27-Hydroxymangiferolic Acid Extends Lifespan and Improves Neurodegeneration in Caenorhabditis elegans by Activating Nuclear Receptors
Abstract
1. Introduction
2. Results
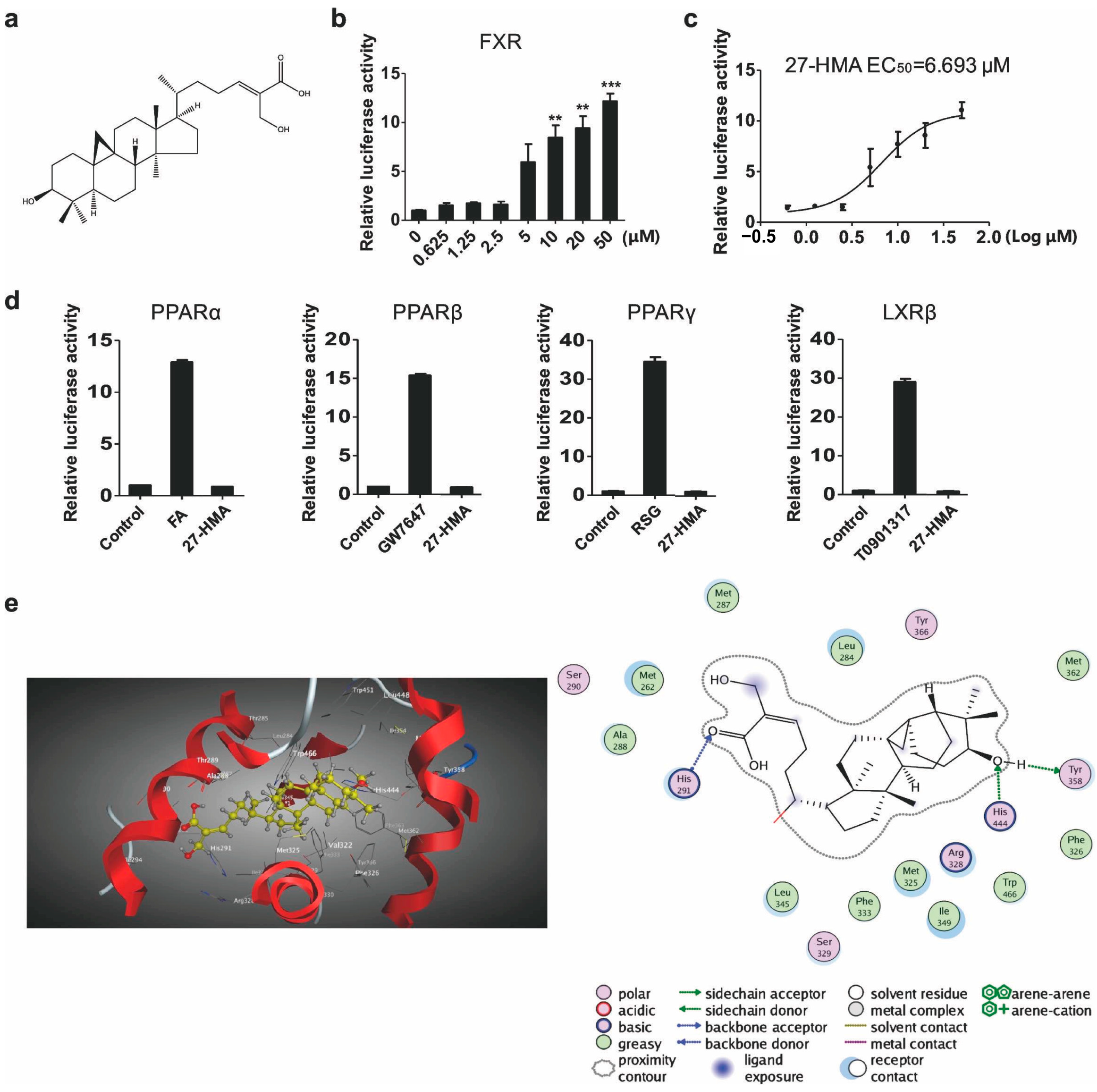
2.1. 27-HMA Is an FXR Agonist
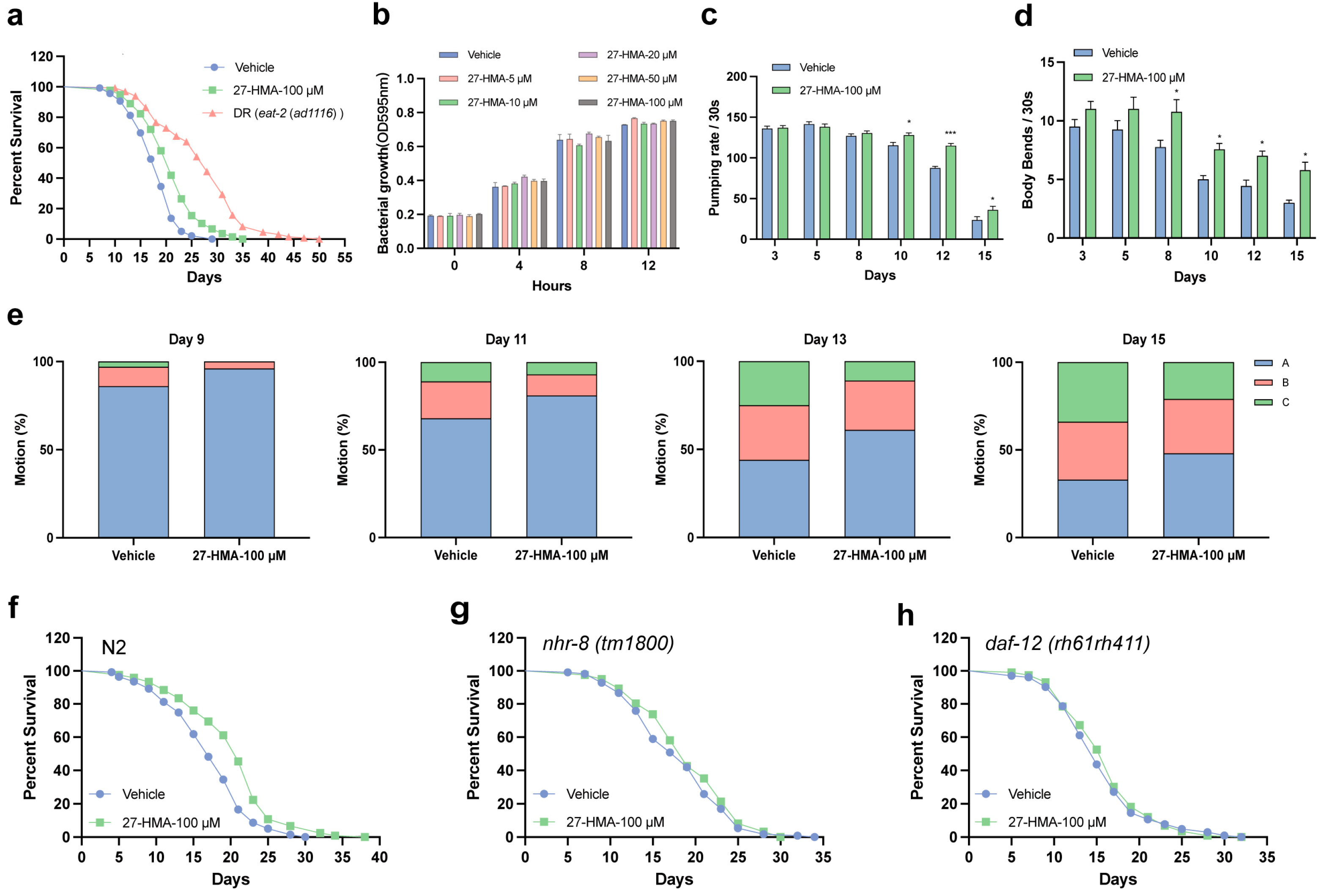
2.2. 27-HMA Extends Lifespan and Enhances Healthspan in C. elegans
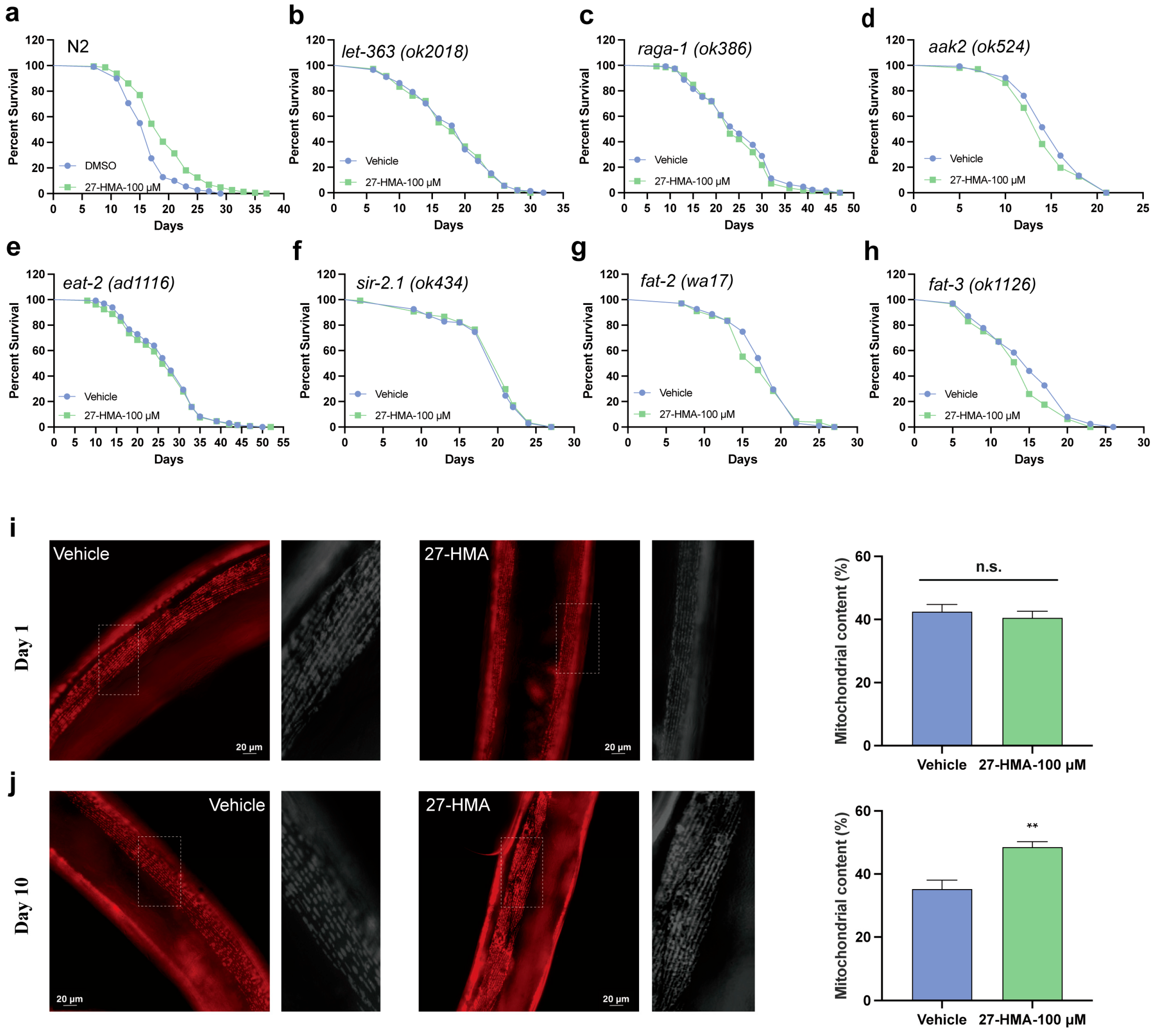
2.3. The Longevity Effect of 27-HMA Is Mediated by Nuclear Receptors in C. elegans
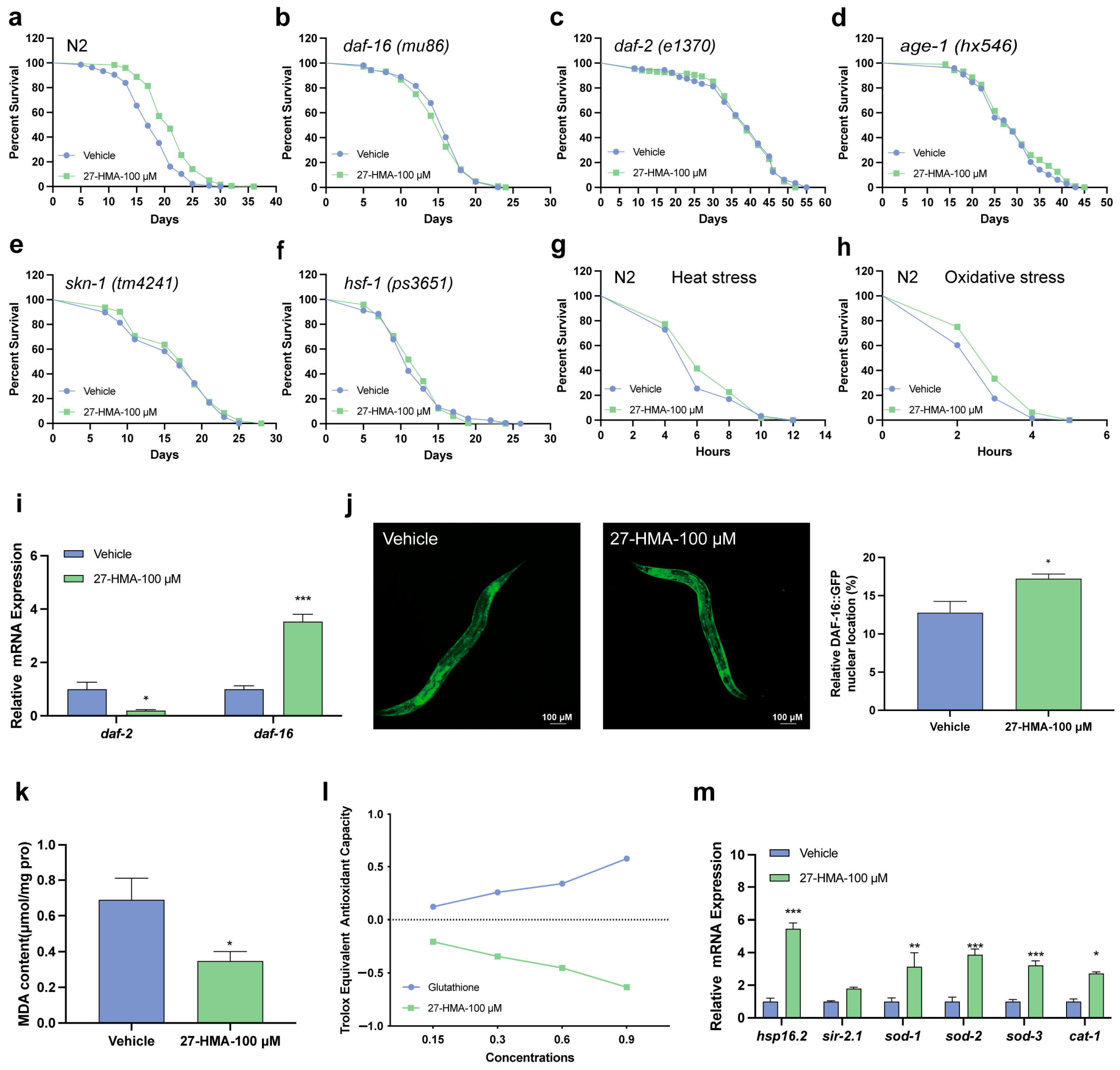
2.4. Lifespan-Extending Effect of 27-HMA Is Related to Insulin/Insulin-like Growth Factor-1 Signaling (IIS) Pathway
2.5. TORC1 Pathway May Contribute to the Lifespan-Extending Effect of 27-HMA
2.6. 27-HMA Activates Xenobiotic Detoxification via Nuclear Receptors in C. elegans
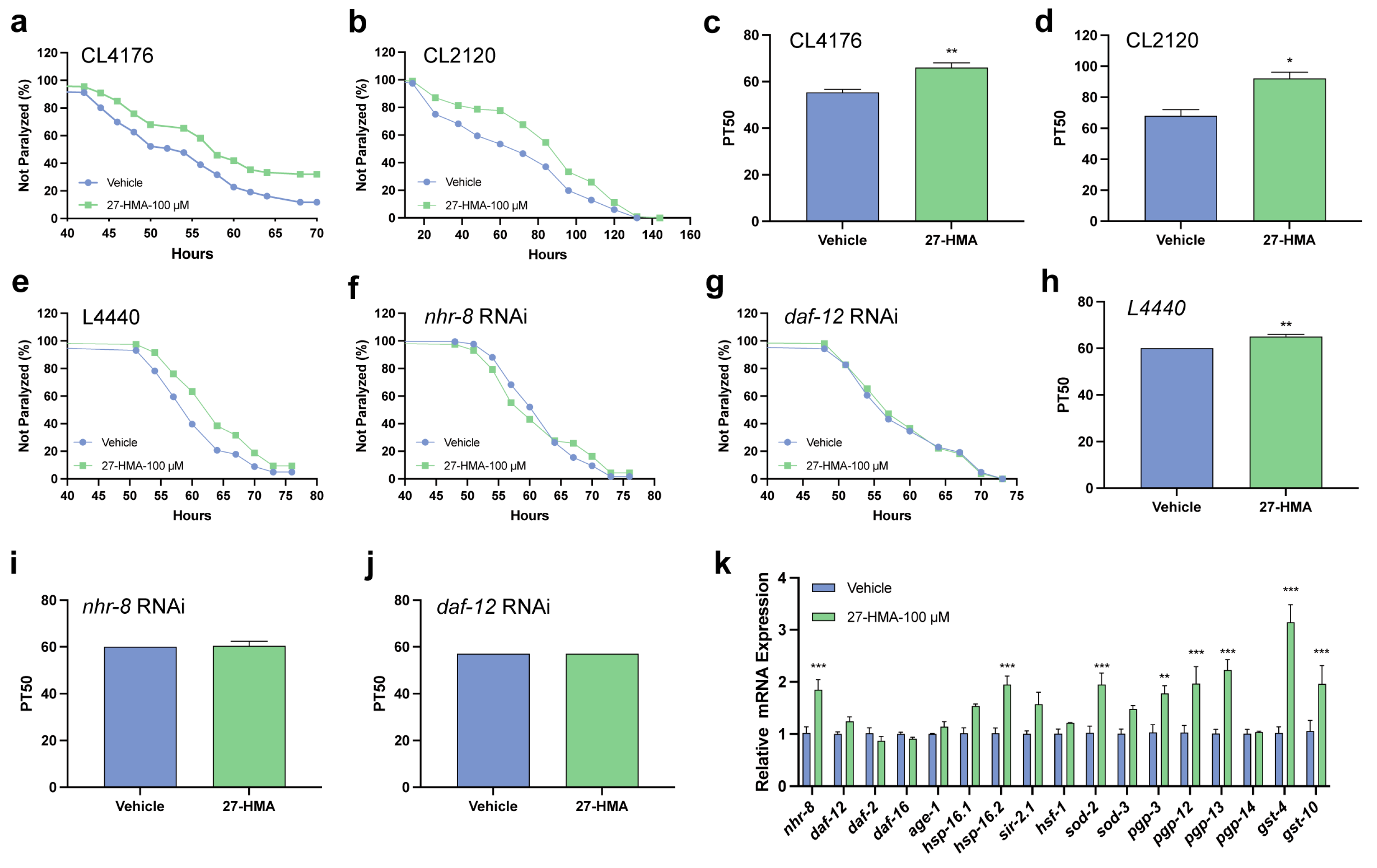
2.7. 27-HMA Alleviates the Paralysis of AD in C. elegans Through Nuclear Receptors
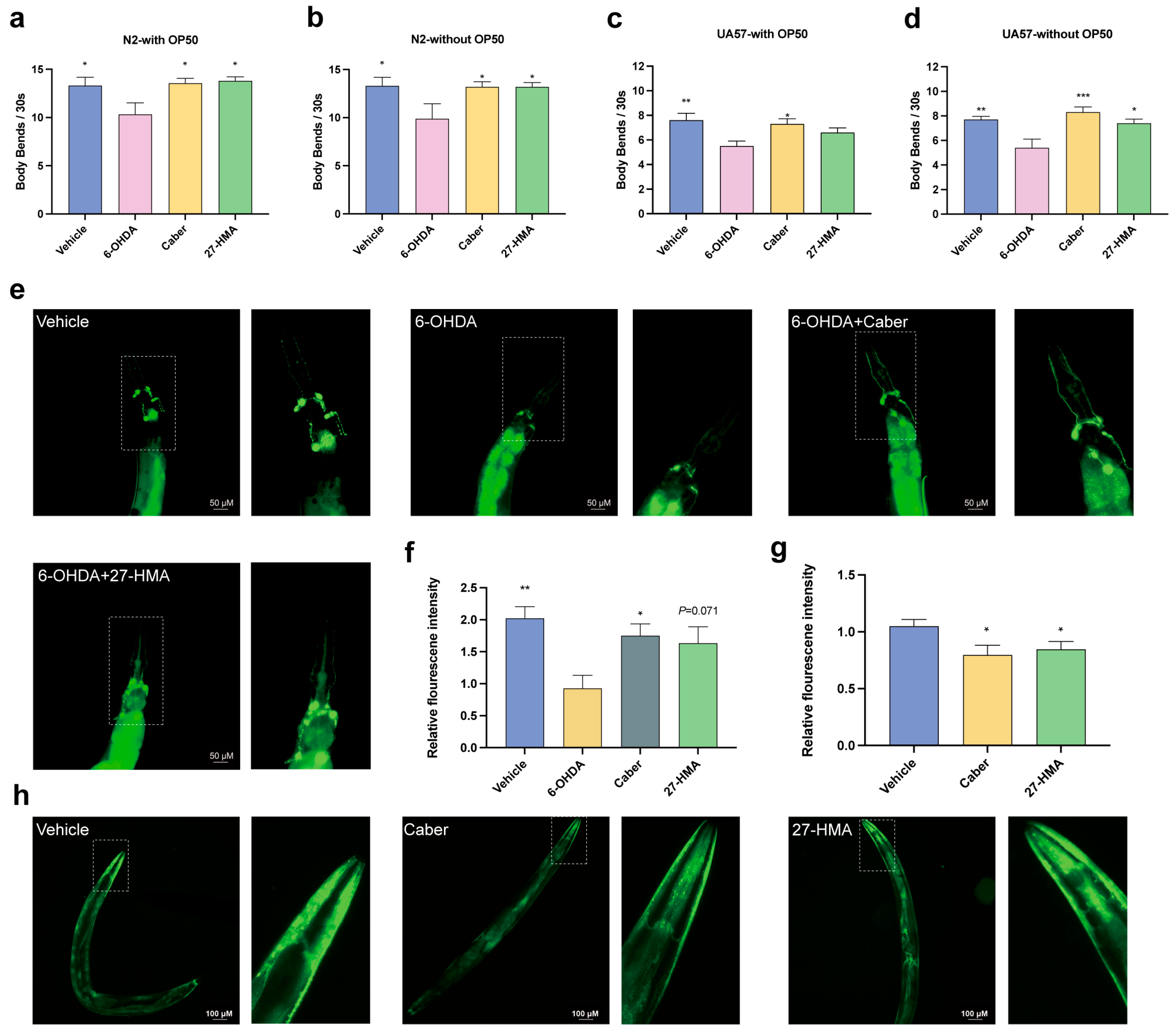
2.8. 27-HMA Improves the Pathology and Behavior of PD in C. elegans
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures and Dual-Luciferase Reporter Assays
4.3. Molecular Modeling Assay
4.4. Strains and Maintenance Conditions
4.5. Lifespan Assays
4.6. Bacterial Growth Assay
4.7. Chemotaxis Assay
4.8. Progeny Production Assay
4.9. Locomotion and Pumping Rate Assays
4.10. DAF-16::GFP Nuclear Localization Assay
4.11. Stress Resistance Assays
4.12. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
4.13. Toxicity Assay
4.14. Mitochondrial Integrity Analysis
4.15. Neurodegenerative Disease Assays
