Antioxidant and Tyrosinase-Inhibitory Activities and Biological Bioactivities of Flavonoid Derivatives from Quercus mongolica Pollen
Abstract
1. Introduction
2. Results and Discussion
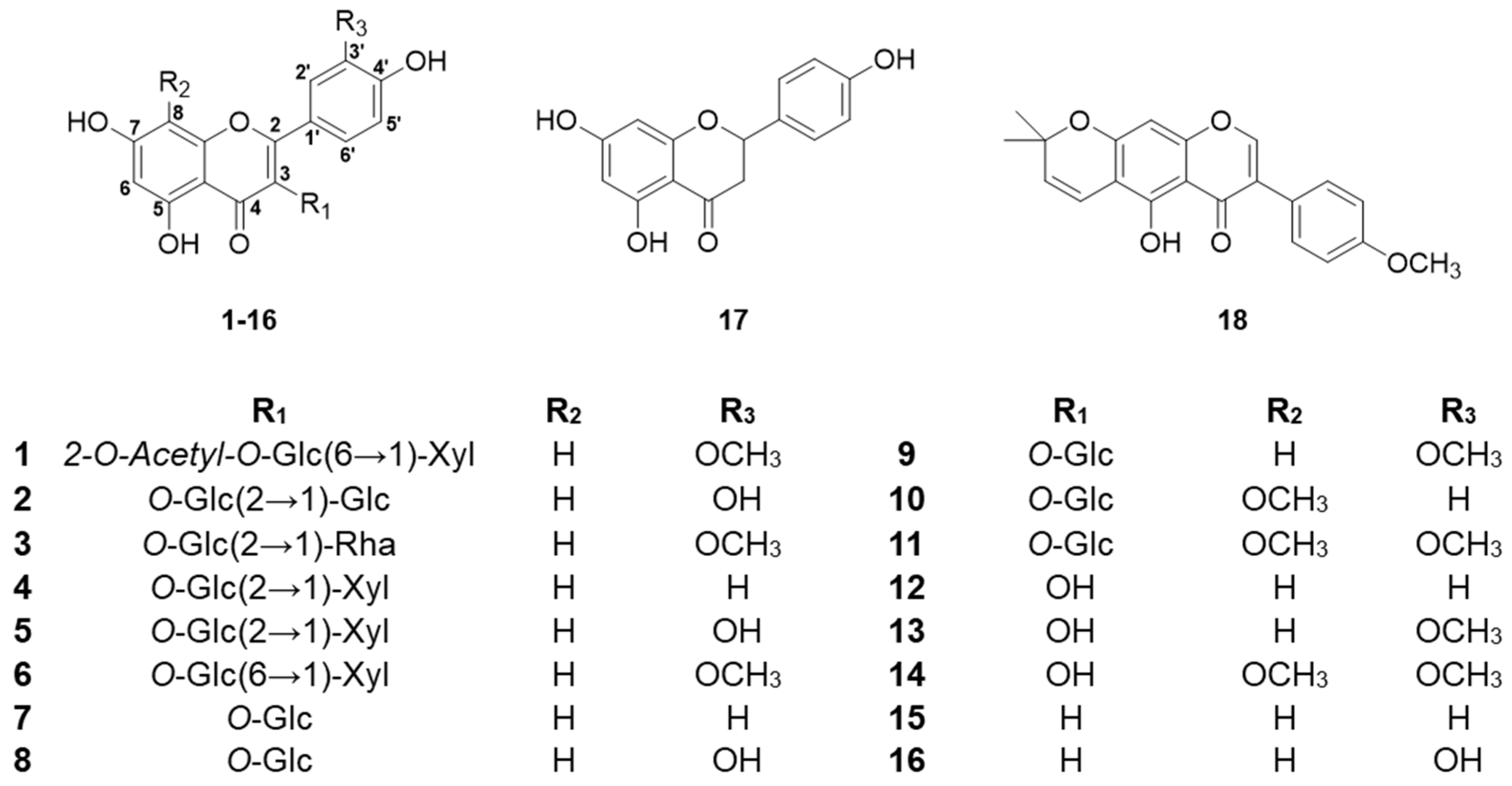
2.1. Structural Determination of New Compound
2.2. Structural Identification of Known Compounds
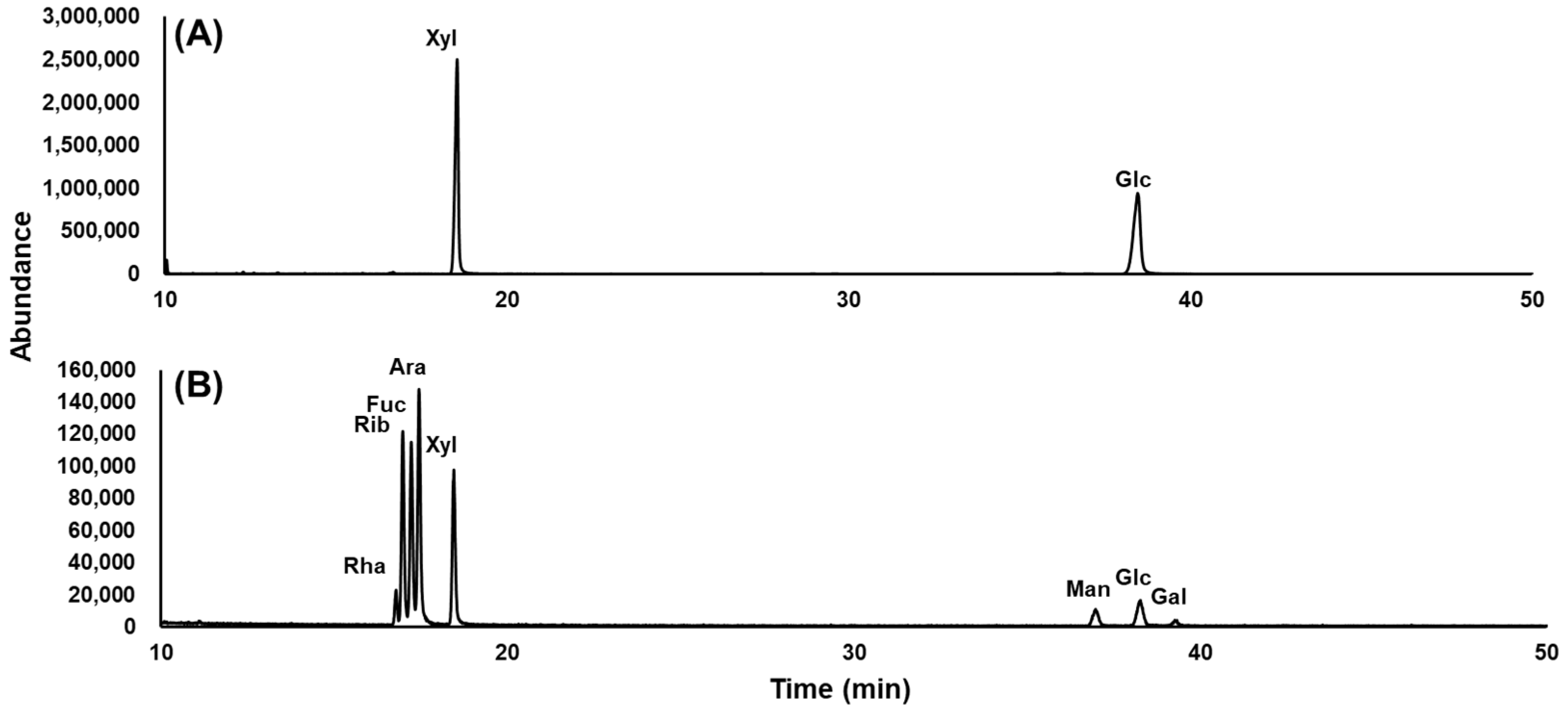
2.3. Monosaccharide Composition Analysis of New Compound
2.4. Tyrosinase-Inhibitory Activity of Isolated Compounds
2.5. Antioxidant Activity of Isolated Compounds
3. Experimental Sections
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation of Flavonoids from Pollen of Q. Mongolica
3.4. New Compound
3.5. Monosaccharide Composition Analysis
3.5.1. Monosaccharide Hydrolysis and GC-MS Analysis for Composition Assay
3.5.2. GC-MS Analytical Conditions
3.6. Tyrosinase Inhibition Assay and Antioxidant Assay
3.6.1. Tyrosinase Inhibition Assay
3.6.2. Antioxidant Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Suh, M.; Lee, D. Stand Structure and Regeneration of Quercus Mongolica Forests in Korea. For. Ecol. Manag. 1998, 106, 27–34. [Google Scholar] [CrossRef]
- Yin, J.; Kim, H.H.; Hwang, I.H.; Kim, D.H.; Lee, M.W. Anti-Inflammatory Effects of Phenolic Compounds Isolated from Quercus Mongolica Fisch. ex Ledeb. on UVB-Irradiated Human Skin Cells. Molecules 2019, 24, 3094. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Nonaka, G.-I.; Nishioka, I. Phenolic glucoside gallates from quercus mongolica and q. acutissima. Phytochemistry 1987, 26, 1147–1152. [Google Scholar] [CrossRef]
- Kim, S.B.; Liu, Q.; Ahn, J.H.; Jo, Y.H.; Turk, A.; Hong, I.P.; Han, S.M.; Hwang, B.Y.; Lee, M.K. Polyamine derivatives from the bee pollen of Quercus mongolica with tyrosinase inhibitory activity. Bioorg. Chem. 2018, 81, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Matsuo, Y.; Maeda, H.; Saito, Y.; Tanaka, T. New ellagitannin and galloyl esters of phenolic glycosides from sapwood of Quercus mongolica var. crispula (Japanese oak). Phytochem. Lett. 2013, 6, 486–490. [Google Scholar] [CrossRef]
- Ishimaru, K.; Ishimatsu, M.; Nonaka, I.; Nishioka, G.-I.; Mihashi, K.; Iwase, Y. Tannins and related compounds. LXXI. Isolation and characterization of mongolicins A and B, novel flavono-ellagitannins from quercus mongolica var grosseserrata. Chem. Pharm. Bull. 1988, 36, 3312–3318. [Google Scholar] [CrossRef]
