Impregnation of Se2S6 into a Nitrogen- and Sulfur-Co-Doped Functional Metal Carbides and Nitrides for High-Performance Li-S Batteries
Abstract
1. Introduction
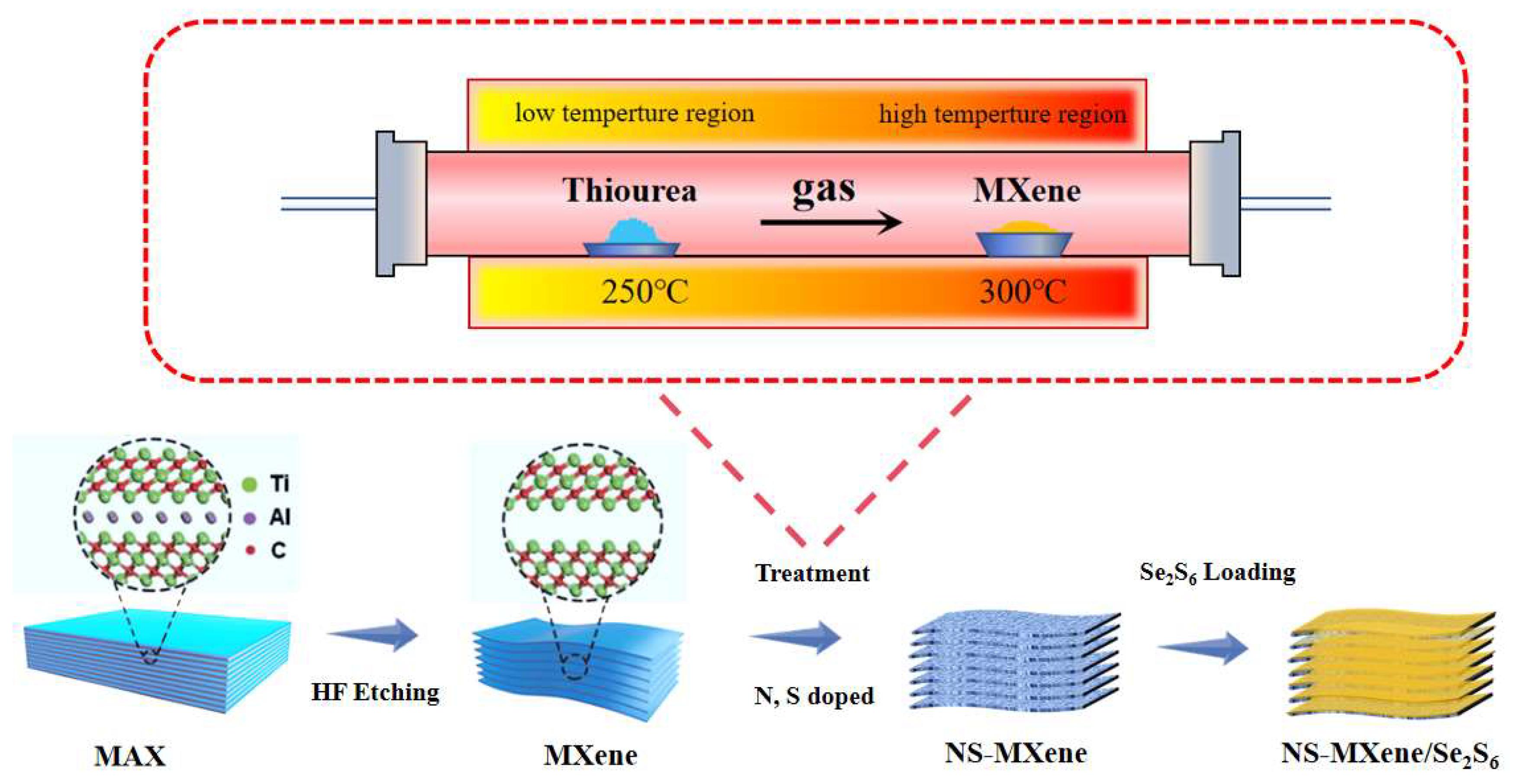
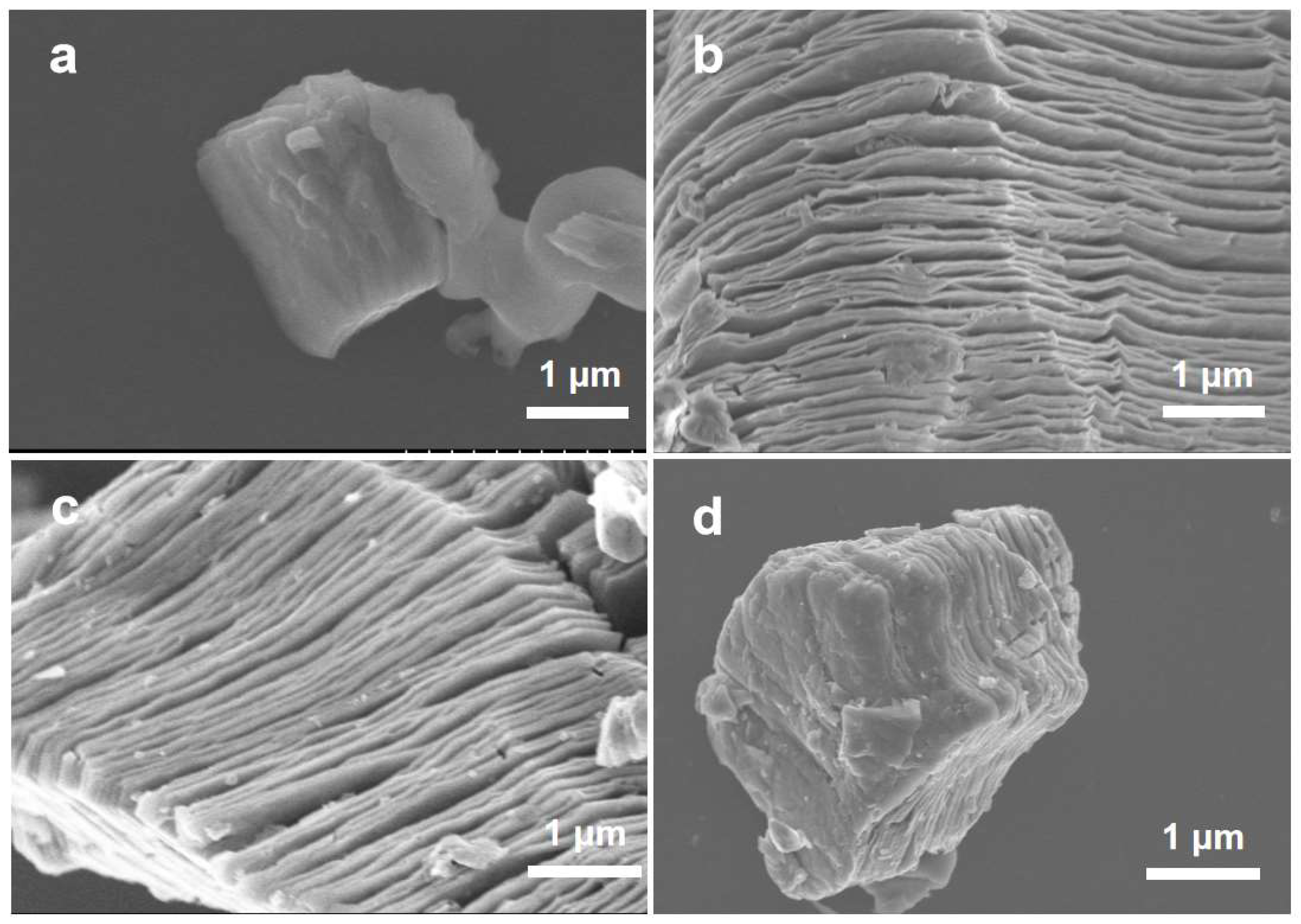
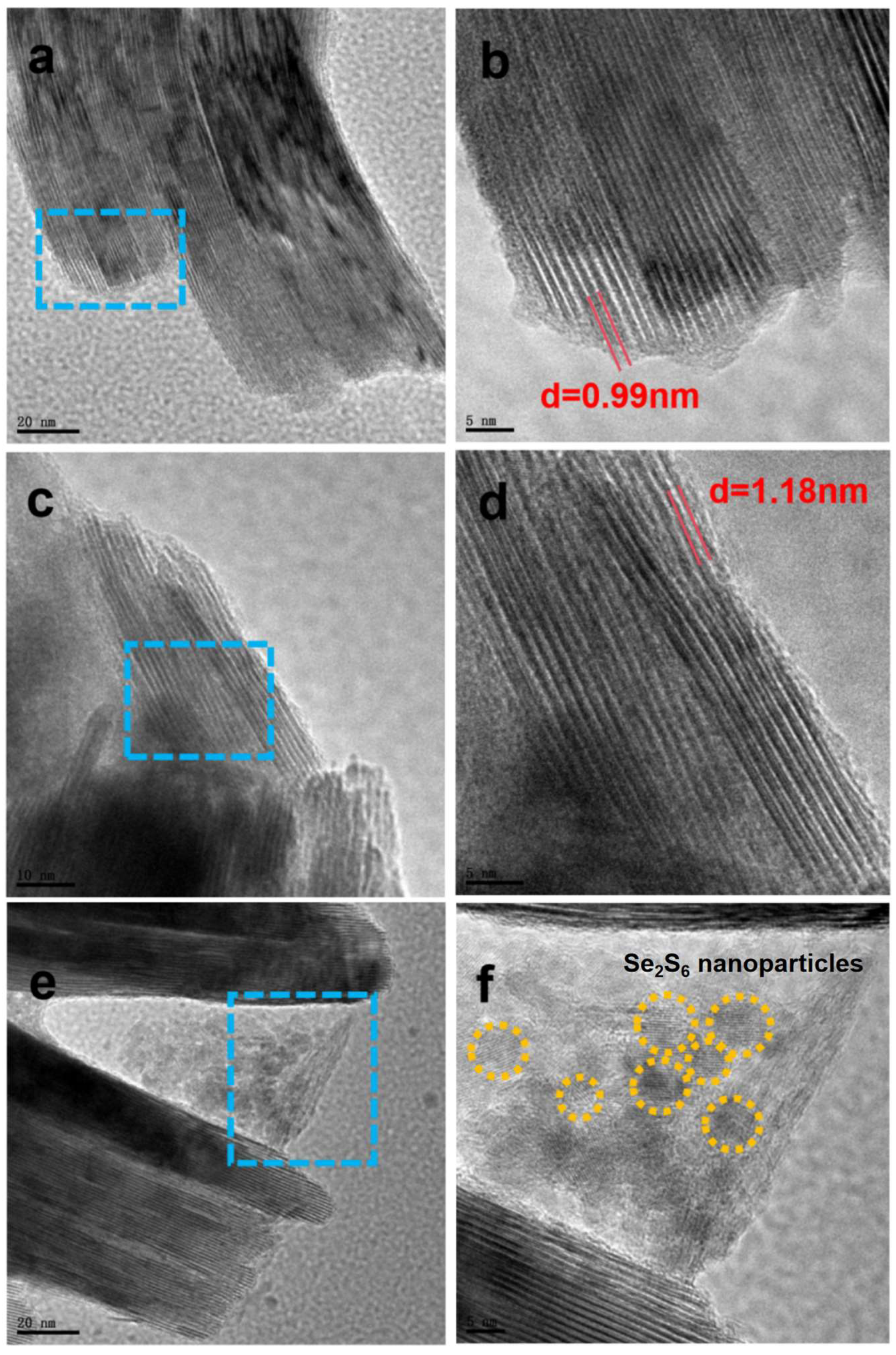
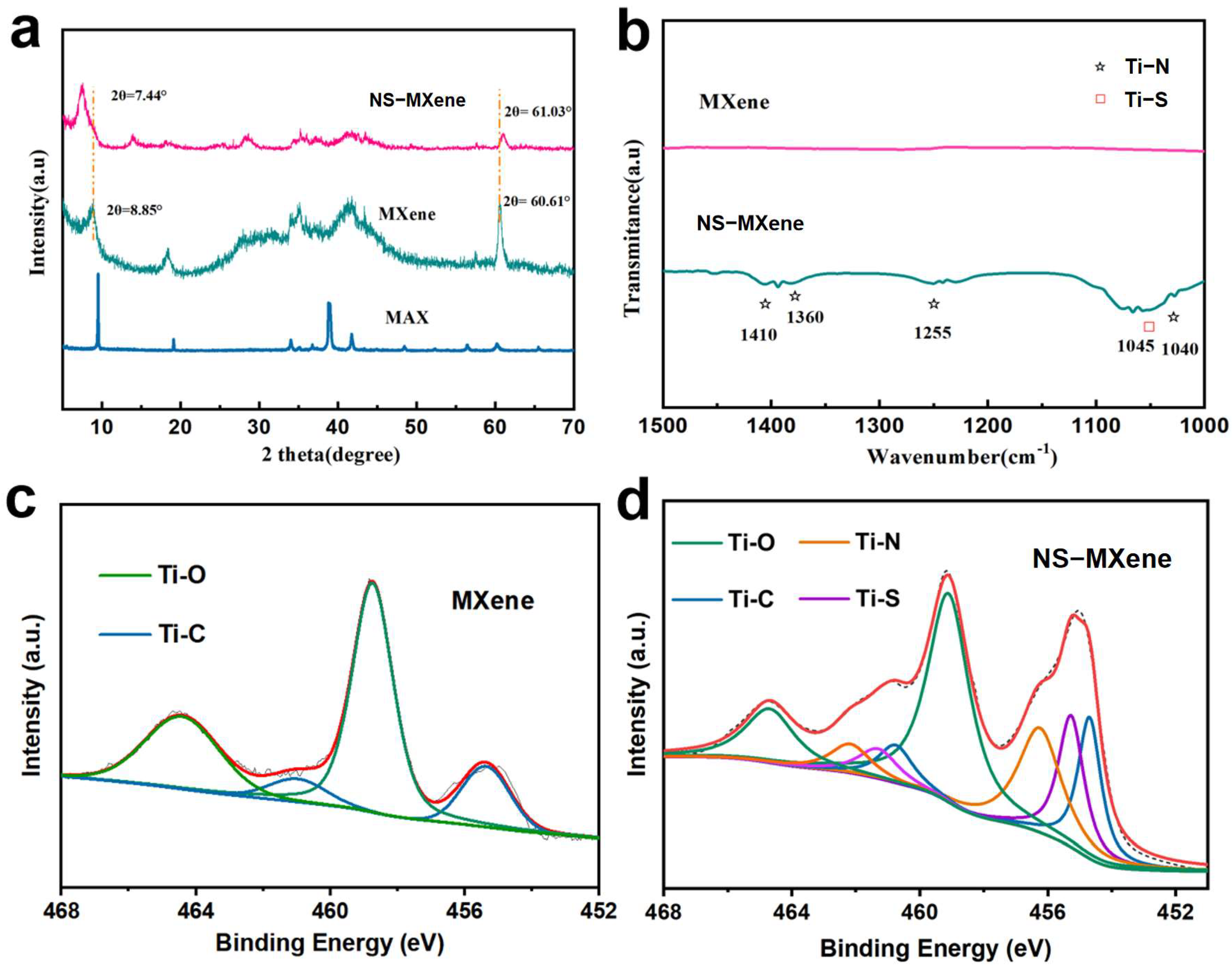
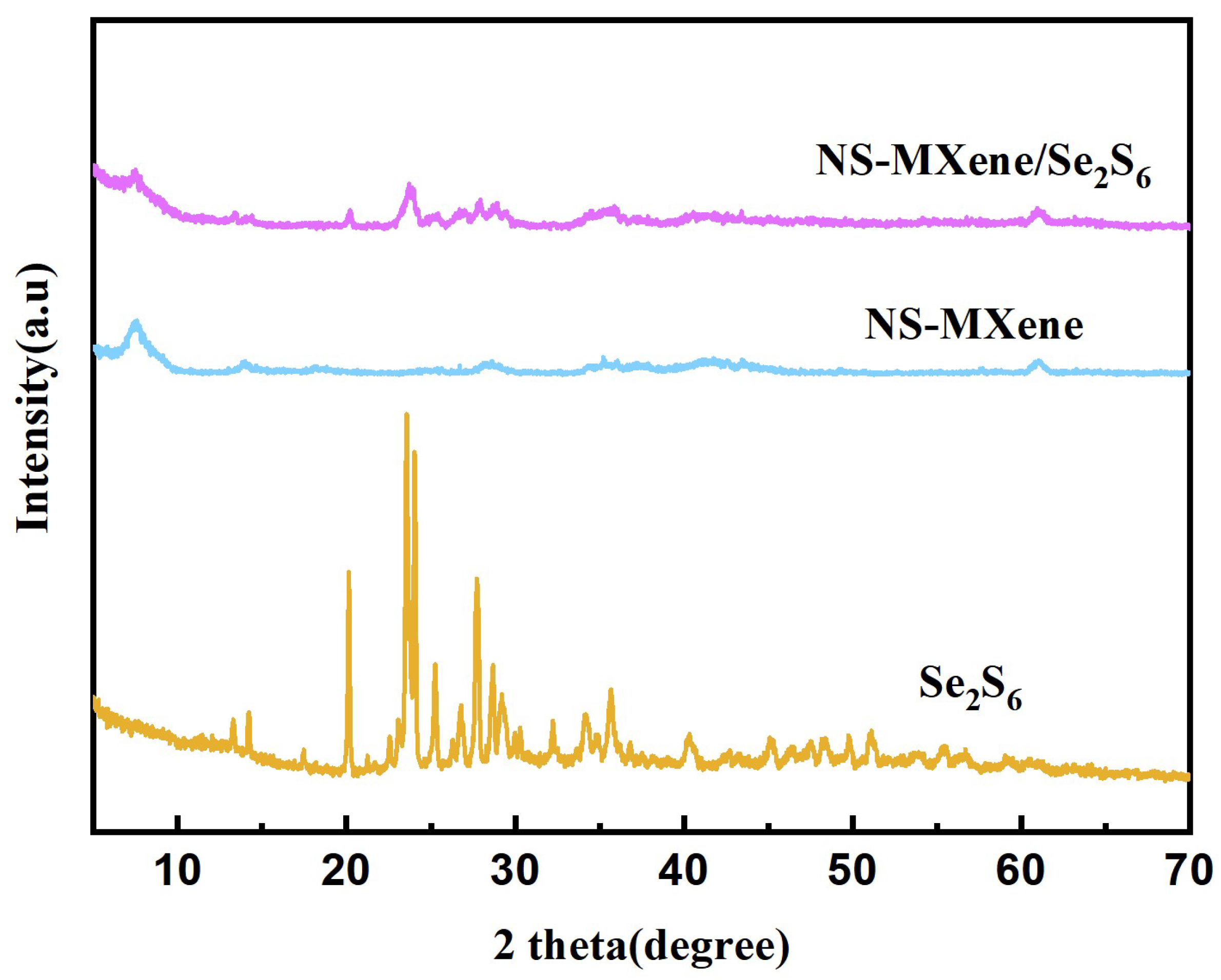
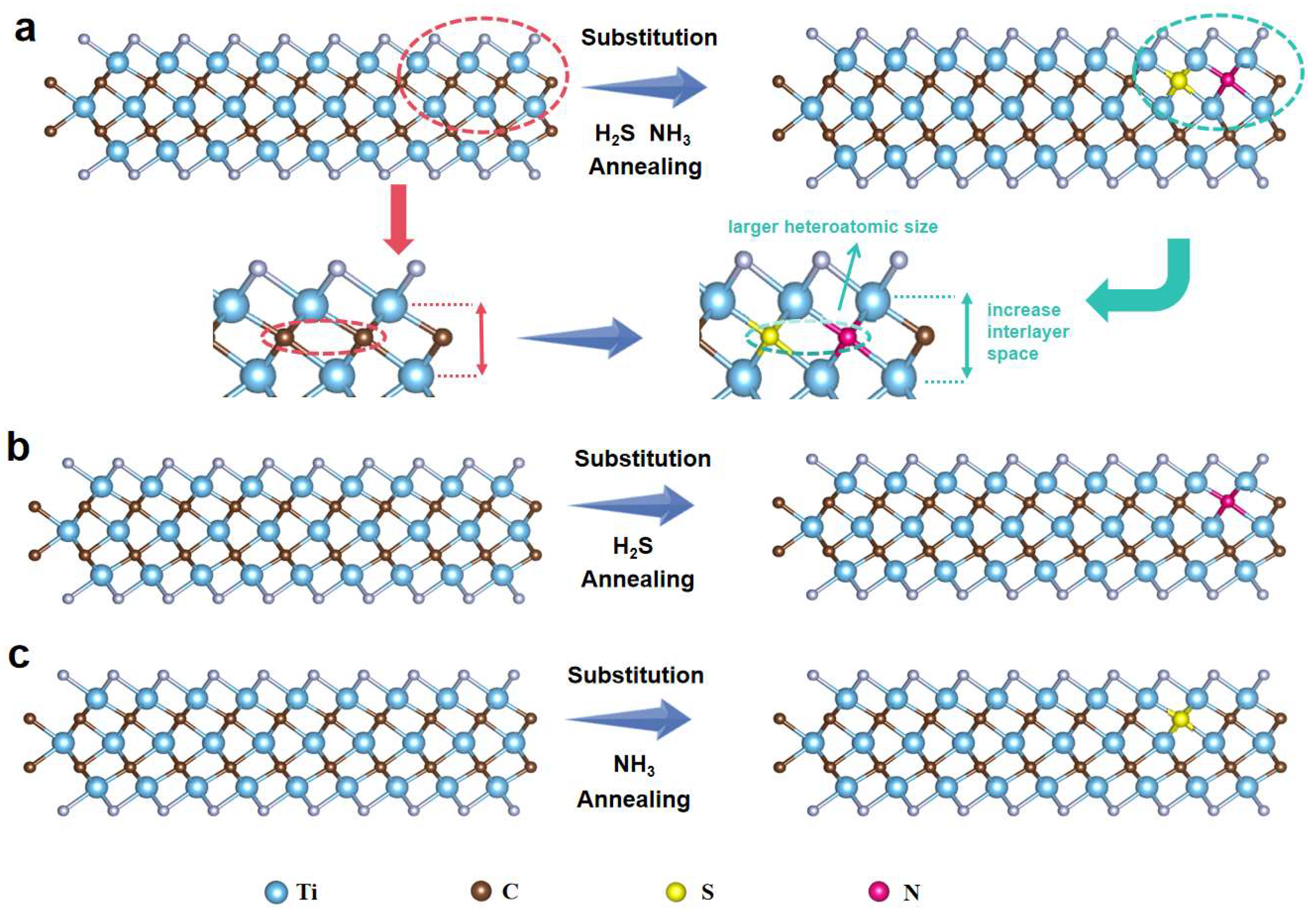
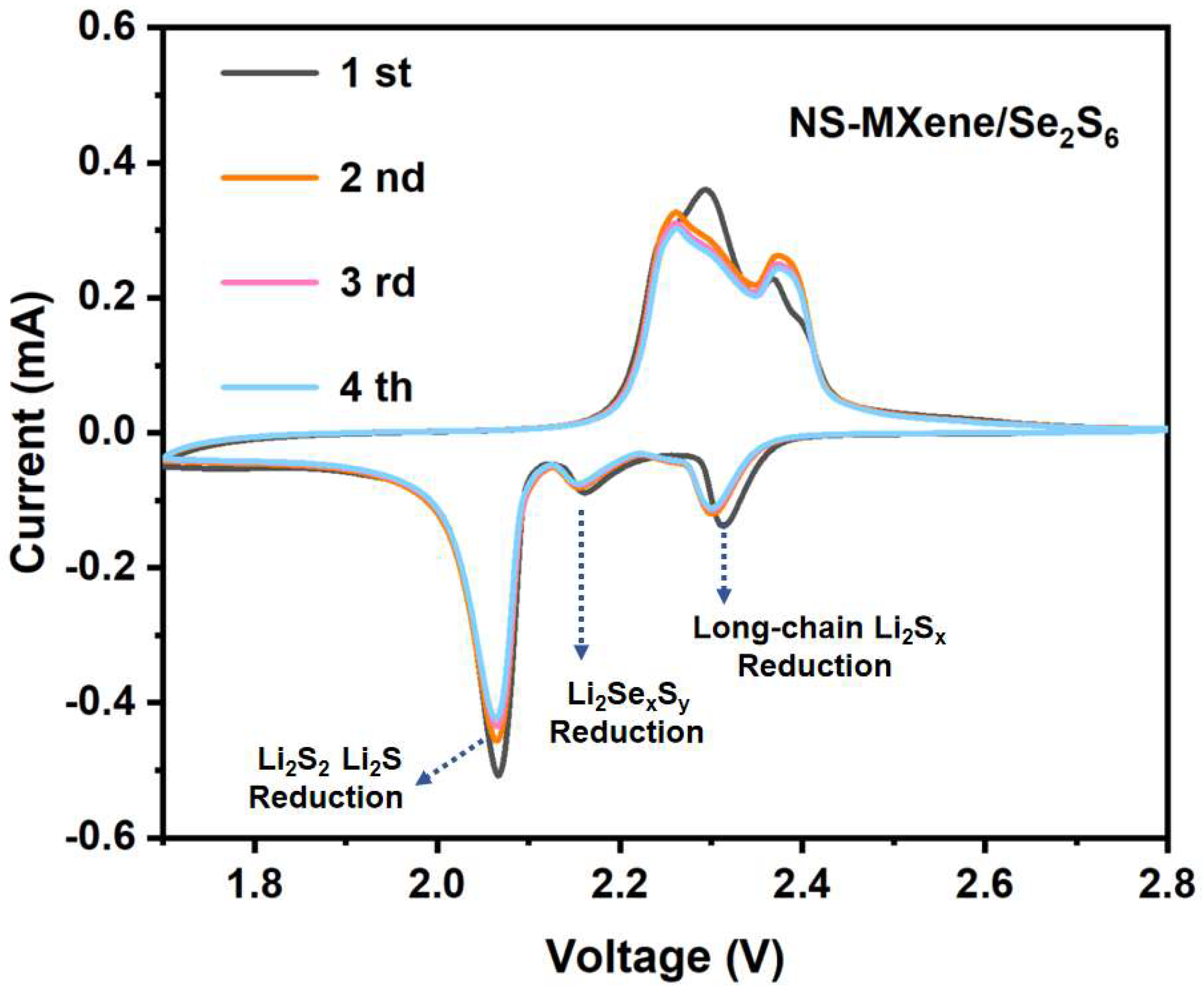
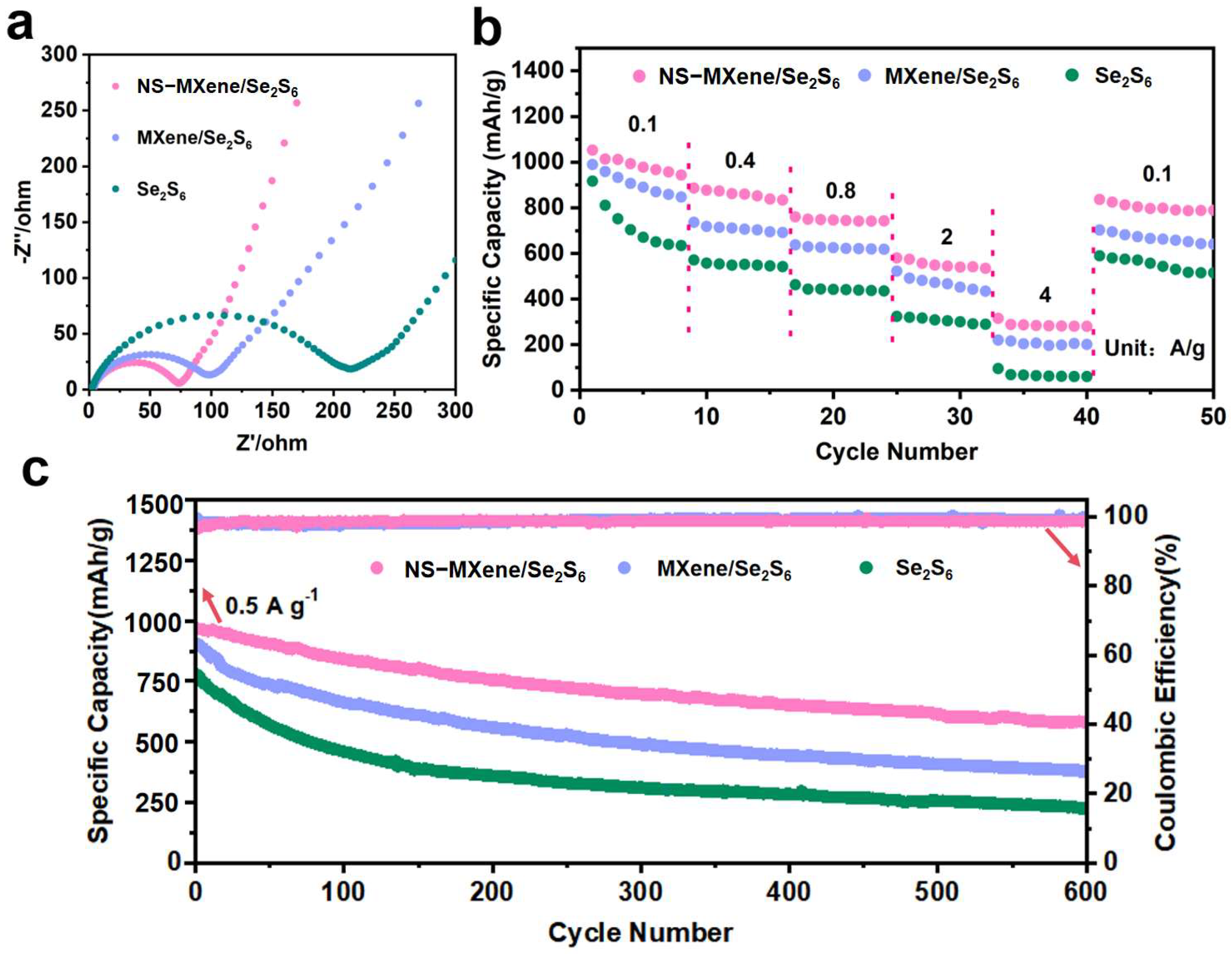
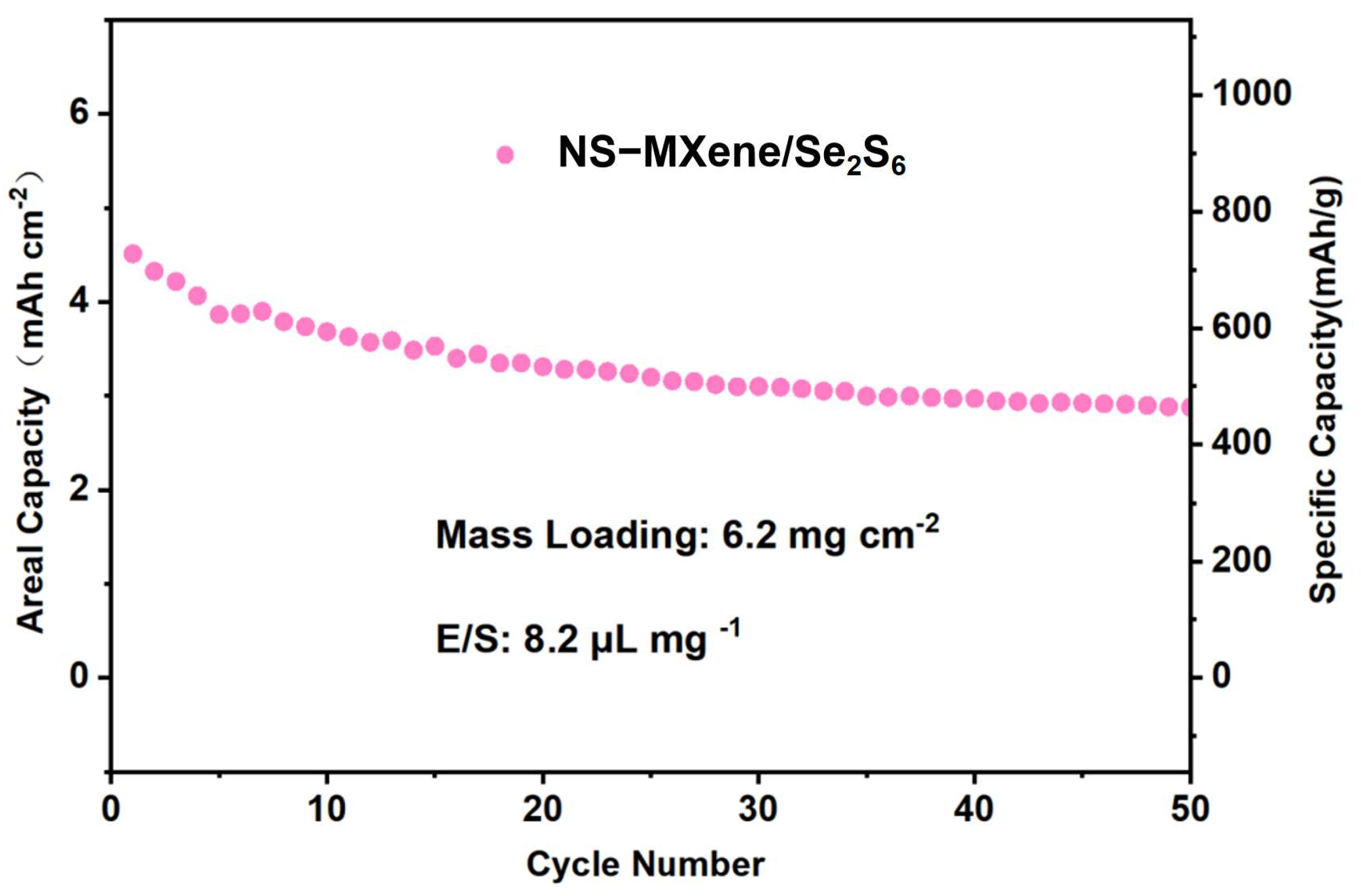
2. Result and Discussion
3. Method
3.1. Experimental Section
3.2. Preparation of MXene
3.3. Preparation of NS-MXene
3.4. Synthesis of the Se2S6
3.5. Synthesis of the NS-MXene/Se2S6
3.6. Material Characterizations
3.7. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Sun, Z.; Wang, L.; Pan, J.; Yi, L.; Zhang, Y.; Han, J.; Yao, Z.; Li, J.; Wen, Z.; et al. Resolving the origins of superior cycling performance of antimony anode in sodium-ion batteries: A comparison with lithium-ion batteries. Angew. Chem. Int. Ed. 2024, 63, e202320183. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.; Cheng, H.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Chen, H.; Cui, Y. Formulating energy density for designing practical lithium-sulfur batteries. Nat. Energy 2022, 7, 312–319. [Google Scholar] [CrossRef]
