Preparation and Optimization of a Polyhydroxyoctanoate–Hydroxyapatite Composite Available to Scaffolds in Implantable Devices
Abstract
1. Introduction
2. Results and Discussions
2.1. Design of Experiments Studies
2.2. Determination of the Effect of Independent Variables
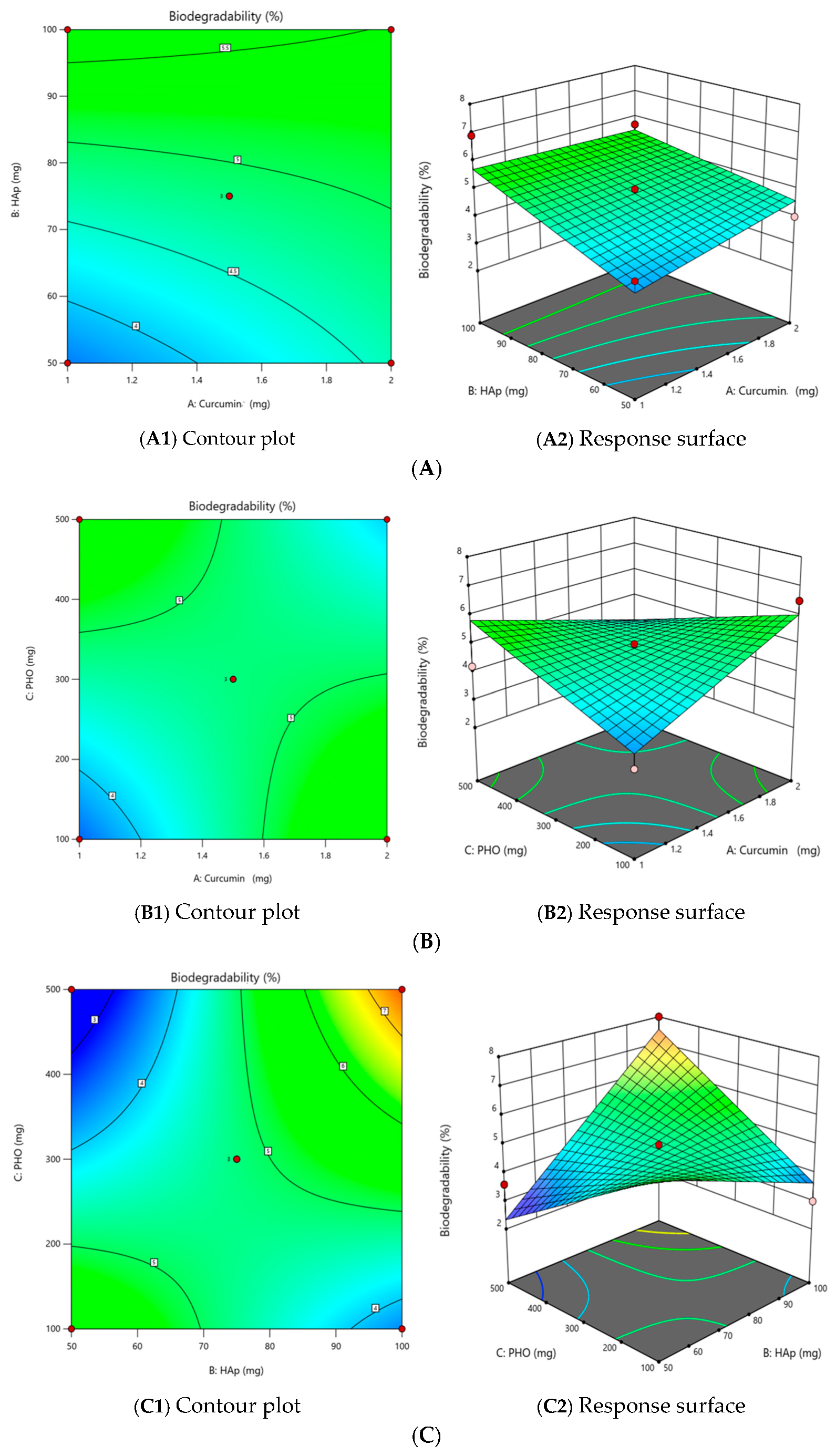
2.2.1. The Effect of Independent Variables on the Degree of Biodegradability (Y1)
2.2.2. The Effect of Independent Variables on the Film Thickness (Y3)
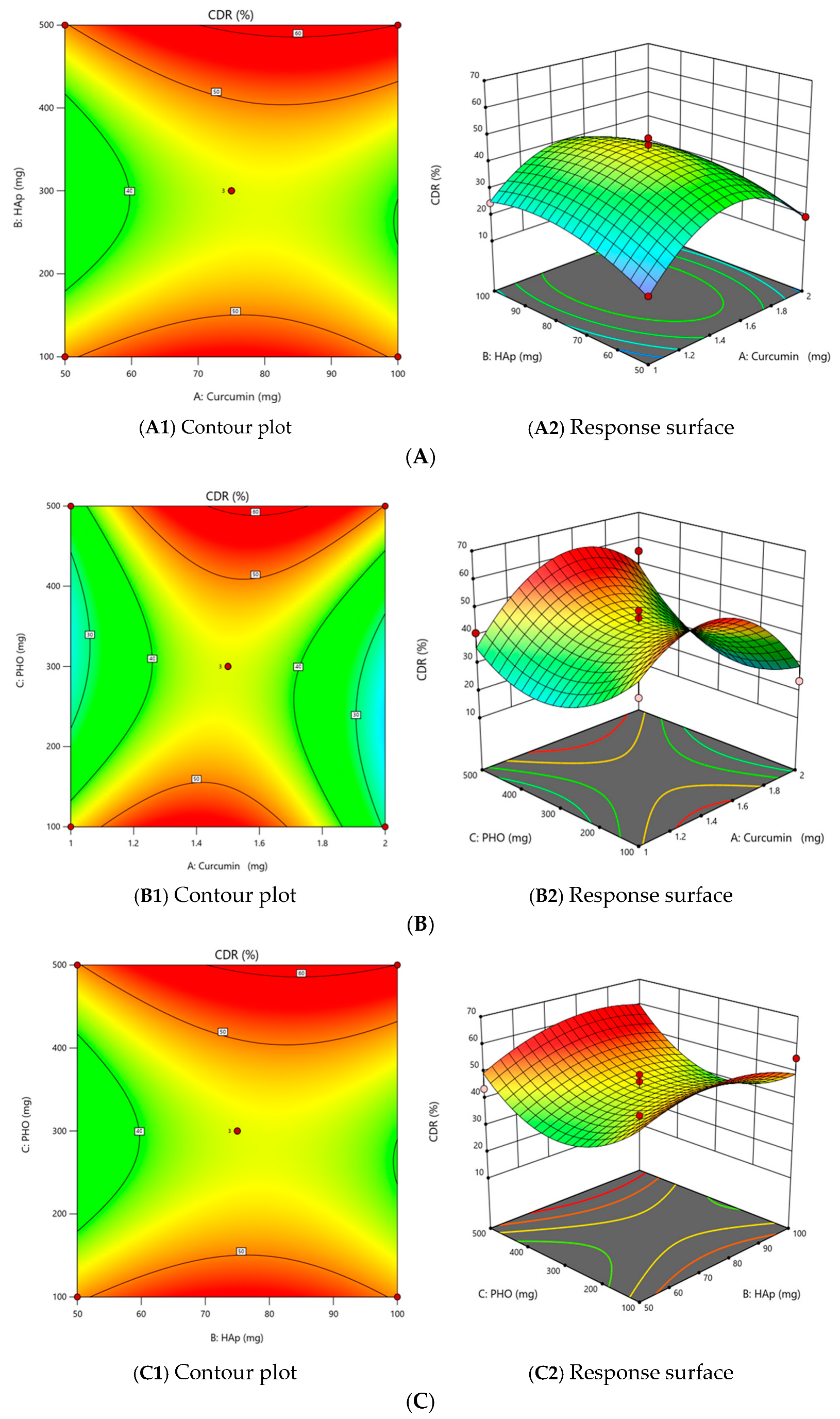
2.2.3. The Effect of Independent Variables on the Cumulative Release of Curcumin (CDR) (Y4)
2.2.4. Film Quality
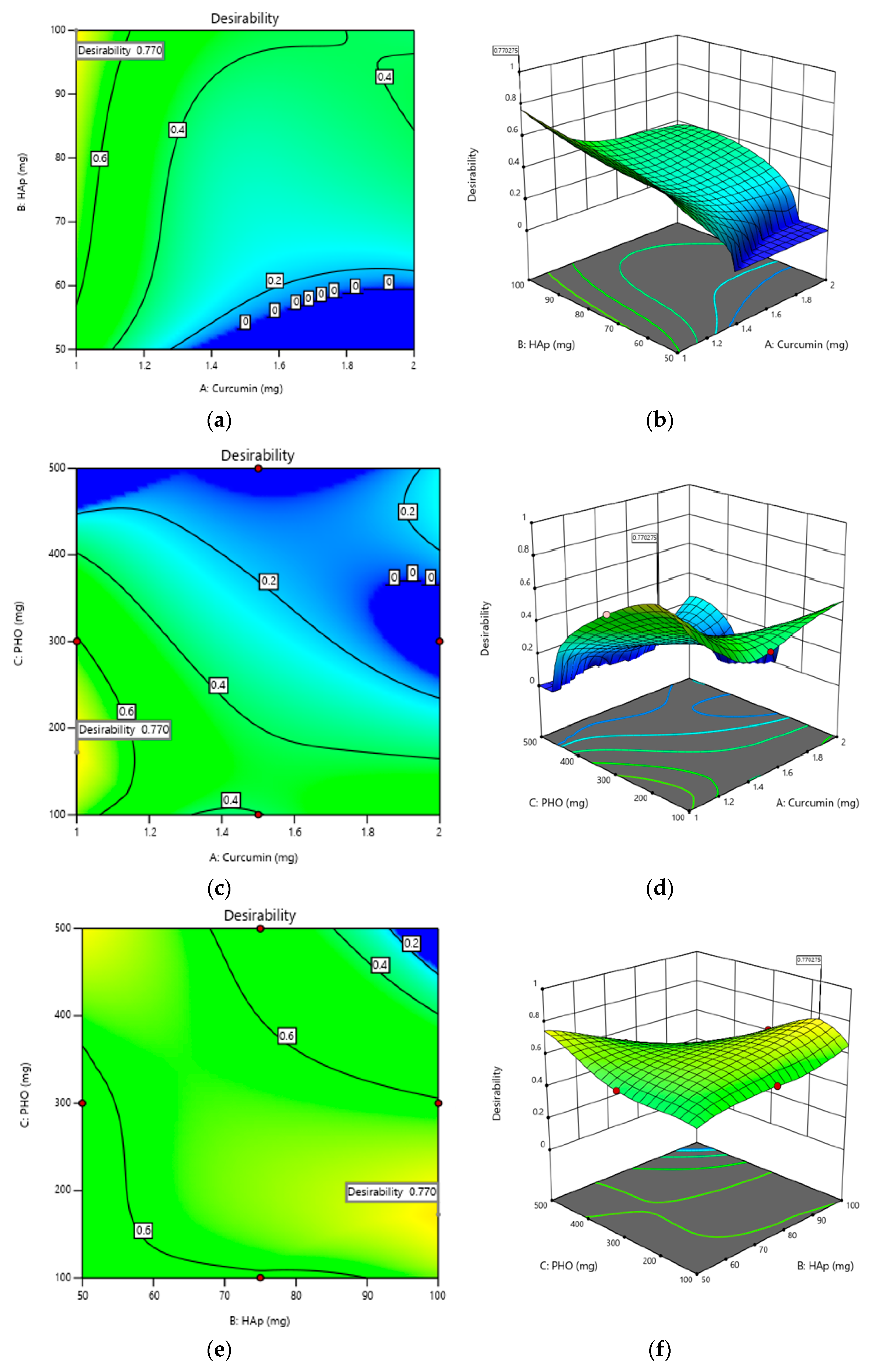
2.3. Optimization of Composite Films
2.4. Characterization of the Optimum Film Obtained
2.4.1. Morphological Evaluation
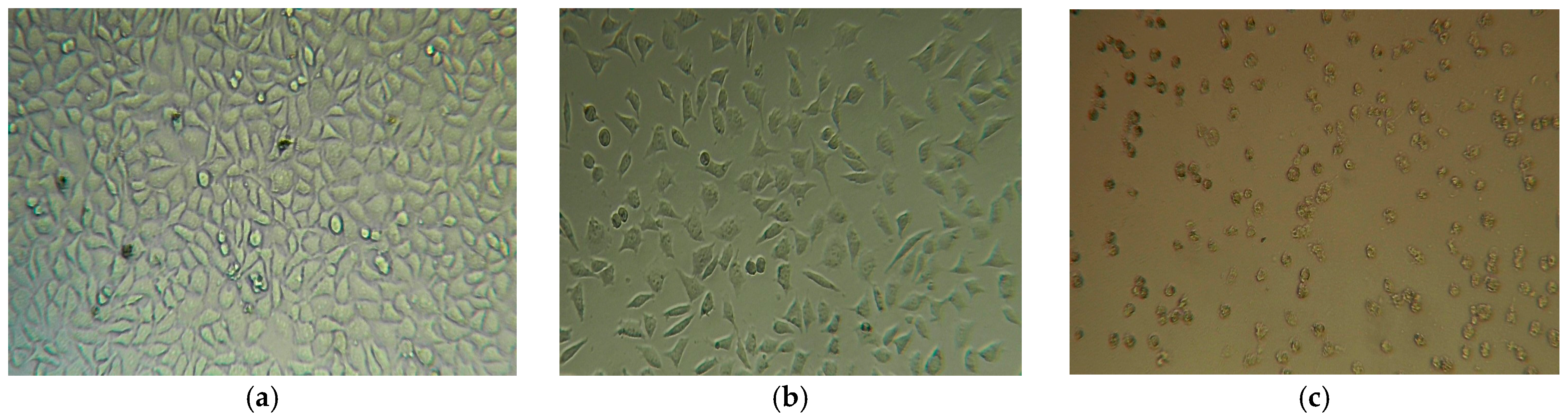
2.4.2. Evaluation of Cytotoxicity
3. Experimental Section
3.1. Materials
3.2. Methods
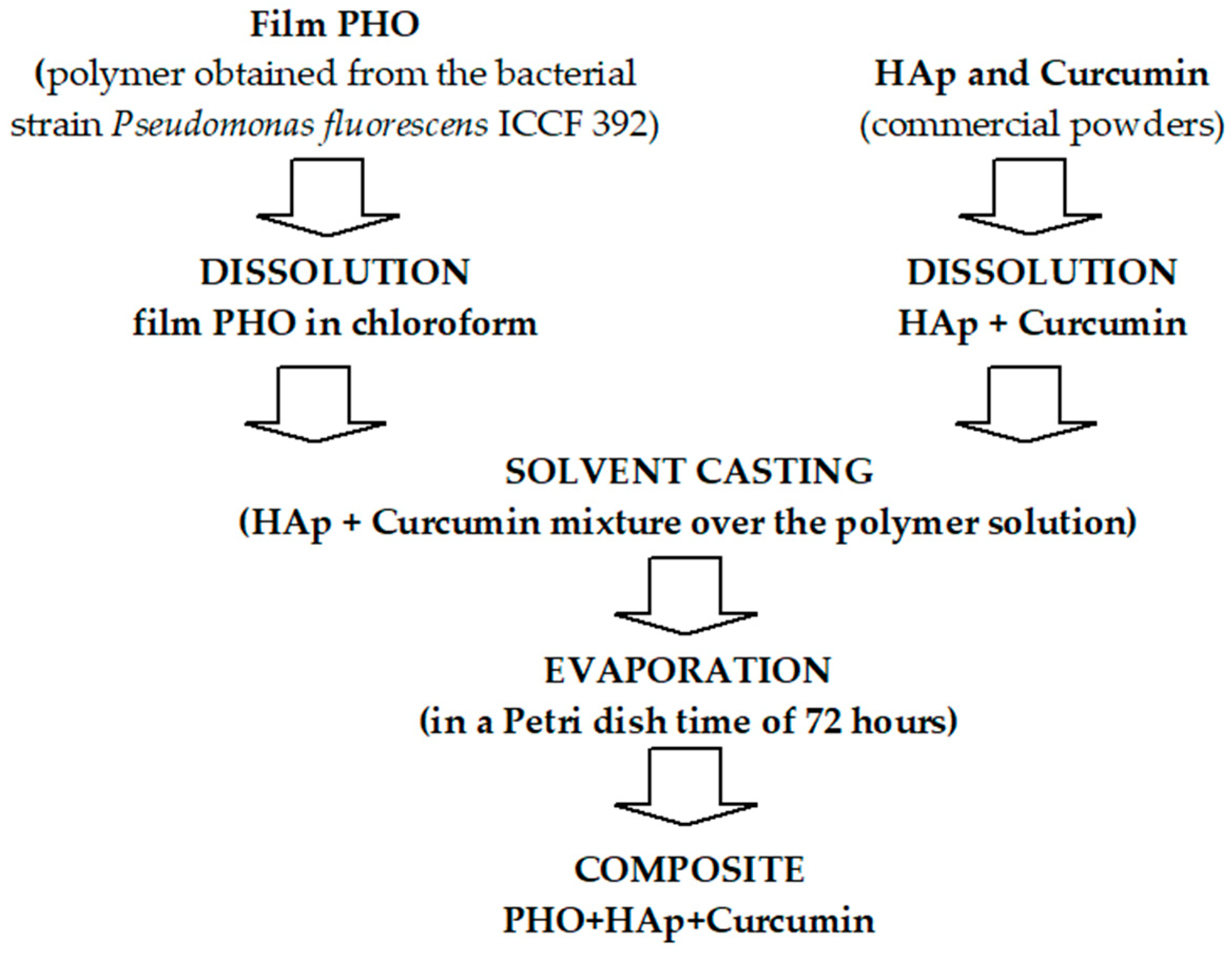
3.2.1. The Preparation of Composite Films
3.2.2. Characterization of Composite Films
Design of Experiment
Evaluation of Film Thickness
Biodegradability Evaluation
Evaluation of Cumulative Release of Curcumin (CDR)
Evaluation of the Cytotoxic Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Kessler, M.W.; Grande, D.A. Tissue engineering and cartilage. Organogenesis 2008, 4, 28–32. [Google Scholar] [CrossRef]
