Characteristic Fragmentation Behavior of Linear and Cyclic O-Linked Glycopeptides and Their Peptide Skeletons in MALDI-TOF/TOF MS
Abstract
1. Introduction
2. Results and Discussion
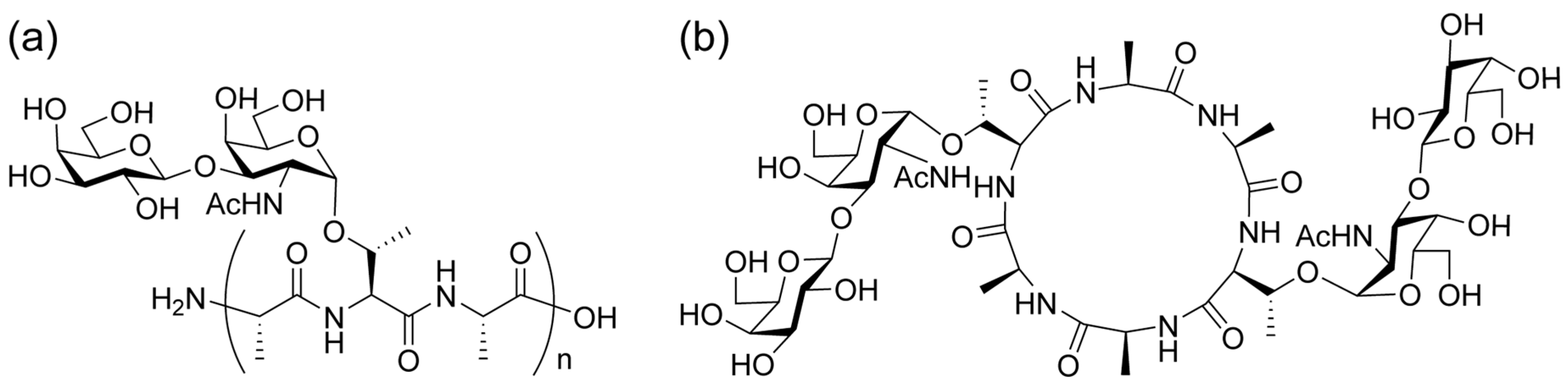
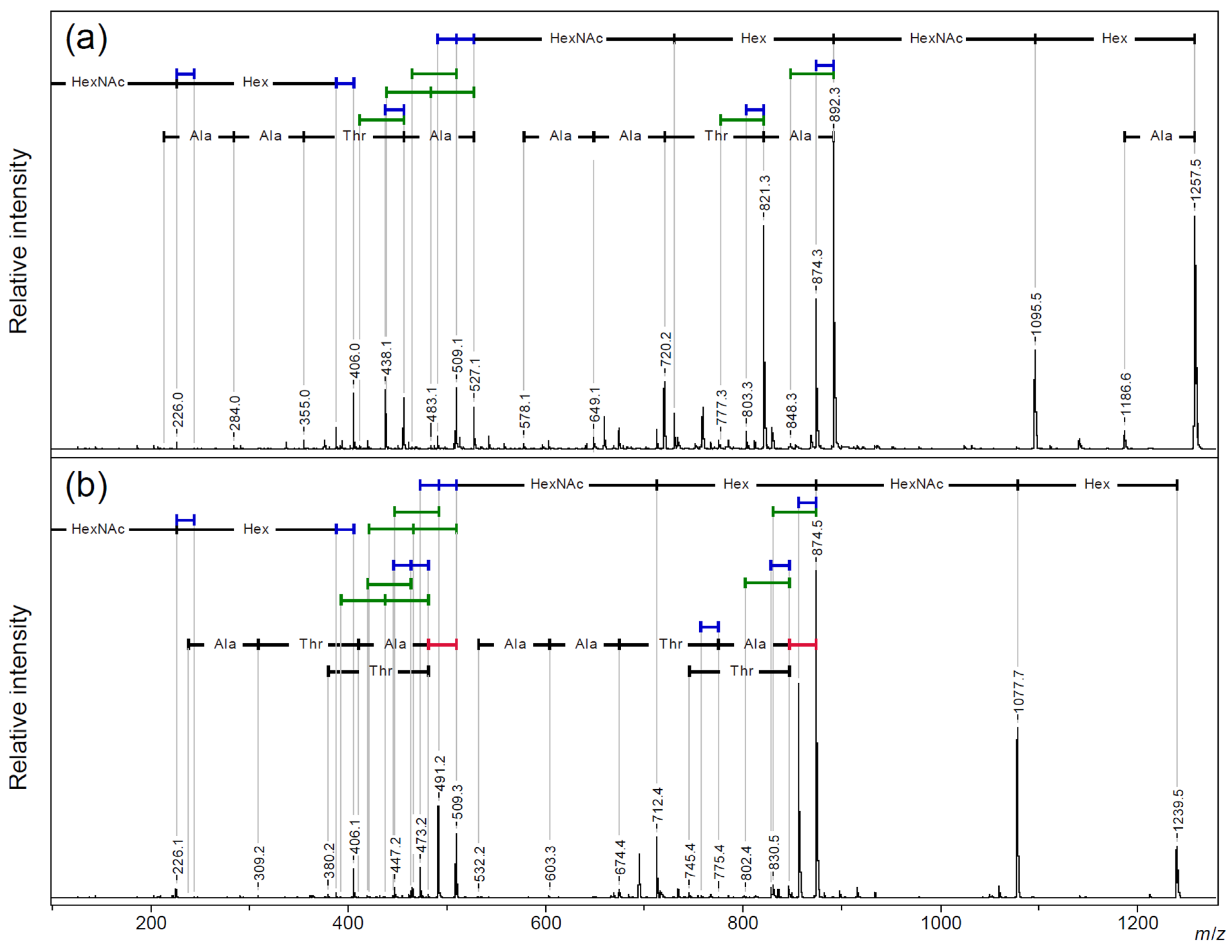
2.1. Comparative MALDI-TOF/TOF MS Analysis of Linear and Cyclic AFGP
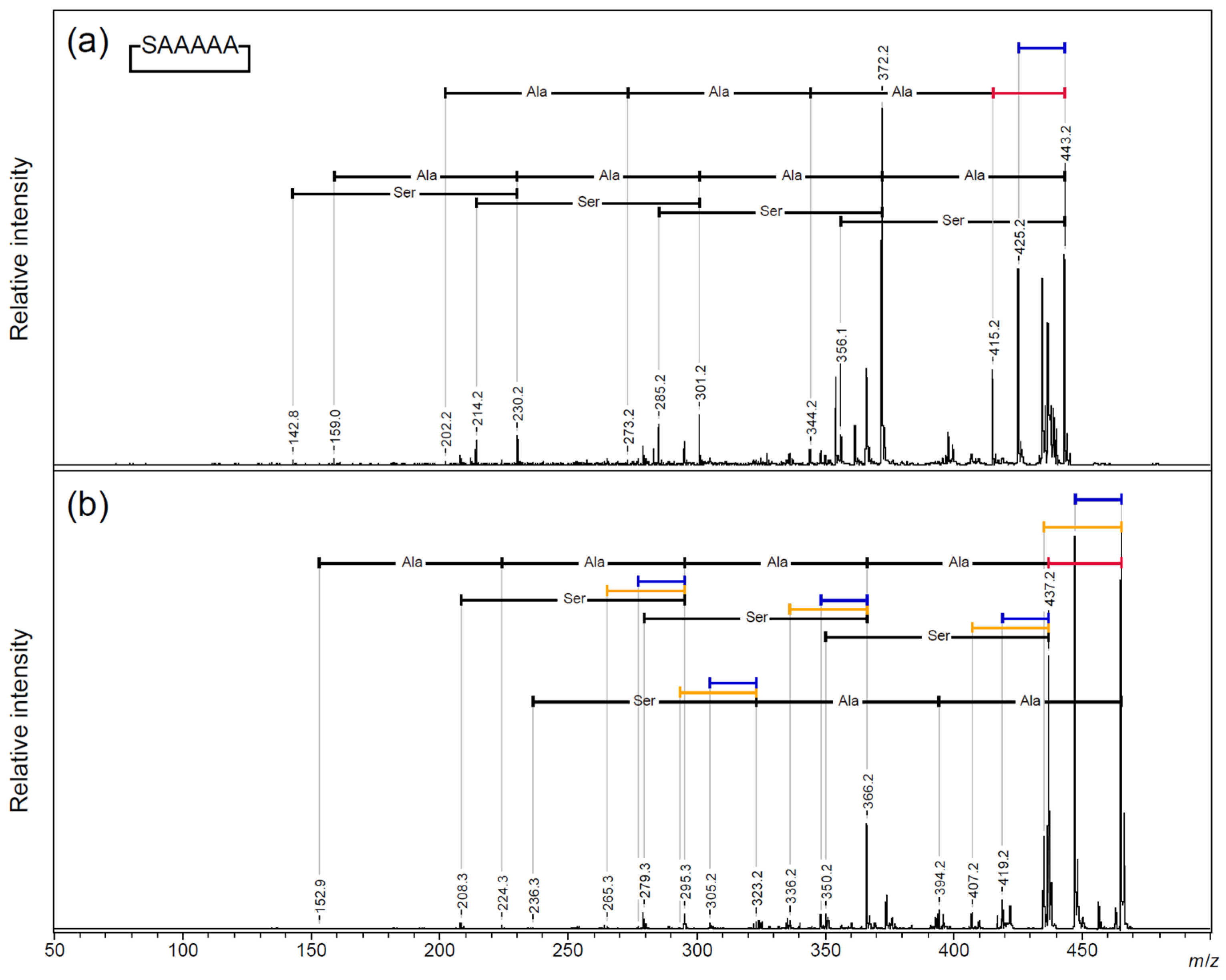
2.2. MALDI-TOF/TOF MS Analysis of Core Cyclic Peptide of Cyclic AFGP
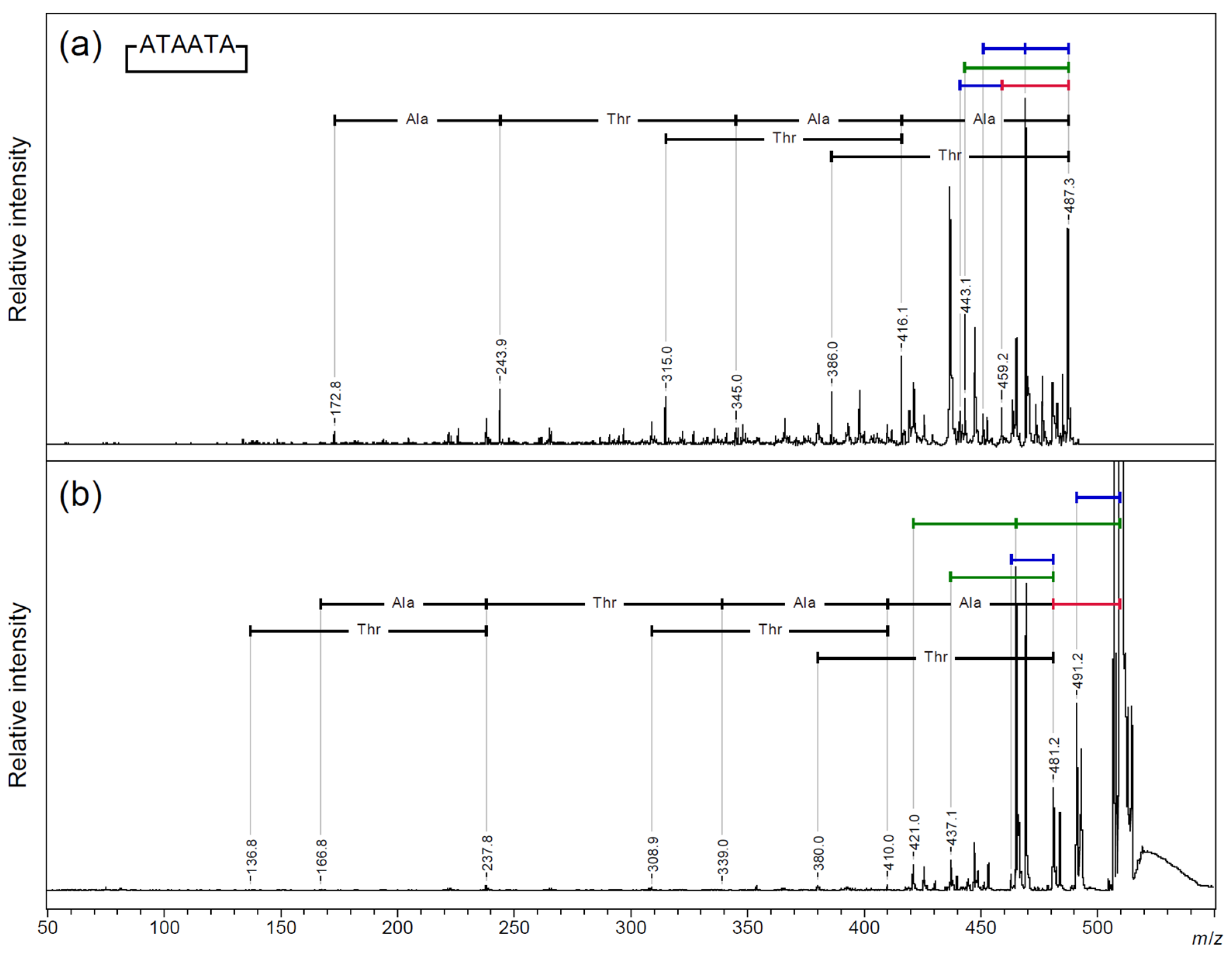
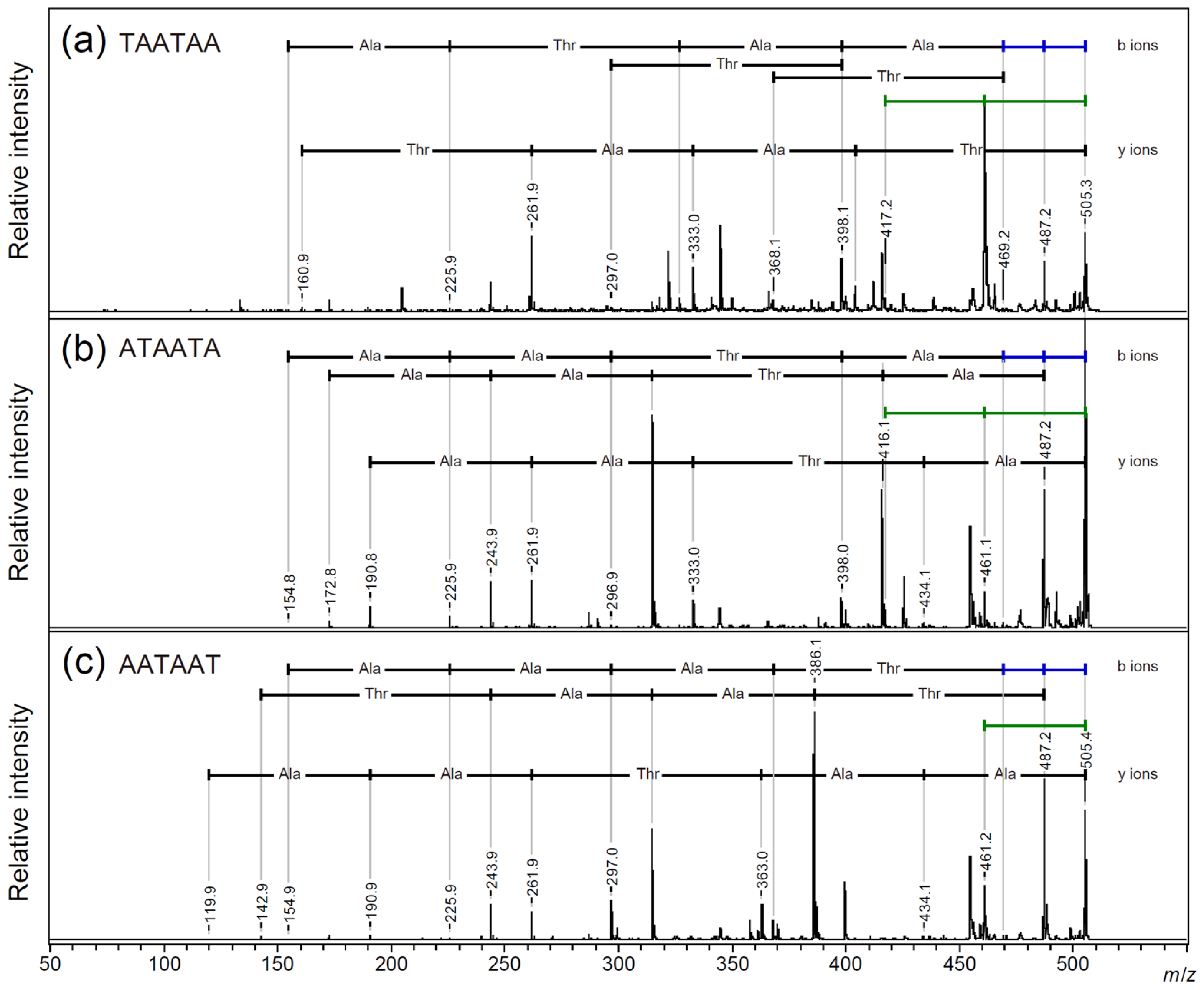
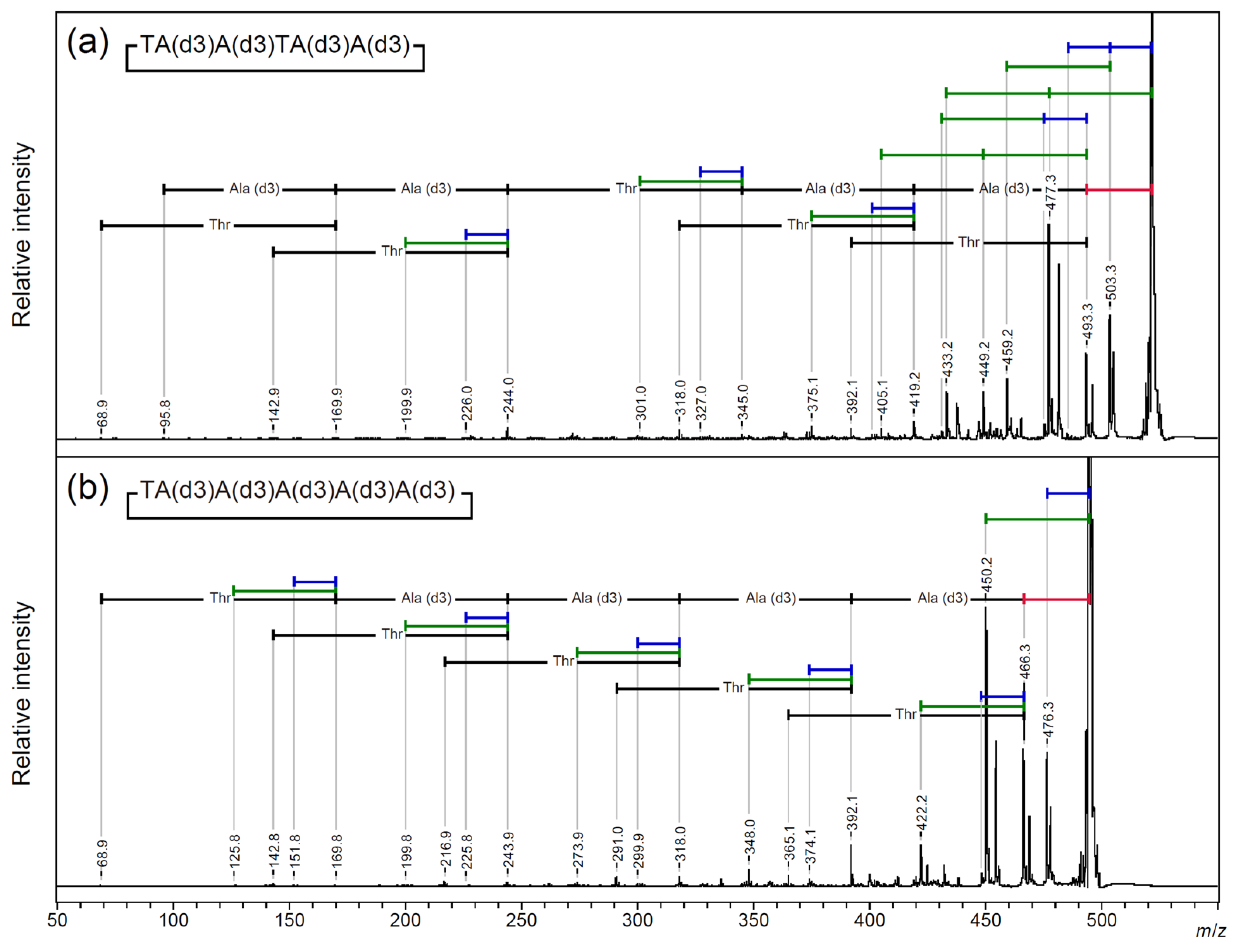
2.3. Searching for a Characteristic Fragmentation Mechanism Using Isotope-Labeled Derivatives
2.4. Side Chain Elimination Mechanism and Adduct Ion Selectivity of Cyclic Peptides
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis and Cyclization of Linear AFGP (n = 2)—Related Peptides
3.3. Synthesis of Linear Peptides and Their Cyclization
3.4. MALDI-TOF/TOF Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Mohnen, D.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology, 4th ed.; Cold Spring Harbor: New York, NY, USA, 2022; pp. 265–278. [Google Scholar]
