Anti-Melanogenic Effects of L-Theanine on B16F10 Cells and Zebrafish
Abstract
1. Introduction
2. Results
2.1. Effects of L-Theanine on the Viability, Melanin Content, and Intracellular Tyrosinase Activity of B16F10 Cells
2.2. Effect of L-Theanine on the Expression Levels of Melanogenesis-Related Proteins in B16F10 Cells
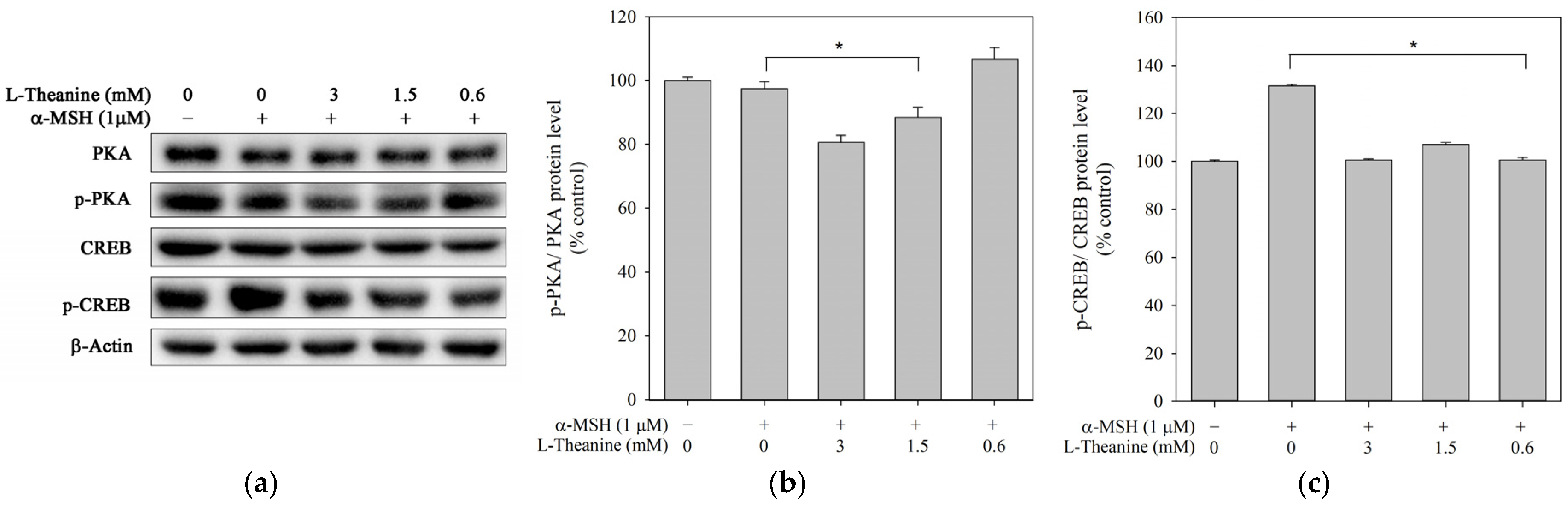
2.3. L-Theanine Reduced Melanogenesis Through the PKA/CREB Signaling Pathway
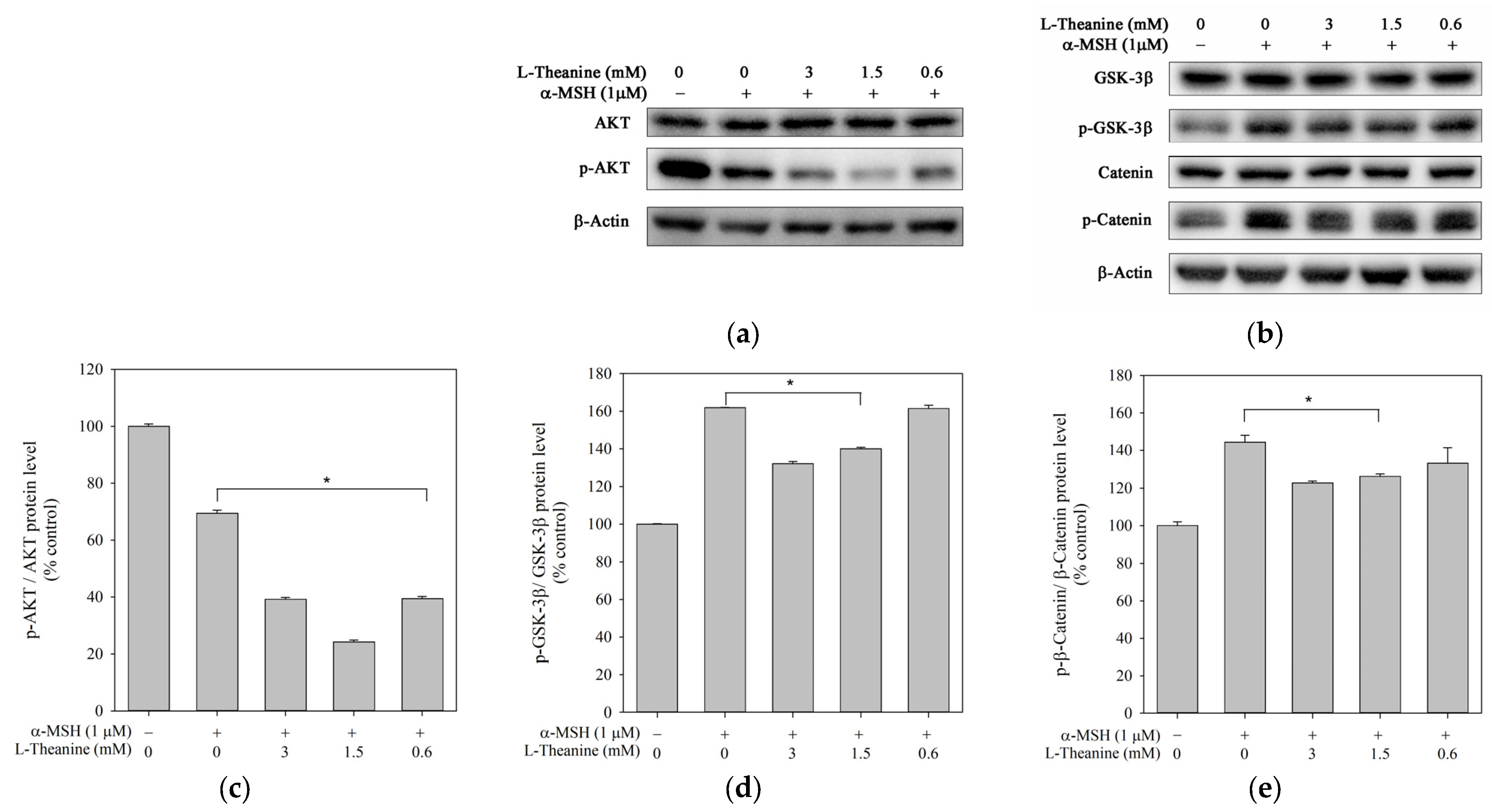
2.4. L-Theanine Reduced Melanogenesis Through the Akt/GSK-3β/β-Catenin Signaling Pathway
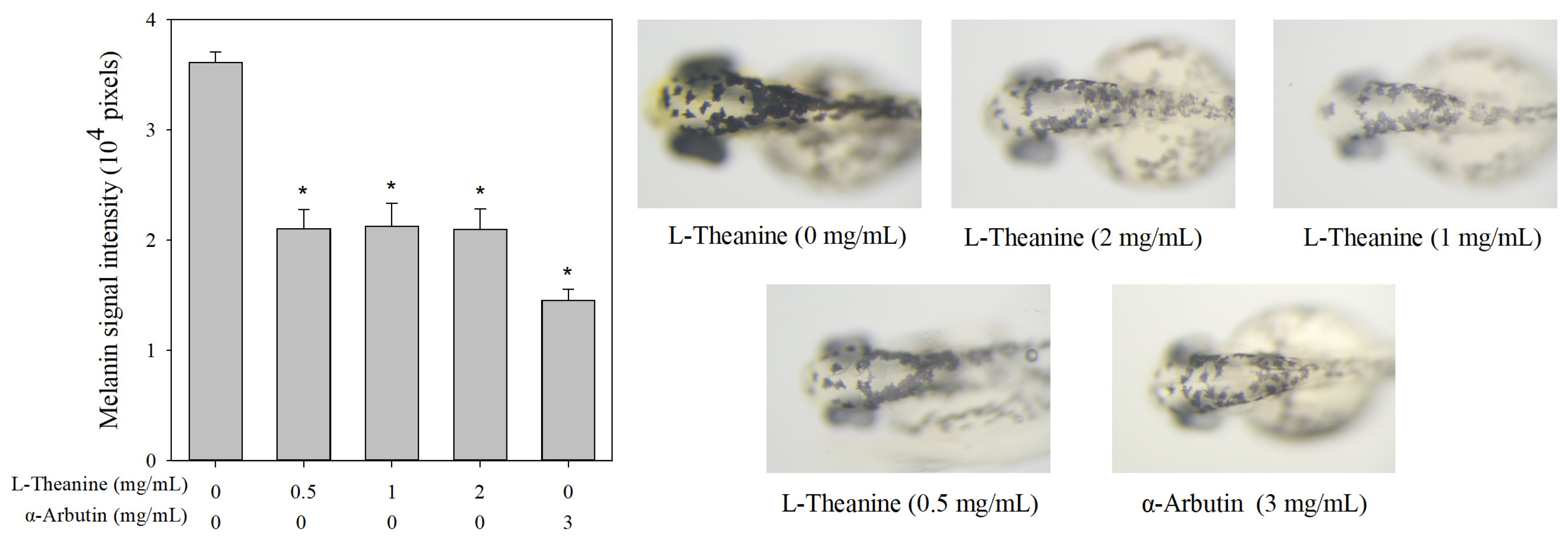
2.5. Effects of L-Theanine on Melanin Pigmentation In Vivo Zebrafish Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Melanin Content and Intracellular Tyrosinase Activity Assay
4.5. Western Blotting Assay
4.6. Determination of Zebrafish Embryo Mortality Rate
4.7. Evaluation of Antimelanogenesis Effect in Zebrafish
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Pollock, S.; Taylor, S.; Oyerinde, O.; Nurmohamed, S.; Dlova, N.; Sarkar, R.; Galadari, H.; Manela-Azulay, M.; Chung, H.S.; Handog, E.; et al. The dark side of skin lightening: An international collaboration and review of a public health issue affecting dermatology. Int. J. Women’s Dermatol. 2021, 7, 158–164. [Google Scholar] [CrossRef]
- Liu, S.-C.; Sheu, M.-L.; Tsai, Y.-C.; Lin, Y.-C.; Chang, C.-W.; Lai, D.-W. Attenuation of in vitro and in vivo melanin synthesis using a Chinese herbal medicine through the inhibition of tyrosinase activity. Phytomedicine 2022, 95, 153876. [Google Scholar] [CrossRef]
- Kim, T.; Kang, J.-K.; Hyun, C.-G. 6-Methylcoumarin promotes melanogenesis through the PKA/CREB, MAPK, AKT/PI3K, and GSK3β/β-catenin signaling pathways. Molecules 2023, 28, 4551. [Google Scholar] [CrossRef]