- Sen, N.B.; Vovk, I.; Kırmızıbekmez, H.; Guzelmeric, E. Phytochemical and Bioactivity Evaluation of Bee Pollen and Androecia of Castanea, Salix, and Quercus Species. Antioxidants 2024, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Kacemi, R.; Campos, M.G. Translational Research on Bee Pollen as a Source of Nutrients: A Scoping Review from Bench to Real World. Nutrients 2023, 15, 2413. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Tomás-Lorente, F.; Ferreres, F.; Garcia-Viguera, C. Flavonoids as biochemical markers of the plant origin of bee pollen. J. Sci. Food Agric. 1989, 47, 337–340. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Lu, Q. Separation and Characterization of Phenolamines and Flavonoids from Rape Bee Pollen, and Comparison of Their Antioxidant Activities and Protective Effects Against Oxidative Stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Heil, S.; Haßlauer, I.; Schmidt, L.; von der Ohe, K.; Theuring, C.; Reinhard, A.; Schreier, P.; Beuerle, T. Pyrrolizidine alkaloids in pollen and pollen products. Mol. Nutr. Food Res. 2010, 54, 292–300. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid chromatographic–mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pólit, C.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Carrera-Pacheco, S.E.; Castillo-Solis, F.; Vallejo-Imbaquingo, R.; Barba-Ostria, C.; Guamán, L.P. Chemical Properties and Biological Activity of Bee Pollen. Molecules 2023, 28, 7768. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Martínez, P.; Yam-Puc, A.; Ramón-Sierra, J.; Hernández-Bolio, G.; Hernández-Núñez, E.; Zamora-Bustillos, R.; Ortiz-Vázquez, E. Antioxidant and Antibacterial Properties of Ethanolic Pot-Pollen Extracts of Melipona beecheii and Determination of the Major Components by GC-MS. Chem. Biodivers. 2024, 21, e202401355. [Google Scholar] [CrossRef]
- Kacemi, R.; Campos, M.G. Bee Pollen as a Source of Pharmaceuticals: Where Are We Now? In Pollen Chemistry & Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 319–336. ISBN 9783031475627. [Google Scholar]