- Hao, N.; Ahmed, S.; An, J.; Sun, H.; Park, G.; Shim, J. Co-Co3O4 embedded in carbon nanotube derived from a zeolitic-imidazolate framework as anode material for lithium-ion batteries. Bull. Korean Chem. Soc. 2021, 42, 1220–1224. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, A.; Asif, M.; Shim, J.; Park, G. Exploring innovative trends and advancements in rechargeable zinc-air batteries. Inorg. Chem. Commun. 2024, 170, 113288. [Google Scholar] [CrossRef]
- Peng, H.; Huang, J.; Zhang, Q. A review of flexible lithium-sulfur and analogous alkali metal-chalcogen rechargeable batteries. Chem. Soc. Rev. 2017, 46, 5237–5288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Amirzadeh, Z.; Marco, R.; Tan, X.; Whittaker, A.; Huang, X.; Wepf, R.; Knibbe, R. In situ techniques for developing robust Li-S batteries. Small Methods 2018, 2, 1800133. [Google Scholar] [CrossRef]
- Abdul, R.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-performance lithium-sulfur batteries enabled by a synergy between sulfur and carbon nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Yan, L.; Gao, X.; Wahid, F.; Ernest, J.; Meng, Y.; Li, Y. A novel epoxy resin-based cathode binder for low cost, long cycling life, and high-energy lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 14315–14323. [Google Scholar] [CrossRef]
- Qi, B.; Zhao, X.; Wang, S.; Chen, K.; Wei, Y.; Chen, G.; Li, F. Mesoporous TiN microspheres as an efficient polysulfide barrier for lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 14359–14366. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile synthesis of crumpled nitrogen-doped MXene nanosheets as a new sulfur host for lithium-sulfur batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.; Nazar, L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Bae, J.; Chung, S.; Zhang, W.; Manthiram, A.; Yu, G. Nanostructured host materials for trapping sulfur in rechargeable Li-S batteries: Structure design and interfacial chemistry. Small Methods 2018, 2, 1700279. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Kang, Y. Yolk-shell structured assembly of bamboo-like nitrogen-doped carbon nanotubes embedded with Co nanocrystals and their application as cathode material for Li-S batteries. Adv. Funct. Mater. 2018, 28, 1705264. [Google Scholar] [CrossRef]