- Lee, A.; Kim, J.Y.; Heo, J.; Cho, D.-H.; Kim, H.-S.; An, I.-S.; An, S.; Bae, S. The inhibition of melanogenesis via the PKA and ERK signaling pathways by Chlamydomonas reinhardtii extract in B16F10 melanoma cells and artificial human skin equivalents. J. Microbiol. Biotechnol. 2018, 28, 2121–2132. [Google Scholar] [CrossRef]
- Jeong, H.; Yu, S.M.; Kim, S.J. Inhibitory effects on melanogenesis by thymoquinone are mediated through the β-catenin pathway in B16F10 mouse melanoma cells. Int. J. Oncol. 2020, 56, 379–389. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Inhibitors of melanogenesis: A patent review (2009–2014). Expert Opin. Ther. Pat. 2015, 25, 775–788. [Google Scholar] [CrossRef]
- Lonati, C.; Gatti, S.; Catania, A. Activation of melanocortin receptors as a potential strategy to reduce local and systemic reactions induced by respiratory viruses. Front. Endocrinol. 2020, 11, 569241. [Google Scholar] [CrossRef] [PubMed]
- Bullock, B.P.; Habener, J.F. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry 1998, 37, 3795–3809. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef]
- Khaled, M.; Larribere, L.; Bille, K.; Aberdam, E.; Ortonne, J.-P.; Ballotti, R.; Bertolotto, C. Glycogen synthase kinase 3β is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem. 2002, 277, 33690–33697. [Google Scholar] [CrossRef]
- Takeda, K.; Yasumoto, K.-I.; Takada, R.; Takada, S.; Watanabe, K.-I.; Udono, T.; Saito, H.; Takahashi, K.; Shibahara, S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000, 275, 14013–14016. [Google Scholar] [CrossRef]
- Ko, H.H.; Chang, Y.T.; Kuo, Y.H.; Lin, C.H.; Chen, Y.F. Oenothera laciniata Hill Extracts Exhibits Antioxidant Effects and Attenuates Melanogenesis in B16-F10 Cells via Downregulating CREB/MITF/Tyrosinase and Upregulating p-ERK and p-JNK. Plants 2021, 10, 727. [Google Scholar] [CrossRef]