- Murthy, N.S.; Damodaran, V.B.; Lee, S.H.; Hwang, A.S.; Sung, H.J. Characterization of thin films for biomedical applications. In Thin Film Coatings for Biomaterials and Biomedical Applications; Griesser, H.J., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 81–115. [Google Scholar]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Kara, A.; Gunes, O.C.; Albayrak, A.Z.; Bilici, G.; Erbil, G.; Havitcioglu, H. Fish scale/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofibrous composite scaffolds for bone regeneration. J. Biomater. Appl. 2020, 34, 1201–1215. [Google Scholar] [CrossRef]
- Senatov, F.; Anisimova, N.; Kiselevskiy, M.; Kopylov, A.; Tcherdyntsev, V.; Maksimkin, A. Polyhydroxybutyrate/Hydroxyapatite Highly Porous Scaffold for Small Bone Defects Replacement in the Nonload-bearing Parts. J. Bionic Eng. 2017, 14, 648–658. [Google Scholar] [CrossRef]
- Araujo, P.L.B.; Ferreira, C.R.P.C.; Araujo, E.S. Biodegradable conductive composites of poly (3-hydroxybutyrate) and polyaniline nanofibers: Preparation, characterization and radiolytic effects. Express Polym. Lett. 2011, 5, 12–22. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, H.C.; Kim, S.Y.; Rhee, Y.H. Molecular characterization of extracellular medium-chain-length poly (3-hydroxyalkanoate) depolymerase genes from Pseudomonas alcaligenes strains. J. Microbiol. 2005, 43, 285–294. [Google Scholar]
