Structural Characterization and Anti-Gouty Nephropathy Potential of Polysaccharides from Atractylodes chinensis
Abstract
1. Introduction
2. Results
2.1. Extraction and Isolation of Polysaccharides
2.2. Purification, Molecular Weight and Monosaccharide Composition of ACP-60
2.3. FT-IR Spectra
2.4. Methylation Analysis
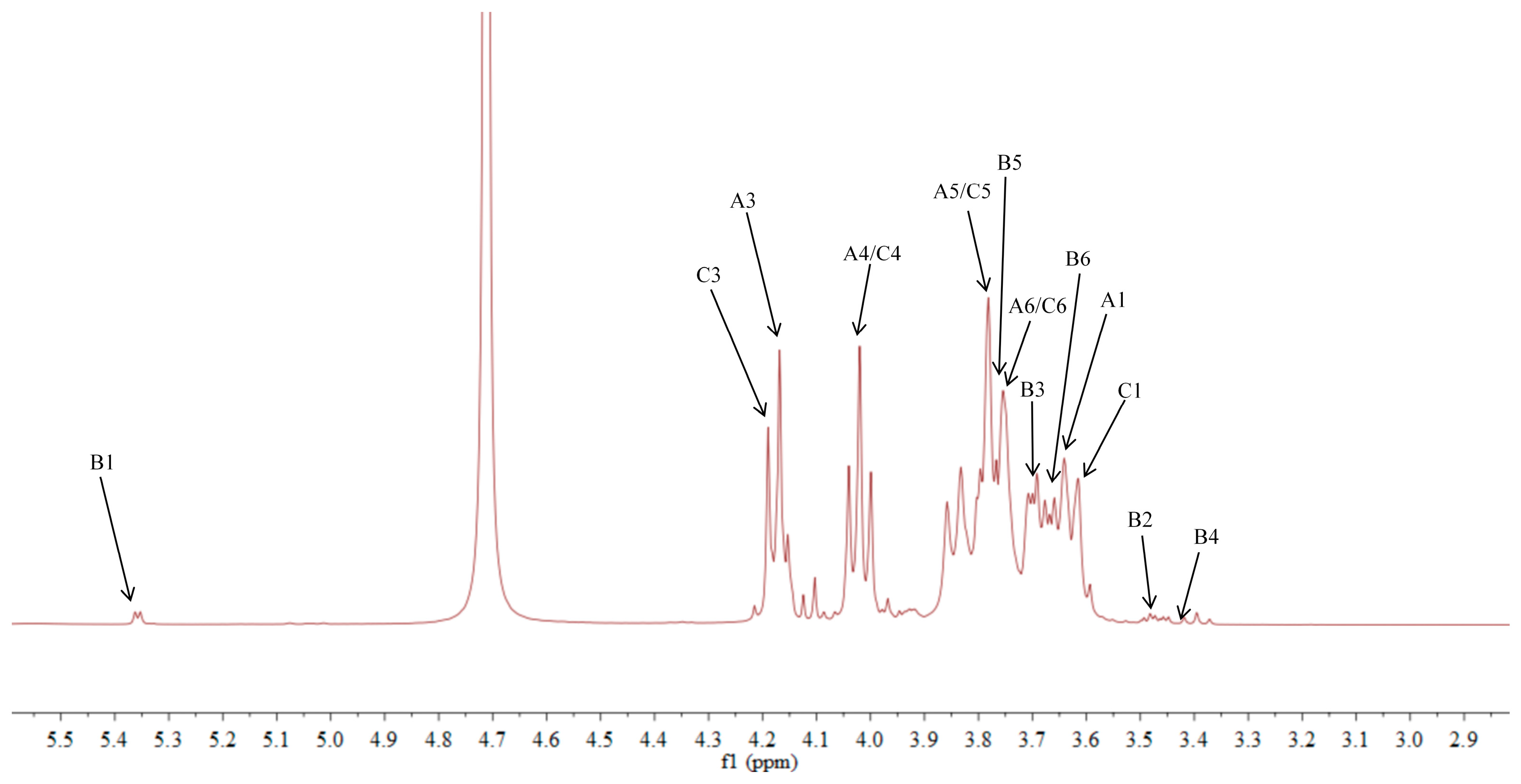
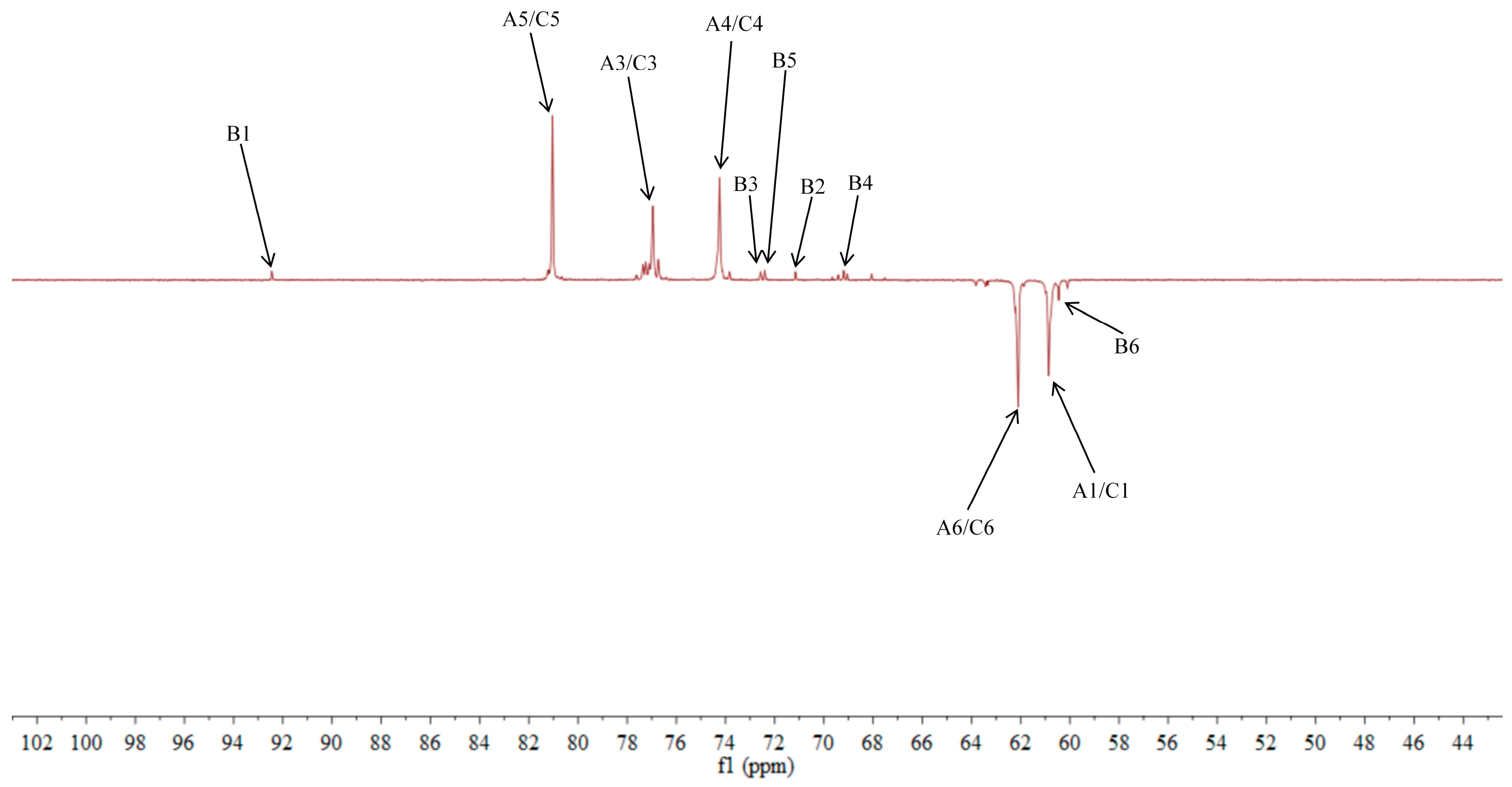
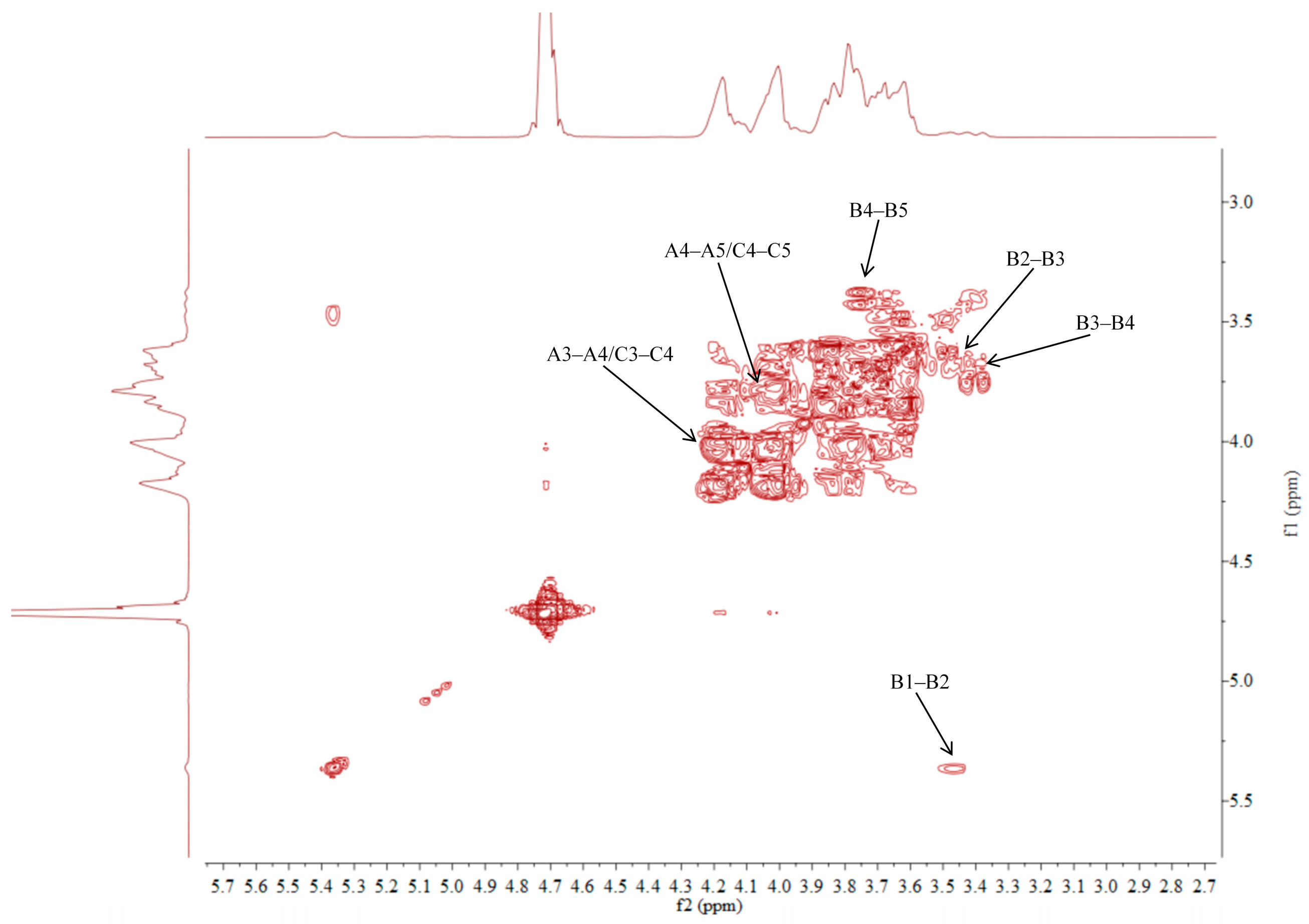
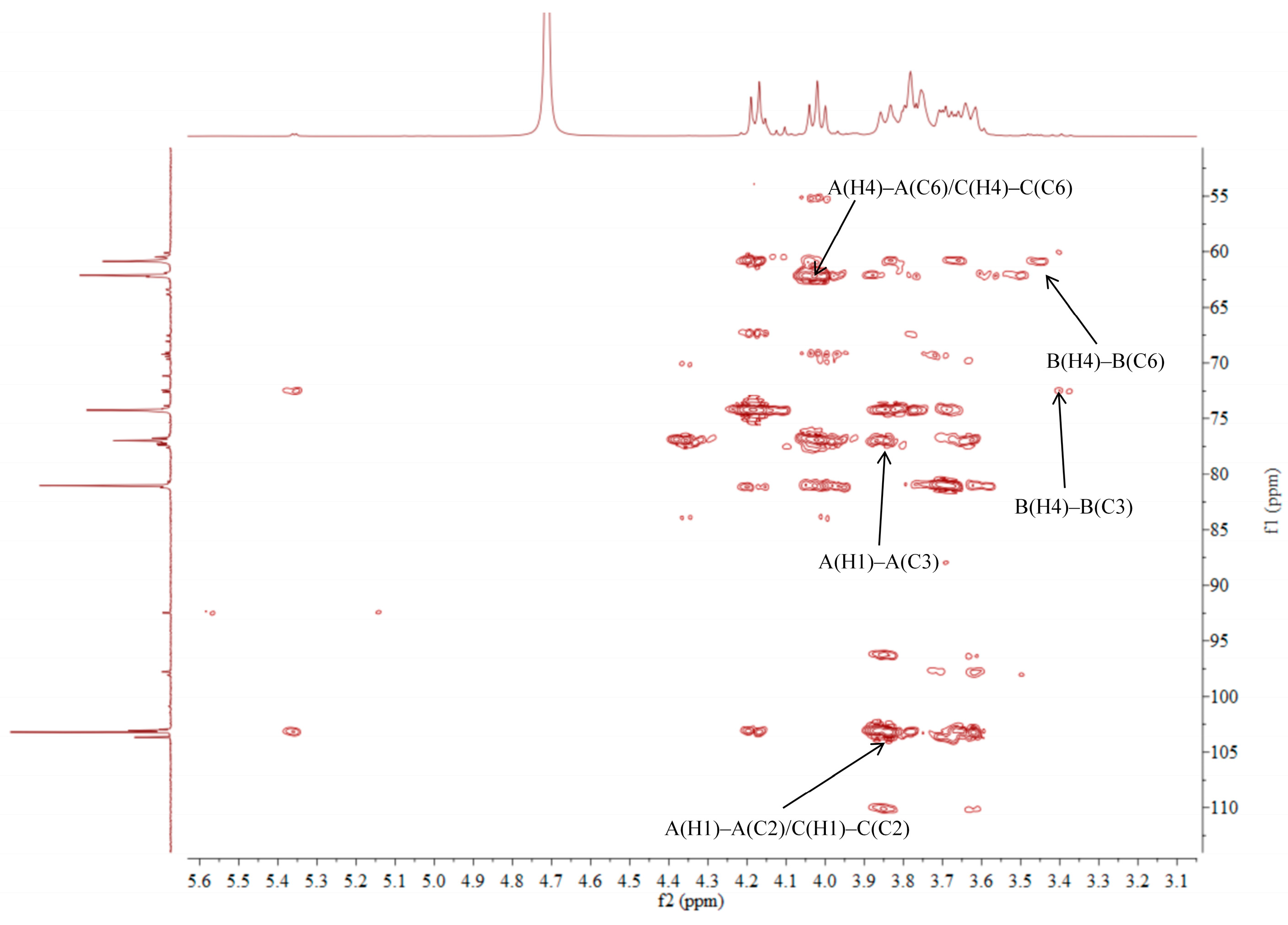
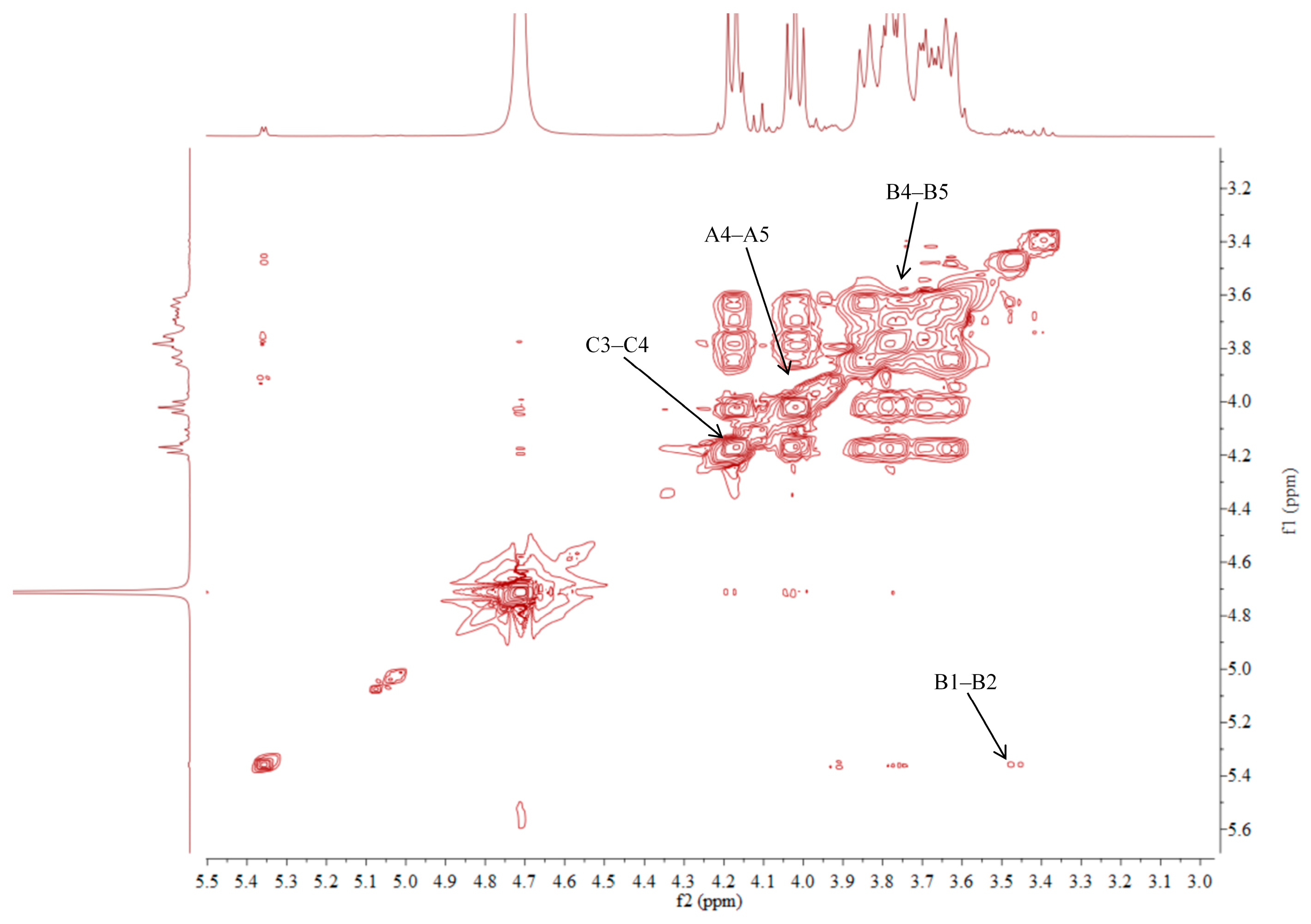
2.5. NMR Analysis of ACP-60-A
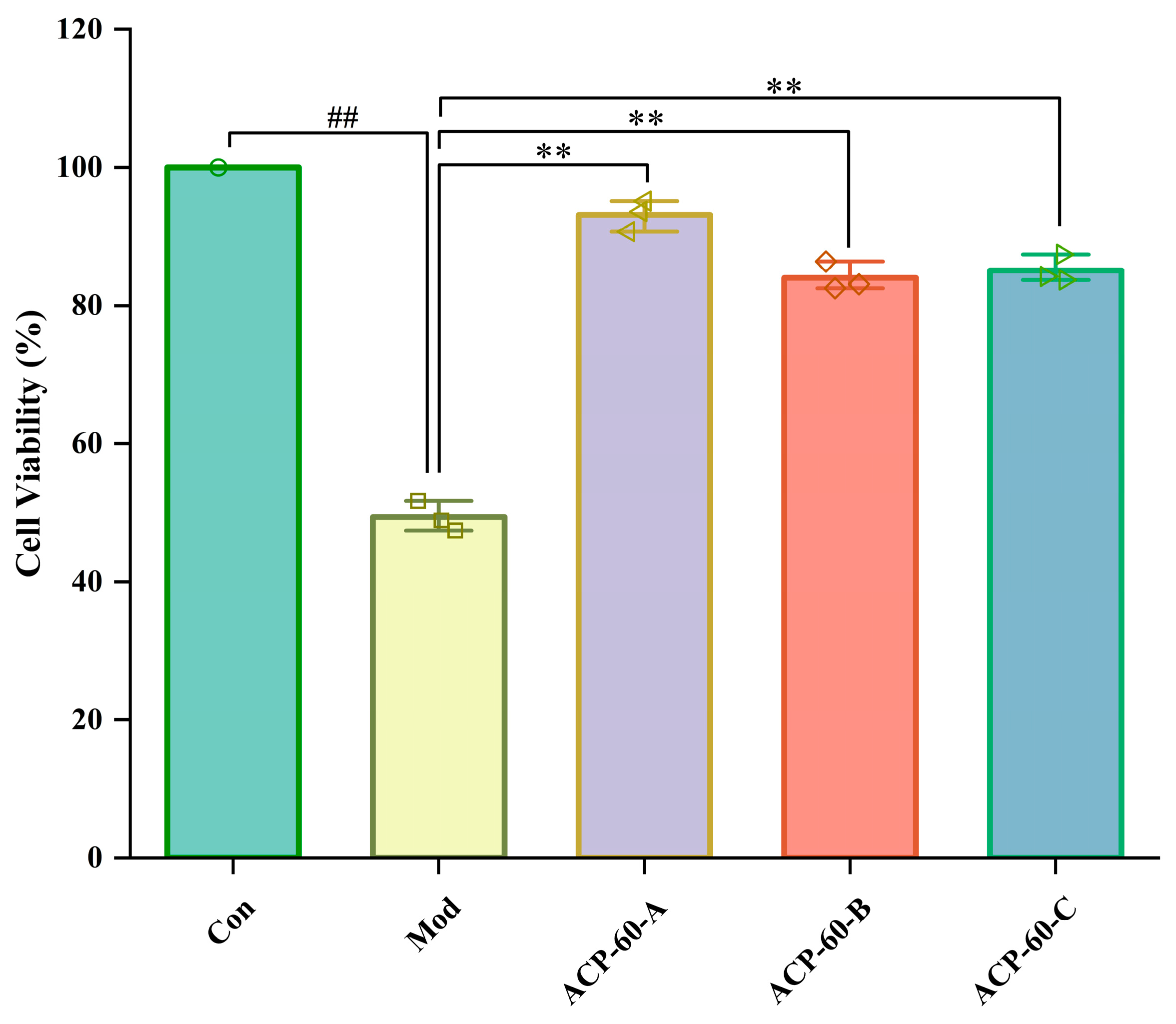
2.6. Effects of ACP-60-A, ACP-60-B and ACP-60-C on HK-2 Cell Viability
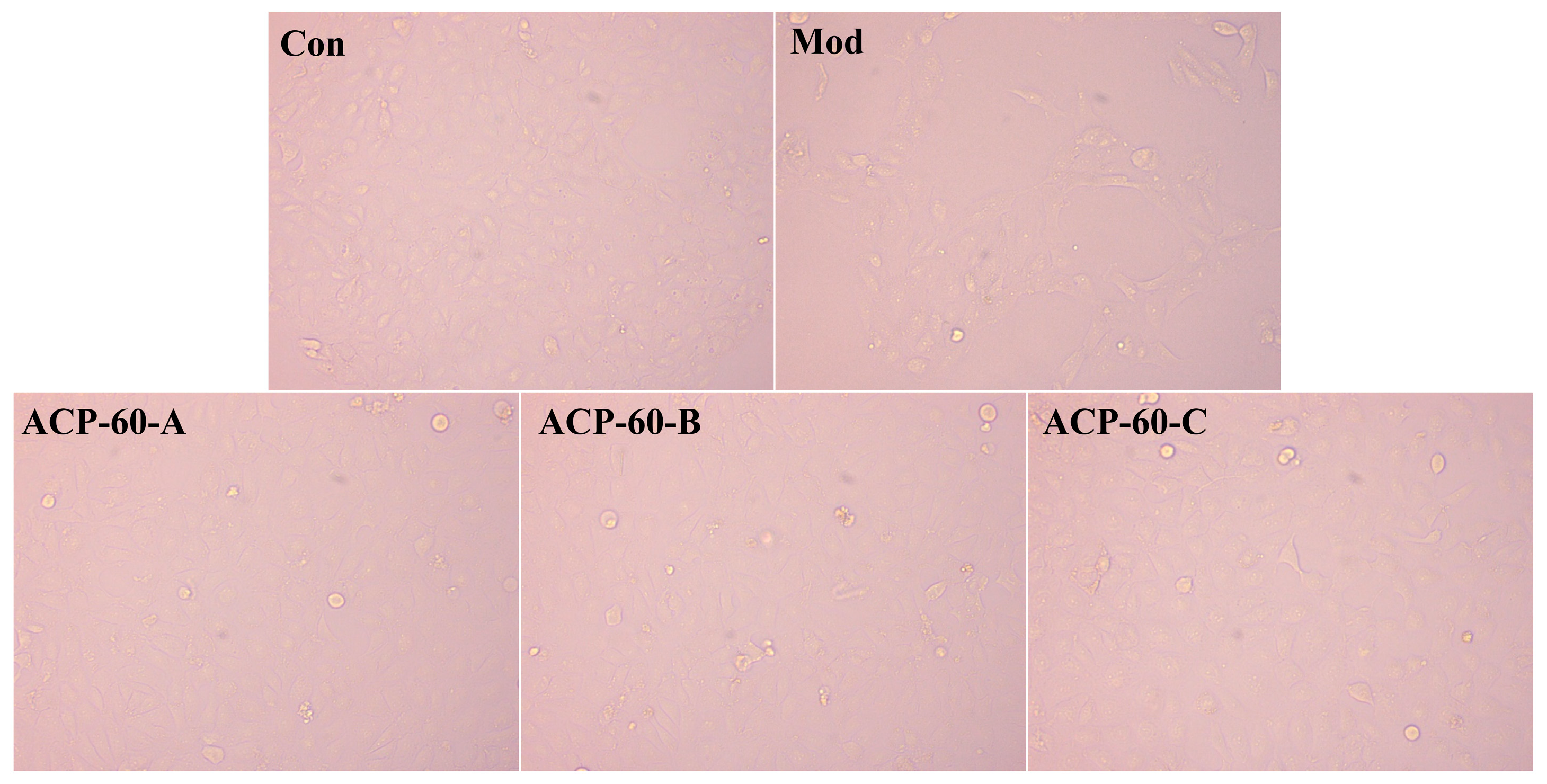
2.7. Observation of HK-2 Cells Morphology
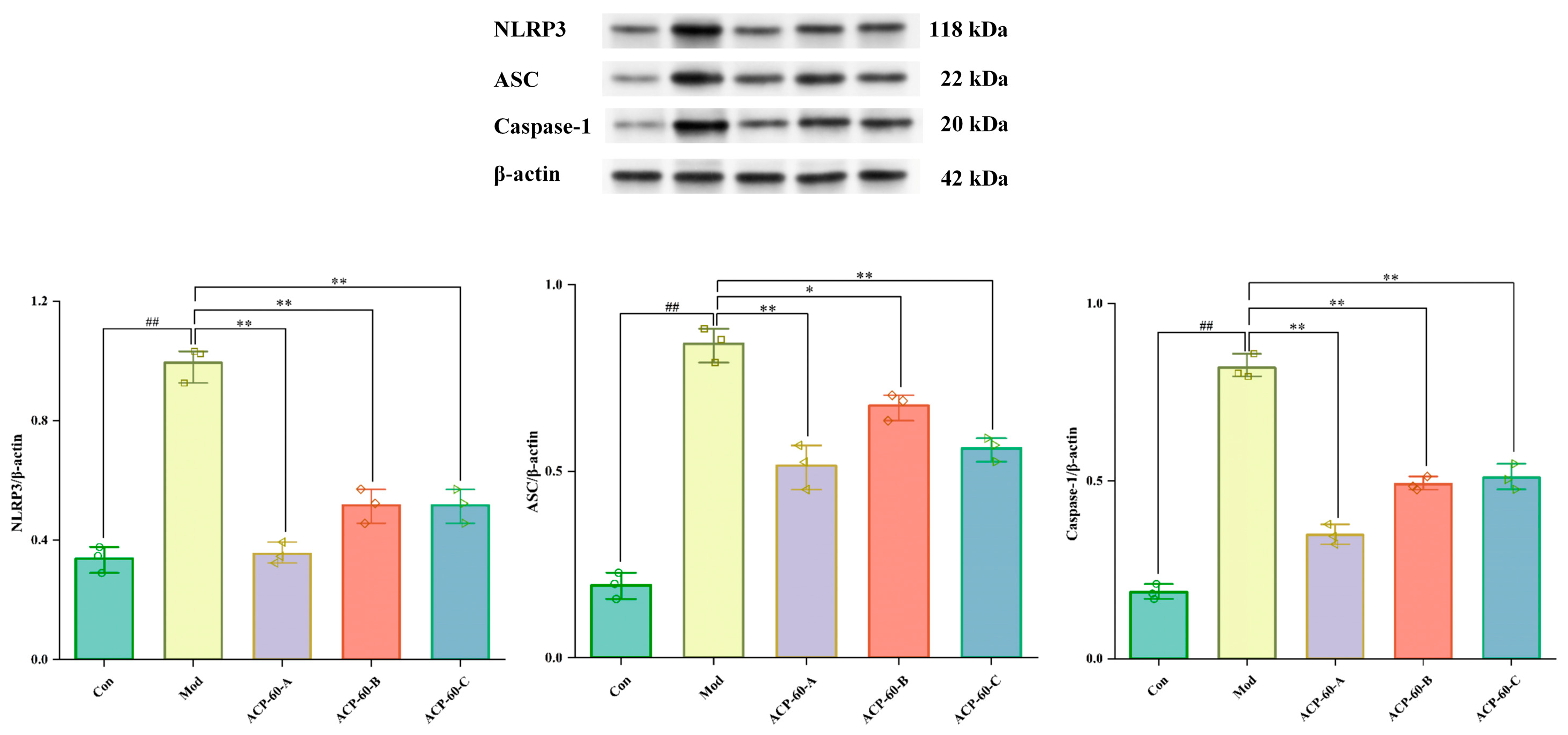
2.8. Protein Expression of NLRP3, ASC, and Caspaes-1 in HK-2 Cells
2.9. Effects of ACP-60 on 24-h Urinary Protein Levels in GN Rats
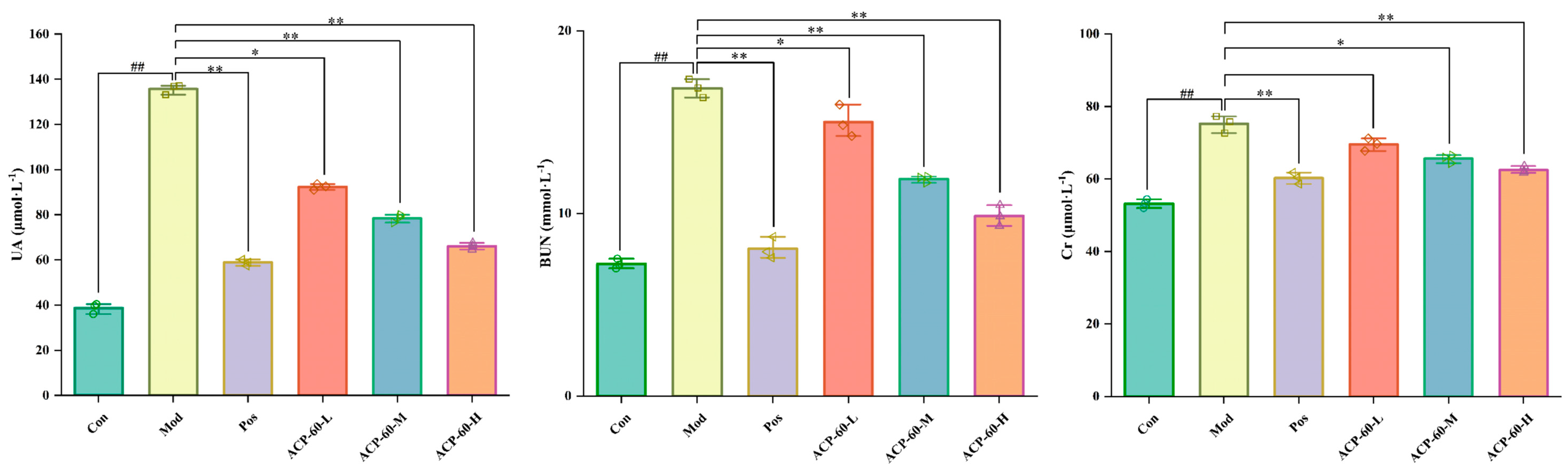
2.10. Effects of ACP-60 on Serum Biochemical Parameters Levels in GN Rats
2.11. Effects of ACP-60 on IL-18 and IL-1β Levels in GN Rats
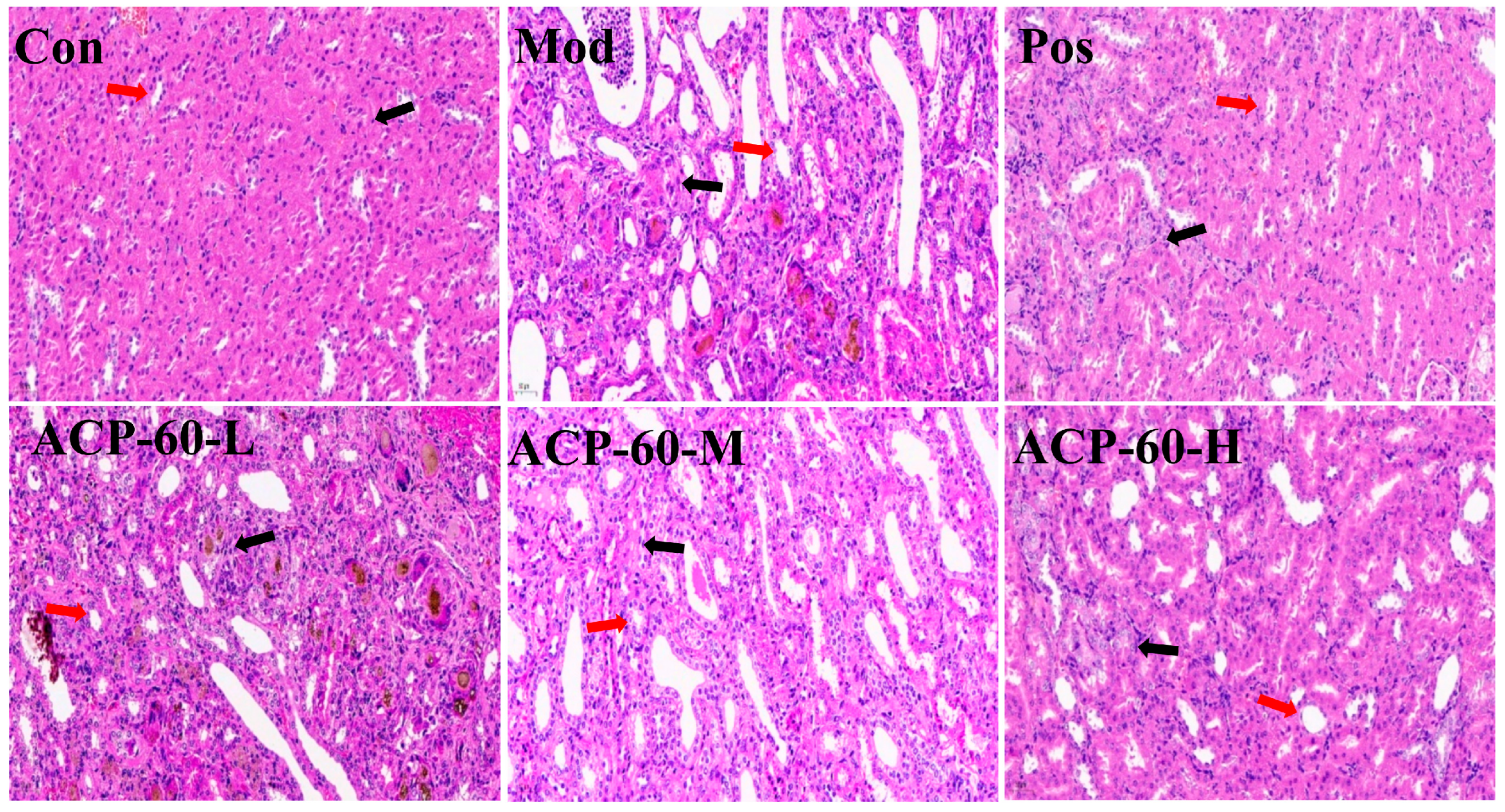
2.12. Histological Analysis (HE Staining)
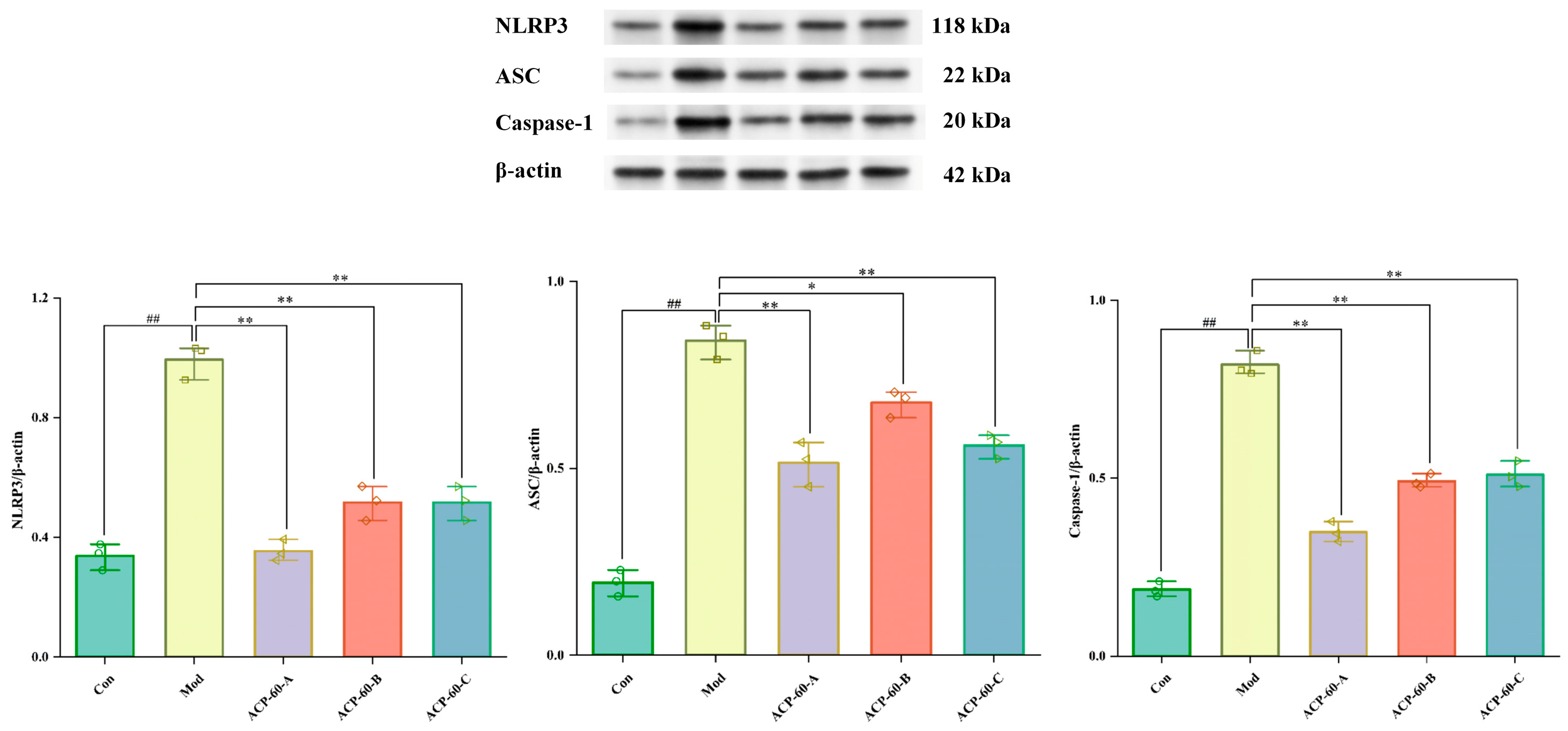
2.13. Protein Expression of NLRP3, ASC, and Caspaes-1 in Renal Tissue
2.14. Effect of ACP-60 on Rats’ mRNA Expression of NLRP3, ASC, and Caspase-1
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction and Isolation of Polysaccharides
3.3. Purification and Structural Analysis of ACP-60
3.3.1. Purification of ACP-60
3.3.2. Molecular Weight Determination
3.3.3. Monosaccharide Composition Analysis
3.3.4. Infrared Spectroscopy (IR) Analysis
3.3.5. Methylation Analysis
3.3.6. NMR Analysis
3.4. Analysis of the Anti-Gouty Nephropathy Activity of ACP-60-A, ACP-60-B and ACP-60-C
3.4.1. Determination of HK-2 Cell Viability
3.4.2. Morphological Observation of HK-2 Cells
3.4.3. Western Blotting Analysis
3.5. Analysis of the Anti-Gouty Nephropathy Activity of ACP-60
3.5.1. Gouty Nephropathy Model Establishment
3.5.2. Determination of 24-h Urinary Protein