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amador-Noguez, D.; Yagi, K.; Venable, S.; Darlington, G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell 2004, 3, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Grandison, R.C.; Piper, M.D.; Partridge, L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 2009, 462, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Antebi, A. Nuclear receptor signal transduction in C. elegans. WormBook 2015, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Frigo, D.E.; Bondesson, M.; Williams, C. Nuclear receptors: From molecular mechanisms to therapeutics. Essays Biochem. 2021, 65, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Bustos, V.; Partridge, L. Good Ol’ Fat: Links between Lipid Signaling and Longevity. Trends Biochem. Sci. 2017, 42, 812–823. [Google Scholar] [CrossRef]
- McElwee, J.J.; Schuster, E.; Blanc, E.; Piper, M.D.; Thomas, J.H.; Patel, D.S.; Selman, C.; Withers, D.J.; Thornton, J.M.; Partridge, L.; et al. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007, 8, R132. [Google Scholar] [CrossRef]
- Hoffmann, J.M.; Partridge, L. Nuclear hormone receptors: Roles of xenobiotic detoxification and sterol homeostasis in healthy aging. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 380–392. [Google Scholar] [CrossRef]
- Fan, S.J.; Yan, Y.X.; Xia, Y.; Zhou, Z.Y.; Luo, L.L.; Zhu, M.N.; Han, Y.L.; Yao, D.Q.; Zhang, L.J.; Fang, M.L.; et al. Pregnane X receptor agonist nomilin extends lifespan and healthspan in preclinical models through detoxification functions. Nat. Commun. 2023, 14, 3368. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell Endocrinol. 2022, 548, 111618. [Google Scholar] [CrossRef]
- Tian, S.Y.; Chen, S.M.; Pan, C.X.; Li, Y. FXR: Structures, biology, and drug development for NASH and fibrosis diseases. Acta Pharmacol. Sin. 2022, 43, 1120–1132. [Google Scholar] [CrossRef]