- Kim, D.S.; Hwang, E.S.; Lee, J.E.; Kim, S.Y.; Kwon, S.B.; Park, K.C. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003, 116, 1699–1706. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, M.-J.; Kang, E.Y.; Kim, Y.B.; Kim, B.S.; Park, S.-M.; Hyun, C.-G. Anti-melanogenic effects of hydroxyectoine via mitf inhibition by jnk, p38, and akt pathways in b16f10 melanoma cells. Nat. Prod. Commun. 2019, 14, 1934578X19858523. [Google Scholar] [CrossRef]
- Bu, J.; Ma, P.C.; Chen, Z.Q.; Zhou, W.Q.; Fu, Y.J.; Li, L.J.; Li, C.R. Inhibition of MITF and tyrosinase by paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am. J. Chin. Med. 2008, 36, 245–263. [Google Scholar] [CrossRef]
- Yu, C.-L.; Wu, H.; Chen, Y.-P.; Chen, F.; Wang, G.-H. Orcinol Inhibits Melanogenesis in B16F10 Cells via the Upregulation of the MAPK/ERK Signaling Pathway. Nat. Prod. Commun. 2023, 18, 1934578X231156704. [Google Scholar] [CrossRef]
- Deng, W.-W.; Ogita, S.; Ashihara, H. Distribution and biosynthesis of theanine in Theaceae plants. Plant Physiol. Biochem. 2010, 48, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Ahmad, R.S.; Sultan, M.T.; Qayyum, M.M.N.; Naz, A. Green tea and anticancer perspectives: Updates from last decade. Crit. Rev. Food Sci. Nutr. 2015, 55, 792–805. [Google Scholar] [CrossRef]
- Cooper, R. Green tea and theanine: Health benefits. Int. J. Food Sci. Nutr. 2012, 63, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef]
- Kim, Y.C.; Choi, S.Y.; Park, E.Y. Anti-melanogenic effects of black, green, and white tea extracts on immortalized melanocytes. J. Vet. Sci. 2015, 16, 135–143. [Google Scholar] [CrossRef]