- Capparelli, S.; Pieracci, Y.; Coppola, F.; Marchioni, I.; Sagona, S.; Felicioli, A.; Pistelli, L.; Pistelli, L. The colors of Tuscan bee pollen: Phytochemical profile and antioxidant activity. Nat. Prod. Res. 2023, 38, 2313–2319. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Brugnerotto, P.; Deolindo, C.T.P.; Blainski, E.; Dortzbach, D.; Santana, B.d.O.; Hoff, R.B.; Gonzaga, L.V.; Costa, A.C.O. LC–MS/MS analysis of pyrrolizidine alkaloids in bee bread and commercial pollen from Brazil. Eur. Food Res. Technol. 2024, 250, 2757–2765. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Gercek, Y.C.; Bayram, N.E. Phenolic Acids in Pollen. In Pollen Chemistry & Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 103–125. ISBN 9783031475627. [Google Scholar]
- Kalaba, M.; Tešić, Ž.; Blagojević, S. Flavonoids in Pollen. In Pollen Chemistry & Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 127–145. ISBN 9783031475627. [Google Scholar]
- Rivest, S.; Muralidhar, M.; Forrest, J.R.K. Pollen chemical and mechanical defences restrict host-plant use by bees. Proc. R. Soc. B Biol. Sci. 2024, 291, 20232298. [Google Scholar] [CrossRef]
- Jiang, F.; Li, M.; Huang, L.; Wang, H.; Bai, Z.; Niu, L.; Zhang, Y. Metabolite Profiling and Biological Activity Assessment of Paeonia ostii Anthers and Pollen Using UPLC-QTOF-MS. Int. J. Mol. Sci. 2024, 25, 5462. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Kilibarda, S. Lipids in Pollen. In Pollen Chemistry & Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 71–84. ISBN 9783031475627. [Google Scholar]
- Kacemi, R.; Campos, M.G. Bee Pollen Phytochemicals and Nutrients as Unequaled Pool of Epigenetic Regulators: Implications for Age-Related Diseases. Foods 2025, 14, 347. [Google Scholar] [CrossRef]
- Bernal, J.; Valverde, S.; Fuente-Ballesteros, A.; Martín-Gómez, B.; Ares, A.M. Other Bioactive Constituents of Pollen. In Pollen Chemistry & Biotechnology; Springer International Publishing: Cham, Switzerland, 2023; pp. 197–227. ISBN 9783031475627. [Google Scholar]
- Zhang, H.; Lu, Q.; Liu, R. Widely targeted metabolomics analysis reveals the effect of fermentation on the chemical composition of bee pollen. Food Chem. 2021, 375, 131908. [Google Scholar] [CrossRef] [PubMed]
- Rivest, S.; Forrest, J.R.K. Defence Compounds in Pollen: Why Do They Occur and How Do They Affect the Ecology and Evolution of Bees? New Phytol. 2020, 225, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Kaźmierczak, J.; Mencner, L.; Olczyk, K. Bee Pollen: Chemical Composition and Therapeutic Application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Vincentini, O.; Pinto, D.; Polo, A.; Maialetti, F.; Porrelli, A.; Gobbetti, M. Nutrients Bioaccessibility and Anti-inflammatory Features of Fermented Bee Pollen: A Comprehensive Investigation. