Potential of the Nano-Encapsulation of Antioxidant Molecules in Wound Healing Applications: An Innovative Strategy to Enhance the Bio-Profile
Abstract
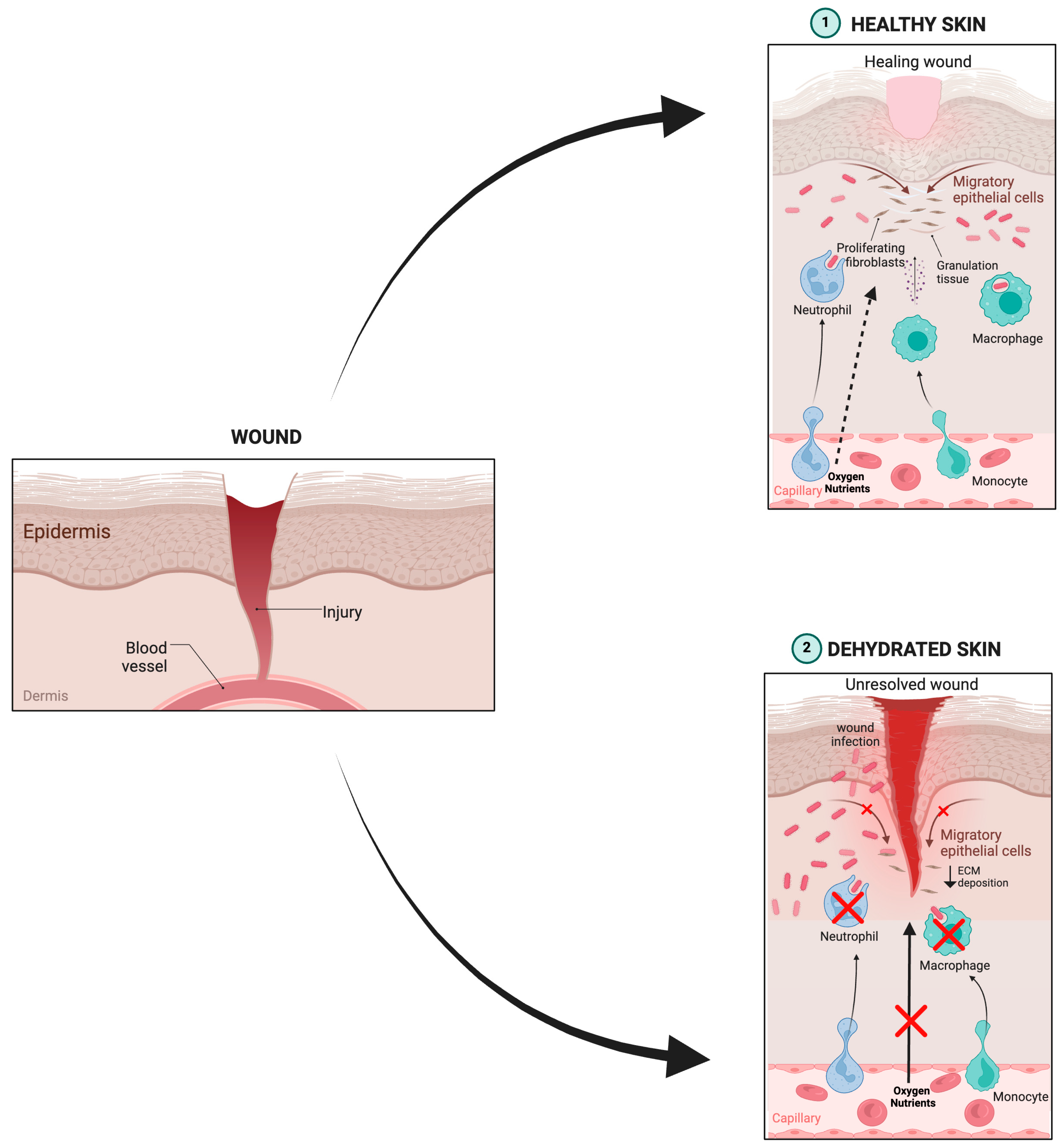
1. Introduction
2. Role of the Lipid in the Skin Permeation
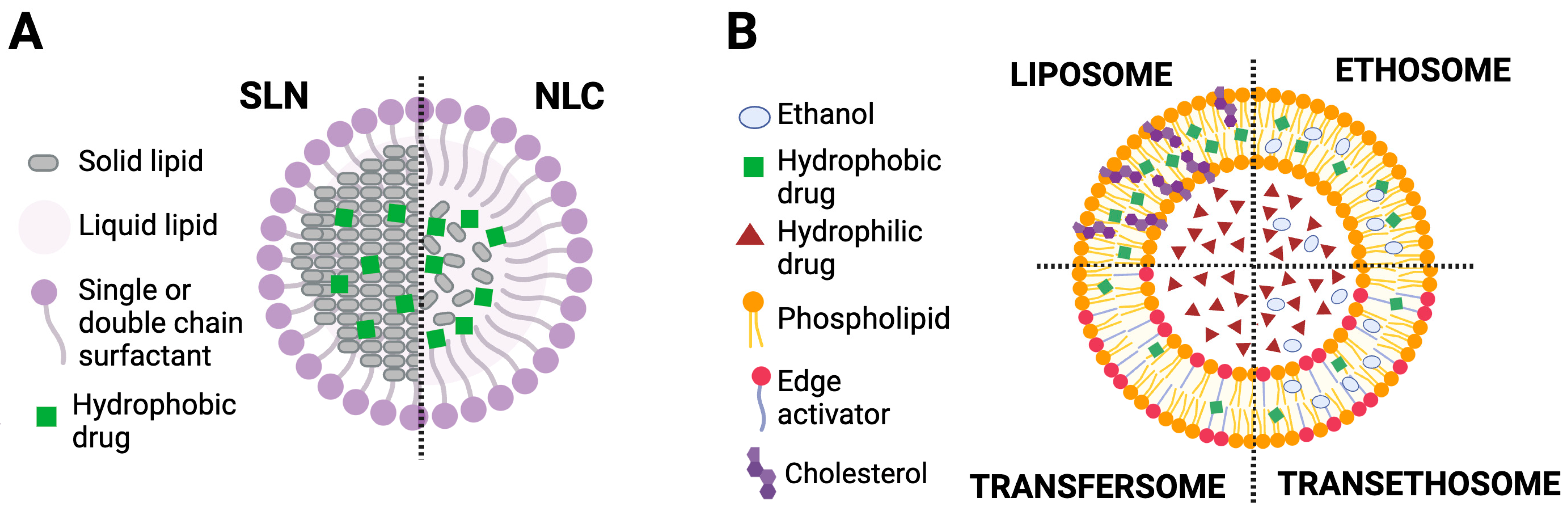
2.1. Lipid Nanoparticles (L-NPs)
2.1.1. Solid Lipid Nanoparticles
2.1.2. Nanostructured Lipid Carriers
2.2. Lipid-Based Deformable Vesicles
3. Polymeric Nano-Transporters
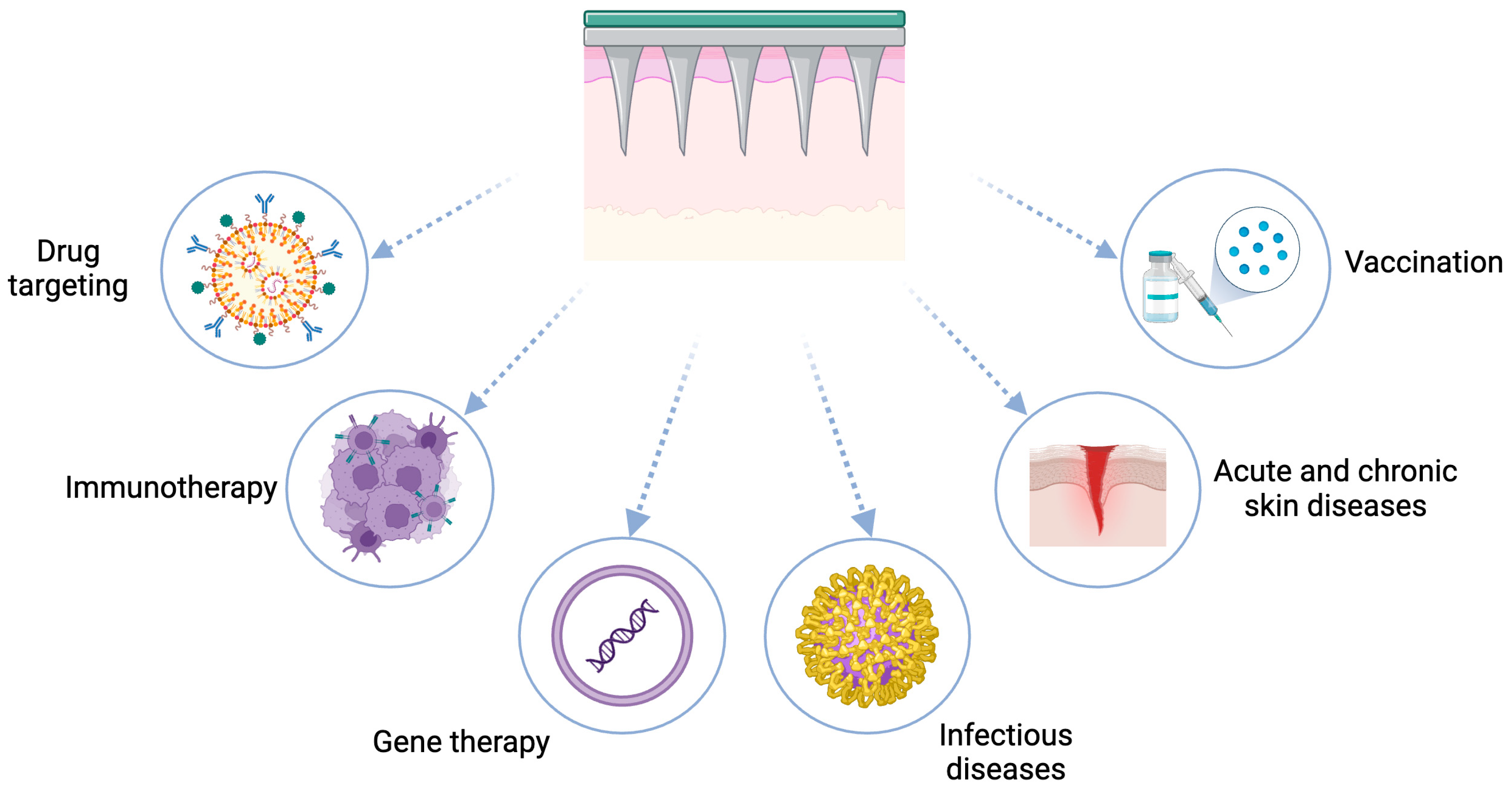
4. Microneedle-Assisted Transdermal Drug Delivery
5. Investigation of the Structural Organization and Physical Aspects of the Lipid Nanosystems
6. Antioxidant Nanosystems and Their in Vivo Wound Healing Management
7. Challenges and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| TNF | Tumor necrosis factor |
| IL-6 | Interleukin 6 |
| SC | Stratum corneum |
| FTIR | Fourier-transform infrared |
| DSC | Differential scanning calorimetry |
| NPs | Nanoparticles |
| L-NPs | Lipid nanoparticles |
| SLNs | Solid lipid nanoparticles |
| NLCs | Nanostructured lipid carrier |
| CMC | Critical Micelle concentration |
| MNLs | Microneedles |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| XRD | X-Ray Diffraction |
| MD | Molecular dynamics |
| DDS | Drug delivery system |
| SAXS | Small-angle X-ray scattering |
References
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Da Ros, R.; Assaloni, R.; Michelli, A.; Brunato, B.; Barro, E.; Meloni, M.; Miranda, C. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs in a Third-Level Center. Diabetology 2024, 5, 141–150. [Google Scholar] [CrossRef]
- Yapislar, H.; Gurler, E.B. Management of Microcomplications of Diabetes Mellitus: Challenges, Current Trends, and Future Perspectives in Treatment. Biomedicines 2024, 12, 1958. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.A.; Rahmani, E.; Kiick, K.L.; Sullivan, M.O. Growth Factors and Growth Factor Gene Therapies for Treating Chronic Wounds. Bioeng. Transl. Med. 2024, 9, e10642. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Safta, D.A.; Bogdan, C.; Moldovan, M.L. Vesicular Nanocarriers for Phytocompounds in Wound Care: Preparation and Characterization. Pharmaceutics 2022, 14, 991. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Sivakumar, A.S.; Lee, C.-H.; Kim, S.J. Diabetes Mellitus and Diabetic Foot Ulcer: Etiology, Biochemical and Molecular Based Treatment Strategies via Gene and Nanotherapy. Biomed. Pharmacother. 2022, 151, 113134. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process: Reactive Oxygen Species and Wound Healing. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Makhmalzade, B.; Chavoshy, F. Polymeric Micelles as Cutaneous Drug Delivery System in Normal Skin and Dermatological Disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef]
- Agner, T. (Ed.) Skin Barrier Function; Current Problems in Dermatology; S. Karger AG: Basel, Switzerland, 2016; Volume 49, ISBN 978-3-318-05585-6. [Google Scholar]
- Maver, T.; Kurečič, M.; Maja Smrke, D.; Stana Kleinschek, K.; Maver, U. Plant-Derived Medicines with Potential Use in Wound Treatment. In Herbal Medicine; Builders, P.F., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-782-6. [Google Scholar]
- Pandey, A.; Yang, T.-S.; Cheng, S.-L.; Huang, C.-S.; Brangule, A.; Kareiva, A.; Yang, J.-C. A Novel One-Pot Synthesis and Characterization of Silk Fibroin/α-Calcium Sulfate Hemihydrate for Bone Regeneration. Polymers 2021, 13, 1996. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS Comprehensive Classification System for Lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhai, G. Advances in Lipid-Based Colloid Systems as Drug Carrier for Topic Delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef]
- Ricci, A.; Stefanuto, L.; Gasperi, T.; Bruni, F.; Tofani, D. Lipid Nanovesicles for Antioxidant Delivery in Skin: Liposomes, Ufasomes, Ethosomes, and Niosomes. Antioxidants 2024, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Liang, Y.Y.; Dong, F.J.; Ma, L.; Tu, Y.; Liu, H.Y.; Jiang, J. Structure of Rat Skin after Application of Electret Characterized by DSC. J. Phys. Conf. Ser. 2011, 301, 012027. [Google Scholar] [CrossRef]
- Obata, Y.; Utsumi, S.; Watanabe, H.; Suda, M.; Tokudome, Y.; Otsuka, M.; Takayama, K. Infrared Spectroscopic Study of Lipid Interaction in Stratum Corneum Treated with Transdermal Absorption Enhancers. Int. J. Pharm. 2010, 389, 18–23. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of Nanoparticles in Percutaneous Delivery of Active Ingredients in Cosmetic Preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Ramteke, K.H.; Joshi, S.A.; Dhole, S.N. Solid Lipid Nanoparticle: A Review. IOSR J. Pharm. 2012, 2, 34–44. [Google Scholar] [CrossRef]
- Asawale, R.H.; Meshram, J.H.; Kumbhar, V.B. Solid Lipid Nanoparticle as Drug Delivery System: An Overview. Pharm. Glob. 2014, 5, 1. [Google Scholar]
- Nikam, S.; Chavan, M.; Sharma, P.H. Solid Lipid Nanoparticles: A Lipid Based Drug Delivery. Nanotechnology 2014, 1, 5. [Google Scholar]
- Akanda, M.; Mithu, M.S.H.; Douroumis, D. Solid Lipid Nanoparticles: An Effective Lipid-Based Technology for Cancer Treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Khatak, S.; Dureja, H. Recent Techniques and Patents on Solid Lipid Nanoparticles as Novel Carrier for Drug Delivery. Recent Pat. Nanotechnol. 2015, 9, 150–177. [Google Scholar] [CrossRef]
- Hernández-Esquivel, R.-A.; Navarro-Tovar, G.; Zárate-Hernández, E.; Aguirre-Bañuelos, P. Solid Lipid Nanoparticles (SLN). In Nanocomposite Materials for Biomedical and Energy Storage Applications; Sharma, A., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-618-5. [Google Scholar]
- Abdel-Mageed, H.M.; Abd El Aziz, A.E.; Mohamed, S.A.; AbuelEzz, N.Z. The Tiny Big World of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: An Updated Review. J. Microencapsul. 2022, 39, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability; SpringerBriefs in Pharmaceutical Science & Drug Development; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-10710-3. [Google Scholar]