- Chena, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a family of natural polymers, and their applications in drug delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Insomphun, C.; Chuah, J.A.; Kobayashi, S.; Fujiki, T.; Numata, K. Influence of hydroxyl groups on the cell viability of polyhydroxyalkanoate(PHA) scaffolds for tissue engineering. ACS Biomater. Sci. Eng. 2017, 3, 3064–3075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Y.Z.; Wu, Q.; Chen, G.Q. Evaluation of three-dimensional scaffolds prepared from poly (3-hydroxybutyrateco-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials 2008, 29, 2858–2868. [Google Scholar] [CrossRef]
- Chamary, S.; Hautcoeur, D.; Hornez, J.; Leriche, A.; Cambier, F. Bio-inspired hydroxyapatite dual core-shell structure for bone substitutes. J. Europ. Ceram. Soc. 2017, 37, 5321–5327. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Mu, T.; Richel, A. Research Progress in 3D Printed Biobased and Biodegradable Polyester/Ceramic Composite Materials: Applications and Challenges in Bone Tissue Engineering. ACS Appl. Mater. Inter. 2025, 17, 2791–2813. [Google Scholar] [CrossRef]
- Goranova, K.L.; Kattenhøj Sloth Overgaard, A.K.; Gitsov, I. Hydroxyapatite-poly (D,L-lactide) Nanografts. Synthesis and Characterization as Bone Cement Additives. Molecules 2021, 26, 424. [Google Scholar] [CrossRef] [PubMed]