3.5.3. Determination of Serum Biochemical Parameters
3.5.4. Determination of IL-18 and IL-1β
3.5.5. Renal Histopathological Analysis
3.5.6. Western Blotting Analysis
3.5.7. qRT-PCR Analysis
3.6. Data Analysis
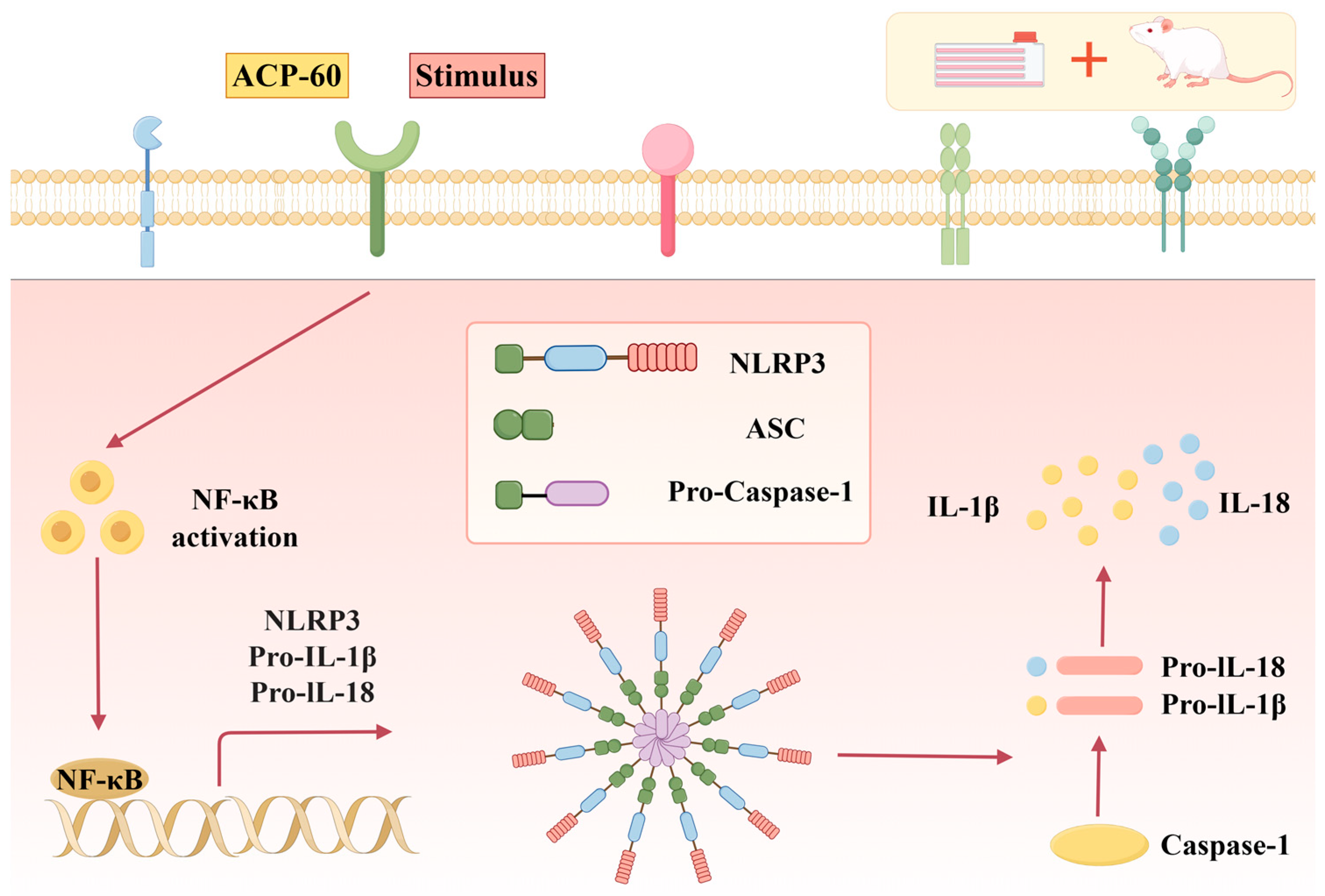
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, L.; Zhang, X.; Shen, J.; Wei, Y.; Zhao, T.; Xiao, N.; Lv, X.; Qin, D.; Xu, Y.; Zhou, Y. Models of gouty nephropathy: Exploring disease mechanisms and identifying potential therapeutic targets. Front. Med. 2024, 11, 1305431. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Bai, Y.; Wan, Y.; Luo, S.; Zhang, L.; Wu, X.; Chen, R.; Yin, Z.; Xie, Y.; Guo, P. DaiTongXiao improves gout nephropathy by inhibiting inflammatory response through the TLR4/MyD88/NF-κB pathway. Front. Pharmacol. 2024, 15, 1447241. [Google Scholar] [CrossRef]
- Ghaemi-Oskouie, F.; Shi, Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr. Rheumatol. Rep. 2011, 13, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Sui, X.L.; Xu, Y.P.; Gu, F.J.; Zhang, A.S.; Chen, J.H. Association between Nod-like receptor protein 3 inflammasome and gouty nephropathy. Exp. Ther. Med. 2020, 20, 195–204. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Wang, R.; Tang, W.; Kong, L.; Wang, W.; Wang, L.; Zhang, Y.; Ma, W. Plantaginis Semen polysaccharides ameliorate renal damage through regulating NLRP3 inflammasome in gouty nephropathy rats. Food Funct. 2021, 12, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ma, G.; Wang, Y.; Wang, L.; Li, P. Therapeutic effects of traditional Chinese medicine on gouty nephropathy: Based on NF-κB signalingpathways. Biomed. Pharmacother. 2023, 158, 114199. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.M.; Sun, Z.H.; Fei, Q. Study on the regulation and renal protective effect of Youguiyin on ROS/NF-κB signal pathway in rats with gouty nephropathy. Chin. J. Integr. Tradit. West. Nephrol. 2021, 22, 106. [Google Scholar]