- Della Sala, F.; Borzacchiello, A.; Dianzani, C.; Muntoni, E.; Argenziano, M.; Capucchio, M.T.; Valsania, M.C.; Bozza, A.; Garelli, S.; Di Muro, M.; et al. Ultrasmall Solid-Lipid Nanoparticles via the Polysorbate Sorbitan Phase-Inversion Temperature Technique: A Promising Vehicle for Antioxidant Delivery into the Skin. Pharmaceutics 2023, 15, 1962. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J. Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders. Cosmetics 2021, 8, 84. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral Delivery of Therapeutic Peptides and Proteins: Technology Landscape of Lipid-Based Nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Ramalho, M.J.; Silva, R.; Silva, V.; Marques-Oliveira, R.; Silva, A.C.; Pereira, M.C.; Loureiro, J.A. Vine Cane Compounds to Prevent Skin Cells Aging through Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Ramalho, M.J.; Silva, R.; Silva, V.; Marques-Oliveira, R.; Silva, A.C.; Pereira, M.C.; Loureiro, J.A. Lipid Nanoparticles Containing Mixtures of Antioxidants to Improve Skin Care and Cancer Prevention. Pharmaceutics 2021, 13, 2042. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, O.M.; Badawy, M.E.I.; Noreldin, A.E.; Abou-Ahmed, H.M.; El-Kammar, M.H.; Elkhenany, H.A. Comparative Evaluation of Propolis Nanostructured Lipid Carriers and Its Crude Extract for Antioxidants, Antimicrobial Activity, and Skin Regeneration Potential. BMC Complement. Med. Ther. 2022, 22, 256. [Google Scholar] [CrossRef]
- Sharma, N.; Vasisht, K.; Kaur, J.; Sandhu, S.K.; Dey, K.; Hameed, B.A.; Bajaj, R.; Kaur, I.P.; Karan, M. Blending Ethnomedicine with Modern Technology—From Conventional to Tailored Products: Modulating Biopharmaceutical Properties of Berberis Extract by Solid Lipid Nanoparticles for Wound Healing. J. Funct. Biomater. 2023, 14, 418. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Kumar, S.; Raut, J.; Singh, M.; Kaur, S.; Sharma, G.; Roldan, T.L.; Trehan, S.; Holloway, J.; Wahler, G.; et al. Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing. Antioxidants 2021, 10, 725. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured Lipid Carrier (NLC) as a Strategy for Encapsulation of Quercetin and Linseed Oil: Preparation and in Vitro Characterization Studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Pulmonary Application: A Review of the State of the Art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Garg, J.; Pathania, K.; Sah, S.P.; Pawar, S.V. Nanostructured Lipid Carriers: A Promising Drug Carrier for Targeting Brain Tumours. Future J. Pharm. Sci. 2022, 8, 25. [Google Scholar] [CrossRef]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for Preparation of Nanostructured Lipid Carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef]
- Sharma, K.; Hallan, S.S.; Lal, B.; Bhardwaj, A.; Mishra, N. Development and Characterization of Floating Spheroids of Atorvastatin Calcium Loaded NLC for Enhancement of Oral Bioavailability. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1448–1456. [Google Scholar] [CrossRef]
- Singh Hallan, S.; Sguizzato, M.; Pavoni, G.; Baldisserotto, A.; Drechsler, M.; Mariani, P.; Esposito, E.; Cortesi, R. Ellagic Acid Containing Nanostructured Lipid Carriers for Topical Application: A Preliminary Study. Molecules 2020, 25, 1449. [Google Scholar] [CrossRef] [PubMed]
- Chander, N.; Morstein, J.; Bolten, J.S.; Shemet, A.; Cullis, P.R.; Trauner, D.; Witzigmann, D. Optimized Photoactivatable Lipid Nanoparticles Enable Red Light Triggered Drug Release. Small 2021, 17, 2008198. [Google Scholar] [CrossRef]
- de Barros, D.P.C.; Santos, R.; Reed, P.; Fonseca, L.P.; Oliva, A. Design of Quercetin-Loaded Natural Oil-Based Nanostructured Lipid Carriers for the Treatment of Bacterial Skin Infections. Molecules 2022, 27, 8818. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.V.d.C.; Leão, K.M.; Speranza, P.; Ribeiro, A.P.B.; Macedo, G.A.; Macedo, J.A. Evaluation of Nanostructured Lipid Carriers Produced with Interesterified Buriti Oil. Food Technol. Biotechnol. (Online) 2020, 58, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Emanet, M.; Şen, Ö.; Pignatelli, F.; Lavarello, C.; Petretto, A.; Ciofani, G. Hazelnut Extract-Loaded Nanostructured Lipid Carriers and Evaluation of Their Antioxidant Properties. Front. Bioeng. Biotechnol. 2022, 10, 953867. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Nanostructured Lipid Carriers (NLCs) Stabilized by Natural or Synthetic Emulsifiers for Lutein Delivery: Improved Physicochemical Stability, Antioxidant Activity, and Bioaccessibility. Food Chem. 2023, 403, 134465. [Google Scholar] [CrossRef]
- Badalkhani, O.; Pires, P.C.; Mohammadi, M.; Babaie, S.; Paiva-Santos, A.C.; Hamishehkar, H. Nanogel Containing Gamma-Oryzanol-Loaded Nanostructured Lipid Carriers and TiO2/MBBT: A Synergistic Nanotechnological Approach of Potent Natural Antioxidants and Nanosized UV Filters for Skin Protection. Pharmaceuticals 2023, 16, 670. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated Linoleic Acid Loaded Nanostructured Lipid Carrier as a Potential Antioxidant Nanocarrier for Food Applications. Food Sci. Nutr. 2020, 8, 4185–4195. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of Oleuropein in Nanostructured Lipid Carriers: Biocompatibility and Antioxidant Efficacy in Lung Epithelial Cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Rohmah, M.; Rahmadi, A.; Raharjo, S. Bioaccessibility and Antioxidant Activity of β-Carotene Loaded Nanostructured Lipid Carrier (NLC) from Binary Mixtures of Palm Stearin and Palm Olein. Heliyon 2022, 8, e08913. [Google Scholar] [CrossRef]
- Saejung, T.; Don-In, J.; Chimsook, T. Preparation of Ethanolic Butterfly Pea Extract Using Microwave Assisted Extraction and Loaded Nanostructured Lipid Carriers: Evaluation of Antioxidant Potential for Stabilization of Fish Oil. Key Eng. Mater. 2021, 873, 1–5. [Google Scholar] [CrossRef]