- Xu, W.; Gong, L.; Haddad, M.M.; Bischof, O.; Campisi, J.; Yeh, E.T.; Medrano, E.E. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp. Cell Res. 2000, 255, 135–143. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Hodi, F.S.; Fisher, D.E. From genes to drugs: Targeted strategies for melanoma. Nat. Rev. Cancer 2012, 12, 349–361. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, T.H.; Lee, W.J.; Shim, W.S.; Yeo, E.J.; Kim, S.; Kim, S.Y. A novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI 3K and ERK pathways and by regulating Ca2+ influx via TRPM 1 channels. Pigment Cell Melanoma Res. 2016, 29, 81–91. [Google Scholar] [CrossRef]
- Han, H.; Hyun, C. Acenocoumarol, an anticoagulant drug, prevents melanogenesis in B16F10 melanoma cells. Pharmaceuticals 2023, 16, 604. [Google Scholar] [CrossRef]
- Qu, J.; Yan, M.; Fang, Y.; Zhao, J.; Xu, T.; Liu, F.; Zhang, K.; He, L.; Jin, L.; Sun, D. Zebrafish in dermatology: A comprehensive review of their role in investigating abnormal skin pigmentation mechanisms. Front. Physiol. 2023, 14, 1296046. [Google Scholar] [CrossRef]
- Mu, W.; Zhang, T.; Jiang, B. An overview of biological production of L-theanine. Biotechnol. Adv. 2015, 33, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lu, Y.; Zhong, Z.; Qu, B.; Wang, M.; Yu, X.; Chen, J. Mitf involved in innate immunity by activating tyrosinase-mediated melanin synthesis in Pteria penguin. Front. Immunol. 2021, 12, 626493. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of α-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef]
- Chung, Y.C.; Hyun, C.G. Inhibitory Effects of Pinostilbene Hydrate on Melanogenesis in B16F10 Melanoma Cells via ERK and p38 Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4732. [Google Scholar] [CrossRef]
- Su, T.-R.; Lin, J.-J.; Tsai, C.-C.; Huang, T.-K.; Yang, Z.-Y.; Wu, M.-O.; Zheng, Y.-Q.; Su, C.-C.; Wu, Y.-J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef]