- Kareem, R.O.; Bulut, N.; Kaygili, O. Hydroxyapatite Biomaterials: A Comprehensive Review of their Properties, Structures, Medical Applications, and Fabrication Methods. J. Chem. Rev. 2023, 6, 1–26. [Google Scholar]
- Astaneh, M.E.; Noori, F.; Fereydouni, N. Curcumin-loaded scaffolds in bone regeneration. Heliyon 2024, 10, e32566. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-T.; Chiang, Y.-C.; Huang, T.-Y.; Chen, P.C.; Chang, P.J.; Lee, C.W. Curcumin nanoparticles are a promising anti-bacterial and anti-inflammatory agent for treating periprosthetic joint infections. Int. J. Nanomed. 2019, 14, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.E.; Gilbert, R.J. Curcumin Release from Biomaterials for Enhanced Tissue Regeneration Following Injury or Disease. Bioengineering 2023, 10, 262. [Google Scholar] [CrossRef]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Thu, N.T.T.; Hoang, L.H.; Cuong, P.K.; Viet-Linh, N.; Nga, T.T.H.; Kim, D.D.; Leong, Y.K.; Nhi-Cong, L.T. Evaluation of polyhydroxyalkanoate (PHA) synthesis by Pichia sp. TSLS24 yeast isolated in Vietnam. Sci. Rep. 2023, 13, 3137. [Google Scholar] [CrossRef] [PubMed]
- Strandars ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO technical committees: Geneva, Switzerland, 2009.
- Strandars ISO 10993-1; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management System. ISO technical committees: Geneva, Switzerland, 2009.
