Purification, Characterization, and Potential Immune-Regulation Mechanism of Polysaccharides from Artemisia odosica Krasch.
Abstract
:1. Introduction
2. Results and Discussion
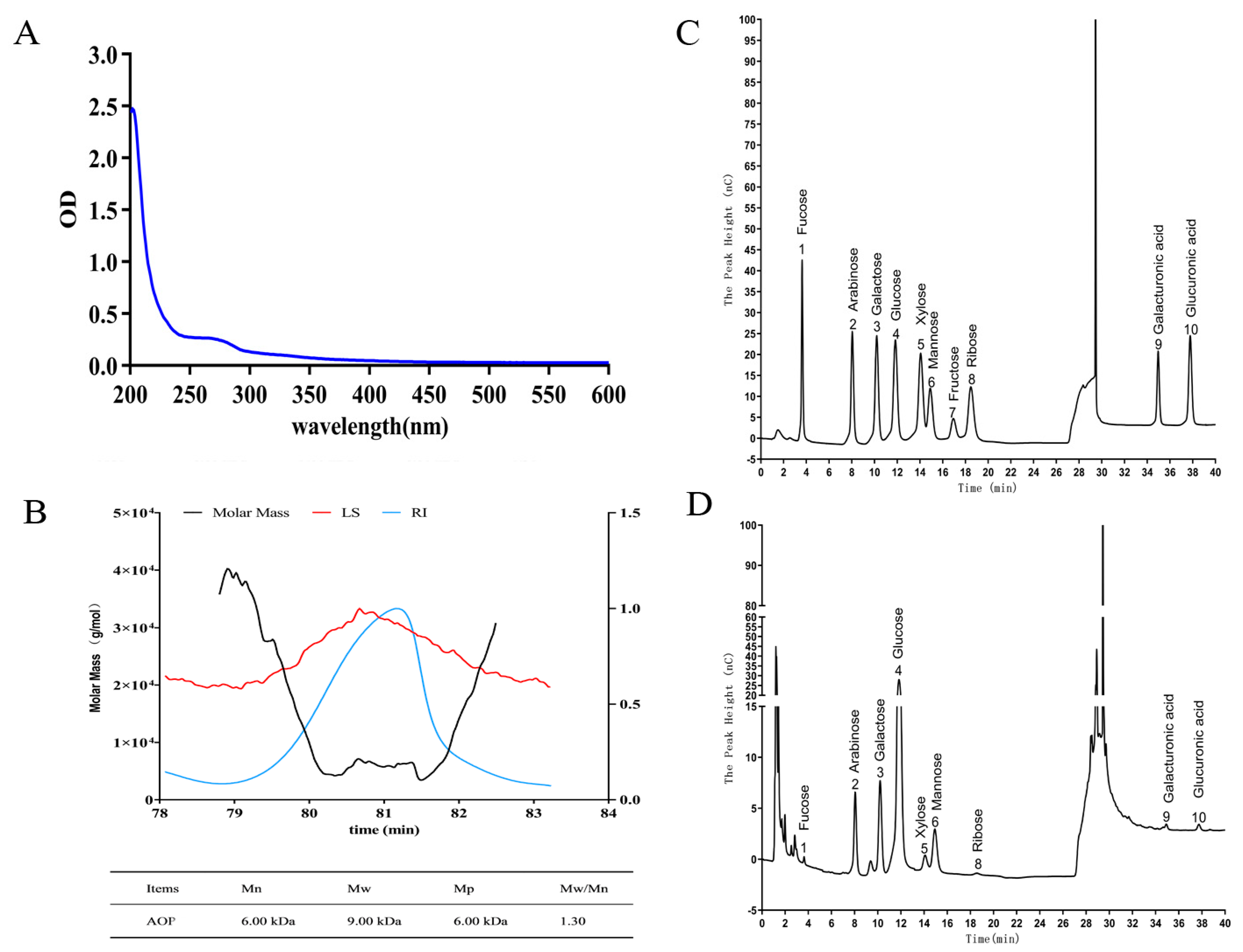
2.1. Purification of AOP
2.2. Molecular Weight, Monosaccharide Composition, and UV-Spectra of AOP
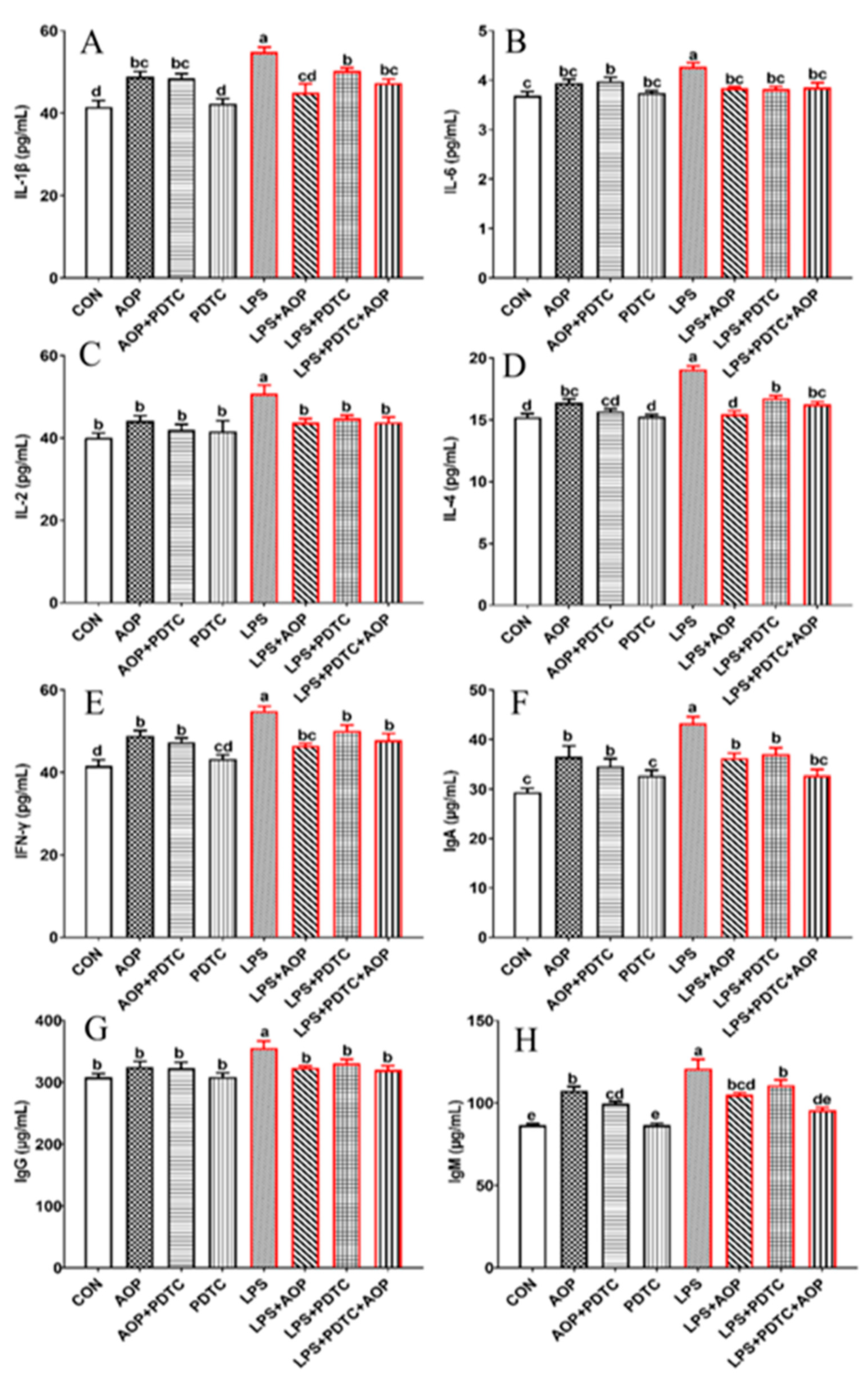
2.3. Immunomodulatory Effects of TLR4 as a Candidate Target of AOP
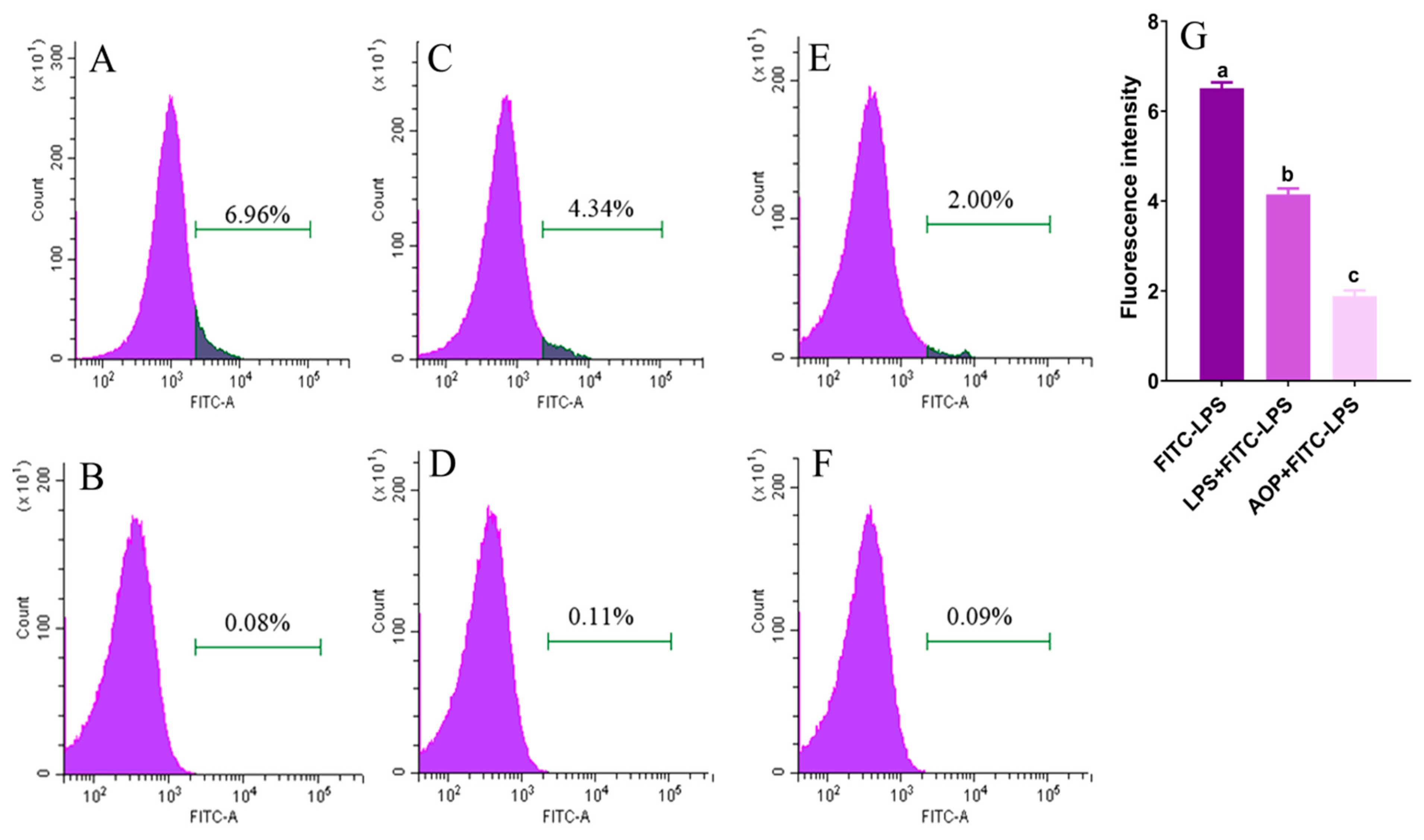
2.4. AOP Interfering with LPS Binding to TLR4
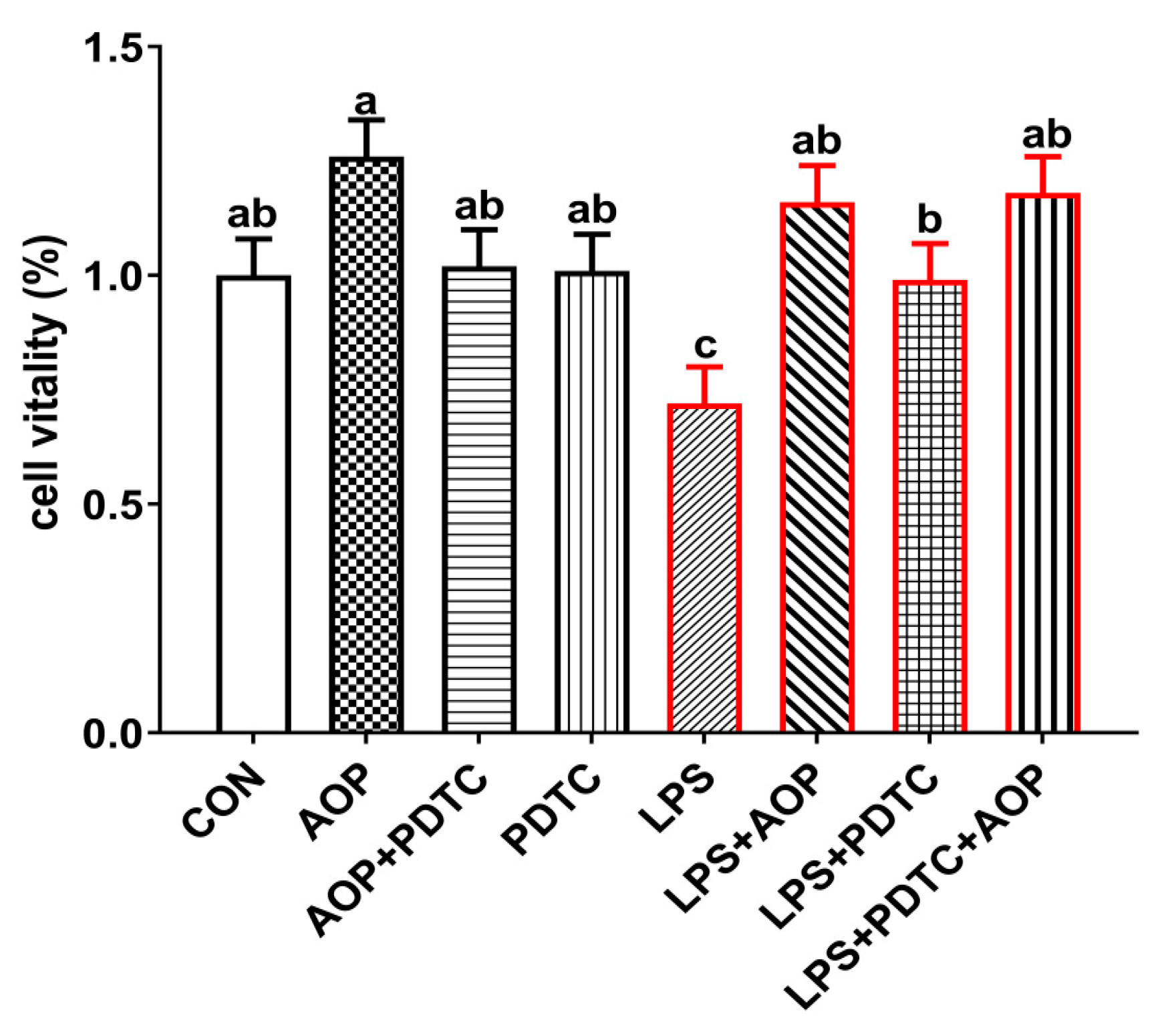
2.5. AOP Attenuates the Immune Stress Through Alleviates the Decrease in Cell Viability of PBLs Induced by LPS
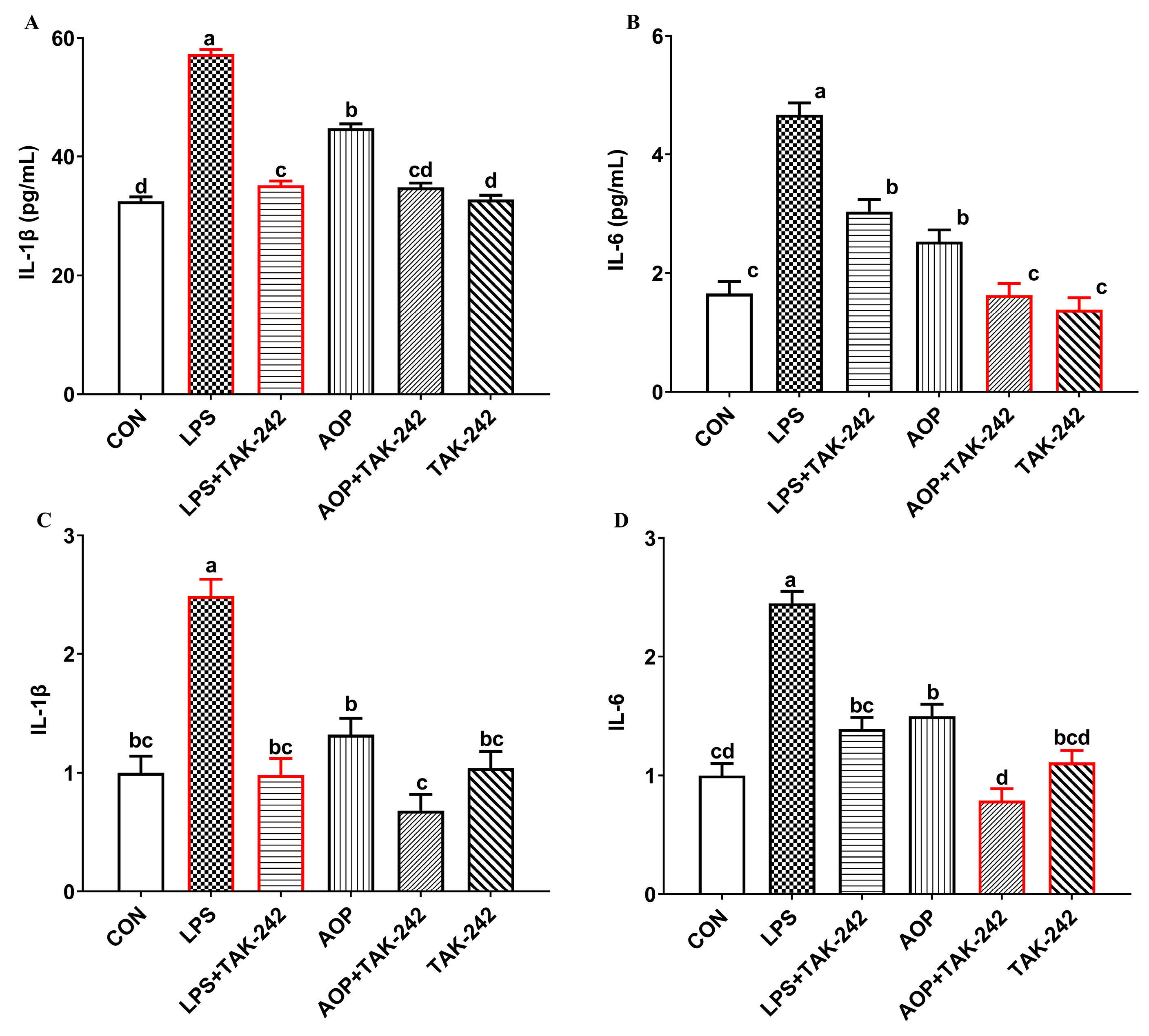
2.6. AOP Attenuates the Immune Stress in PBLs Induced by LPS Through Suppressing the Over-Expression of NF-κB Signaling Pathway
3. Materials and Methods
3.1. Preparation of AOP
3.2. Characterization of AOP
3.2.1. Molecular Weight
3.2.2. Monosaccharide Composition
3.2.3. UV Spectral Scanning Analysis
3.3. Isolation and Culture of Peripheral Blood Lymphocytes
3.4. Treatment of PBLs with Receptor and Signaling Molecules Inhibitors
3.5. Cell Viability Measurement
3.6. Assay of Immune Indices in Cell Sample
3.7. RNA Preparation and Fluorescence Quantitative Real-Time PCR
3.8. Data Processing and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araújo-Rodrigues, H.; Sousa, A.S.; Relvas, J.B.; Tavaria, F.K.; Pintado, M. An overview on mushroom polysaccharides: Health-promoting properties, prebiotic and gut microbiota modulation effects and structure-function correlation. Carbohydr. Polym. 2024, 333, 121978. [Google Scholar] [CrossRef] [PubMed]
