N 20°36′52.5″ E | September 2022 | Fg/A.S/2022/09/1 |
| S8 | 50°02′50.7″ N 19°57′28.8″ E | August 2023 | Fg/A.S/2023/08/1 | |
| S9 | 50°02′27.5″ N 19°52′51.4″ E | August 2020 | Fg/A.S/2020/08/1 | |
| Blitum bonus-henricus (L.) Rchb. (syn. Chenopodium bonus-henricus L.) | S10 | 50°00′44.0″ N 19°59′38.6″ E | August 2022 | Fg/B.BH/2022/08/1 |
| S11 | 50°00′44.1″ N 19°59′40.2″ E | August 2020 | Fg/B.BH/2020/08/1 | |
| S12 | 49°49′26.2″ N 19°05′05.4″ E | September 2019 | Fg/B.BH/2019/09/1 | |
| Chenopodiastrum hybridum (L.) S.Fuentes, Uotila, and Borsch (syn. Chenopodium hybridum L.) | S13 | 50°02′20.1″ N 19°55′26.3″ E | August 2021 | Fg/Ch.H/2021/08/1 |
| S14 | 50°02′19.3″ N 19°55′28.1″ E | September 2024 | Fg/Ch.H/2024/09/1 | |
| S15 | 50°02′14.4″ N 19°49′18.9″ E | September 2023 | Fg/Ch.H/2023/09/1 | |
| Chenopodium album L. | S16 | 49°54′53.1″ N 20°11′51.7″ E | August 2024 | Fg/Ch.A/2024/08/4 |
| S17 | 49°56′54.7″ N 20°09′55.4″ E | August 2020 | Fg/Ch.A/2020/08/1 | |
| S18 | 50°02′21.7″ N 19°49′26.6″ E | August 2023 | Fg/Ch.A/2023/08/2 | |
| Chenopodium strictum Roth (syn. Ch. betaceum Andrz.) | S19 | 49°54′53.1″ N 20°11′51.7″ E | August 2024 | Fg/Ch.S/2024/08/1 |
| S20 | 50°00′42.7″ N 19°59′42.5″ E | July 2019 | Fg/Ch.S/2019/07/2 | |
| S21 | 50°00′43.2″ N 19°59′40.6″ E | August 2021 | Fg/Ch.S/2021/08/1 | |
| Lipandra polysperma (L.) S.Fuentes, Uotila, and Borsch (syn. Chenopodium polyspermum L.) | S22 | 49°55′02.1″ N 20°12′02.1″ E | July 2024 | Fg/L.P/2024/07/1 |
| S23 | 50°02′19.1″ N 19°55′27.5″ E | August 2023 | Fg/L.P/2023/08/1 | |
| S24 | 50°02′19.1″ N 19°55′27.5″ E | August 2022 | Fg/L.P/2022/08/2 | |
| Oxybasis glauca (L.) S.Fuentes, Uotila, and Borsch (syn. Chenopodium glaucum L.) | S25 | 49°55′02.1″ N 20°12′02.1″ E | September 2024 | Fg/O.G/2024/09/1 |
| S26 | 50°01′42.1″ N 19°47′49.9″ E | August 2023 | Fg/O.G/2023/08/1 | |
| S27 | 50°05′33.9″ N 19°54′43.9″ E | October 2020 | Fg/O.G/2020/10/1 | |
| Oxybasis rubra (L.) S.Fuentes, Uotila, and Borsch (syn. Chenopodium rubrum L.) | S28 | 50°08′47.9″ N 20°34′34.4″ E | August 2017 | Fg/O.R/2017/08/1 |
| S29 | 50°05′33.9″ N 19°54′43.9″ E | July 2022 | Fg/O.R/2022/07/1 | |
| S30 | 50°08′45.7″ N 20°34′36.9″ E | August 2020 | Fg/O.R/2020/08/1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowska, K.; Żmudzki, P.; Galanty, A.; Podolak, I. Simultaneous Quantification of Bioactive Triterpene Saponins Calenduloside E and Chikusetsusaponin IVa in Different Plant Parts of Ten Amaranthaceae Species by UPLC-ESI-MS/MS Method. Molecules 2025, 30, 1088. https://doi.org/10.3390/molecules30051088
Grabowska K, Żmudzki P, Galanty A, Podolak I. Simultaneous Quantification of Bioactive Triterpene Saponins Calenduloside E and Chikusetsusaponin IVa in Different Plant Parts of Ten Amaranthaceae Species by UPLC-ESI-MS/MS Method. Molecules. 2025; 30(5):1088. https://doi.org/10.3390/molecules30051088
Chicago/Turabian StyleGrabowska, Karolina, Paweł Żmudzki, Agnieszka Galanty, and Irma Podolak. 2025. "Simultaneous Quantification of Bioactive Triterpene Saponins Calenduloside E and Chikusetsusaponin IVa in Different Plant Parts of Ten Amaranthaceae Species by UPLC-ESI-MS/MS Method" Molecules 30, no. 5: 1088. https://doi.org/10.3390/molecules30051088
APA StyleGrabowska, K., Żmudzki, P., Galanty, A., & Podolak, I. (2025). Simultaneous Quantification of Bioactive Triterpene Saponins Calenduloside E and Chikusetsusaponin IVa in Different Plant Parts of Ten Amaranthaceae Species by UPLC-ESI-MS/MS Method. Molecules, 30(5), 1088. https://doi.org/10.3390/molecules30051088