- Jiang, Y.; Jin, J.; Iakova, P.; Hernandez, J.C.; Jawanmardi, N.; Sullivan, E.; Guo, G.L.; Timchenko, N.A.; Darlington, G.J. Farnesoid X receptor directly regulates xenobiotic detoxification genes in the long-lived Little mice. Mech. Ageing Dev. 2013, 134, 407–415. [Google Scholar] [CrossRef] [PubMed]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, C.; Fan, S. Mangiferin and organ fibrosis: A mini review. Biofactors 2021, 47, 59–68. [Google Scholar] [CrossRef]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- So, S.; Tokumaru, T.; Miyahara, K.; Ohshima, Y. Control of lifespan by food bacteria, nutrient limitation and pathogenicity of food in C. elegans. Mech. Ageing Dev. 2011, 132, 210–212. [Google Scholar] [CrossRef]
- Fisher, A.L.; Lithgow, G.J. The nuclear hormone receptor DAF-12 has opposing effects on Caenorhabditis elegans lifespan and regulates genes repressed in multiple long-lived worms. Aging Cell 2006, 5, 127–138. [Google Scholar] [CrossRef]
- Lazakovitch, E.; Kalb, J.M.; Gronostajski, R.M. Lifespan extension and increased pumping rate accompany pharyngeal muscle-specific expression of nfi-1 in C. elegans. Dev. Dyn. 2008, 237, 2100–2107. [Google Scholar] [CrossRef]
- Thondamal, M.; Witting, M.; Schmitt-Kopplin, P.; Aguilaniu, H. Steroid hormone signalling links reproduction to lifespan in dietary-restricted Caenorhabditis elegans. Nat. Commun. 2014, 5, 4879. [Google Scholar] [CrossRef]
- Chamoli, M.; Singh, A.; Malik, Y.; Mukhopadhyay, A. A novel kinase regulates dietary restriction-mediated longevity in Caenorhabditis elegans. Aging Cell 2014, 13, 641–655. [Google Scholar] [CrossRef]