- Wiemann, S.; Keck, C.M. Are Lipid Nanoparticles Really Superior? A Holistic Proof of Concept Study. Drug Deliv. Transl. Res. 2022, 12, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Mazur, F.; Bally, M.; Städler, B.; Chandrawati, R. Liposomes and Lipid Bilayers in Biosensors. Adv. Colloid Interface Sci. 2017, 249, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668, IN2–IN10. [Google Scholar] [CrossRef] [PubMed]
- Mirzavi, F.; Barati, M.; Soleimani, A.; Vakili-Ghartavol, R.; Jaafari, M.R.; Soukhtanloo, M. A Review on Liposome-Based Therapeutic Approaches against Malignant Melanoma. Int. J. Pharm. 2021, 599, 120413. [Google Scholar] [CrossRef]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design Considerations for Liposomal Vaccines: Influence of Formulation Parameters on Antibody and Cell-Mediated Immune Responses to Liposome Associated Antigens. Vaccine 2012, 30, 2256–2272. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Gawne, P.J.; de Rosales, R.T.M. Nuclear Imaging of Liposomal Drug Delivery Systems: A Critical Review of Radiolabelling Methods and Applications in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. Predicting Hydrophilic Drug Encapsulation inside Unilamellar Liposomes. Int. J. Pharm. 2012, 423, 410–418. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Fransen, G.J.; Salemink, P.J.M. Stability of Liposomes on Storage. In Targeting of Drugs with Synthetic Systems; Gregoriadis, G., Senior, J., Poste, G., Eds.; Springer: Boston, MA, USA, 1986; pp. 277–287. ISBN 978-1-4684-5187-0. [Google Scholar]
- Ezzat, H.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Improved Oral Bioavailability of the Anticancer Drug Catechin Using Chitosomes: Design, in-Vitro Appraisal and in-Vivo Studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Mertins, O.; Dimova, R. Binding of Chitosan to Phospholipid Vesicles Studied with Isothermal Titration Calorimetry. Langmuir 2011, 27, 5506–5515. [Google Scholar] [CrossRef]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current Advances in Niosomes Applications for Drug Delivery and Cancer Treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef]
- Mann, J.F.S.; Scales, H.E.; Shakir, E.; Alexander, J.; Carter, K.C.; Mullen, A.B.; Ferro, V.A. Oral Delivery of Tetanus Toxoid Using Vesicles Containing Bile Salts (Bilosomes) Induces Significant Systemic and Mucosal Immunity. Methods 2006, 38, 90–95. [Google Scholar] [CrossRef]
- Yang, L.; Tucker, I.G.; Østergaard, J. Effects of Bile Salts on Propranolol Distribution into Liposomes Studied by Capillary Electrophoresis. J. Pharm. Biomed. Anal. 2011, 56, 553–559. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as Versatile and Flexible Nano-Vesicular Carriers in Skin Cancer Therapy: The State of the Art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef]
- Cevc, G.; Schätzlein, A.; Richardsen, H. Ultradeformable Lipid Vesicles Can Penetrate the Skin and Other Semi-Permeable Barriers Unfragmented. Evidence from Double Label CLSM Experiments and Direct Size Measurements. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1564, 21–30. [Google Scholar] [CrossRef]
- Rother, M.; Lavins, B.J.; Kneer, W.; Lehnhardt, K.; Seidel, E.J.; Mazgareanu, S. Efficacy and Safety of Epicutaneous Ketoprofen in Transfersome (IDEA-033) versus Oral Celecoxib and Placebo in Osteoarthritis of the Knee: Multicentre Randomised Controlled Trial. Ann. Rheum. Dis. 2007, 66, 1178–1183. [Google Scholar] [CrossRef]
- Fesq, H.; Lehmann, J.; Kontny, A.; Erdmann, I.; Theiling, K.; Rother, M.; Ring, J.; Cevc, G.; Abeck, D. Improved Risk-Benefit Ratio for Topical Triamcinolone Acetonide in TransfersomeR in Comparison with Equipotent Cream and Ointment: A Randomized Controlled Trial. Br. J. Dermatol. 2003, 149, 611–619. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and Characterization of Ethosomes for Transdermal Delivery of Caffeic Acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel Vesicular Carriers for Enhanced Delivery: Characterization and Skin Penetration Properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Abdul Karim Khan, N.; Abou Assi, R.; Ali Khan, A. Ethosomal Nanocarriers: The Impact of Constituents and Formulation Techniques on Ethosomal Properties, in Vivo Studies, and Clinical Trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Himeno, T.; Konno, Y.; Naito, N. Liposomes for Cosmetics. In Cosmetic Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 539–549. ISBN 978-0-12-802005-0. [Google Scholar]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research Progress on Liposomes: Application in Food, Digestion Behavior and Absorption Mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Henne, W.M.; Reese, M.L.; Goodman, J.M. The Assembly of Lipid Droplets and Their Roles in Challenged Cells. EMBO J. 2019, 38, e101816. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Sriwidodo; Umar, A.K.; Wathoni, N.; Zothantluanga, J.H.; Das, S.; Luckanagul, J.A. Liposome-Polymer Complex for Drug Delivery System and Vaccine Stabilization. Heliyon 2022, 8, e08934. [Google Scholar] [CrossRef]
- Hallan, S.S.; Marchetti, P.; Bortolotti, D.; Sguizzato, M.; Esposito, E.; Mariani, P.; Trapella, C.; Rizzo, R.; Cortesi, R. Design of Nanosystems for the Delivery of Quorum Sensing Inhibitors: A Preliminary Study. Molecules 2020, 25, 5655. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, X.; Lu, B.; Zhang, J. Preparation of Blueberry Anthocyanin Liposomes and Changes of Vesicle Properties, Physicochemical Properties, in Vitro Release, and Antioxidant Activity before and after Chitosan Modification. Food Sci. Nutr. 2022, 10, 75–87. [Google Scholar] [CrossRef]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef] [PubMed]