- Campo-Grande, G.C.; Luz, B.B.D.; Maria-Ferreira, D.; Werner, M.F.D.P.; Cipriani, T.R. Water-soluble polysaccharides from Piper regnellii (Pariparoba) leaves: Structural characterization and antinociceptive and anti-inflammatory activities. Carbohydr. Polym. 2023, 319, 121142. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Y.; Jiao, H.X.; Sun, J.Z.; Okoye, C.O.; Zhang, H.X.; Li, Y.; Lu, X.C.; Wang, Q.Q.; Liu, J. Structure-activity relationships of bioactive polysaccharides extracted from macroalgae towards biomedical application: A review. Carbohydr. Polym. 2024, 324, 121533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chang, S.L.; Tian, Y.M.; Li, W.; Ren, J.L. Glucan polysaccharides isolated from Lactarius hatsudake Tanaka mushroom: Structural characterization and in vitro bioactivities. Carbohydr. Polym. 2024, 337, 122171. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Wu, J.P.; Zhao, X.Y.; Li, Z.X.; Jie, Y.; Shao, T.L.; Hou, X.F.; Zhou, L.T.; Wang, C.F.; Wang, G.D.; et al. Structural elucidation of an active polysaccharide from Radix Puerariae lobatae and its protection against acute alcoholic liver disease. Carbohydr. Polym. 2024, 325, 121565. [Google Scholar]
- Li, J.H.; Gu, F.T.; Yang, Y.; Zhao, Z.C.; Huang, L.X.; Zhu, Y.Y.; Chen, S.G.; Wu, J.Y. Simulated human digestion and fermentation of a high-molecular-weight polysaccharide from Lentinula edodes mushroom and protective effects on intestinal barrier. Carbohydr. Polym. 2024, 343, 122478. [Google Scholar] [CrossRef]