- Pascual, E.; Perdiguero, M. Gout, diuretics and the kidney. Ann. Rheum. Dis. 2006, 65, 981–982. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, G.Y.; Dai, R.H.; Ma, Y.P.; Zhang, K.; Zhang, C.; Li, X.; Wang, J.H. Two new polyacetylenic compounds from Atractylodes chinensis (DC.) Koidz. J. Asian Nat. Prod. Res. 2011, 13, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.F.; Wu, S.H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci. Technol. 2006, 17, 313–323. [Google Scholar] [CrossRef]
- Nakai, Y.; Kido, T.; Hashimoto, K.; Kase, Y.; Sakakibara, I.; Higuchi, M.; Sasaki, H. Effect of the rhizomes of Atractylodes lancea and its constituents on the delay of gastric emptying. J. Ethnopharmacol. 2003, 84, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Resch, M.; Heilmann, J.; Steigel, A.; Bauer, R. Further phenols and polyacetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activity. Planta Med. 2001, 67, 437–442. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Su, Y.; Li, J.; Ren, W.C.; Wang, Q.H.; Kuang, H.X. Probiotics with anti-type 2 diabetes mellitus properties: Targets of polysaccharides from traditional Chinese medicine. Chin. J. Nat. Med. 2022, 20, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Corrie, L.; Gulati, M.; Awasthi, A.; Vishwas, S.; Kaur, J.; Khursheed, R.; Porwal, O.; Alam, A.; Parveen, S.R.; Singh, H. Harnessing the dual role of polysaccharides in treating gastrointestinal diseases: As therapeutics and polymers for drug delivery. Chem. Biol. Interact. 2022, 368, 110238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Yu, H.; Zhou, S.; Zhang, Z.; Wu, D.; Yan, M.; Tang, Q.; Zhang, J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr. Polym. 2017, 167, 337–344. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, C.; Guo, Y.; Zhou, W.; Zhang, Y. Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016, 149, 186–206. [Google Scholar] [CrossRef]