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Kačániová, M.; Máriássyová, M.; Rovná, K.; Stričík, M. Antioxidant and antimicrobial properties of monofloral bee pollen. J. Environ. Sci. Health Part B 2013, 48, 133–138. [Google Scholar] [CrossRef]
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Ceska, O.; Styles, E. Flavonoids from Zea mays pollen. Phytochemistry 1984, 23, 1822–1823. [Google Scholar] [CrossRef]
- Strack, D.; Meurer, B.; Wray, V.; Grotjahn, L.; Austenfeld, F.; Wiermann, R. Quercetin 3-glucosylgalactoside from pollen of Corylus avellana. Phytochemistry 1984, 23, 2970–2971. [Google Scholar] [CrossRef]
- Meurer, B.; Wray, V.; Grotjahn, L.; Wiermann, R.; Strack, D. Hydroxycinnamic acid spermidine amides from pollen of Corylus avellana L. Phytochemistry 1986, 25, 433–435. [Google Scholar] [CrossRef]
- Kim, S.B.; Jo, Y.H.; Liu, Q.; Ahn, J.H.; Hong, I.P.; Han, S.M.; Hwang, B.Y.; Lee, M.K. Optimization of Extraction Condition of Bee Pollen Using Response Surface Methodology: Correlation between Anti-Melanogenesis, Antioxidant Activity, and Phenolic Content. Molecules 2015, 20, 19764–19774. [Google Scholar] [CrossRef] [PubMed]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobiş, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Taylor, L.P.; Strenge, D.; Miller, K.D. The Role of Glycosylation in Flavonol-Induced Pollen Germination. Adv. Exp. Med. Biol. 1998, 439, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Feng, Z.; Zhang, Y.; Xiao, X.; Dong, J.; Haubruge, E.; Zhang, H. Phenolamide and flavonoid glycoside profiles of 20 types of monofloral bee pollen. Food Chem. 2022, 405, 134800. [Google Scholar] [CrossRef]
- Campos, M.; Markham, K.R.; Mitchell, K.A.; da Cunha, A.P. An Approach to the Characterization of Bee Pollens via Their Flavonoid/Phenolic Profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship1. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment. Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Chang, T.-S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Odonbayar, B.; Murata, T.; Batkhuu, J.; Yasunaga, K.; Goto, R.; Sasaki, K. Antioxidant Flavonols and Phenolic Compounds from Atraphaxis frutescens and Their Inhibitory Activities against Insect Phenoloxidase and Mushroom Tyrosinase. J. Nat. Prod. 2016, 79, 3065–3071. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Das, R.; Banerjee, E.R. Role of free radicals in human inflammatory diseases. AIMS Biophys. 2017, 4, 596–614. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Frigerio, C.; Lopes, J.; Bogdanov, S. What Is the Future of Bee-Pollen? J. Apiproduct Apimedical Sci. 2010, 2, 131–144. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; Cianci, R.; Caldarelli, M.; Leggeri, G.; Raffaelli, G.; Pizzocaro, E.; Cirillo, M.; De Lorenzo, A. Exploring the Exposome Spectrum: Unveiling Endogenous and Exogenous Factors in Non-Communicable Chronic Diseases. Diseases 2024, 12, 176. [Google Scholar] [CrossRef]
- Noor, S.N.M.; Musa, M.; Azlina, A.; Gan, S.H.; Thirumulu, K.P. Polyphenols in bee products and prevention of cell senescence. BioMedicine 2024, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.-J.; Song, N.-Y.; Shrestha, S.; Chung, I.-S.; Kim, J.-Y.; Jeong, T.-S.; Baek, N.-I. Flavonoid glycosides from cowpea seeds (Vigna sinensis K.) inhibit LDL oxidation. Food Sci. Biotechnol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.; Zhu, C.; Han, X.; Yu, B. Synthesis of tamarixetin and isorhamnetin 3-O-neohesperidoside. Carbohydr. Res. 2005, 340, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Toki, K.; Honda, T.; Tatsuzawa, F. ChemInform Abstract: Floral Pigments Isolated from the Sky-Blue Flowers of Oxypetalum caeruleum. ChemInform 2012, 43. [Google Scholar] [CrossRef]
- Webby, R.F. A flavonol triglycoside from Actinidia arguta var. Giraldii. Phytochemistry 1991, 30, 2443–2444. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Lorente, F.; Garcia-Grau, M.M.; Nieto, J.L.; Tomás-Barberán, F.A. Flavonoids from Cistus ladanifer bee pollen. Phytochemistry 1992, 31, 2027–2029. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-T.; Bang, M.-H.; Chun, O.-K.; Kim, D.-O.; Lee, C.-Y.; Baek, N.-I. Flavonol glycosides from the aerial parts ofAceriphyllum rossii and their antioxidant activities. Arch. Pharmacal. Res. 2004, 27, 390–395. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, H.S.; Shin, K.H.; Kim, B.-K.; Lee, S. Constituents of the Halophyte Salicornia herbacea. Arch. Pharmacal. Res. 2004, 27, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Rayyan, S.; Fossen, T.; Nateland, H.S.; Andersen, Ø.M. Isolation and Identification of Flavonoids, Including Flavone Rotamers, from the Herbal Drug ‘Crataegi Folium Cum Flore’ (Hawthorn). Phytochem. Anal. 2005, 16, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Roj, J.M. Phenolic constituents of the inflorescences of Sorbus torminalis (L.) Crantz. Phytochem. Lett. 2011, 4, 151–157. [Google Scholar] [CrossRef]