- Badhe, Y.; Sharma, P.; Gupta, R.; Rai, B. Elucidating Collective Translocation of Nanoparticles across the Skin Lipid Matrix: A Molecular Dynamics Study. Nanoscale Adv. 2023, 5, 1978–1989. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Rai, B.; Mitragotri, S. Molecular Mechanism of the Skin Permeation Enhancing Effect of Ethanol: A Molecular Dynamics Study. RSC Adv. 2020, 10, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Liposomes Increase Skin Penetration of Entrapped and Non-Entrapped Hydrophilic Substances into Human Skin: A Skin Penetration and Confocal Laser Scanning Microscopy Study. Eur. J. Pharm. Biopharm. 2003, 55, 271–277. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Balata, G.F.; Faisal, M.M.; Elghamry, H.A.; Sabry, S.A. Preparation and Characterization of Ivabradine HCl Transfersomes for Enhanced Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 101921. [Google Scholar] [CrossRef]
- Hua, S. Lipid-Based Nano-Delivery Systems for Skin Delivery of Drugs and Bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J. Structure of the Skin Barrier and Its Modulation by Vesicular Formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Geusens, B.; Strobbe, T.; Bracke, S.; Dynoodt, P.; Sanders, N.; Gele, M.V.; Lambert, J. Lipid-Mediated Gene Delivery to the Skin. Eur. J. Pharm. Sci. 2011, 43, 199–211. [Google Scholar] [CrossRef]
- Kajimoto, K.; Yamamoto, M.; Watanabe, M.; Kigasawa, K.; Kanamura, K.; Harashima, H.; Kogure, K. Noninvasive and Persistent Transfollicular Drug Delivery System Using a Combination of Liposomes and Iontophoresis. Int. J. Pharm. 2011, 403, 57–65. [Google Scholar] [CrossRef]
- Lin, H.; Xie, Q.; Huang, X.; Ban, J.; Wang, B.; Wei, X.; Chen, Y.; Lu, Z. Increased Skin Permeation Efficiency of Imperatorin via Charged Ultradeformable Lipid Vesicles for Transdermal Delivery. Int. J. Nanomed. 2018, 13, 831–842. [Google Scholar] [CrossRef]
- Lőrincz, A.; Mihály, J.; Németh, C.; Wacha, A.; Bóta, A. Effects of Ursolic Acid on the Structural and Morphological Behaviours of Dipalmitoyl Lecithin Vesicles. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2015, 1848, 1092–1098. [Google Scholar] [CrossRef]
- Altunayar-Unsalan, C. DSC and FTIR Study on the Interaction between Pentacyclic Triterpenoid Lupeol and DPPC Membrane. J. Bioenerg. Biomembr. 2024, 56, 553–561. [Google Scholar] [CrossRef]
- Abboud, R.; Charcosset, C.; Greige-Gerges, H. Tetra- and Penta-Cyclic Triterpenes Interaction with Lipid Bilayer Membrane: A Structural Comparative Study. J. Membr. Biol. 2016, 249, 327–338. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Tao, W.; Yang, X.; Li, J.; Wang, R.; Zhao, Y.; Peng, C.; Zhang, C. Preparation of Paeoniflorin-Glycyrrhizic Acid Complex Transethosome Gel and Its Preventive and Therapeutic Effects on Melasma. Eur. J. Pharm. Sci. 2024, 192, 106664. [Google Scholar] [CrossRef] [PubMed]
- Le Guyader, G.; Do, B.; Rietveld, I.B.; Coric, P.; Bouaziz, S.; Guigner, J.-M.; Secretan, P.-H.; Andrieux, K.; Paul, M. Mixed Polymeric Micelles for Rapamycin Skin Delivery. Pharmaceutics 2022, 14, 569. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.; Rady, M.; Gomaa, I.; Syrovets, T.; Simmet, T.; Fayad, W.; Abdel-Kader, M. Ethosomes and Lipid-Coated Chitosan Nanocarriers for Skin Delivery of a Chlorophyll Derivative: A Potential Treatment of Squamous Cell Carcinoma by Photodynamic Therapy. Int. J. Pharm. 2019, 568, 118528. [Google Scholar] [CrossRef] [PubMed]
- Šmejkalová, D.; Muthný, T.; Nešporová, K.; Hermannová, M.; Achbergerová, E.; Huerta-Angeles, G.; Svoboda, M.; Čepa, M.; Machalová, V.; Luptáková, D.; et al. Hyaluronan Polymeric Micelles for Topical Drug Delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Go, Y.K.; Leal, C. Polymer–Lipid Hybrid Materials. Chem. Rev. 2021, 121, 13996–14030. [Google Scholar] [CrossRef] [PubMed]
- Jangde, R.; Elhassan, G.O.; Khute, S.; Singh, D.; Singh, M.; Sahu, R.K.; Khan, J. Hesperidin-Loaded Lipid Polymer Hybrid Nanoparticles for Topical Delivery of Bioactive Drugs. Pharmaceuticals 2022, 15, 211. [Google Scholar] [CrossRef]
- Schulz, M.; Binder, W.H. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromol. Rapid Commun. 2015, 36, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Bin Jardan, Y.A.; Ahad, A.; Raish, M.; Al-Jenoobi, F.I. Preparation and Characterization of Transethosome Formulation for the Enhanced Delivery of Sinapic Acid. Pharmaceutics 2023, 15, 2391. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hofni, A.; Abourehab, M.A.; Abdel-Rahman, I.A. Ginger Extract–Loaded Transethosomes for Effective Transdermal Permeation and Anti-Inflammation in Rat Model. Int. J. Nanomed. 2023, 18, 1259–1280. [Google Scholar] [CrossRef]
- Le Meins, J.-F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid Polymer/Lipid Vesicles: State of the Art and Future Perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Larrañeta, E.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Microneedles: A New Frontier in Nanomedicine Delivery. Pharm. Res. 2016, 33, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.J.; Jornet-Mollá, E.; Landsberg, N.; Milián-Guimerá, C.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Cyanocobalamin Ultraflexible Lipid Vesicles: Characterization and In Vitro Evaluation of Drug-Skin Depth Profiles. Pharmaceutics 2021, 13, 418. [Google Scholar] [CrossRef]
- Rodgers, A.M.; Cordeiro, A.S.; Kissenpfennig, A.; Donnelly, R.F. Microneedle Arrays for Vaccine Delivery: The Possibilities, Challenges and Use of Nanoparticles as a Combinatorial Approach for Enhanced Vaccine Immunogenicity. Expert Opin. Drug Deliv. 2018, 15, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Mooney, K.; Mccrudden, M.T.C.; Vicente-Pérez, E.M.; Belaid, L.; González-Vázquez, P.; Mcelnay, J.C.; David Woolfson, A. Hydrogel-Forming Microneedles Increase in Volume During Swelling in Skin, but Skin Barrier Function Recovery Is Unaffected. J. Pharm. Sci. 2014, 103, 1478–1486. [Google Scholar] [CrossRef]
- Kamila, M.Z.; Helena, R. The Effectiveness of Ferulic Acid and Microneedling in Reducing Signs of Photoaging: A Split-face Comparative Study. Dermatol. Ther. 2020, 33, e14000. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Wu, Y.-W.; Hung, J.-I.; Chen, M.-C. Epigallocatechin Gallate/L-Ascorbic Acid–Loaded Poly-γ-Glutamate Microneedles with Antioxidant, Anti-Inflammatory, and Immunomodulatory Effects for the Treatment of Atopic Dermatitis. Acta Biomater. 2021, 130, 223–233. [Google Scholar] [CrossRef]