- Li, L.F.; Wong, T.L.; Han, Q.B. Difficulties in research of Chinese medicine polysaccharides. Chin. J. Nat. Med. 2019, 17, 883–886. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.; Dong, Y.; Yuan, S.; Wei, X.; Zhang, Y.; Dong, L.; Wang, F.; Bai, T.; Yang, Y. Polysaccharides from Chinese herbal medicine: A review on the hepatoprotective and molecular mechanism. Chin. J. Nat. Med. 2024, 22, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, Y.; Wang, B.; Yang, J.; Chen, Y.; Luo, H.; Chen, T.; Xiao, C.; Weng, L. Structural characterization of the polysaccharides from Atractylodes chinensis (DC.) Koidz. and the protective effection against alcohol-induced intestinal injury in rats. Int. J. Biol. Macromol. 2024, 282, 136641. [Google Scholar] [CrossRef]
- Liang, M.; Wu, Y.; Sun, J.; Zhao, Y.; Liu, L.; Zhao, R.; Wang, Y. Ultrasound-Assisted Extraction of Atractylodes chinensis (DC.) Koidz. Polysaccharides and the Synergistic Antigastric Cancer Effect in Combination with Oxaliplatin. ACS Omega 2024, 9, 18375–18384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Q.; Jia, L.; He, H.; Zhang, T.; Jia, W.; Zhu, L.; Qi, W.; Wang, N. Effects of in vitro fermentation of Atractylodes chinensis (DC.) Koidz. polysaccharide on fecal microbiota and metabolites in patients with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2023, 253, 126860. [Google Scholar] [CrossRef]
- Hu, T.G.; Wu, H.; Yu, Y.S.; Xu, Y.J.; Li, E.N.; Liao, S.T.; Wen, P.; Zou, Y.X. Preparation, structural characterization and prebiotic potential of mulberry leaf oligosaccharides. Food Funct. 2022, 13, 5287–5298. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Hong, R.; Zhang, R.; Yi, Y.; Dong, L.; Liu, L.; Jia, X.; Ma, Y.; Zhang, M. Physicochemical and biological properties of longan pulp polysaccharides modified by Lactobacillus fermentum fermentation. Int. J. Biol. Macromol. 2019, 125, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhu, X.; Yang, G.; Zhang, J.; Wang, R.; Shen, Y.; Li, H.; Gatasheh, M.K.; Abbasi, A.M.; Yang, X. Ultrasonic extraction of Moringa oleifera seeds polysaccharides: Optimization, purification, and anti-inflammatory activities. Int. J. Biol. Macromol. 2024, 258, 128833. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, K.; Wang, Y.; Zhou, Z.; Wang, L.; Zhao, H.; Zhang, Y. Pharmacodynamic structure of deer antler base protein and its mammary gland hyperplasia inhibition mechanism by mediating Raf-1/MEK/ERK signaling pathway activation. Food Funct. 2023, 14, 3319–3331. [Google Scholar] [CrossRef] [PubMed]