- Pang, Y.R.; Peng, Z.G.; Ding, K. An in-depth review: Unraveling the extraction, structure, bio-functionalities, target molecules, and applications of pectic polysaccharides. Carbohydr. Polym. 2024, 343, 122457. [Google Scholar] [CrossRef]
- Du, H.D.; Xing, Y.Y.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Dietary Artemisia ordosica polysaccharide enhances spleen and intestinal immune response of broiler chickens. Biology 2023, 12, 1390. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica polysaccharide ameliorated LPS-induced growth inhibition and intestinal injury in broilers through enhancing immune regulation and antioxidant capacity. J. Nutr. Biochem. 2024, 115, 109284. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Y.; Zheng, Y.K.; Yang, S.; Zhang, L.H.; Guo, S.W.; Shi, L.L.; Xu, Y.Q.; Jin, X.; Yan, S.M.; Shi, B.L. Artemisia ordosica polysaccharide alleviated lipopolysaccharide-induced oxidative stress of broilers via Nrf2/Keap1 and TLR4/NF-κB pathway. Ecotoxicol. Environ. Saf. 2021, 223, 112566. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Chen, X.; Yu, G.; Fan, S.; Bian, M.; Ma, H.; Lu, J.; Jin, L.Q. Sargassum fusiforme polysaccharide activates nuclear factor kappa-B and induces cytokine production via Toll-like receptors. Carbohydr. Polym. 2014, 105, 113–120. [Google Scholar] [CrossRef]