- Melero, A.; Garrigues, T.M.; Almudever, P.; Villodre, A.M.; Lehr, C.M.; Schäfer, U. Nortriptyline Hydrochloride Skin Absorption: Development of a Transdermal Patch. Eur. J. Pharm. Biopharm. 2008, 69, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, H.; Wang, Z.; Yang, X.; Zhang, M.; Liu, X.; Wang, B.; Wu, Z.; Chen, D. Preparation and Characterization of Dissolving Hyaluronic Acid Composite Microneedles Loaded Micelles for Delivery of Curcumin. Drug Deliv. Transl. Res. 2020, 10, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, C.; Li, X.; Wen, T.; Wu, Q.; Zhang, A.; Hu, P.; Wu, C.; Pan, X.; Huang, Z.; et al. Demonstrating Biological Fate of Nanoparticle-Loaded Dissolving Microneedles with Aggregation-Caused Quenching Probes: Influence of Application Sites. Pharmaceutics 2023, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Azim, H.; Tekko, I.A.; Ali, A.; Ramadan, A.; Nafee, N.; Khalafallah, N.; Rahman, T.; Mcdaid, W.; Aly, R.G.; Vora, L.K.; et al. Hollow Microneedle Assisted Intradermal Delivery of Hypericin Lipid Nanocapsules with Light Enabled Photodynamic Therapy against Skin Cancer. J. Control. Release 2022, 348, 849–869. [Google Scholar] [CrossRef]
- Prabhu, A.; Jose, J.; Kumar, L.; Salwa, S.; Vijay Kumar, M.; Nabavi, S.M. Transdermal Delivery of Curcumin-Loaded Solid Lipid Nanoparticles as Microneedle Patch: An In Vitro and In Vivo Study. AAPS PharmSciTech 2022, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Prabahar, K.; Udhumansha, U.; Elsherbiny, N.; Qushawy, M. Microneedle Mediated Transdermal Delivery of β-Sitosterol Loaded Nanostructured Lipid Nanoparticles for Androgenic Alopecia. Drug Deliv. 2022, 29, 3022–3034. [Google Scholar] [CrossRef]
- Gorantla, S.; Dabholkar, N.; Sharma, S.; Rapalli, V.K.; Alexander, A.; Singhvi, G. Chitosan-Based Microneedles as a Potential Platform for Drug Delivery through the Skin: Trends and Regulatory Aspects. Int. J. Biol. Macromol. 2021, 184, 438–453. [Google Scholar] [CrossRef]
- Cui, Y.; Mo, Y.; Zhang, Q.; Tian, W.; Xue, Y.; Bai, J.; Du, S. Microneedle-Assisted Percutaneous Delivery of Paeoniflorin-Loaded Ethosomes. Molecules 2018, 23, 3371. [Google Scholar] [CrossRef] [PubMed]
- Vladár, A.E.; Hodoroaba, V.-D. Characterization of Nanoparticles by Scanning Electron Microscopy. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. ISBN 978-0-12-814182-3. [Google Scholar]
- Dubes, A.; Parrot-Lopez, H.; Abdelwahed, W.; Degobert, G.; Fessi, H.; Shahgaldian, P.; Coleman, A.W. Scanning Electron Microscopy and Atomic Force Microscopy Imaging of Solid Lipid Nanoparticles Derived from Amphiphilic Cyclodextrins. Eur. J. Pharm. Biopharm. 2003, 55, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.A.; Ali, N.; Hamzah, S.; Yatim, N.I. Physicochemical Characteristics of Liposome Encapsulation of Stingless Bees’ Propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef]
- Robson, A.-L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and Limitations of Current Imaging Techniques for Characterizing Liposome Morphology. Front. Pharmacol. 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, J.S.; Milne, J.L.; Subramaniam, S. Electron Tomography in Nanoparticle Imaging and Analysis. Nanomedicine 2008, 3, 125–131. [Google Scholar] [CrossRef]
- Stewart, P.L. Cryo-electron Microscopy and Cryo-electron Tomography of Nanoparticles. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1417. [Google Scholar] [CrossRef]
- Gupta, K.M.; Das, S.; Chow, P.S.; Macbeath, C. Encapsulation of Ferulic Acid in Lipid Nanoparticles as Antioxidant for Skin: Mechanistic Understanding through Experiment and Molecular Simulation. ACS Appl. Nano Mater. 2020, 3, 5351–5361. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical Characterization of Liposomes and Other Lipid Nanoparticles for Drug Delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Aanisah, N.; Sulistiawati, S.; Djabir, Y.Y.; Asri, R.M.; Sumarheni, S.; Chabib, L.; Hamzah, H.; Permana, A.D. Development of Solid Lipid Nanoparticle-Loaded Polymeric Hydrogels Containing Antioxidant and Photoprotective Bioactive Compounds of Safflower (Carthamus tinctorius L.) for Improved Skin Delivery. Langmuir 2023, 39, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, W.; Wu, B.; Ji, S.; Xia, Q. Preparation and in Vitro Characterization Studies of Astaxanthin-Loaded Nanostructured Lipid Carriers with Antioxidant Properties. J. Biomater. Appl. 2023, 38, 292–301. [Google Scholar] [CrossRef]
- Shaji, J.; Varkey, D. Silica-Coated Solid Lipid Nanoparticles Enhance Antioxidant and Antiradical Effects of Meloxicam. J. Pharm. Investig. 2013, 43, 405–416. [Google Scholar] [CrossRef]
- Trapani, A.; Esteban, M.Á.; Curci, F.; Manno, D.E.; Serra, A.; Fracchiolla, G.; Espinosa-Ruiz, C.; Castellani, S.; Conese, M. Solid Lipid Nanoparticles Administering Antioxidant Grape Seed-Derived Polyphenol Compounds: A Potential Application in Aquaculture. Molecules 2022, 27, 344. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Yang, S.; Ding, H.; Wang, R.; Dai, X.; Shi, X.; Qiao, Y. Molecular Dynamics Simulation Studies of Transmembrane Transport of Chemical Components in Chinese Herbs and the Function of Platycodin D in a Biological Membrane. J. Tradit. Chin. Med. Sci. 2017, 4, 174–183. [Google Scholar] [CrossRef]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, D.P.; de Vries, A.H. The MARTINI Force Field: Coarse Grained Model for Biomolecular Simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Valacchi, G.; Cortesi, R. Lipid Nanostructures for Antioxidant Delivery: A Comparative Preformulation Study. Beilstein J. Nanotechnol. 2019, 10, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, F.; Menziani, M.C. Computational Insight on the Interaction of Common Blood Proteins with Gold Nanoparticles. Int. J. Mol. Sci. 2021, 22, 8722. [Google Scholar] [CrossRef]