- Wandall, H.H.; Nielsen, M.A.I.; King-Smith, S.; de Haan, N.; Bagdonaite, I. Global functions of O-glycosylation: Promises and challenges in O-glycobiology. FEBS J. 2021, 288, 7183–7212. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Nielsen, A.L.; Heinis, C. Cyclic Peptides for Drug Development. Angew. Chem. Int. Ed. Engl. 2024, 63, e202308251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, S. Cyclic peptide drugs approved in the last two decades (2001–2021). RSC Chem. Biol. 2022, 3, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef]
- Tanaka, K. The origin of macromolecule ionization by laser irradiation (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2003, 42, 3860–3870. [Google Scholar] [CrossRef]
- Fabre, B.; Combier, J.P.; Plaza, S. Recent advances in mass spectrometry-based peptidomics workflows to identify short-open-reading-frame-encoded peptides and explore their functions. Curr. Opin. Chem. Biol. 2021, 60, 122–130. [Google Scholar] [CrossRef]
- Grabarics, M.; Lettow, M.; Kirschbaum, C.; Greis, K.; Manz, C.; Pagel, K. Mass Spectrometry-Based Techniques to Elucidate the Sugar Code. Chem. Rev. 2022, 122, 7840–7908. [Google Scholar] [CrossRef]
- Seidler, J.; Zinn, N.; Boehm, M.E.; Lehmann, W.D. De novo sequencing of peptides by MS/MS. Proteomics 2010, 10, 634–649. [Google Scholar] [CrossRef]