- Hayat, J.; Akodad, M.; Moumen, A.; Baghour, M.; Skalli, A.; Ezrari, S.; Belmalha, S. Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon 2020, 6, e05609. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, N.; Deng, Y. Purification and structural investigation of a water-soluble polysaccharide from Flammulina velutipes. Carbohydr. Polym. 2012, 87, 2279–2283. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Shi, S. An inulin-type fructan isolated from Artemisia japonica and its anti-arthritic effects. J. Funct. Foods 2017, 29, 2929–2936. [Google Scholar] [CrossRef]
- Chen, J.; Cheong, K.l.; Song, Z.; Shi, Y.; Huang, X. Structure and protective effect on UVB-induced keratinocyte damage of fructan from white garlic. Carbohydr. Polym. 2013, 92, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, Z.; Zhao, B. Structural characterization and antioxidant activity in aging mice of a homogenous polysaccharide isolated from Ecklonia kurome. J. Mol. Struct. 2024, 1318, 139111. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Chen, L. Structural characterization and anti-inflammatory effects of polysaccharides from fruit calyx of Physalis alkekengi L. var. franchetii (Mast.) Makino. J. Mol. Struct. 2025, 1321, 139789. [Google Scholar] [CrossRef]
- Hu, T.G.; Zhu, W.L.; Yu, Y.S.; Zou, B.; Xu, Y.J.; Xiao, G.S.; Wu, J.J. The variation on structure and immunomodulatory activity of polysaccharide during the longan pulp fermentation. Int. J. Biol. Macromol. 2022, 222, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.G.; Wang, T.L.; Yu, S.M.; Liang, J.; Kuang, H.X. Structural characteristics and hepatoprotective potential of Aralia elata root bark polysaccharides and their effects on SCFAs produced by intestinal flora metabolism. Carbohydr. Polym. 