High-Power Ultrasound and High-Voltage Electrical Discharge-Assisted Extractions of Bioactive Compounds from Sugar Beet (Beta vulgaris L.) Waste: Electron Spin Resonance and Optical Emission Spectroscopy Analysis
Abstract
1. Introduction
2. Results and Discussion
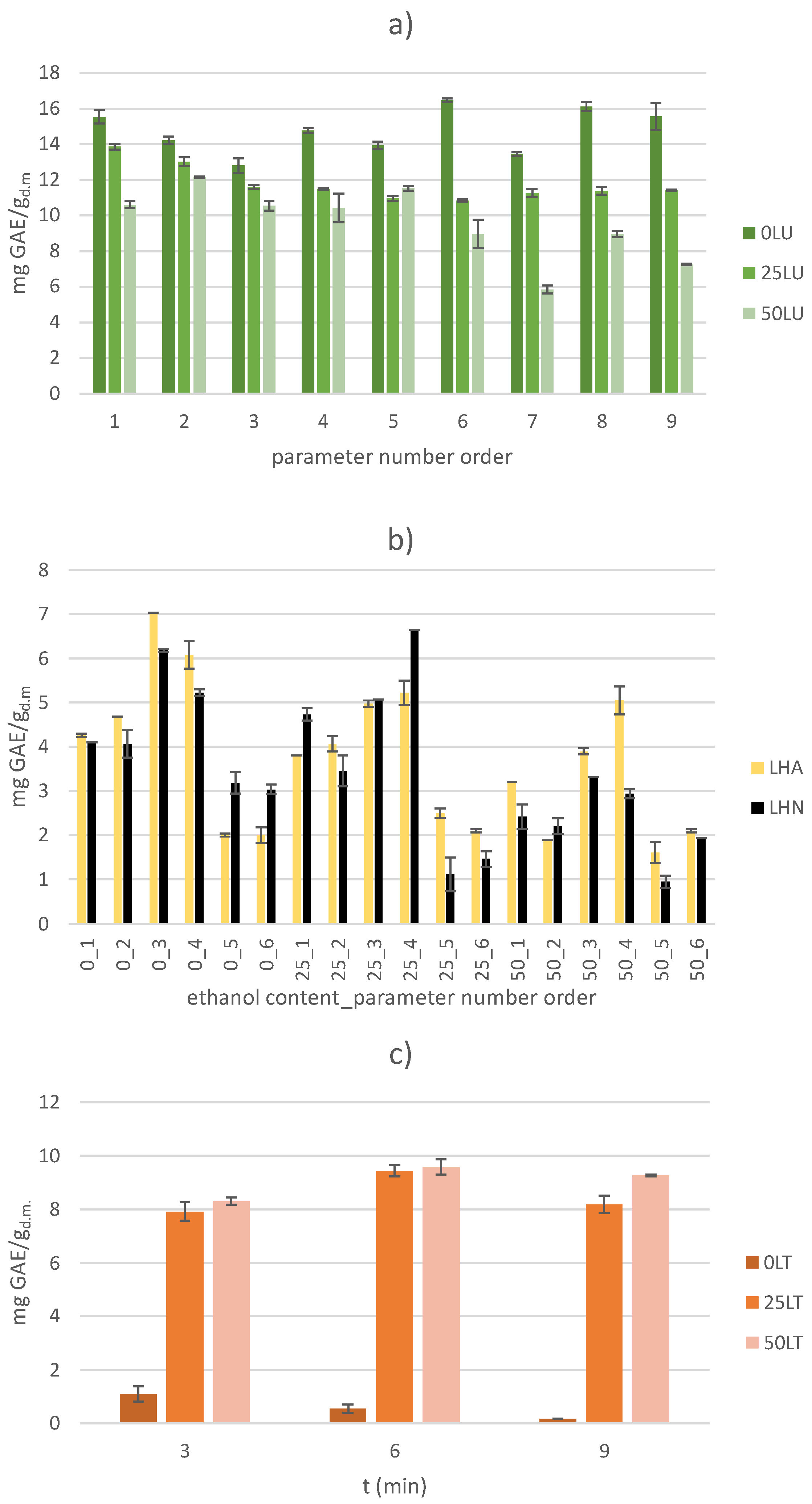
2.1. Total Phenolic Content (TPC)
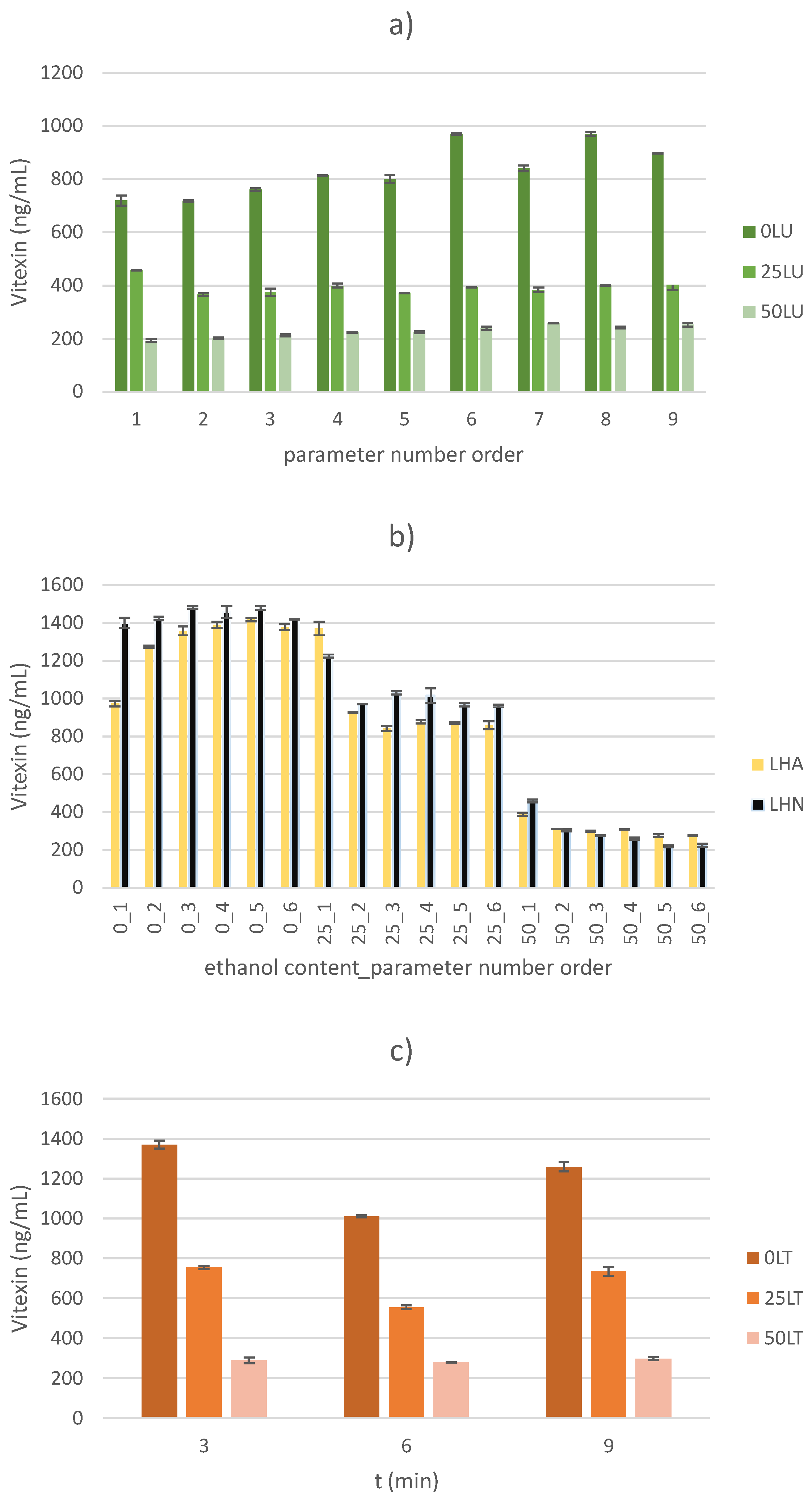
2.2. Vitexin Content
2.3. Determination of Antioxidant Capacity (AC)
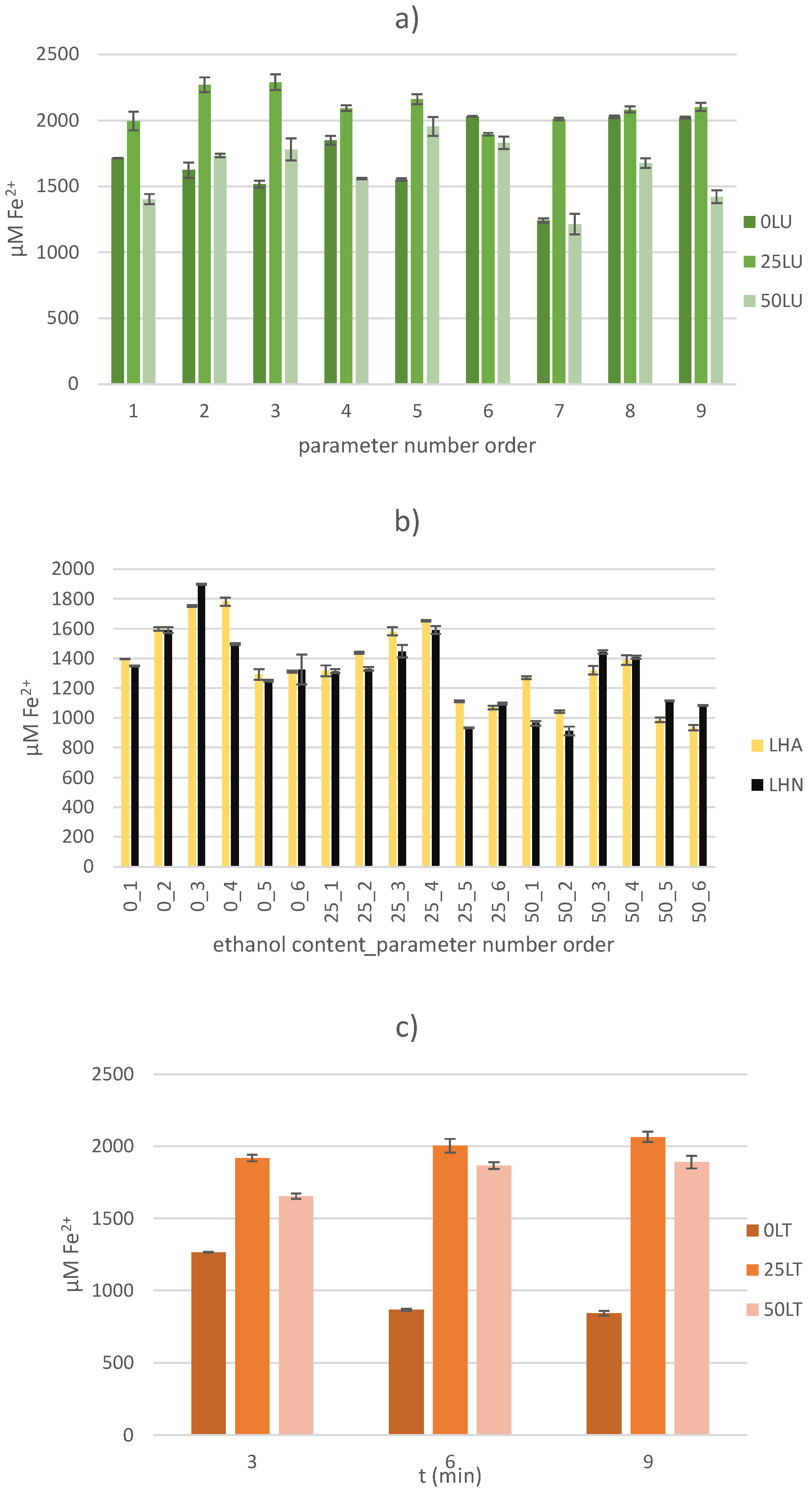
2.3.1. Ferric Reducing Antioxidant Power (FRAP) Assay
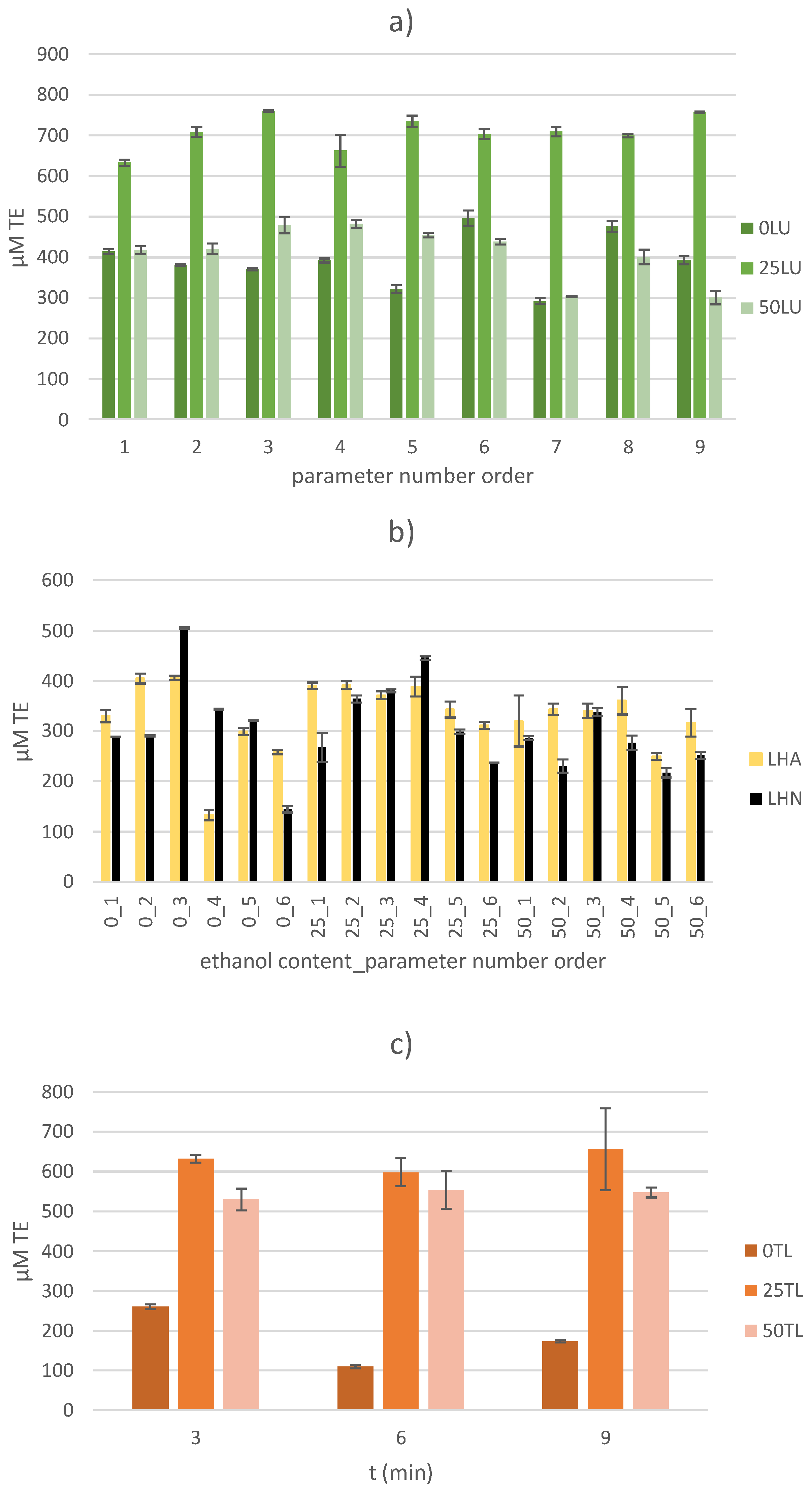
2.3.2. DPPH (2,2-Diphenyl-2-picrylhydrazyl) Free Radical Assay
2.3.3. Determination of Antioxidant Capacity by Electron Spin Resonance (ESR) Spectroscopy
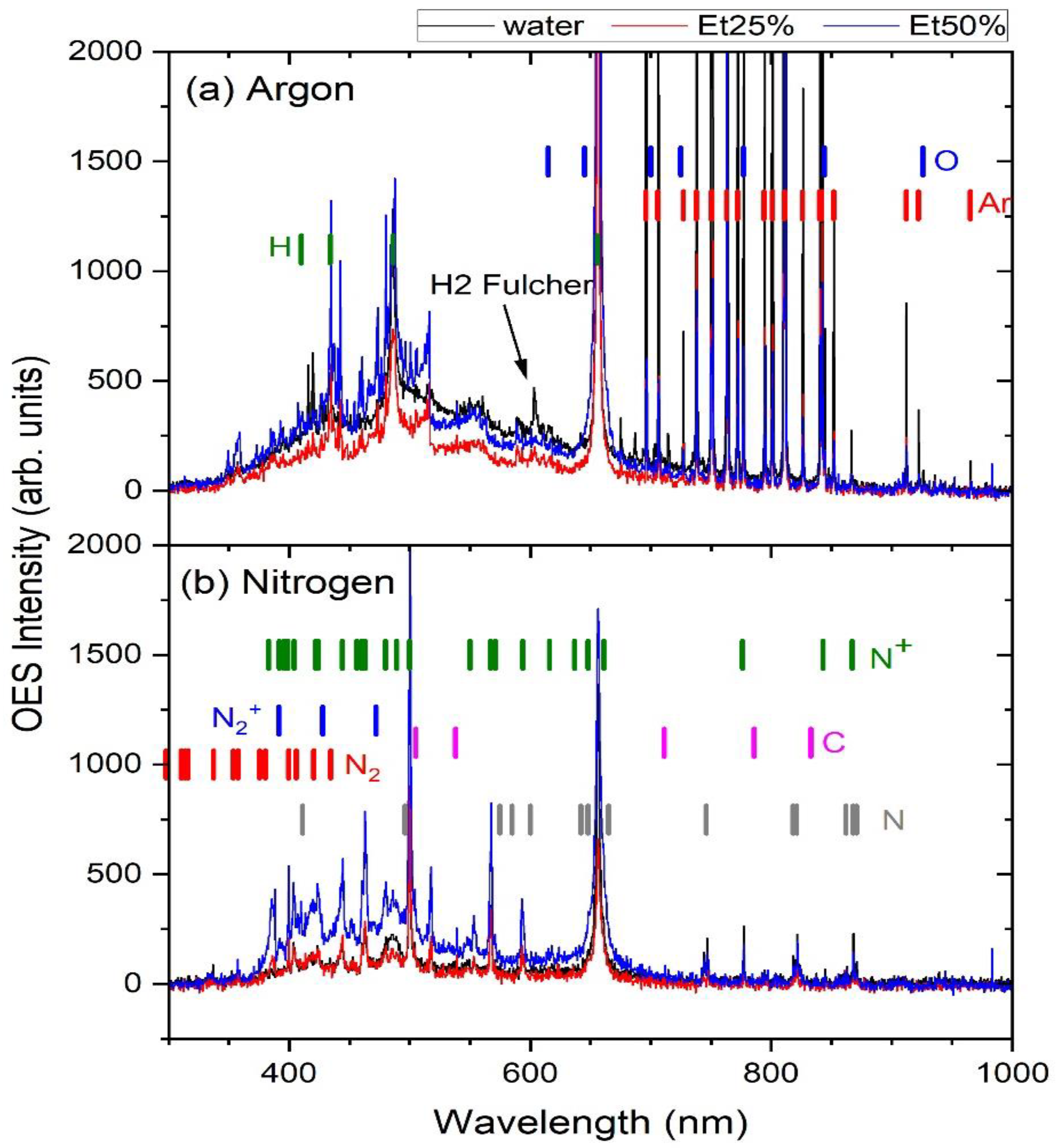
2.4. Plasma Characterization by Optical Emission Spectroscopy (OES)
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction Methods and Sample Labeling
3.3.1. High-Power Ultrasound Assisted Extraction (US)
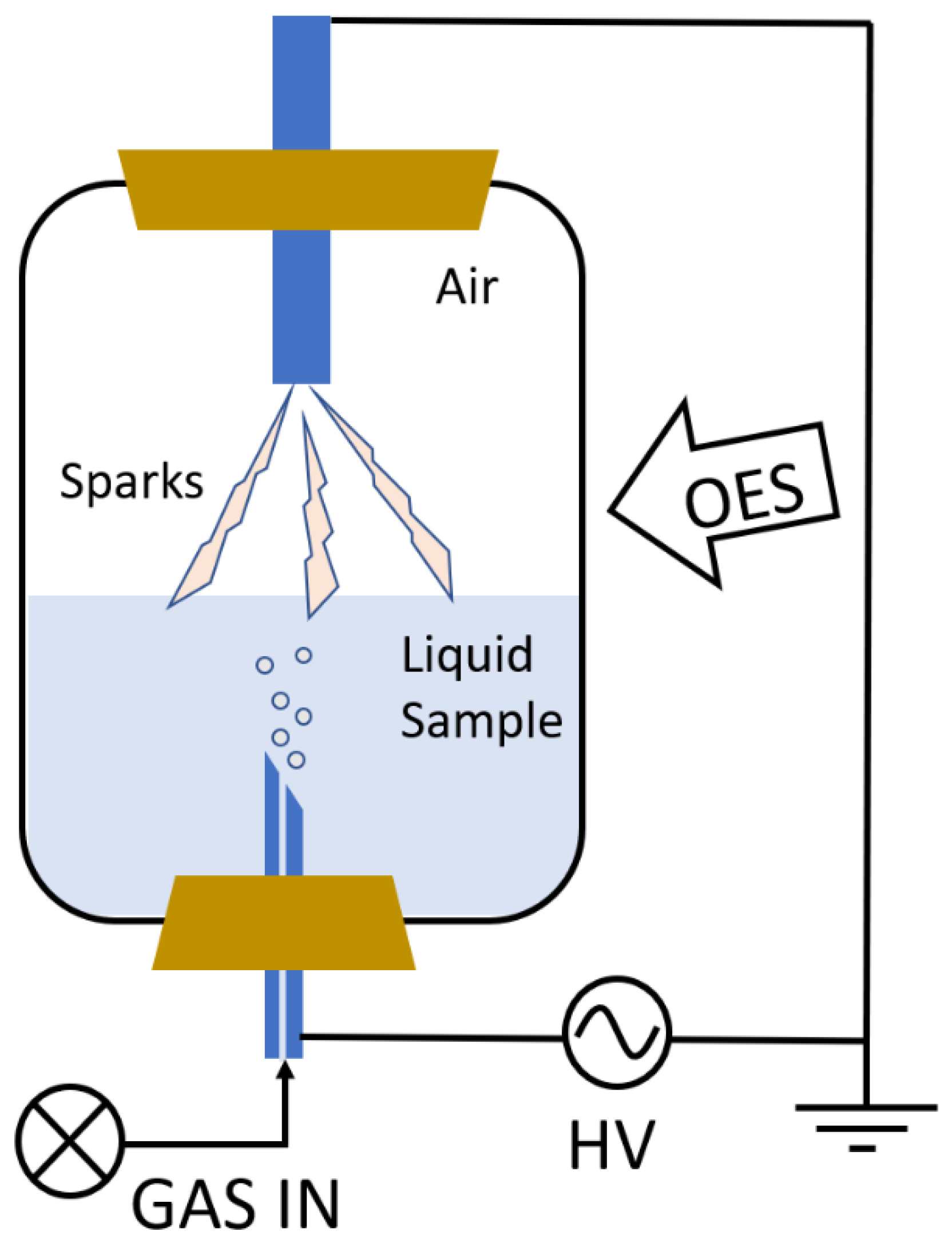
3.3.2. High-Voltage Electrical Discharge-Assisted Extraction (HVED)
3.3.3. Conventional Thermal Extraction
3.4. Analysis
3.4.1. Determination of Total Phenolic Content (TPC)
3.4.2. Determination of Vitexin Content
Standard Solutions and Samples for HPLC-MS/MS Determination
Chromatographic and Mass Spectrometric Conditions
Method Validation
3.4.3. Determination of Antioxidant Capacity (AC)
Ferric Reducing Antioxidant Power (FRAP) Assay
DPPH (2,2-Diphenyl-2-picrylhydrazyl) Free Radical Assay
Determination of Antioxidant Capacity by Electron Spin Resonance (ESR) Spectroscopy
3.4.4. Plasma Characterization by Optical Emission Spectroscopy (OES)
3.4.5. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adam, D. How Far Will Global Population Rise? Researchers Can’t Agree. Nature 2021, 597, 462–465. [Google Scholar] [CrossRef]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.W.; Aadil, R.M. Pulsed Electric Field: A Potential Alternative towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P.K.; Mason-D’Croz, D.; Palmer, J.; Bodirsky, B.L.; Pradhan, P.; Barrett, C.B.; Benton, T.G.; Hall, A.; Pikaar, I.; et al. Articulating the Effect of Food Systems Innovation on the Sustainable Development Goals. Lancet. Planet Health 2021, 5, e50–e62. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.T.; Wei, Y.H.; Chi-Wei Lan, J. Recent Advances on the Sustainable Approaches for Conversion and Reutilization of Food Wastes to Valuable Bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar] [CrossRef]
- Burm, K.T.A.L. Plasma: The Fourth State of Matter. Plasma Chem. Plasma Process. 2012, 32, 401–407. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Hori, M. Radical-Controlled Plasma Processes. Rev. Mod. Plasma Phys. 2022, 6, 36. [Google Scholar] [CrossRef]
- Shao, T.; Wang, R.; Zhang, C.; Yan, P. Atmospheric-Pressure Pulsed Discharges and Plasmas: Mechanism, Characteristics and Applications. High Volt. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Wang, T.-y; Li, Q.; Bi, K.-s. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative Stress Mitigation by Antioxidants—An Overview on Their Chemistry and Influences on Health Status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Mihaylova, D.; Marangon, C.M.; Grigoletto, L.; Lante, A. Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves. Molecules 2022, 27, 8110. [Google Scholar] [CrossRef] [PubMed]
- Dukić, J.; Hunić, M.; Nutrizio, M.; Režek Jambrak, A. Influence of High-Power Ultrasound on Yield of Proteins and Specialized Plant Metabolites from Sugar Beet Leaves (Beta vulgaris subsp. vulgaris var. altissima) . Appl. Sci. 2022, 12, 8949. [Google Scholar] [CrossRef]
- Tenorio, A.T. Sugar Beet Leaves for Functional Ingredients. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Zewail, R.M.Y.; El-Gmal, I.S.; Khaitov, B.; El-Desouky, H.S.A. Micronutrients through Foliar Application Enhance Growth, Yield and Quality of Sugar Beet (Beta vulgaris L.). J. Plant Nutr. 2020, 43, 2275–2285. [Google Scholar] [CrossRef]
- El-gengaihi, S.E.; Hamed, M.A.; Aboubaker, D.H.; Mossa, A.H. FLAVONOIDS FROM SUGAR BEET LEAVES AS HEPATOPROTECTIVE AGENT. Int. J. Pharm. Sci. 2016, 8, 281–286. [Google Scholar]