- Strandars ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO Technical Committees: Geneva, Switzerland, 2009.
- Lupescu, I.; Stanescu, P.O.; Eremia, M.C.; Savoiu, G.; Petrescu, M.M.; Stefaniu, A.; Spiridon, M. Process for Obtaining Poly 3-Hydroxyoctanoic Acid, Published in the State Office for Inventions and Trademarks (OSIM). RO Patent 130766 B1, 30 March 2020. [Google Scholar]
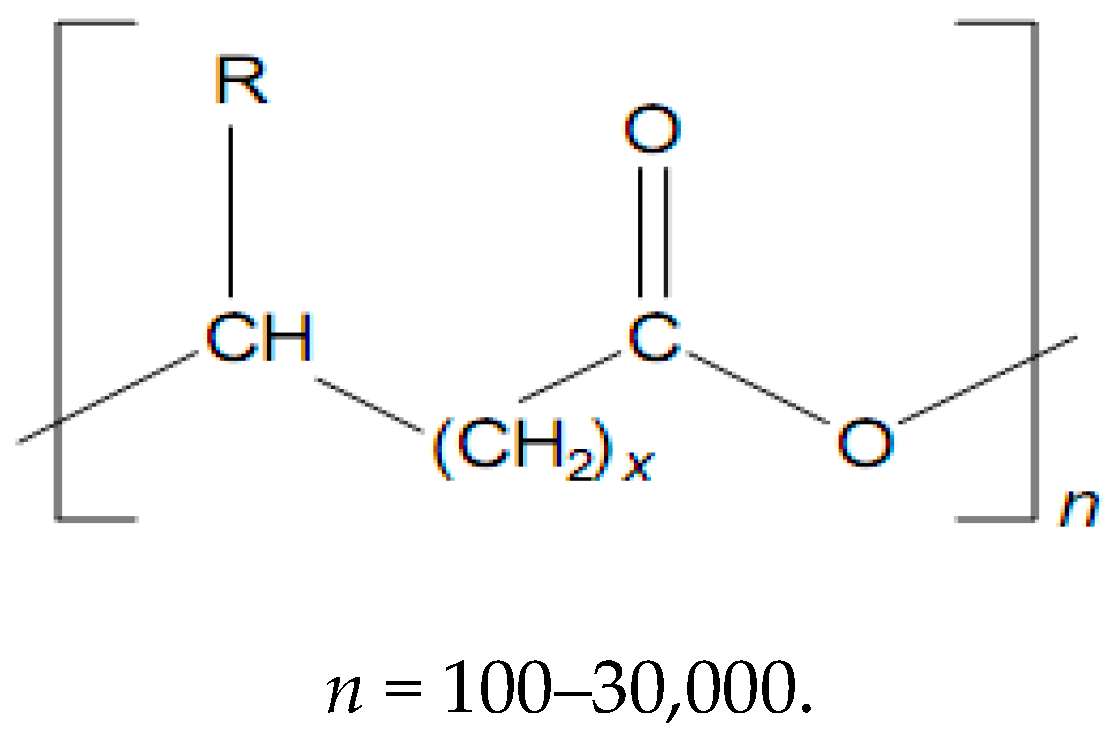



| x | R | Polymer Name |
|---|---|---|
| 1 | H | Poly-(3-hydroxypropionate) |
| CH3– | Poly-(3-hydroxybutyrate) | |
| CH3–CH2– | Poly-(3-hydroxyvalerate) | |
| CH3–CH2–CH2– | Poly-(3-hydroxyhexanoate) | |
| CH3–(CH2)3–CH2– | Poly-(3-hydroxyoctanoate) | |
| CH3–(CH2)7–CH2– | Poly-(3-hydroxydodecanoate) | |
| 2 | H | Poly-(4-hydroxybutyrate) |
| 3 | H | Poly-(5-hydroxyvalerate) |
| Independent Variables. | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| The amount of curcumin (X1), mg | 1 | 1.5 | 2 |
| The amount of HAp (X2), mg | 50 | 75 | 100 |
| The amount of PHO (X3), mg | 100 | 300 | 500 |
| Std | Run | Level X1 | Level X2 | Level X3 | X1 (mg) | X2 (mg) | X3 (mg) |
|---|---|---|---|---|---|---|---|
| P1 | 2 | −1 | −1 | 0 | 1 | 50 | 300 |
| P2 | 7 | −1 | 1 | 0 | 2 | 50 | 300 |
| P3 | 6 | 1 | 1 | 0 | 1 | 100 | 300 |
| P4 | 11 | 0 | −1 | −1 | 2 | 100 | 300 |
| P5 | 13 | 0 | −1 | 1 | 1 | 75 | 100 |
| P6 | 1 | −1 | 0 | −1 | 2 | 75 | 100 |
| P7 | 8 | −1 | 0 | 1 | 1 | 75 | 500 |