- Liu, W.T.; Ng, J.; Meluzzi, D.; Bandeira, N.; Gutierrez, M.; Simmons, T.L.; Schultz, A.W.; Linington, R.G.; Moore, B.S.; Gerwick, W.H.; et al. Interpretation of tandem mass spectra obtained from cyclic nonribosomal peptides. Anal. Chem. 2009, 81, 4200–4209. [Google Scholar] [CrossRef]
- Suckau, D.; Cornett, D.S. Protein sequencing by ISD and PSD MALDI-TOF MS. Analusis 1998, 26, M18–M21. [Google Scholar] [CrossRef][Green Version]
- Schilling, B.; Wang, W.; McMurray, J.S.; Medzihradszky, K.F. Fragmentation and sequencing of cyclic peptides by matrix-assisted laser desorption/ionization post-source decay mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 2174–2179. [Google Scholar] [CrossRef]
- Jeric, I.; Versluis, C.; Horvat, S.; Heck, A.J. Tracing glycoprotein structures: Electrospray ionization tandem mass spectrometric analysis of sugar-peptide adducts. J. Mass Spectrom. 2002, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Villones, L.L., Jr.; Ludwig, A.K.; Kikuchi, S.; Ochi, R.; Nishimura, S.I.; Gabius, H.J.; Kaltner, H.; Hinou, H. Altering the modular architecture of galectins affects its binding with synthetic alpha-dystroglycan O-Mannosylated Core M1 Glycoconjugates In situ. Chembiochem 2023, 24, e202200783. [Google Scholar] [CrossRef]