- Maravić, N.; Teslić, N.; Nikolić, D.; Dimić, I.; Šereš, Z.; Pavlić, B. From Agricultural Waste to Antioxidant-Rich Extracts: Green Techniques in Extraction of Polyphenols from Sugar Beet Leaves. Sustain. Chem. Pharm. 2022, 28, 100728. [Google Scholar] [CrossRef]
- Drăghici-Popa, A.M.; Boscornea, A.C.; Brezoiu, A.M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of Extraction Process Factors on the Composition and Antioxidant Activity of Blackthorn (Prunus spinosa L.) Fruit Extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Bayram, I.; Mihaylova, D.; Lante, A. A Strategy to Minimize the Chlorophyll Content in the Phenolic Extract of Sugar Beet Leaves: Can This Extract Work as a Natural Antioxidant in Vegetable Oils? Food Bioprocess. Tech. 2024, 18, 2493–2506. [Google Scholar] [CrossRef]
- Hemmati, V.; Garavand, F.; Khorshidian, N.; Cacciotti, I.; Goudarzi, M.; Chaichi, M.; Tiwari, B.K. Impact of Cold Atmospheric Plasma on Microbial Safety, Total Phenolic and Flavonoid Contents, Antioxidant Activity, Volatile Compounds, Surface Morphology, and Sensory Quality of Green Tea Powder. Food Biosci. 2021, 44, 101348. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Snoussi, A.; Bouacida, S.; Mitić, M.; Arsić, B.; Koubaier, H.B.H.; Chouaibi, M.; Janković, S.; Zlatanović, I.; Mrmošanin, J.; Stojanović, G.; et al. Thermal Degradation Kinetics of Myrtle Leaves Ethanol Extract (Myrtus communis L.): Effect on Phenolic Compounds Content and Antioxidant Activity. J. Food Meas. Charact. 2022, 16, 2119–2130. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Q.; Zhao, X.; Wang, D.; Li, W.; Sui, X.; Zhang, Y.; Jiang, S.; Wang, Q.; Gu, C. Preparation and Characterization of Vitexin Powder Micronized by a Supercritical Antisolvent (SAS) Process. Powder Technol. 2012, 228, 47–55. [Google Scholar] [CrossRef]
- Razak, L.T. Optimization of Vitexin and Isovitexin Compounds Extracted from Dried Mas Cotek Leaves Using One-Factor-at-a-Time (OFAT) Approach in Aqueous Extraction. Int. Food Res. J. 2018, 25, 2560–2571. [Google Scholar]