- Lee, J.B.; Tanikawa, T.; Hayashi, K.; Asagi, M.; Kasahara, Y.; Hayashi, T. Characterization and biological effects of two polysaccharides isolated from Acanthopanax sciadophylloides. Carbohydr. Polym. 2015, 116, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, J.F.; Zha, X.Q.; Cui, S.H.; Cao, L.; Luo, J.P. Immunomodulatory activity on macrophage of a purified polysaccharide extracted from Laminaria japonica. Carbohydr. Polym. 2015, 134, 66–73. [Google Scholar] [CrossRef]
- Fan, X.Q.; Xiao, X.J.; Yu, W.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, J.Q.; Luo, Y.H.; Yan, H.; et al. Yucca schidigera purpurea-sourced arabinogalactan polysaccharides augment antioxidant capacity facilitating intestinal antioxidant functions. Carbohydr. Polym. 2024, 326, 121613. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, X.; Wen, J.; Chen, S.; Wu, X.; Ma, W.; Cui, S.W.; Xie, M.; Nie, S. Innate immune receptors co-recognition of polysaccharides initiates multi-pathway synergistic immune response. Carbohydr. Polym. 2023, 305, 120533. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wu, Q.; Gao, N.; Wang, Z.; Yang, Y.; Shan, A. Exopolysaccharides of Lactobacillus rhamnosus GG ameliorate Salmonella typhimurium-induced intestinal inflammation via the TLR4/NF-κB/MAPK pathway. J. Anim. Sci. Biotechnol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Li, C.X.; Liu, Y.; Zhang, Y.Z.; Li, J.C. Astragalus polysaccharide: A review of its immunomodulatory effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an inducible regulator of inflammation in the central nervous system. Cells 2024, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Bai, Y.P.; Zhang, Z.D.; Cai, W.L.; Del Rio Flores, A. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Chang, H.; Cai, B.; Gao, H.J.; Chen, G.Y.; Hou, W.; Jappar, Z.; Yan, Y.Z. Research progress on extraction, purification, structure, and biological activity of Dendrobium officinale polysaccharides. Front. Nutr. 2022, 18, 965073. [Google Scholar] [CrossRef] [PubMed]
- Yahaya, N.; Mohamed, A.H.; Sajid, M.; Zain, N.N.M.; Liao, P.C.; Chew, K.W. Deep eutectic solvents as sustainable extraction media for polysaccharides from natural sources: Status, challenges, and prospects. Carbohydr. Polym. 2024, 338, 122199. [Google Scholar] [CrossRef]
- Yin, C.M.; Li, C.; Ma, K.; Fan, X.Z.; Yao, F.; Shi, D.F.; Wu, W.J.; Qiu, J.H.; Hu, G.Y.; Gao, H. The physicochemical, antioxidant, hypoglycemic, and prebiotic properties of γ-irradiated polysaccharides extracted from Lentinula edodes. Food Sci. Biotechnol. 2023, 32, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical modification of polysaccharides: A review of synthetic approaches, biological activity, and structure-activity relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef]
- Tang, Z.Z.; Lin, W.J.; Chen, Y.S.; Feng, S.L.; Qin, Y.H.; Xiao, Y.R.; Chen, H.; Liu, Y.T.; Chen, H.; Bu, T.L.; et al. Extraction, purification, physicochemical properties, and activity of a new polysaccharide from Cordyceps cicadae. Front. Nutr. 2022, 9, 911310. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Hua, K.F.; Lin, C.C.; Hsu, H.Y.; Wong, C.H. Extract of Reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. J. Immunol. 2004, 173, 5989–5999. [Google Scholar] [CrossRef]