- Lv, L.; Guo, C.; Sun, W.; Wang, Y. Strong surface-bound sulfur in carbon nanotube bridged hierarchical Mo2C-based MXene nanosheets for lithium-sulfur batteries. Small 2019, 15, 1804338. [Google Scholar] [CrossRef]
- Chen, L.; He, T.; Liao, K.; Lu, H.; Ma, J.; Feng, Y.; Meng, S.; Zhang, C.; Yang, J. A Ternary (P, Se, S) Covalent Inorganic Framework as a shuttle effect-free cathode for Li-S Batteries. Adv. Mater. 2024, 36, 2308587. [Google Scholar] [CrossRef]
- Feng, Y.; Zu, L.; Yang, S.; Chen, L.; Liao, K.; Meng, S.; Zhang, C.; Yang, J. ultrahigh-content Co-P Cluster as a dual-atom-site electrocatalyst for accelerating polysulfides conversion in Li-S batteries. Adv. Funct. Mater. 2022, 32, 2207579. [Google Scholar] [CrossRef]
- Liang, X.; Rangom, Y.; Kwok, C. Interwoven MXene nanosheet/carbon nanotube composites as Li-S cathode hosts. Adv. Mater. 2017, 29, 1603040. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zheng, S.; Qin, J. All-MXene-based integrated electrode constructed by Ti3C2 nanoribbon framework host and nanosheet interlayer for high-energy-density Li-S batteries. ACS Nano 2018, 12, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lei, T.; Wu, C.; Deng, M.; Gong, C.; Hu, K.; Ma, Y.; Dai, L.; Lv, W.; He, W.; et al. Designing safe electrolyte systems for a high-stability lithium-sulfur battery. Adv. Energy Mater. 2018, 8, 1702348. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J. Highly stable lithium-sulfur batteries based on laponite nanosheet-coated celgard separators. Adv. Energy Mater. 2018, 8, 7070–7081. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xie, J.; Xu, N.; Li, Y. Sulfur-carbon nano-composite as cathode for rechargeable lithium battery based on gel electrolyte. Electrochem. Commun. 2002, 4, 499–502. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Ling, Z.; Yang, J.; Wan, C.; Jiang, C. Polymer lithium cells with sulfur composites as cathode materials. Electrochim. Acta 2003, 48, 1861–1867. [Google Scholar] [CrossRef]
- Zu, C.; Manthiram, A.; Hydroxylated, A. Graphene-sulfur nanocomposites for high rate lithium-sulfur batteries. Adv. Energy Mater. 2013, 3, 1008–1012. [Google Scholar] [CrossRef]
- Wang, C.; Su, K.; Wan, W.; Guo, H.; Zhou, H.; Chen, J.; Zhang, X.; Huang, Y. High sulfur loading composite wrapped by 3D nitrogen-doped graphene as a cathode material for lithium-sulfur batteries. J. Mater. Chem. A 2014, 2, 5018–5023. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, W.; Wang, J.; Zhou, G.; Zhuang, Z.; Luo, J.; Huang, H.; Gan, Y.; Liang, C.; Xia, Y.; et al. Facilitation of sulfur evolution reaction by pyridinic nitrogen doped carbon nanoflakes for highly-stable lithium-sulfur batteries. Energy Storage Mater. 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Feng, J.; Liu, W.; Shi, C.; Zhang, C.; Zhao, X.; Wang, T.; Chen, S.; Li, Q.; Song, J. Enabling fast diffusion/conversion kinetics by thiourea-induced wrinkled N, S co-doped functional MXene for lithium-sulfur battery. Energy Storage Mater. 2024, 67, 103328. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Yang, D.; Soomro, R.; Xu, B. Flexible carbon dots-intercalated MXene film electrode with outstanding volumetric performance for supercapacitors. Adv. Funct. Mater. 2023, 33, 2209918. [Google Scholar] [CrossRef]
- Zhang, P.; Soomro, R.; Guan, Z.; Sun, N.; Xu, B. 3D carbon-coated MXene architectures with high and ultrafast lithium/sodium-ion storage. Energy Storage Mater. 2020, 29, 163–171. [Google Scholar] [CrossRef]