2019, 207, 256. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shi, Y.; Chen, H.; Tao, M.; Zhou, X.; Li, J.; Ma, X.; Wang, Y.; Liu, N. Blockade of Autophagy Prevents the Progression of Hyperuricemic Nephropathy Through Inhibiting NLRP3 Inflammasome-Mediated Pyroptosis. Front. Immunol. 2022, 13, 858494. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, S.; Song, M.; Zhang, H.; Zhao, H.; Wu, L.; Zhao, H.; Qiu, H.; Zhang, Y. Correction: Shen et al. The Isolation, Structural Characterization and Anti-Inflammatory Potentials of Neutral Polysaccharides from the Roots of Isatis indigotica Fort. Molecules 2024, 29, 2683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, L.; Chen, L.; Wu, H.; Jia, L.; Zhu, H. Dendrobium officinale Polysaccharides as a Natural Functional Component for Acetic-Acid-Induced Gastric Ulcers in Rats. Molecules 2024, 29, 880. [Google Scholar] [CrossRef]
- Du, Z.; Liu, H.; Zhang, Z.; Li, P. Antioxidant and anti-inflammatory activities of Radix Isatidis polysaccharide in murine alveolar macrophages. Int. J. Biol. Macromol. 2013, 58, 329–335. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, X.; Zhang, D.; Zhang, B.; Lu, J.; Wang, X. Structural Characteristics, Antioxidant, and Immunostimulatory Activities of an Acidic Polysaccharide from Raspberry Pulp. Molecules 2022, 27, 4385. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Y.; Paulsen, S.B. Prospects of Codonopsis pilosula polysaccharides: Structural features and bioactivities diversity. Trends Food Sci. Technol. 2020, 103, 1–11. [Google Scholar] [CrossRef]
- Liang, X.; Liu, M.; Wei, Y.; Tong, L.; Guo, S.; Kang, H.; Zhang, W.; Yu, Z.; Zhang, F.; Duan, J.A. Structural characteristics and structure-activity relationship of four polysaccharides from Lycii fructus. Int. J. Biol. Macromol. 2023, 253, 127256. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Hu, J.; Wu, Y.; Zhang, B.; Zheng, G. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Hugo, E.; Esmeralda, J.U.; Norma, M. The role of agave fructans in health and food applications: A review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar]