- Sarangapani, C.; O’Toole, G.; Cullen, P.J.; Bourke, P. Atmospheric Cold Plasma Dissipation Efficiency of Agrochemicals on Blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Liu, J.; Gu, H.; Yang, L. An Efficient Approach for the Extraction of Orientin and Vitexin from Trollius Chinensis Flowers Using Ultrasonic Circulating Technique. Ultrason. Sonochem. 2017, 37, 267–278. [Google Scholar] [CrossRef]
- Zu, G.; Zhang, R.; Yang, L.; Ma, C.; Zu, Y.; Wang, W.; Zhao, C. Ultrasound-Assisted Extraction of Carnosic Acid and Rosmarinic Acid Using Ionic Liquid Solution from Rosmarinus officinalis. Int. J. Mol. Sci. 2012, 13, 11027–11043. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Malta, L.G.; Liu, R.H. Analyses of Total Phenolics, Total Flavonoids, and Total Antioxidant Activities in Foods and Dietary Supplements. In Encyclopedia of Agriculture and Food Systems; Academic Press: Cambridge, MA, USA, 2014; pp. 305–314. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant Measurements. Physiol. Meas. 2007, 28, R41. [Google Scholar] [CrossRef]
- Nilsson, J.; Pillai, D.; Önning, G.; Persson, C.; Nilsson, Å.; Åkesson, B. Comparison of the 2,2′-Azinobis-3-Ethylbenzotiazo-Line-6-Sulfonic Acid (ABTS) and Ferric Reducing Anti-Oxidant Power (FRAP) Methods to Asses the Total Antioxidant Capacity in Extracts of Fruit and Vegetables. Mol. Nutr. Food Res. 2005, 49, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons Ltd.: New York, NY, USA, 2017; pp. 77–106. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. Applications of Ultrasound in Food and Bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xu, Z.; Xiang, C.; Liu, J.; Zhou, L.; Li, T.; Yang, Z.; Ding, C. Comparative Evaluation of Maceration and Ultrasonic-Assisted Extraction of Phenolic Compounds from Fresh Olives. Ultrason. Sonochem. 2017, 37, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and Quantification of Polyphenols from Kinnow (Citrus reticulate L.) Peel Using Ultrasound and Maceration Techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Nutrizio, M.; Pataro, G.; Carullo, D.; Carpentieri, S.; Mazza, L.; Ferrari, G.; Chemat, F.; Banović, M.; Jambrak, A.R. High Voltage Electrical Discharges as an Alternative Extraction Process of Phenolic and Volatile Compounds from Wild Thyme (Thymus serpyllum L.): In Silico and Experimental Approaches for Solubility Assessment. Molecules 2020, 25, 4131. [Google Scholar] [CrossRef] [PubMed]