| P8 | 9 | 1 | 0 | 1 | 2 | 75 | 500 |
| P9 | 10 | 0 | 1 | −1 | 1.5 | 50 | 100 |
| P10 | 14 | 0 | 1 | 1 | 1.5 | 100 | 100 |
| P11 | 3 | 1 | −1 | 0 | 1.5 | 50 | 500 |
| P12 | 5 | 1 | 0 | −1 | 1.5 | 100 | 500 |
| P13 | 4 | 0 | 0 | 0 | 1.5 | 75 | 300 |
| P14 | 15 | 0 | 0 | 0 | 1.5 | 75 | 300 |
| P15 | 12 | 0 | 0 | 0 | 1.5 | 75 | 300 |
| Std | Run | X1 (mg) | X2 (mg) | X3 (mg) | Y1 Biodegradability (%) | Y2 Film Quality | Y3 Thickness (mm) | Y4 CDR (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flexibility (%) | Adhesion (%) | Peeling Mode (%) | Smoothing (%) | Total Film Quality (%) | ||||||||
| P1 | 2 | 1 | 50 | 300 | 4 | 25 | 12.5 | 25 | 25 | 87.5 | 0.5 | 14.4 |
| P2 | 7 | 2 | 50 | 300 | 4 | 25 | 25 | 25 | 25 | 100 | 0.46 | 19.3 |
| P3 | 6 | 1 | 100 | 300 | 6.9 | 25 | 12.5 | 25 | 25 | 87.5 | 0.73 | 24.6 |
| P4 | 11 | 2 | 100 | 300 | 5.7 | 25 | 12.5 | 25 | 12.5 | 75 | 0.35 | 18 |
| P5 | 13 | 1 | 75 | 100 | 3 | 25 | 25 | 25 | 25 | 100 | 1.1 | 39.8 |
| P6 | 1 | 2 | 75 | 100 | 6.5 | 25 | 12.5 | 12.5 | 0 | 50 | 0.4 | 23.7 |
| P7 | 8 | 1 | 75 | 500 | 4.2 | 25 | 25 | 25 | 25 | 100 | 0.96 | 41.1 |
| P8 | 9 | 2 | 75 | 500 | 3.4 | 25 | 25 | 25 | 25 | 100 | 0.87 | 55 |
| P9 | 10 | 1.5 | 50 | 100 | 6 | 25 | 12.5 | 12.5 | 12.5 | 62.5 | 0.37 | 53.9 |
| P10 | 14 | 1.5 | 100 | 100 | 3 | 25 | 25 | 25 | 25 | 100 | 0.94 | 55 |
| P11 | 3 | 1.5 | 50 | 500 | 3.6 | 25 | 25 | 25 | 25 | 100 | 0.64 | 43.8 |