- Fernández-Lainez, C.; Aande, S.M.; Silva-Lagos, L.A.; López-Velázquez, G.; de Vos, P. β(2 → 1)-β(2 → 6) branched graminan-type fructans and β(2 → 1) linear fructans impact mucus-related and endoplasmic reticulum stress-related genes in goblet cells and attenuate inflammatory responses in a fructan dependent fashion. Food Funct. 2023, 14, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Schaefer, L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. 2014, 25, 1387–1400. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, M.; Lu, G.; Yang, Z.; Ji, H.; Hu, Q. Emodinol ameliorates urate nephropathy by regulating renal organic ion transporters and inhibiting immune inflammatory responses in rats. Biomed. Pharmacother. 2017, 96, 727–735. [Google Scholar] [CrossRef]
- Broz, P. Immunology: Caspase target drives pyroptosis. Nature 2015, 526, 642–643. [Google Scholar] [CrossRef]
- Mei, Y.; Dong, B.; Geng, Z.; Xu, L. Excess Uric Acid Induces Gouty Nephropathy Through Crystal Formation: A Review of Recent Insights. Front. Endocrinol. 2022, 13, 911968. [Google Scholar] [CrossRef] [PubMed]
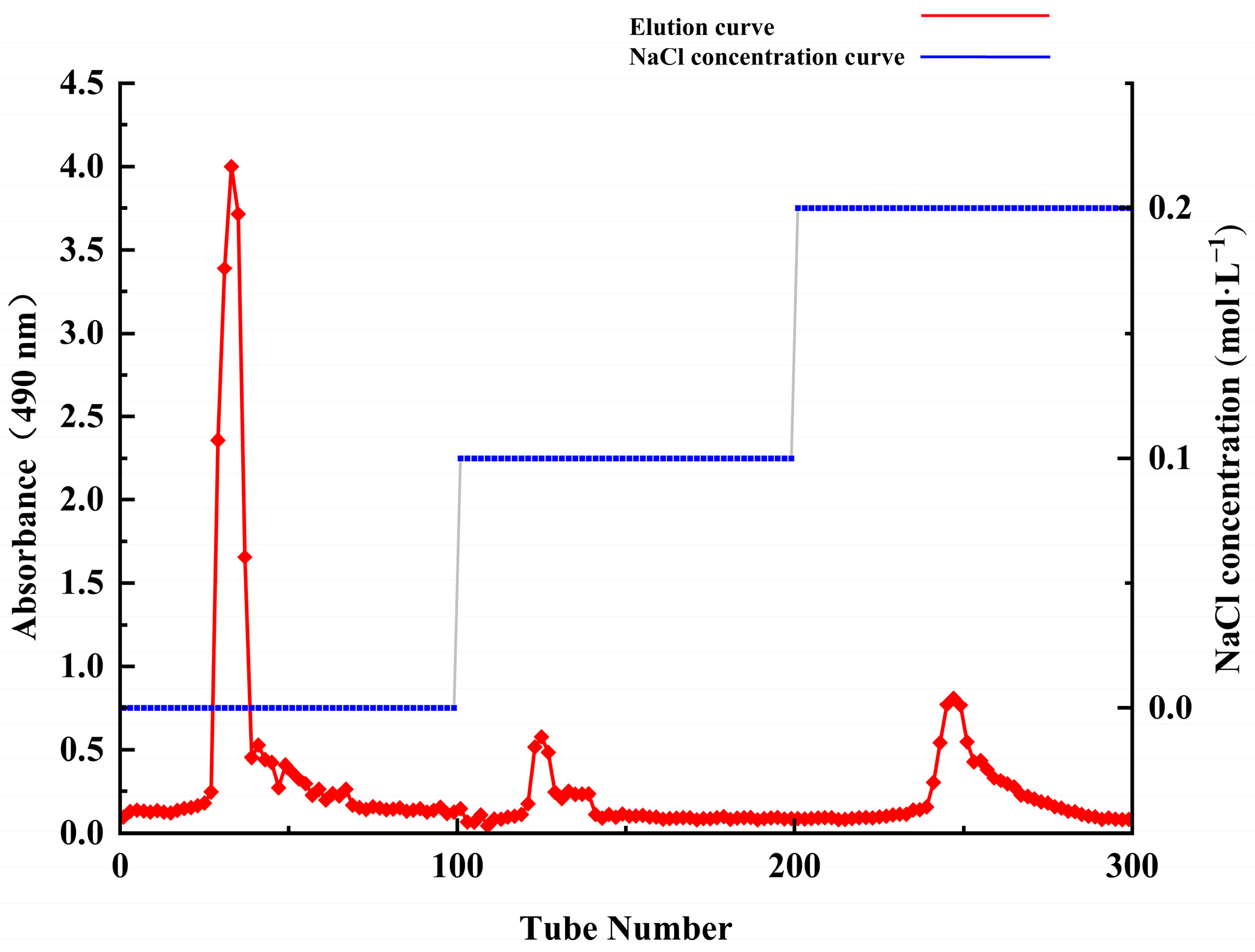
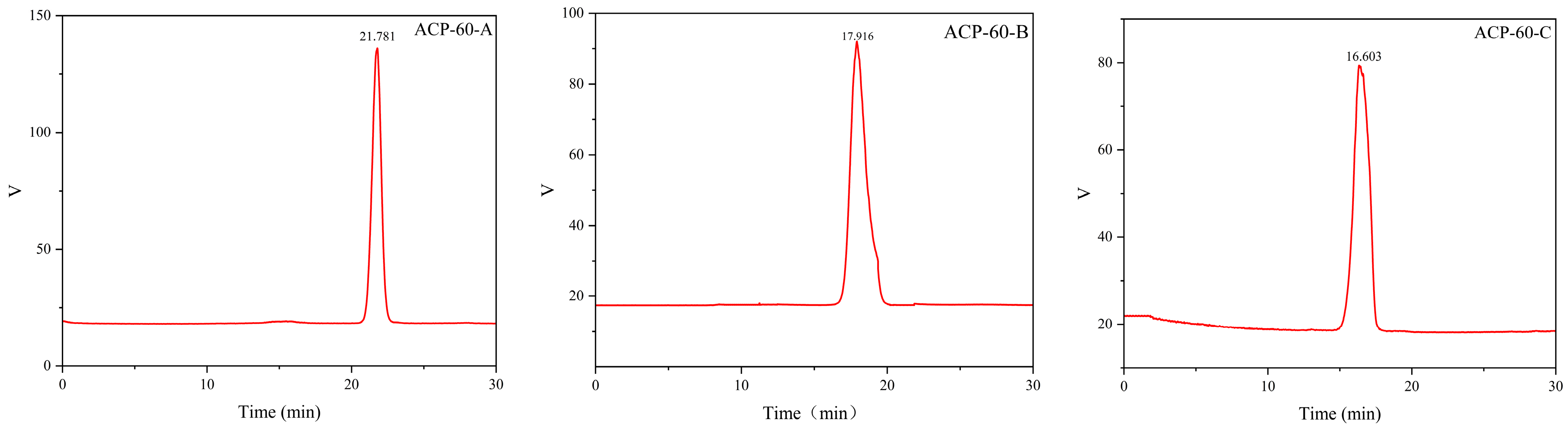
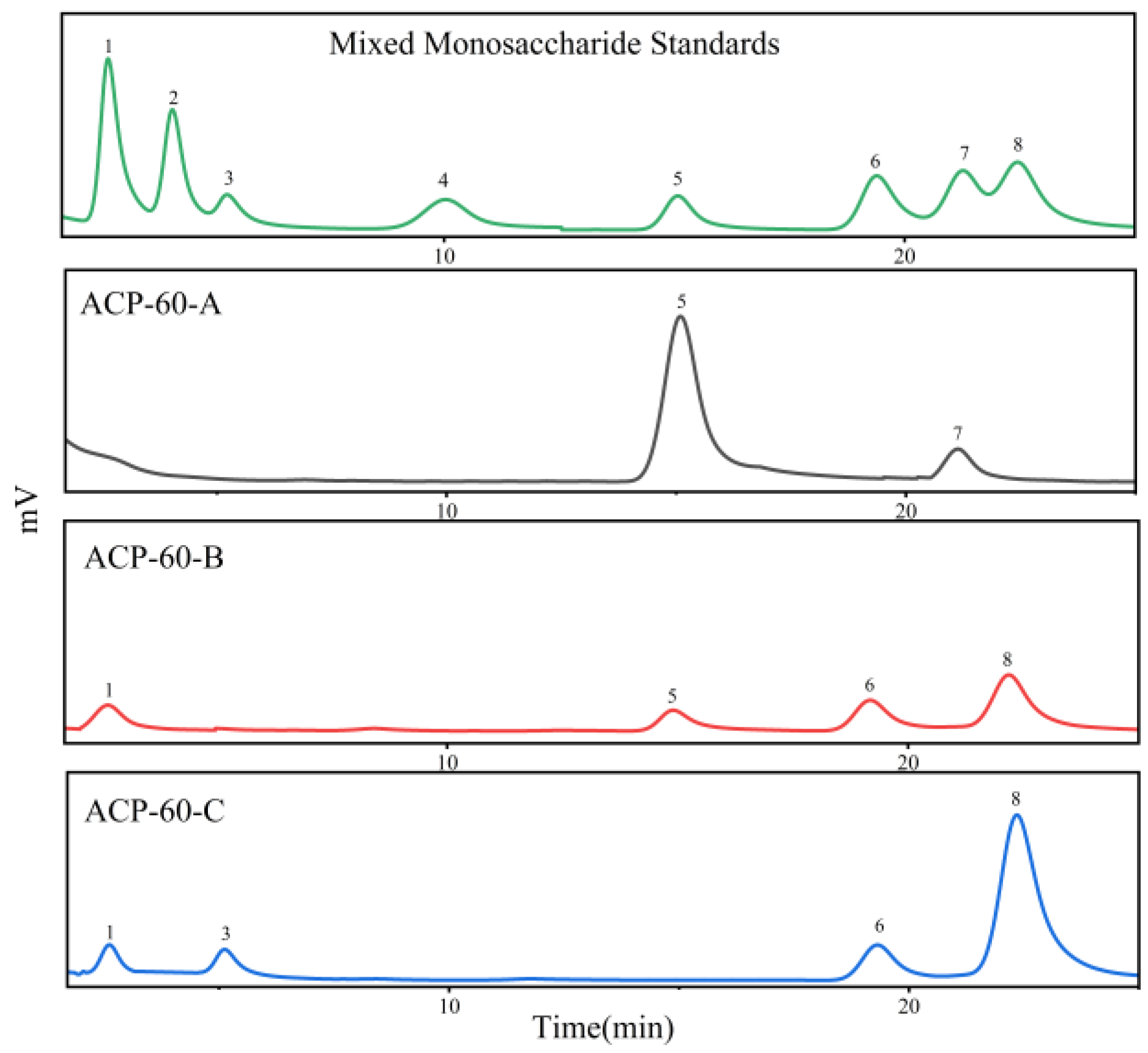
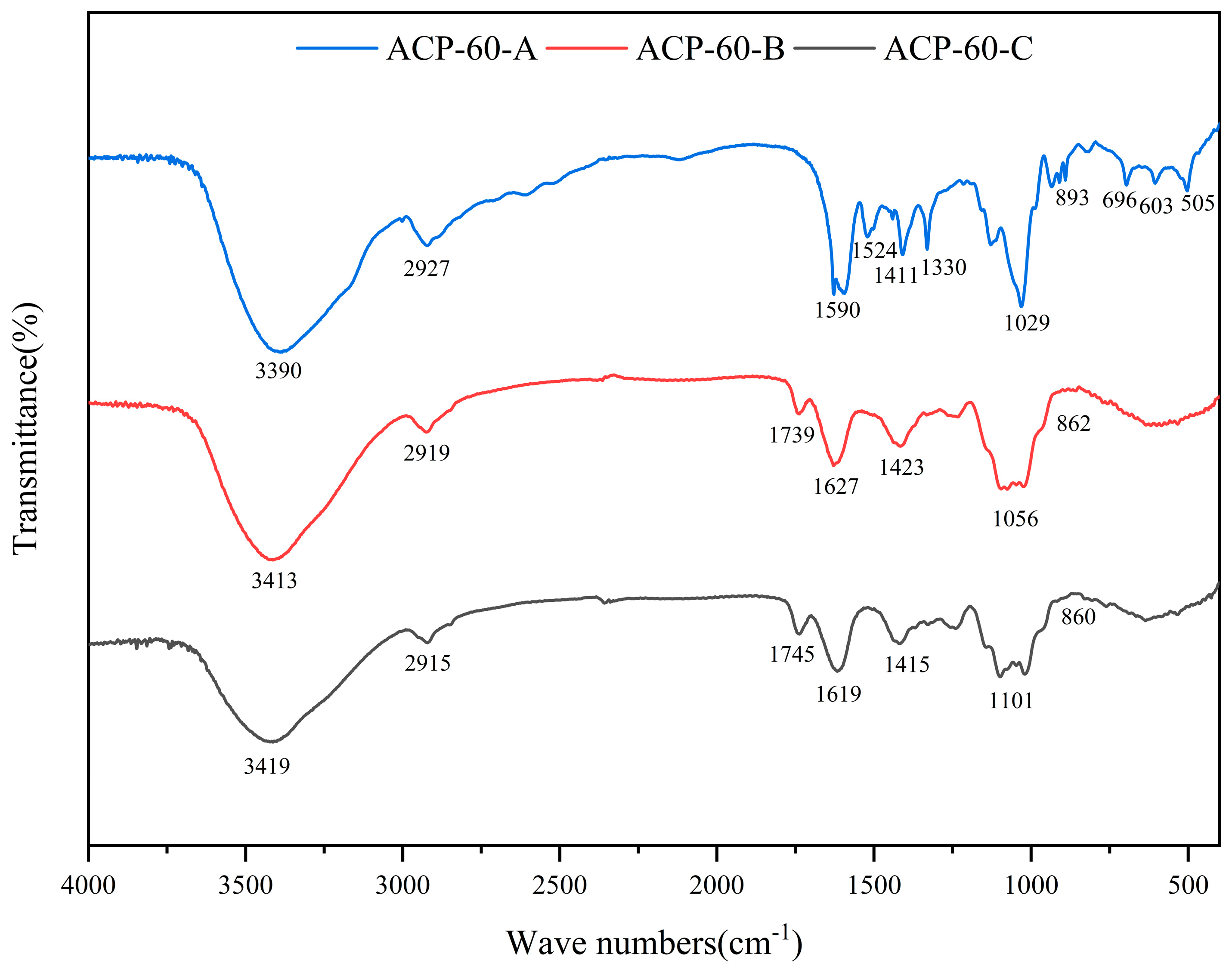




| ACP-30 | ACP-40 | ACP-50 | ACP-60 | ACP-70 | ACP-80 | |
|---|---|---|---|---|---|---|
| Extraction rate (wt%) | 7.63 ± 1.21 | 12.16 ± 1.34 | 10.05 ± 1.18 | 20.89 ± 2.13 | 39.06 ± 1.95 | 10.21 ± 1.52 |
| Sugar content (%) | 57.45 ± 1.82 | 75.16 ± 2.15 | 73.07 ± 1.79 | 77.21 ± 2.41 | 74.81 ± 2.37 | 72.44 ± 1.91 |
| Sample | Rha | Fuc | Xyl | Ara | Fru | Man | Glc | Gal |
|---|---|---|---|---|---|---|---|---|
| ACP-60-A | - | - | - | - | 1.00 | - | 0.15 | - |
| ACP-60-B | 0.05 | - | - | - | 0.24 | 0.14 | - | 0.20 |
| ACP-60-C | 0.11 | - | 0.53 | - | - | 0.29 | - | 1.04 |
| Deduced Residues | Derivatives | mol% |
| Fruf-(2→ | 2,5-Di-O-acety-(2-deuterio)-1,3,4,6-tetra-O-methyl hexitol (mannitol, glucitol) | 23.38 |