- Ding, H.; Lin, H.; Teng, C.; Wu, Y. Phytochemical and Pharmacological Studies on Chinese Paeonia Species. J. Chin. Chem. Soc. 2000, 47, 381–388. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, H.-J.; Lee, E.-O.; Ko, S.-G.; Bae, H.-S.; Kim, C.-H.; Ahn, K.-S.; Lu, J.; Kim, S.-H. Mitochondria-Cytochrome C-Caspase-9 Cascade Mediates Isorhamnetin-Induced Apoptosis. Cancer Lett. 2008, 270, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Tsukayama, M.; Kawamura, Y.; Seno, M.; Yamamoto, S. Studies of the Selective O-Alkylation and Dealkylation of Flavonoids. XI. A New Convenient Method for Synthesizing 3,5,7-Trihydroxy-8-Methoxyflavones from 7-Hydroxy-3,5,8-Trimethoxyflavones. Bull. Chem. Soc. Jpn. 1988, 61, 441–447. [Google Scholar] [CrossRef]
- Miyazawa, M.; Hisama, M. Antimutagenic Activity of Flavonoids from Chrysanthemum morifolium. Biosci. Biotechnol. Biochem. 2003, 67, 2091–2099. [Google Scholar] [CrossRef]
- Park, Y.; Moon, B.-H.; Lee, E.; Lee, Y.; Yoon, Y.; Ahn, J.-H.; Lim, Y. 1H and 13C-NMR Data of Hydroxyflavone Derivatives. Magn. Reson. Chem. 2007, 45, 674–679. [Google Scholar] [CrossRef]
- Khan, M.K.; Rakotomanomana, N.; Loonis, M.; Dangles, O. Chemical Synthesis of Citrus Flavanone Glucuronides. J. Agric. Food Chem. 2010, 58, 8437–8443. [Google Scholar] [CrossRef]
- Han, X.H.; Hong, S.S.; Hwang, J.S.; Jeong, S.H.; Hwang, J.H.; Lee, M.H.; Lee, M.K.; Lee, D.; Ro, J.S.; Hwang, B.Y. Monoamine oxidase inhibitory constituents from the fruits of Cudrania tricuspidata. Arch. Pharmacal. Res. 2005, 28, 1324–1327. [Google Scholar] [CrossRef]

| Compound 1 a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | δH, m (J in Hz) | δC | Type | Position | δH, m (J in Hz) | δC | Type | ||
| 1 | - | 1′′ | 5.58, d | (8.0) | 100.80 | CH | |||
| 2 | 158.44 | C | 2′′ | 4.99, dd | (9.4, 8.0) | 75.64 | CH | ||
| 3 | 134.71 | C | 3′′ | 3.67, dd | (9.4, 9.0) | 75.64 | CH | ||
| 4 | 178.85 | C | 4′′ | 3.41, dd | (9.9, 9.0) | 71.53 | CH | ||
| 5 | 162.76 | C | 5′′ | 3.53, m | 77.49 | CH | |||
| 6 | 6.13, s | 99.80 | CH | 6′′ | 3.96, dd | (11.4, 1.9) | 69.38 | CH2 | |