- Žuntar, I.; Putnik, P.; Kovačević, D.B.; Nutrizio, M.; Šupljika, F.; Poljanec, A.; Dubrović, I.; Barba, F.J.; Jambrak, A.R. Phenolic and Antioxidant Analysis of Olive Leaves Extracts (Olea europaea L.) Obtained by High Voltage Electrical Discharges (HVED). Foods 2019, 8, 248. [Google Scholar] [CrossRef]
- Dukić, J.; Košpić, K.; Kelava, V.; Mavrić, R.; Nutrizio, M.; Balen, B.; Butorac, A.; Halil Öztop, M.; Režek Jambrak, A. Alternative Methods for RuBisCO Extraction from Sugar Beet Waste: A Comparative Approach of Ultrasound and High Voltage Electrical Discharge. Ultrason. Sonochem. 2023, 99, 106535. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Sirichan, T.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Optimization of Ultrasound Extraction of Functional Compound from Makiang Seed by Response Surface Methodology and Antimicrobial Activity of Optimized Extract with Its Application in Orange Juice. Ultrason. Sonochem. 2022, 83, 105916. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioproc. Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of Valuable Biocompounds Assisted by High Voltage Electrical Discharges: A Review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Cui, W.S.; Zhao, X.H. Composition and Activity Changes of the Soluble Water and Ethanol Extracts from White Mulberry (Morus alba L.) Fruits in Response to Thermal Treatment. J. Food Meas. Charact. 2020, 14, 838–848. [Google Scholar] [CrossRef]
- Režek Jambrak, A.; Ojha, S.; Šeremet, D.; Nutrizio, M.; Maltar-Strmečki, N.; Valić, S.; Gajdoš Kljusurić, J.; Tiwari, B. Free Radical Detection in Water after Processing by Means of High Voltage Electrical Discharges and High Power Ultrasound. J. Food Process. Preserv. 2021, 45, e15176. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Shen, Y.; Fan, X.H.; García Martín, J.F.; Wang, X.; Song, Y. Free Radical Generation Induced by Ultrasound in Red Wine and Model Wine: An EPR Spin-Trapping Study. Ultrason. Sonochem. 