| Glcp-(1→ | 1,5-Di-O-acetyl-(1-deuterio)-2,3,4,6-tetra-O-methyl glucitol | 15.90 |
| →1)-Fruf-(2→ | 1,2,5-Tri-O-acetyl-(2-deuterio)-3,4,6-tri-O-methyl hexitol (mannitol, glucitol) | 60.72 |
| Sugar Residues | Chemical Shifts, δ (ppm) | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| →1)-β-Fruf-(2→A | 3.85, 3.62/60.87 | -/103.67 | 4.18/76.97 | 4.02/74.26 | 3.77/81.05 | 3.75, 3.68/62.11 |
| α-Glcp-(1→B | 5.36/92.46 | 3.45/71.16 | 3.68/72.58 | 3.39/69.21 | 3.76/72.41 | 3.66/60.45 |
| β-Fruf-(2→C | 3.85, 3.62/60.87 | -/103.21 | 4.18/76.97 | 4.02/74.26 | 3.77/81.05 | 3.75, 3.68/62.11 |
| Gene | Primary Sequence |
| NLRP3 | 5′-GATTTCTCCACAACTCACCCAA-3′ 5′-AGTCTGGAAGAACAGGCAACAT-3′ |
| ASC | 5′-ACTATCTGGAGGGGTATGGCTT-3′ 5′-CAATGAGTGCTTGCCTGTGTT-3′ |
| Caspase-1 | 5′-TGCCTGGTCTTGTGACTTGGAG-3′ 5′-TGTCCTGGGAAGAGGTAGAAACG-3′ |
| GADPH | 5′-CTGGAGAAACCTGCCAAGTATG-3′ 5′-GGTGGAAGAATGGGAGTTGCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Jia, R.; Zhang, K.; Sun, S.; Mei, M.; Zhao, H.; Shen, Y.; Wang, Y.; Zhang, Y. Structural Characterization and Anti-Gouty Nephropathy Potential of Polysaccharides from Atractylodes chinensis. Molecules 2025, 30, 757. https://doi.org/10.3390/molecules30040757
Chen X, Jia R, Zhang K, Sun S, Mei M, Zhao H, Shen Y, Wang Y, Zhang Y. Structural Characterization and Anti-Gouty Nephropathy Potential of Polysaccharides from Atractylodes chinensis. Molecules. 2025; 30(4):757. https://doi.org/10.3390/molecules30040757
Chicago/Turabian StyleChen, Xue, Ruipu Jia, Kai Zhang, Shiqing Sun, Mei Mei, Hong Zhao, Yu Shen, Yuliang Wang, and Yu Zhang. 2025. "Structural Characterization and Anti-Gouty Nephropathy Potential of Polysaccharides from Atractylodes chinensis" Molecules 30, no. 4: 757. https://doi.org/10.3390/molecules30040757
APA StyleChen, X., Jia, R., Zhang, K., Sun, S., Mei, M., Zhao, H., Shen, Y., Wang, Y., & Zhang, Y. (2025). Structural Characterization and Anti-Gouty Nephropathy Potential of Polysaccharides from Atractylodes chinensis. Molecules, 30(4), 757. https://doi.org/10.3390/molecules30040757