- Wakui, H.; Yokoi, Y.; Horidome, C.; Ose, T.; Yao, M.; Tanaka, Y.; Hinou, H.; Nishimura, S.I. Structural and molecular insight into antibody recognition of dynamic neoepitopes in membrane tethered MUC1 of pancreatic cancer cells and secreted exosomes. RSC Chem. Biol. 2023, 4, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Fletcher, G.L.; Fujitani, N.; Tsuda, S.; Monde, K.; Nishimura, S. Antifreeze glycoproteins: Elucidation of the structural motifs that are essential for antifreeze activity. Angew. Chem. Int. Ed. Engl. 2004, 43, 856–862. [Google Scholar] [CrossRef]
- Devries, A.L.; Wohlschlag, D.E. Freezing Resistance in Some Antarctic Fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef]
- Hachisu, M.; Hinou, H.; Takamichi, M.; Tsuda, S.; Koshida, S.; Nishimura, S. One-pot synthesis of cyclic antifreeze glycopeptides. Chem. Commun. 2009, 13, 1641–1643. [Google Scholar] [CrossRef]
- Urakami, S.; Hinou, H. Sodium-Doped 3-Amino-4-hydroxybenzoic Acid: Rediscovered matrix for direct MALDI glycotyping of O-linked glycopeptides and intact mucins. Int. J. Mol. Sci. 2023, 24, 16836. [Google Scholar] [CrossRef]
- Kurogochi, M.; Matsushita, T.; Nishimura, S. Post-translational modifications on proteins: Facile and efficient procedure for the identification of O-glycosylation sites by MALDI-LIFT-TOF/TOF mass spectrometry. Angew. Chem. Int. Ed. Engl. 2004, 43, 4071–4075. [Google Scholar] [CrossRef]