- Xie, C.; Lin, X.; Hu, J.; Wang, S.; Wu, J.; Xiong, W.; Wu, L. The polysaccharide from Camellia oleifera fruit shell enhances immune responses via activating MAPKs and NF-κB signaling pathways in RAW264.7 macrophages. Food Nutr. Res. 2022, 66, 8963. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Z.; Wu, Q.; Er-Bu, A.; Liang, X.; He, C.; Yin, L.; Xu, F.; Sang, G.; Car, R. Response surface methodology for optimization of the extraction of polysaccharide from the roots of Onosma hookeri Clarke var. longiforum Duthie and its antioxidant capacity and immune activity. Prep. Biochem. Biotechnol. 2023, 53, 923–930. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, D.; Ma, X.; Wang, H. Lotus flower and lotus seedpod-derived polysaccharide: Structural characterization and biological activity. Polymers 2023, 15, 3828. [Google Scholar] [CrossRef]
- Xu, X.; Rui, S.; Chen, C.; Zhang, G.C.; Li, Z.; Wang, J.H.; Luo, Y.P.; Zhu, H.P.; Ma, X.M. Protective effects of Astragalus polysaccharide nanoparticles on septic cardiac dysfunction through inhibition of TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 153, 977–985. [Google Scholar] [CrossRef]
- Zhang, X.H.; Gao, M.; Zhao, X.R.; Qi, Y.; Xu, L.N.; Yin, L.H.; Peng, J.Y. Purification and structural characterization of two polysaccharides with anti-inflammatory activities from Plumbago zeylanica L. Int. J. Biol. Macromol. 2024, 260, 129455. [Google Scholar] [CrossRef] [PubMed]