- Chamoli, M.; Rane, A.; Foulger, A.; Chinta, S.J.; Shahmirzadi, A.A.; Kumsta, C.; Nambiar, D.K.; Hall, D.; Holcom, A.; Angeli, S.; et al. A drug-like molecule engages nuclear hormone receptor DAF-12/FXR to regulate mitophagy and extend lifespan. Nat. Aging 2023, 3, 1529–1543. [Google Scholar] [CrossRef]
- Broughton, S.; Partridge, L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem. J. 2009, 418, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span--from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Dumas, K.J.; Guo, C.; Shih, H.J.; Hu, P.J. Influence of steroid hormone signaling on life span control by Caenorhabditis elegans insulin-like signaling. G3 2013, 3, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Penkov, S.; Kaptan, D.; Erkut, C.; Sarov, M.; Mende, F.; Kurzchalia, T.V. Integration of carbohydrate metabolism and redox state controls dauer larva formation in Caenorhabditis elegans. Nat. Commun. 2015, 6, 8060. [Google Scholar] [CrossRef]
- Friedman, D.B.; Johnson, T.E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 1988, 118, 75–86. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88 Pt B, 290–301. [Google Scholar] [CrossRef]
- Sural, S.; Liang, C.Y.; Wang, F.Y.; Ching, T.T.; Hsu, A.L. HSB-1/HSF-1 pathway modulates histone H4 in mitochondria to control mtDNA transcription and longevity. Sci. Adv. 2020, 6, eaaz4452b. [Google Scholar] [CrossRef]
- Fernandez, J.; Lopez, A.B.; Wang, C.; Mishra, R.; Zhou, L.; Yaman, I.; Snider, M.D.; Hatzoglou, M. Transcriptional control of the arginine/lysine transporter, cat-1, by physiological stress. J. Biol. Chem. 2003, 278, 50000–50009. [Google Scholar] [CrossRef]
- Shi, Y.C.; Pan, T.M.; Liao, V.H. Monascin from Monascus-Fermented Products Reduces Oxidative Stress and Amyloid-beta Toxicity via DAF-16/FOXO in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 7114–7120. [Google Scholar] [CrossRef]