- Deguchi, K.; Ito, H.; Baba, T.; Hirabayashi, A.; Nakagawa, H.; Fumoto, M.; Hinou, H.; Nishimura, S. Structural analysis of O-glycopeptides employing negative- and positive-ion multi-stage mass spectra obtained by collision-induced and electron-capture dissociations in linear ion trap time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 691–698. [Google Scholar] [CrossRef]
- Shiratori, K.; Yokoi, Y.; Wakui, H.; Hirane, N.; Otaki, M.; Hinou, H.; Yoneyama, T.; Hatakeyama, S.; Kimura, S.; Ohyama, C.; et al. Selective reaction monitoring approach using structure-defined synthetic glycopeptides for validating glycopeptide biomarkers pre-determined by bottom-up glycoproteomics. RSC Adv. 2022, 12, 21385–21393. [Google Scholar] [CrossRef] [PubMed]
- Malaker, S.A. Glycoproteomics: Charting new territory in mass spectrometry and glycobiology. J. Mass Spectrom. 2024, 59, e5034. [Google Scholar] [CrossRef] [PubMed]
- Helms, A.; Brodbelt, J.S. Mass Spectrometry Strategies for O-Glycoproteomics. Cells 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Barada, E.; Hinou, H. BOA/DHB/Na: An Efficient UV-MALDI Matrix for High-Sensitivity and Auto-Tagging Glycomics. Int. J. Mol. Sci. 2022, 23, 12510. [Google Scholar] [CrossRef]