| 7 | 165.74 | C | 3.58, dd | (11.4, 6.0) | |||||
| 8 | 6.35, s | 94.90 | CH | 1′′′ | 4.06, d | (7.5) | 105.02 | CH | |
| 9 | 158.18 | C | 2′′′ | 3.05, dd | (9.1, 7.5) | 74.72 | CH | ||
| 10 | 105.84 | C | 3′′′ | 3.15, t | (8.9) | 77.49 | CH | ||
| 1′ | 123.10 | C | 4′′′ | 3.37, m | 71.02 | CH | |||
| 2′ | 7.96, d | (2.1) | 114.50 | CH | 5′′′ | 3.65, dd | (11.3, 5.3) | 66.50 | CH2 |
| 3′ | 148.32 | CH | 2.90, dd | (11.3, 10.0) | |||||
| 4′ | 150.64 | C | OCH3-3′ | 3.99, s | 56.84 | CH3 | |||
| 5′ | 6.90, d | (8.4) | 116.00 | C | OAc-2′′ | 2.16, s | 21.20 | CH3 | |
| 6′ | 7.60, dd | (8.4, 2.1) | 123.60 | CH | 2′′-COO- | - | 172.45 | C | |
| Compounds | Tyrosinase-Inhibitory Activity | Antioxidant Activity |
|---|---|---|
| IC50 Values (µM) | ||
| 1 | >100 | >100 |
| 2 | >100 | 34.3 |
| 3 | >100 | >100 |
| 4 | >100 | >100 |
| 5 | >100 | 18.4 |
| 6 | >100 | >100 |
| 7 | >100 | >100 |
| 8 | >100 | 28.5 |
| 9 | >100 | >100 |
| 10 | >100 | >100 |
| 11 | >100 | >100 |
| 12 | 20.9 | 42.4 |
| 13 | >100 | 25.5 |
| 14 | >100 | 52.3 |
| 15 | >100 | >100 |
| 16 | 38.8 | 9.7 |
| 17 | >100 | >100 |
| 18 | >100 | >100 |
| PC a | 36.4 | 28.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, Y.; Seo, Y.H.; Lee, S.; Shin, E.; Yeon, S.W.; Kim, S.B.; Lee, M.K. Antioxidant and Tyrosinase-Inhibitory Activities and Biological Bioactivities of Flavonoid Derivatives from Quercus mongolica Pollen. Molecules 2025, 30, 794. https://doi.org/10.3390/molecules30040794
Joo Y, Seo YH, Lee S, Shin E, Yeon SW, Kim SB, Lee MK. Antioxidant and Tyrosinase-Inhibitory Activities and Biological Bioactivities of Flavonoid Derivatives from Quercus mongolica Pollen. Molecules. 2025; 30(4):794. https://doi.org/10.3390/molecules30040794
Chicago/Turabian StyleJoo, Yerim, Young Ho Seo, Sangmin Lee, Eunbeen Shin, Sang Won Yeon, Seon Beom Kim, and Mi Kyeong Lee. 2025. "Antioxidant and Tyrosinase-Inhibitory Activities and Biological Bioactivities of Flavonoid Derivatives from Quercus mongolica Pollen" Molecules 30, no. 4: 794. https://doi.org/10.3390/molecules30040794
APA StyleJoo, Y., Seo, Y. H., Lee, S., Shin, E., Yeon, S. W., Kim, S. B., & Lee, M. K. (2025). Antioxidant and Tyrosinase-Inhibitory Activities and Biological Bioactivities of Flavonoid Derivatives from Quercus mongolica Pollen. Molecules, 30(4), 794. https://doi.org/10.3390/molecules30040794