- Zhou, X.; Oh, J.H.; Karadeniz, F.; Yang, J.; Lee, H.; Seo, Y.; Kong, C.-S. Anti-melanogenesis effect of Rosa rugosa on α-MSH-induced B16F10 cells via PKA/CREB pathway activation. Appl. Sci. 2022, 13, 184. [Google Scholar] [CrossRef]
- Zhu, P.-Y.; Yin, W.-H.; Wang, M.-R.; Dang, Y.-Y.; Ye, X.-Y. Andrographolide suppresses melanin synthesis through Akt/GSK3β/β-catenin signal pathway. J. Dermatol. Sci. 2015, 79, 74–83. [Google Scholar] [CrossRef]
- Ko, G.-A.; Cho, S.K. Ethyl linoleate inhibits α-MSH-induced melanogenesis through Akt/GSK3β/β-catenin signal pathway. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2018, 22, 53. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Catricalà, C.; Larue, L.; Picardo, M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment Cell Melanoma Res. 2011, 24, 309–325. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Liu, I.-H.; Huang, X.-Z.; Chen, H.-J.; Chang, S.-T.; Chang, M.-L.; Ho, Y.-T.; Chang, H.-T. Antimelanogenesis effects of leaf extract and phytochemicals from ceylon olive (Elaeocarpus serratus) in zebrafish model. Pharmaceutics 2021, 13, 1059. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.-L.; Pang, H.; Run, Z.; Wang, G.-H. Anti-Melanogenic Effects of L-Theanine on B16F10 Cells and Zebrafish. Molecules 2025, 30, 956. https://doi.org/10.3390/molecules30040956
Yu C-L, Pang H, Run Z, Wang G-H. Anti-Melanogenic Effects of L-Theanine on B16F10 Cells and Zebrafish. Molecules. 2025; 30(4):956. https://doi.org/10.3390/molecules30040956
Chicago/Turabian StyleYu, Chih-Li, Haiyue Pang, Zhao Run, and Guey-Horng Wang. 2025. "Anti-Melanogenic Effects of L-Theanine on B16F10 Cells and Zebrafish" Molecules 30, no. 4: 956. https://doi.org/10.3390/molecules30040956
APA StyleYu, C.-L., Pang, H., Run, Z., & Wang, G.-H. (2025). Anti-Melanogenic Effects of L-Theanine on B16F10 Cells and Zebrafish. Molecules, 30(4), 956. https://doi.org/10.3390/molecules30040956