- Kubackova, J.; Holas, O.; Zbytovska, J.; Vranikova, B.; Zeng, G.; Pavek, P.; Mullertz, A. Oligonucleotide Delivery across the Caco-2 Monolayer: The Design and Evaluation of Self-Emulsifying Drug Delivery Systems (SEDDS). Pharmaceutics 2021, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A Game Changing Approach for the Oral Administration of Hydrophilic Macromolecular Drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Le-Vinh, B.; Le, N.-M.N.; Phan, T.N.Q.; Lam, H.T.; Bernkop-Schnürch, A. Effects of Excipients on the Interactions of Self-Emulsifying Drug Delivery Systems with Human Blood Plasma and Plasma Membranes. Drug Deliv. Transl. Res. 2024, 14, 3200–3211. [Google Scholar] [CrossRef]
- Driscoll, D.F. Commercial Lipid Emulsions and All-in-One Mixtures for Intravenous Infusion—Composition and Physicochemical Properties. In World Review of Nutrition and Dietetics; Calder, P.C., Waitzberg, D.L., Koletzko, B., Eds.; S. Karger AG: Basel, Switzerland, 2015; Volume 112, pp. 48–56. ISBN 978-3-318-02752-5. [Google Scholar]
- Benson, S.P.; Pleiss, J. Molecular Dynamics Simulations of Self-Emulsifying Drug-Delivery Systems (SEDDS): Influence of Excipients on Droplet Nanostructure and Drug Localization. Langmuir 2014, 30, 8471–8480. [Google Scholar] [CrossRef]
- Chaban, V.V.; Khandelia, H. Lipid Structure in Triolein Lipid Droplets. J. Phys. Chem. B 2014, 118, 10335–10340. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, B.; Miao, S. Improved Emulsion Stability and Modified Nutrient Release by Structuring O/W Emulsions Using Konjac Glucomannan. Food Hydrocoll. 2018, 81, 120–128. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Y.; Gao, C.; Meng, L.; Feng, X.; Wang, Z.; Shen, X.; Tang, X. Preparation and Characterization of Starch/PBAT Film Containing Hydroxypropyl-β-Cyclodextrin/Ethyl Lauroyl Arginate/Cinnamon Essential Oil Microcapsules and Its Application in the Preservation of Strawberry. Int. J. Biol. Macromol. 2024, 259, 129204. [Google Scholar] [CrossRef]
- Fixman, M. Radius of Gyration of Polymer Chains. II. Segment Density and Excluded Volume Effects. J. Chem. Phys. 1962, 36, 3123–3129. [Google Scholar] [CrossRef]
- Weerapol, Y.; Manmuan, S.; Chaothanaphat, N.; Limmatvapirat, S.; Sirirak, J.; Tamdee, P.; Tubtimsri, S. New Approach for Preparing Solid Lipid Nanoparticles with Volatile Oil-Loaded Quercetin Using the Phase-Inversion Temperature Method. Pharmaceutics 2022, 14, 1984. [Google Scholar] [CrossRef]
- Parchekani, J.; Allahverdi, A.; Taghdir, M.; Naderi-Manesh, H. Design and Simulation of the Liposomal Model by Using a Coarse-Grained Molecular Dynamics Approach towards Drug Delivery Goals. Sci. Rep. 2022, 12, 2371. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.P.; Thakur, N.; Pour, N.G.; Eddy, M.T. Dual Mechanisms of Cholesterol-GPCR Interactions That Depend on Membrane Phospholipid Composition. Structure 2023, 31, 836–847. [Google Scholar] [CrossRef]
- Chaban, V.V.; Khandelia, H. Distribution of Neutral Lipids in the Lipid Droplet Core. J. Phys. Chem. B 2014, 118, 11145–11151. [Google Scholar] [CrossRef] [PubMed]
- Urimi, D.; Hellsing, M.; Mahmoudi, N.; Söderberg, C.; Widenbring, R.; Gedda, L.; Edwards, K.; Loftsson, T.; Schipper, N. Structural Characterization Study of a Lipid Nanocapsule Formulation Intended for Drug Delivery Applications Using Small-Angle Scattering Techniques. Mol. Pharm. 2022, 19, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diez, R.; Gollwitzer, C.; Krumrey, M.; Varga, Z. Size Determination of a Liposomal Drug by Small-Angle X-Ray Scattering Using Continuous Contrast Variation. Langmuir 2016, 32, 772–778. [Google Scholar] [CrossRef]
- Nele, V.; Holme, M.N.; Kauscher, U.; Thomas, M.R.; Doutch, J.J.; Stevens, M.M. Effect of Formulation Method, Lipid Composition, and PEGylation on Vesicle Lamellarity: A Small-Angle Neutron Scattering Study. Langmuir 2019, 35, 6064–6074. [Google Scholar] [CrossRef] [PubMed]
- Castelletto, V.; Seitsonen, J.; de Mello, L.R.; Hamley, I.W. Interaction of Arginine-Rich Surfactant-like Peptide Nanotubes with Liposomes. Biomacromolecules 2024, 25, 7410–7420. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Maroto, A.; Rubio, J.E.F.; Prévost, S.; Maestro, A.; Rubio, R.G.; Ortega, F.; Guzmán, E. Probing the Effect of the Capping Polyelectrolyte on the Internal Structure of Layer-by-Layer Decorated Nanoliposomes. J. Colloid Interface Sci. 2023, 640, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, F.; Moretti, P.; Perinelli, D.R.; Corucci, G.; Piergiovanni, P.; Amenitsch, H.; Sancini, G.A.; Franzese, G.; Blasi, P. Small-Angle X-Ray Scattering Unveils the Internal Structure of Lipid Nanoparticles. J. Colloid Interface Sci. 2024, 662, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, M. Evaluation of the Supramolecular Structure of Drug Delivery Carriers Using Synchrotron X-Ray Scattering. Polym. J. 2021, 53, 1335–1344. [Google Scholar] [CrossRef]
- Ausili, A.; Torrecillas, A.; de Godos, A.M.; Corbalán-García, S.; Gómez-Fernández, J.C. Phenolic Group of α-Tocopherol Anchors at the Lipid–Water Interface of Fully Saturated Membranes. Langmuir 2018, 34, 3336–3348. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Siejak, P.; Pietralik, Z.; Kozak, M.; Polewski, K. Disruptive Effect of Tocopherol Oxalate on DPPC Liposome Structure: DSC, SAXS, and Fluorescence Anisotropy Studies. Chem. Phys. Lipids 2018, 216, 104–113. [Google Scholar] [CrossRef]
- Clemente, I.; Bonechi, C.; Rodolfi, L.; Bacia-Verloop, M.; Rossi, C.; Ristori, S. Lipids from Algal Biomass Provide New (Nonlamellar) Nanovectors with High Carrier Potentiality for Natural Antioxidants. Eur. J. Pharm. Biopharm. 2021, 158, 410–416. [Google Scholar] [CrossRef]
- Fernandes, E.; Benfeito, S.; Cagide, F.; Gonçalves, H.; Bernstorff, S.; Nieder, J.B.; CD Real Oliveira, M.E.; Borges, F.; Lúcio, M. Lipid Nanosystems and Serum Protein as Biomimetic Interfaces: Predicting the Biodistribution of a Caffeic Acid-Based Antioxidant. Nanotechnol. Sci. Appl. 2021, 14, 7–27. [Google Scholar] [CrossRef]