- Nelson, C.R.; Abutokaikah, M.T.; Harrison, A.G.; Bythell, B.J. Proton Mobility in b Ion Formation and Fragmentation Reactions of Histidine-Containing Peptides. J. Am. Soc. Mass Spectr. 2016, 27, 487–497. [Google Scholar] [CrossRef]
- Farrugia, J.M.; O’Hair, R.A.J.; Reid, G.E. Do all b ions have oxazolone structures? Multistage mass spectrometry and ab initio studies on protonated-acyl amino acid methyl ester model systems. Int. J. Mass Spectrom. 2001, 210, 71–87. [Google Scholar] [CrossRef]
- Polfer, N.C.; Oomens, J.; Suhai, S.; Paizs, B. Spectroscopic and theoretical evidence for oxazolone ring formation in collision-induced dissociation of peptides. J. Am. Chem. Soc. 2005, 127, 17154–17155. [Google Scholar] [CrossRef]
- Seipert, R.R.; Dodds, E.D.; Lebrilla, C.B. Exploiting differential dissociation chemistries of O-linked glycopeptide ions for the localization of mucin-type protein glycosylation. J. Proteome Res. 2009, 8, 493–501. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, B.; Wei, Z.L.; Cao, Y.W.; Guan, X.S.; Guo, X.H. Characteristic neutral loss of CH3CHO from Thr-containing sodium-associated peptides. J. Mass Spectrom. 2015, 50, 488–494. [Google Scholar] [CrossRef]