- Cui, Y.; Abouimrane, A.; Lu, J.; Bolin, T.; Ren, Y.; Weng, W.; Sun, C.; Maroni, V.; Amine, S.; Amine, K. (De)Lithiation mechanism of Li/SeSx (x = 0–7) batteries determined by in situ synchrotron x-ray diffraction and x-ray absorption spectroscopy. J. Am. Chem. Soc. 2013, 135, 8047–8055. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zeng, W.; Jiang, Y.; Wei, X.; Li, W.; Yang, C.; Zhu, Y.; Yu, Y. A flexible porous carbon nanofibers-selenium cathode with superior electrochemical performance for both Li-Se and Na-Se batteries. Adv. Energy Mater. 2015, 5, 1401377. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.; Chen, X.; Li, N.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Li, Z.; Sami, I.; Yang, J.; Li, J.; Kumar, R.; Chhowalla, M. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium-sulfur batteries. Nat. Energy 2023, 8, 84–93. [Google Scholar] [CrossRef]
- Pei, F.; Lin, L.; Ou, D.; Zheng, Z.; Mo, S.; Fang, X.; Zheng, N. Self-supporting sulfur cathodes enabled by two-dimensional carbon yolk-shell nanosheets for high-energy-density lithium-sulfur batteries. Nat. Commun. 2017, 8, 482–489. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zheng, Z.; Meng, S.; Wu, W.; Zhou, W.; Yang, S.; Liao, K.; Zuo, Y.; He, T. Impregnation of Se2S6 into a Nitrogen- and Sulfur-Co-Doped Functional Metal Carbides and Nitrides for High-Performance Li-S Batteries. Molecules 2025, 30, 1070. https://doi.org/10.3390/molecules30051070
Chen L, Zheng Z, Meng S, Wu W, Zhou W, Yang S, Liao K, Zuo Y, He T. Impregnation of Se2S6 into a Nitrogen- and Sulfur-Co-Doped Functional Metal Carbides and Nitrides for High-Performance Li-S Batteries. Molecules. 2025; 30(5):1070. https://doi.org/10.3390/molecules30051070
Chicago/Turabian StyleChen, Lu, Zhongyuan Zheng, Shuo Meng, Wenwei Wu, Weicheng Zhou, Shanshan Yang, Kexuan Liao, Yuanhui Zuo, and Ting He. 2025. "Impregnation of Se2S6 into a Nitrogen- and Sulfur-Co-Doped Functional Metal Carbides and Nitrides for High-Performance Li-S Batteries" Molecules 30, no. 5: 1070. https://doi.org/10.3390/molecules30051070
APA StyleChen, L., Zheng, Z., Meng, S., Wu, W., Zhou, W., Yang, S., Liao, K., Zuo, Y., & He, T. (2025). Impregnation of Se2S6 into a Nitrogen- and Sulfur-Co-Doped Functional Metal Carbides and Nitrides for High-Performance Li-S Batteries. Molecules, 30(5), 1070. https://doi.org/10.3390/molecules30051070