2015, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.D.; Zhang, Q.A.; Zheng, H.R. Roles of Free Radical on the Formation of Acetaldehyde in Model Wine Solutions under Different Ultrasound Parameters: A Key Bridge-Link Compound for Red Wine Coloration during Ageing. Ultrason. Sonochem. 2021, 79, 105757. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of Reactive Species Using Various Gas Plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Uhm, H.S. Generation of Various Radicals in Nitrogen Plasma and Their Behavior in Media. Phys. Plasmas 2015, 22, 123506. [Google Scholar] [CrossRef]
- Alide, T.; Wangila, P.; Kiprop, A. Effect of Cooking Temperature and Time on Total Phenolic Content, Total Flavonoid Content and Total in Vitro Antioxidant Activity of Garlic. BMC Res. Notes 2020, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Atomic Spectra Database|NIST. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 5 November 2024).
- Krstulović, N.; Labazan, I.; Miloević, S.; Cvelbar, U.; Vesel, A.; Mozeti, M. Optical Emission Spectroscopy Characterization of Oxygen Plasma during Treatment of a PET Foil. J. Phys. D Appl. Phys. 2006, 39, 3799. [Google Scholar] [CrossRef]
- Cvelbar, U.; Krstulović, N.; Milošević, S.; Mozetič, M. Inductively Coupled RF Oxygen Plasma Characterization by Optical Emission Spectroscopy. Vacuum 2007, 82, 224–227. [Google Scholar] [CrossRef]
- Jurov, A.; Popović, D.; Šrut Rakić, I.; Delač Marion, I.; Filipič, G.; Kovač, J.; Cvelbar, U.; Krstulović, N. Atmospheric Pressure Plasma Jet–Assisted Impregnation of Gold Nanoparticles into PVC Polymer for Various Applications. Int. J. Adv. Manuf. Technol. 2019, 101, 927–938. [Google Scholar] [CrossRef]
- Qayyum, A.; Zeb, S.; Naveed, M.A.; Rehman, N.U.; Ghauri, S.A.; Zakaullah, M. Optical Emission Spectroscopy of Ar–N2 Mixture Plasma. J. Quant. Spectrosc. Radiat. Transf. 2007, 107, 361–371. [Google Scholar] [CrossRef]
- Nutrizio, M.; Kljusurić, J.G.; Marijanović, Z.; Dubrović, I.; Viskić, M.; Mikolaj, E.; Chemat, F.; Jambrak, A.R. The Potential of High Voltage Discharges for Green Solvent Extraction of Bioactive Compounds and Aromas from Rosemary (Rosmarinus officinalis L.)—Computational Simulation and Experimental Methods. Molecules 2020, 25, 3711. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 107–115. ISBN 9781119135388. [Google Scholar]