- Caillot, A.R.C.; Bezerra, I.D.L.; Palhares, L.C.G.F.; Santana-Filho, A.P.; Chavante, S.F.; Sassaki, G.L. Structural characterization of blackberry wine polysaccharides and immunomodulatory effects on LPS-activated RAW 264.7 macrophages. Food Chem. 2018, 257, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lou, Q.; Song, X.J.; Kang, F.; Liu, A.; Zheng, C.; Li, Y.; Wang, D.; Qun, S.; Zhang, Z.; et al. Microglia govern the extinction of acute stress-induced anxiety-like behaviors in male mice. Nat. Commun. 2024, 15, 449. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Liu, Y.; Wu, D.; Cai, P.; Pan, Y. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide isolated from Botrychium ternatum. Carbohydr. Polym. 2017, 171, 136–142. [Google Scholar] [CrossRef]
- Martins, V.M.R.; Simoes, J.; Ferreira, I.; Cruz, M.T.; Domingues, M.R.; Coimbra, M.A. In vitro macrophage nitric oxide production by Pterospartum tridentatum (L.) Willk. inflorescence polysaccharides. Carbohydr. Polym. 2017, 157, 176–184. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar]
- Afonina, I.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation: The negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, J.E.; Nelson, E.D.; Sinnott, R.A. Immunomodulatory dietary polysaccharides: A systematic review of the literature. Nutr. J. 2010, 9, 54. [Google Scholar] [CrossRef]
- Ying, Y.; Hao, W. Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: A review. Front. Immunol. 2023, 9, 141147641. [Google Scholar] [CrossRef]