- Hinou, H.; Hyugaji, K.; Garcia-Martin, F.; Nishimura, S.I.; Albericio, F. H-bonding promotion of peptide solubility and cyclization by fluorinated alcohols. RSC Adv. 2012, 2, 2729–2731. [Google Scholar] [CrossRef]
- Barchi, J.J.; Strain, C.N. The effect of a methyl group on structure and function: Serine vs. threonine glycosylation and phosphorylation. Front. Mol. Biosci. 2023, 10, 1117850. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, E.A.; Delaforge, E.; Hartmann-Petersen, R.; Skriver, K.; Kragelund, B.B. How phosphorylation impacts intrinsically disordered proteins and their function. Essays Biochem. 2022, 66, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Matsushita, T.; Yokoi, Y.; Wakui, H.; Garcia-Martin, F.; Hinou, H.; Matsuoka, K.; Nouso, K.; Kamiyama, T.; Taketomi, A.; et al. Impaired O-glycosylation at consecutive threonine TTX motifs in mucins generates conformationally restricted cancer neoepitopes. Biochemistry 2020, 59, 1221–1241. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Matsushita, T.; Fujitani, N.; Naruchi, K.; Shimizu, H.; Tsuda, S.; Hinou, H.; Nishimura, S. Microwave-assisted solid-phase synthesis of antifreeze glycopeptides. Chemistry 2013, 19, 3913–3920. [Google Scholar] [CrossRef]