| P12 | 5 | 1.5 | 100 | 500 | 8 | 25 | 12.5 | 12.5 | 0 | 50 | 0.45 | 55 |
| P13 | 4 | 1.5 | 75 | 300 | 4.7 | 25 | 12.5 | 25 | 12.5 | 75 | 0.56 | 46.5 |
| P14 | 15 | 1.5 | 75 | 300 | 5 | 25 | 12.5 | 25 | 12.5 | 75 | 0.56 | 49.1 |
| P15 | 12 | 1.5 | 75 | 300 | 4.7 | 25 | 12.5 | 12.5 | 12.5 | 62.5 | 0.45 | 36.6 |
| Characteristic | Score (%) | ||
|---|---|---|---|
| Poor Score (0%) | Average Score (12.5%) | Good Score (25%) | |
| The flexibility of the film | Resistance < 20 ori | Resistance 20–30 | Resistance > 30 ori |
| Adhesion | Very sticky film, cannot be processed easily | Slightly sticky but could be easily worked | Non-sticky, processable film |
| Peeling mode | Very adherent | Slightly adherent | Non-adherent |
| Smoothing | The surface is not smooth, with irregularities or voids | Smooth surface with some irregularities | Smooth surface |
| Answer | Aim | Justification |
|---|---|---|
| Y1—The degree of biodegradability | Minimize | Minimizing the degree of biodegradation to maintain the structural integrity of the composite films over time, thus to ensure their long-term stability and durability in the biological environment. |
| Y3—Film thickness | Maximize | A higher film thickness will imply better film quality and improved mechanical properties. |