- Clifford, B.L.; Sedgeman, L.R.; Williams, K.J.; Morand, P.; Cheng, A.; Jarrett, K.E.; Chan, A.P.; Brearley-Sholto, M.C.; Wahlstrom, A.; Ashby, J.W.; et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021, 33, 1671–1684.e4. [Google Scholar] [CrossRef]
- Takahara, T.; Maeda, T. Evolutionarily conserved regulation of TOR signalling. J. Biochem. 2013, 154, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tatebe, H.; Shiozaki, K. Evolutionary Conservation of the Components in the TOR Signaling Pathways. Biomolecules 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.J.; Zhang, Z.K.; Guo, Y.J.; Zhou, H.H.; Gu, Q.; Xu, J. Rubidium Chloride Increases Life Span Through an AMPK/FOXO-Dependent Pathway in Caenorhabditis elegans. J. Gerontol. A Biol. 2022, 77, 1517–1524. [Google Scholar] [CrossRef]
- Long, X.; Spycher, C.; Han, Z.S.; Rose, A.M.; Müller, F.; Avruch, J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr. Biol. 2002, 12, 1448–1461. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Sewell, A.K.; Wu, Z.; Han, M. TOR Signaling in Caenorhabditis elegans Development, Metabolism, and Aging. Genetics 2019, 213, 329–360. [Google Scholar] [CrossRef]
- McKay, J.P.; Raizen, D.M.; Gottschalk, A.; Schafer, W.R.; Avery, L. eat-2 and eat-18 are required for nicotinic neurotransmission in the Caenorhabditis elegans pharynx. Genetics 2004, 166, 161–169. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ookuma, S.; Nishida, E. Lifespan extension by suppression of autophagy genes in Caenorhabditis elegans. Genes. Cells 2009, 14, 717–726. [Google Scholar] [CrossRef]