- Yan, C.; Liu, H.; Lin, L. Simultaneous Determination of Vitexin and Isovitexin in Rat Plasma after Oral Administration of Santalum Album L. Leaves Extract by Liquid Chromatography Tandem Mass Spectrometry. Biomed. Chromatogr. 2013, 27, 228–232. [Google Scholar] [CrossRef]
- Luo, L.; Kang, J.; Zhao, W.; Qi, Y.; Liang, S. Validated LC-MS/MS Method for Simultaneous Quantification of Seven Components of Naodesheng in Rat Serum after Oral Administration and Its Application to a Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2019, 174, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zu, Y.; Liu, W.; Zhang, L.; Tong, M.; Efferth, T.; Kong, Y.; Hou, C.; Chen, L. Determination of Vitexin and Isovitexin in Pigeonpea Using Ultrasonic Extraction Followed by LC-MS. J. Sep. Sci. 2008, 31, 268–275. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nutrizio, M.; Maltar-Strmečki, N.; Chemat, F.; Duić, B.; Jambrak, A.R. High-Voltage Electrical Discharges in Green Extractions of Bioactives from Oregano Leaves (Origanum vulgare L.) Using Water and Ethanol as Green Solvents Assessed by Theoretical and Experimental Procedures. Food Eng. Rev. 2021, 13, 161–174. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Škevin, D.; Valić, S.; Majetić, V.; Petričević, S.; Ljubenkov, I. The Antioxidant Capacity and Oxidative Stability of Virgin Olive Oil Enriched with Phospholipids. Food Chem. 2008, 111, 121–126. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Blagović, B.; Valić, S. Electron Spin Resonance Measurement of Radical Scavenging Activity of Aronia Melanocarpa Fruit Juice. Pharmacogn. Mag. 2012, 8, 171–174. [Google Scholar] [CrossRef] [PubMed]

| Ethanol Content (%) | US-Treated | Parameter Number | Amplitude (%) | Treatment Time (min) |
|---|---|---|---|---|
| 0 25 50 | LU | 1 | 75 | 6 |
| 2 | 75 | 3 | ||
| 3 | 50 | 6 | ||
| 4 | 50 | 9 | ||
| 5 | 75 | 9 | ||
| 6 | 100 | 9 | ||
| 7 | 50 | 3 | ||
| 8 | 100 | 6 | ||
| 9 | 100 | 3 |
| Ethanol Content (%) | HVED-Treated | Applied Gas (A/N) | Parameter Number | Voltage (kV) | Treatment Time (min) |