- Suckau, D.; Resemann, A.; Schuerenberg, M.; Hufnagel, P.; Franzen, J.; Holle, A. A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Anal. Bioanal. Chem. 2003, 376, 952–965. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushi, K.; Urakami, S.; Hinou, H. Characteristic Fragmentation Behavior of Linear and Cyclic O-Linked Glycopeptides and Their Peptide Skeletons in MALDI-TOF/TOF MS. Molecules 2025, 30, 711. https://doi.org/10.3390/molecules30030711
Fukushi K, Urakami S, Hinou H. Characteristic Fragmentation Behavior of Linear and Cyclic O-Linked Glycopeptides and Their Peptide Skeletons in MALDI-TOF/TOF MS. Molecules. 2025; 30(3):711. https://doi.org/10.3390/molecules30030711
Chicago/Turabian StyleFukushi, Kohki, Shogo Urakami, and Hiroshi Hinou. 2025. "Characteristic Fragmentation Behavior of Linear and Cyclic O-Linked Glycopeptides and Their Peptide Skeletons in MALDI-TOF/TOF MS" Molecules 30, no. 3: 711. https://doi.org/10.3390/molecules30030711
APA StyleFukushi, K., Urakami, S., & Hinou, H. (2025). Characteristic Fragmentation Behavior of Linear and Cyclic O-Linked Glycopeptides and Their Peptide Skeletons in MALDI-TOF/TOF MS. Molecules, 30(3), 711. https://doi.org/10.3390/molecules30030711