- Chen, N.; Hu, M.F.; Jiang, T.Y.; Xiao, P.; Duan, J. Insights into the molecular mechanisms, structure-activity relationships, and application prospects of polysaccharides by regulating Nrf2-mediated antioxidant response. Carbohydr. Polym. 2024, 333, 122003. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Chen, R.; Yuan, H.; Liu, Y.; Wu, Y.; Wang, J.; Xia, C. De novo transcriptomic analysis of peripheral blood lymphocytes from the Chinese goose: Gene discovery and immune system pathway description. PLoS ONE 2015, 10, e0121015. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Zheng, Y.; Zhang, J.; Chen, L.; Xu, Y.; Jin, X.; Hong, L.; Yan, S.; Shi, B. Purification, Characterization, and Potential Immune-Regulation Mechanism of Polysaccharides from Artemisia odosica Krasch. Molecules 2025, 30, 675. https://doi.org/10.3390/molecules30030675
Xing Y, Zheng Y, Zhang J, Chen L, Xu Y, Jin X, Hong L, Yan S, Shi B. Purification, Characterization, and Potential Immune-Regulation Mechanism of Polysaccharides from Artemisia odosica Krasch. Molecules. 2025; 30(3):675. https://doi.org/10.3390/molecules30030675
Chicago/Turabian StyleXing, Yuanyuan, Yankai Zheng, Jing Zhang, Lu Chen, Yuanqing Xu, Xiao Jin, Lei Hong, Sumei Yan, and Binlin Shi. 2025. "Purification, Characterization, and Potential Immune-Regulation Mechanism of Polysaccharides from Artemisia odosica Krasch." Molecules 30, no. 3: 675. https://doi.org/10.3390/molecules30030675
APA StyleXing, Y., Zheng, Y., Zhang, J., Chen, L., Xu, Y., Jin, X., Hong, L., Yan, S., & Shi, B. (2025). Purification, Characterization, and Potential Immune-Regulation Mechanism of Polysaccharides from Artemisia odosica Krasch. Molecules, 30(3), 675. https://doi.org/10.3390/molecules30030675