- Horikawa, M.; Nomura, T.; Hashimoto, T.; Sakamoto, K. Elongation and desaturation of fatty acids are critical in growth, lipid metabolism and ontogeny of Caenorhabditis elegans. J. Biochem. 2008, 144, 149–158. [Google Scholar] [CrossRef]
- Melhuish Beaupre, L.M.; Brown, G.M.; Goncalves, V.F.; Kennedy, J.L. Melatonin’s neuroprotective role in mitochondria and its potential as a biomarker in aging, cognition and psychiatric disorders. Transl. Psychiatry 2021, 11, 339. [Google Scholar] [CrossRef]
- Jurcau, A. Insights into the Pathogenesis of Neurodegenerative Diseases: Focus on Mitochondrial Dysfunction and Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 11847. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wan, J.; Liu, A.; Sun, J. Melatonin regulates Abeta production/clearance balance and Abeta neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020, 132, 110887. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Yamujala, R.; Wang, Y.; Wang, H.; Wu, W.H.; Lawton, M.A.; Long, C.; Di, R. Acetylcholineestarase-inhibiting alkaloids from Lycoris radiata delay paralysis of amyloid beta-expressing transgenic C. elegans CL4176. PLoS ONE 2013, 8, e63874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Wang, Z.; Gong, H.; Ma, L.; Sun, D.; Yang, C.; Li, Y.; Cheng, B.; Petersen, R.B.; et al. Menadione sodium bisulfite inhibits the toxic aggregation of amyloid-beta(1-42). Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Garcia, B.; Bannon, M.J.; Martínez-Dávila, I.A.; Soto-Rojas, L.O.; Reyes-Corona, D.; Escobedo, L.; Maldonado-Berny, M.; Gutierrez-Castillo, M.E.; Espadas-Alvarez, A.J.; Fernandez-Parrilla, M.A.; et al. Unilateral rNurr1-V5 transgene expression in nigral dopaminergic neurons mitigates