- Fornasier, M.; Dessì, F.; Pireddu, R.; Sinico, C.; Carretti, E.; Murgia, S. Lipid Vesicular Gels for Topical Administration of Antioxidants. Colloids Surf. B Biointerfaces 2022, 213, 112388. [Google Scholar] [CrossRef]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, Biocompatibility and Antioxidant Activity of PEG-Modified Liposomes Containing Resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Cardile, V.; Panico, A.M.; Crascì, L.; Offerta, A.; Caggia, S.; Drechsler, M.; Mariani, P.; Cortesi, R.; Esposito, E. Evaluation of Monooleine Aqueous Dispersions as Tools for Topical Administration of Curcumin: Characterization, In Vitro and Ex-Vivo Studies. J. Pharm. Sci. 2013, 102, 2349–2361. [Google Scholar] [CrossRef]
- Esposito, E.; Mariani, P.; Ravani, L.; Contado, C.; Volta, M.; Bido, S.; Drechsler, M.; Mazzoni, S.; Menegatti, E.; Morari, M.; et al. Nanoparticulate Lipid Dispersions for Bromocriptine Delivery: Characterization and in Vivo Study. Eur. J. Pharm. Biopharm. 2012, 80, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Drechsler, M.; Mariani, P.; Panico, A.M.; Cardile, V.; Crascì, L.; Carducci, F.; Graziano, A.C.E.; Cortesi, R.; Puglia, C. Nanostructured Lipid Dispersions for Topical Administration of Crocin, a Potent Antioxidant from Saffron (Crocus sativus L.). Mater. Sci. Eng. C 2017, 71, 669–677. [Google Scholar] [CrossRef]
- Li, J.; Chang, C.; Zhai, J.; Yang, Y.; Yu, H. Ascorbyl Palmitate Effects on the Stability of Curcumin-Loaded Soybean Phosphatidylcholine Liposomes. Food Biosci. 2021, 41, 100923. [Google Scholar] [CrossRef]
- Mottola, M.; Wilke, N.; Benedini, L.; Oliveira, R.G.; Fanani, M.L. Ascorbyl Palmitate Interaction with Phospholipid Monolayers: Electrostatic and Rheological Preponderancy. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 2496–2505. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, R.; Kathpalia, M.; Ahmed, B.; Dua, K.; Gulati, M.; Singh, S.; Singh, P.J.; Kumar, S.; Shah, R.M.; et al. Innovative Approaches to Wound Healing: Insights into Interactive Dressings and Future Directions. J. Mater. Chem. B 2024, 12, 7977–8006. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Almoshari, Y. Novel Hydrogels for Topical Applications: An Updated Comprehensive Review Based on Source. Gels 2022, 8, 174. [Google Scholar] [CrossRef]
- Hsueh, Y.-S.; Shyong, Y.-J.; Yu, H.-C.; Jheng, S.-J.; Lin, S.-W.; Wu, H.-L.; Tsai, J.-C. Nanostructured Lipid Carrier Gel Formulation of Recombinant Human Thrombomodulin Improve Diabetic Wound Healing by Topical Administration. Pharmaceutics 2021, 13, 1386. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, P.; ZhuGe, D.; Zhu, Q.; Jin, B.; Shen, B.; Xiao, J.; Zhao, Y. Liposomes with Silk Fibroin Hydrogel Core to Stabilize BFGF and Promote the Wound Healing of Mice with Deep Second-Degree Scald. Adv. Healthc. Mater. 2017, 6, 1700344. [Google Scholar] [CrossRef]
- El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Massik, M.A.; Abdallah, O.Y. Novel Curcumin-Loaded Gel-Core Hyaluosomes with Promising Burn-Wound Healing Potential: Development, in-Vitro Appraisal and in-Vivo Studies. Int. J. Pharm. 2015, 486, 88–98. [Google Scholar] [CrossRef]
- Pandey, S.; Shamim, A.; Shaif, M.; Kushwaha, P. Development and Evaluation of Resveratrol-Loaded Liposomes in Hydrogel-Based Wound Dressing for Diabetic Foot Ulcer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1811–1825. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Mohamad, S.A.; Abdelnaser, M.; Yahia, R.; Mokhtar, F.A.; Alsenani, F.; Badr, M.Y.; Almaghrabi, S.Y.; Altemani, F.H.; Alzubaidi, M.A.; et al. Vitis Vinifera Leaf Extract Liposomal Carbopol Gel Preparation’s Potential Wound Healing and Antibacterial Benefits: In Vivo, Phytochemical, and Computational Investigation. Food Funct. 2023, 14, 7156–7175. [Google Scholar] [CrossRef]
- Karatas, O.; Gevrek, F. Gallic Acid Liposome and Powder Gels Improved Wound Healing in Wistar Rats. Ann. Med. Res. 2019, 26, 2720. [Google Scholar] [CrossRef]
- Fatima, F.; Aleemuddin, M.; Ahmed, M.M.; Anwer, M.K.; Aldawsari, M.F.; Soliman, G.A.; Mahdi, W.A.; Jafar, M.; Hamad, A.M.; Alshehri, S. Design and Evaluation of Solid Lipid Nanoparticles Loaded Topical Gels: Repurpose of Fluoxetine in Diabetic Wound Healing. Gels 2022, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Rahimnia, S.M.; Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Ghasemi, M.; Abootorabi, S.; Negarandeh, R.; Eghbali, M.; Jafarkhani, B.; Boskabadi, M.; et al. Green Preparation, Characterization, Wound Healing Assessment, and Histopathological Evaluation of Vitamin A Encapsulated in Niosome and Solid Lipid Nanoparticle. Pharm. Sci. 2024, 30, 70–84. [Google Scholar] [CrossRef]
- Gupta, B.; Sharma, G.; Sharma, P.; Sandhu, S.K.; Kaur, I.P. Self-Gelling Solid Lipid Nanoparticle Hydrogel Containing Simvastatin as Suitable Wound Dressing: An Investigative Study. Gels 2022, 8, 58. [Google Scholar] [CrossRef]
- Ibrahim, S.A. Spray-on Transdermal Drug Delivery Systems. Expert Opin. Drug Deliv. 2015, 12, 195–205. [Google Scholar] [CrossRef]
- Umar, A.K.; Sriwidodo, S.; Maksum, I.P.; Wathoni, N. Film-Forming Spray of Water-Soluble Chitosan Containing Liposome-Coated Human Epidermal Growth Factor for Wound Healing. Molecules 2021, 26, 5326. [Google Scholar] [CrossRef]
- Dawoud, M.H.; Yassin, G.E.; Ghorab, D.M.; Morsi, N.M. Response Surface Optimization and In-Vitro Evaluation of Sustained Release Topical Insulin Liposomal Spray for Wound Healing. J. Appl. Pharm. Sci. 2018, 8, 22–29. [Google Scholar] [CrossRef]
- Vaghasiya, K.; Sharma, A.; Kumar, K.; Ray, E.; Adlakha, S.; Katare, O.P.; Hota, S.K.; Verma, R.K. Heparin-Encapsulated Metered-Dose Topical “Nano-Spray Gel” Liposomal Formulation Ensures Rapid On-Site Management of Frostbite Injury by Inflammatory Cytokines Scavenging. ACS Biomater. Sci. Eng. 2019, 5, 6617–6631. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef]