| Y4—CDR | Minimize | Minimizing the cumulative release of curcumin will lead to the efficient release of curcumin in a controlled and sustained manner at the implant site. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miu, D.-M.; Pavaloiu, R.D.; Sha’at, F.; Vladu, M.-G.; Neagu, G.; Manoiu, V.-S.; Eremia, M.-C. Preparation and Optimization of a Polyhydroxyoctanoate–Hydroxyapatite Composite Available to Scaffolds in Implantable Devices. Molecules 2025, 30, 730. https://doi.org/10.3390/molecules30030730
Miu D-M, Pavaloiu RD, Sha’at F, Vladu M-G, Neagu G, Manoiu V-S, Eremia M-C. Preparation and Optimization of a Polyhydroxyoctanoate–Hydroxyapatite Composite Available to Scaffolds in Implantable Devices. Molecules. 2025; 30(3):730. https://doi.org/10.3390/molecules30030730
Chicago/Turabian StyleMiu, Dana-Maria, Ramona Daniela Pavaloiu, Fawzia Sha’at, Mariana-Gratiela Vladu, Georgeta Neagu, Vasile-Sorin Manoiu, and Mihaela-Carmen Eremia. 2025. "Preparation and Optimization of a Polyhydroxyoctanoate–Hydroxyapatite Composite Available to Scaffolds in Implantable Devices" Molecules 30, no. 3: 730. https://doi.org/10.3390/molecules30030730
APA StyleMiu, D.-M., Pavaloiu, R. D., Sha’at, F., Vladu, M.-G., Neagu, G., Manoiu, V.-S., & Eremia, M.-C. (2025). Preparation and Optimization of a Polyhydroxyoctanoate–Hydroxyapatite Composite Available to Scaffolds in Implantable Devices. Molecules, 30(3), 730. https://doi.org/10.3390/molecules30030730