|---|---|---|---|---|---|
| 0 25 50 | LH | A (Argon) N (Nitrogen) | 1 | 25 | 6 |
| 2 | 20 | 6 | |||
| 3 | 20 | 9 | |||
| 4 | 25 | 9 | |||
| 5 | 20 | 3 | |||
| 6 | 25 | 3 |
| Sample Name | Ethanol Content (%) | Treatment Time (min) |
|---|---|---|
| 0LT3 | 0 | 3 |
| 0LT6 | 0 | 6 |
| 0LT9 | 0 | 9 |
| 25LT3 | 25 | 3 |
| 25LT6 | 3 | 6 |
| 25LT9 | 25 | 9 |
| 50LT3 | 50 | 3 |
| 50LT6 | 50 | 6 |
| 50LT9 | 50 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dukić, J.; Režek Jambrak, A.; Jurec, J.; Merunka, D.; Valić, S.; Radičić, R.; Krstulović, N.; Nutrizio, M.; Dubrović, I. High-Power Ultrasound and High-Voltage Electrical Discharge-Assisted Extractions of Bioactive Compounds from Sugar Beet (Beta vulgaris L.) Waste: Electron Spin Resonance and Optical Emission Spectroscopy Analysis. Molecules 2025, 30, 796. https://doi.org/10.3390/molecules30040796
Dukić J, Režek Jambrak A, Jurec J, Merunka D, Valić S, Radičić R, Krstulović N, Nutrizio M, Dubrović I. High-Power Ultrasound and High-Voltage Electrical Discharge-Assisted Extractions of Bioactive Compounds from Sugar Beet (Beta vulgaris L.) Waste: Electron Spin Resonance and Optical Emission Spectroscopy Analysis. Molecules. 2025; 30(4):796. https://doi.org/10.3390/molecules30040796
Chicago/Turabian StyleDukić, Josipa, Anet Režek Jambrak, Jurica Jurec, Dalibor Merunka, Srećko Valić, Rafaela Radičić, Nikša Krstulović, Marinela Nutrizio, and Igor Dubrović. 2025. "High-Power Ultrasound and High-Voltage Electrical Discharge-Assisted Extractions of Bioactive Compounds from Sugar Beet (Beta vulgaris L.) Waste: Electron Spin Resonance and Optical Emission Spectroscopy Analysis" Molecules 30, no. 4: 796. https://doi.org/10.3390/molecules30040796
APA StyleDukić, J., Režek Jambrak, A., Jurec, J., Merunka, D., Valić, S., Radičić, R., Krstulović, N., Nutrizio, M., & Dubrović, I. (2025). High-Power Ultrasound and High-Voltage Electrical Discharge-Assisted Extractions of Bioactive Compounds from Sugar Beet (Beta vulgaris L.) Waste: Electron Spin Resonance and Optical Emission Spectroscopy Analysis. Molecules, 30(4), 796. https://doi.org/10.3390/molecules30040796