- Caselli, L.; Conti, L.; De Santis, I.; Berti, D. Small-Angle X-Ray and Neutron Scattering Applied to Lipid-Based Nanoparticles: Recent Advancements across Different Length Scales. Adv. Colloid Interface Sci. 2024, 327, 103156. [Google Scholar] [CrossRef]
- Ranade, S.; Bajaj, A.; Londhe, V.; Babul, N.; Kao, D. Fabrication of Topical Metered Dose Film Forming Sprays for Pain Management. Eur. J. Pharm. Sci. 2017, 100, 132–141. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Wang, H.; Fan, Y.-Y.; Wang, H.; Gao, X.; Niu, B.; Gong, X. Preparation, Characterization, Release and Antioxidant Activity of Curcumin-Loaded Amorphous Calcium Phosphate Nanoparticles. J. Non-Cryst. Solids 2018, 500, 317–325. [Google Scholar] [CrossRef]
- Mori, N.M.; Patel, P.; Sheth, N.R.; Rathod, L.V.; Ashara, K.C. Fabrication and Characterization of Film-Forming Voriconazole Transdermal Spray for the Treatment of Fungal Infection. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 41–51. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S.; Al-Dhubiab, B.; Attimarad, M.; Harsha, S. Basic Considerations in the Dermatokinetics of Topical Formulations. Braz. J. Pharm. Sci. 2013, 49, 423–434. [Google Scholar] [CrossRef]




| Lipids | Active Molecule | EE (%) (± s.d.) | Remarks | Ref. | |
|---|---|---|---|---|---|
| Solid | Liquid | ||||
| Myristic acid | Sunflower, olive, corn, castor, and coconut oil | Quercetin | 99.90 ± 0.01 |
| [46] |
| Compritol 888 ATO | Buriti oil | Amazonian fruit pulp extract | not available |
| [47] |
| Glyceryl dibehenate | Oleic acid | Hazelnut (Corylus avellana) extract | 70.0 ± 0.23 |
| [48] |
| Precirol® ATO | Miglyol® 812 | Lutein | 94.73 ± 0.03 |
| [49] |
| Shea butter and beeswax | Carrot seed oil | Gamma— Oryzanol | 90.00 ± not available |
| [50] |
| GMS and capric acid | Lecithin | Propolis extract | 83.29 ± 0.47 87.21 ± 0.79 |
| [35] |
| Cocoa butter | Conjugated linoleic acid | Conjugated linoleic acid | 98.2 ± not available |
| [51] |
| Precirol | Olive oil | Oleuropein | 99.12 ± 0.70 |
| [52] |
| Palm stearin | Palm olein | β-carotene | 91.20 ± 0.15 |
| [53] |
| Compritol 888 ATO | Miglyol® 812 | Butterfly Pea Extract | 72.51 ± 1.11 |
| [54] |
| Drug | Dosage From | Mean Size (nm ± s.d.) | Penetration Potential | Ref. |
|---|---|---|---|---|
| Carboxyfluorescein (hydrophilic molecule) | Liposomes | 70 ± 2.6 to 90 ± 2.9 | Vesicles are not capable of delivering the payload into the depth of the skin layers. After drying, these vesicles may deposit in or on the SC in the form of a lipid layer. | [91] |
| 1,1-dioctadecyl-3,3,3,3 -tetramethylindocarbo- cyanine perchlorate (lipophilic molecule) | Liposomes | 58 ± 9.0 | Highest deposition in viable epidermal and dermal layers of the payload can be expected | [92] |
| Ivabradine HCl | Transfersomes | 206.7 ± 15.3 | Can deliver drugs up to some extent into deeper skin layers | [93] |
| Not available | Not available | 6–36 ± not available | The uptake can occur across both through lipidic trans-epidermal routes or aqueous pores | [94,95] |
| Insulin | Liposome and iontophoresis | 100–400 ± not available | Drug molecules can be delivered via the trans-follicular route | [96,97] |
| Carrier | Cargo | Composition | Remarks | Ref. |
|---|---|---|---|---|
| Lipid mesophases | Curcumin | Lipids extracted from the marine microalga Nanno chloropsis sp. |
| [165] |
| Lipid-based nanosystem | Caffeic acid | 1 DMPC, 2 DPPC and 3 BPL |
| [166] |
| Liposomes/giant vesicles/crystalline Cubic Phase | Catechin | 4 DOPC/5 POPC/lipid monoolein and DOPC, chitosan |
| [167] |
| Liposomes | Resveratrol | PEGylated phospholipids |
| [168] |
| 6 MAD | Curcumin | Glyceryl monooleate poloxamer 407/sodium cholate-sodium caseinate (alternate surfactants) |
| [169,170] |
| Crocin | Glyceryl monooleate, Sodium cholate Mixture of sodium cholate with sodium caseinate |
| [171] | |
| Liposomes | Curcumin | 7 SPC, Ascorbyl palmitate (AP) |
| [164,172,173] |
| DDS | Gelling Agent | Cargo | In-Vivo Model | Remarks | Ref. |
|---|---|---|---|---|---|
| NLC | Carbopol 940 | Recombinant human thrombomodulin | Streptozotocin-induced diabetic mice |
| [177] |
| Liposomes | Silk Fibroin | Basic fibroblast growth factor (bFGF) | Deep second-degree scald |
| [178] |
| Hyalurosomes | Hyaluronic acid | Curcumin | Burn-wound model |
| [179] |
| Liposomes | Carbopol 940 | Resveratrol | Wound-induced via biopsy punch in diabetic rat |
| [180] |
| Liposomes | Carbopol | Vitis vinifera Leaf Extract | Wound infection model, and peritonitis infection model |
| [181] |
| Liposome | Glycerin and alcohol | Gallic acid | Defects were created via a metal punch in rats |
| [182] |
| SLN | Carbopol 940 | Fluoxetine | Wound excised with sterile toothed forceps and sharp pointed scissors |
| [183] |
| SLN & niosomes | Carbopol 941 | Vitamin A | Full-thickness wound model |
| [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallan, S.S.; Ferrara, F.; Cortesi, R.; Sguizzato, M. Potential of the Nano-Encapsulation of Antioxidant Molecules in Wound Healing Applications: An Innovative Strategy to Enhance the Bio-Profile. Molecules 2025, 30, 641. https://doi.org/10.3390/molecules30030641
Hallan SS, Ferrara F, Cortesi R, Sguizzato M. Potential of the Nano-Encapsulation of Antioxidant Molecules in Wound Healing Applications: An Innovative Strategy to Enhance the Bio-Profile. Molecules. 2025; 30(3):641. https://doi.org/10.3390/molecules30030641
Chicago/Turabian StyleHallan, Supandeep Singh, Francesca Ferrara, Rita Cortesi, and Maddalena Sguizzato. 2025. "Potential of the Nano-Encapsulation of Antioxidant Molecules in Wound Healing Applications: An Innovative Strategy to Enhance the Bio-Profile" Molecules 30, no. 3: 641. https://doi.org/10.3390/molecules30030641
APA StyleHallan, S. S., Ferrara, F., Cortesi, R., & Sguizzato, M. (2025). Potential of the Nano-Encapsulation of Antioxidant Molecules in Wound Healing Applications: An Innovative Strategy to Enhance the Bio-Profile. Molecules, 30(3), 641. https://doi.org/10.3390/molecules30030641