bilateral neuropathology and behavioral deficits in parkinsonian rats with α-synucleinopathy. Neural Regen. Res. 2024, 19, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Sophronea, T.; Agrawal, S.; Kumari, N.; Mishra, J.; Walecha, V.; Luthra, P.M. A(2A)R antagonists triggered the AMPK/m-TOR autophagic pathway to reverse the calcium-dependent cell damage in 6-OHDA induced model of PD. Neurochem. Int. 2024, 178, 105793. [Google Scholar] [CrossRef]
- Cheon, S.M.; Jang, I.; Lee, M.H.; Kim, D.K.; Jeon, H.; Cha, D.S. Sorbus alnifolia protects dopaminergic neurodegeneration in Caenorhabditis elegans. Pharm. Biol. 2017, 55, 481–486. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Chen, Z.; Jiang, X.; Zhou, Z.; Yuan, J.; Li, F.; Zhang, Y.; Huang, X.; Fan, S.; et al. Pepper component 7-ethoxy-4-methylcoumarin, a novel dopamine D2 receptor agonist, ameliorates experimental Parkinson’s disease in mice and Caenorhabditis elegans. Pharmacol. Res. 2021, 163, 105220. [Google Scholar] [CrossRef]
- Chalorak, P.; Sanguanphun, T.; Limboonreung, T.; Meemon, K. Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease. Molecules 2021, 26, 4843. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar]
- Chen, Y.; Onken, B.; Chen, H.; Xiao, S.; Liu, X.; Driscoll, M.; Cao, Y.; Huang, Q. Mechanism of longevity extension of Caenorhabditis elegans induced by pentagalloyl glucose isolated from eucalyptus leaves. J. Agric. Food Chem. 2014, 62, 3422–3431. [Google Scholar] [CrossRef]
- Hibshman, J.D.; Webster, A.K.; Baugh, L.R. Liquid-culture protocols for synchronous starvation, growth, dauer formation, and dietary restriction of Caenorhabditis elegans. STAR Protoc. 2021, 2, 100276. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, C.M.; Li, H.J.; Zhang, Z.P.; Cui, W.B.; An, F.L.; Zhang, Z.X.; Wang, D.S.; Fei, D.Q. Ethyl caffeate attefnuates Abeta-induced toxicity in Caenorhabditis elegans AD models via the insulin/insulin-like growth factor-1 signaling pathway. Bioorganic Chem. 2023, 139, 106714. [Google Scholar] [CrossRef] [PubMed]
- Boelen, R.; Temmerman, L. Large-scale sampling of C. elegans on E. coli HT115 RNAi-compatible bacteria. MicroPublication Biol. 2023, 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Yu, J.; Li, Y.; Shi, H.; Zhang, L.; Fang, M.; Liu, Y.; Huang, C.; Fan, S. 27-Hydroxymangiferolic Acid Extends Lifespan and Improves Neurodegeneration in Caenorhabditis elegans by Activating Nuclear Receptors. Molecules 2025, 30, 1010. https://doi.org/10.3390/molecules30051010
Gao X, Yu J, Li Y, Shi H, Zhang L, Fang M, Liu Y, Huang C, Fan S. 27-Hydroxymangiferolic Acid Extends Lifespan and Improves Neurodegeneration in Caenorhabditis elegans by Activating Nuclear Receptors. Molecules. 2025; 30(5):1010. https://doi.org/10.3390/molecules30051010
Chicago/Turabian StyleGao, Xiaoyan, Jing Yu, Yin Li, Hang Shi, Lijun Zhang, Minglv Fang, Ying Liu, Cheng Huang, and Shengjie Fan. 2025. "27-Hydroxymangiferolic Acid Extends Lifespan and Improves Neurodegeneration in Caenorhabditis elegans by Activating Nuclear Receptors" Molecules 30, no. 5: 1010. https://doi.org/10.3390/molecules30051010
APA StyleGao, X., Yu, J., Li, Y., Shi, H., Zhang, L., Fang, M., Liu, Y., Huang, C., & Fan, S. (2025). 27-Hydroxymangiferolic Acid Extends Lifespan and Improves Neurodegeneration in Caenorhabditis elegans by Activating Nuclear Receptors. Molecules, 30(5), 1010. https://doi.org/10.3390/molecules30051010





