The Role of the Functionalization of Biomedical Fabrics on Their Ability to Adsorb and Release Drugs
Abstract
1. Introduction
2. Results and Discussion
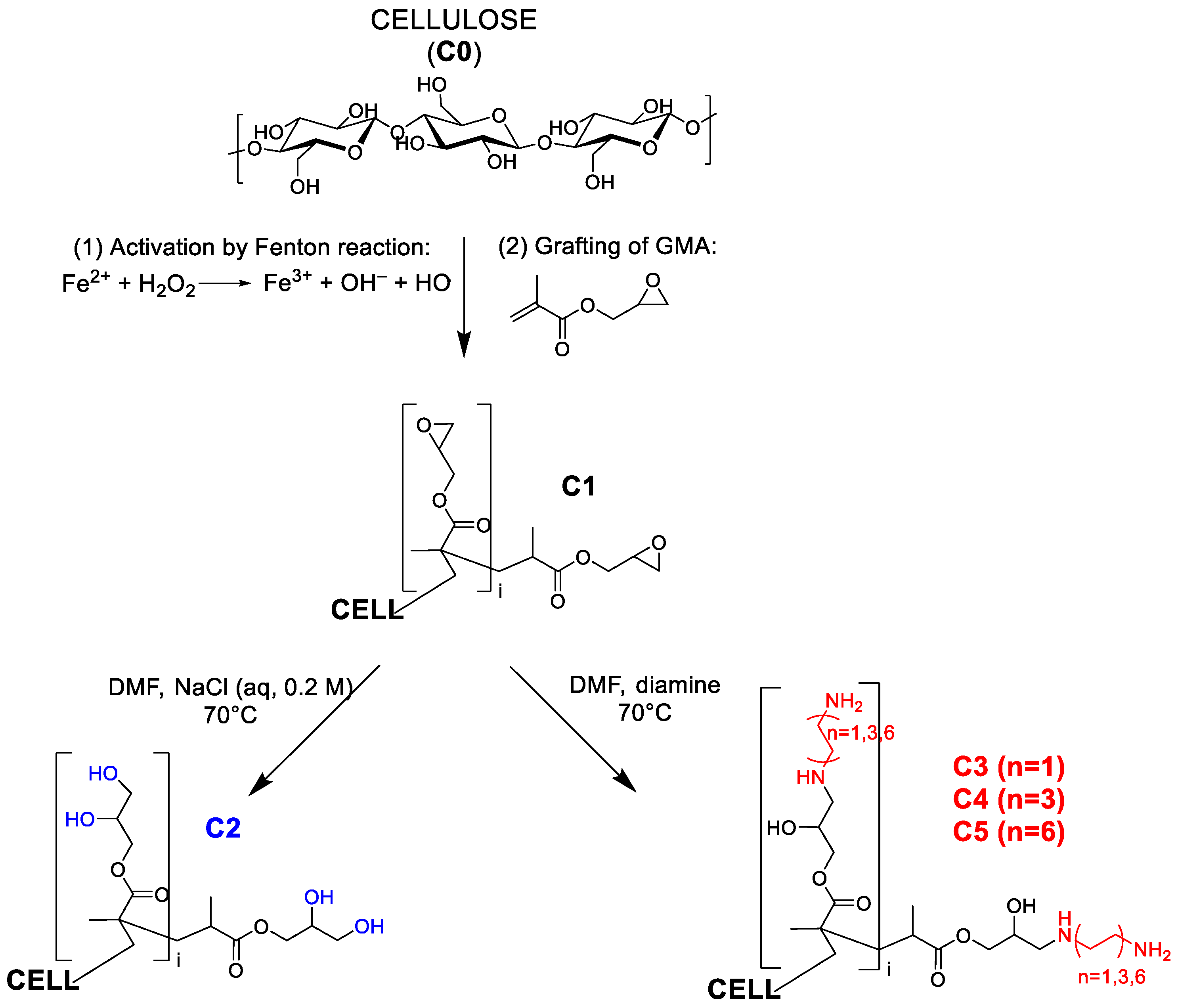
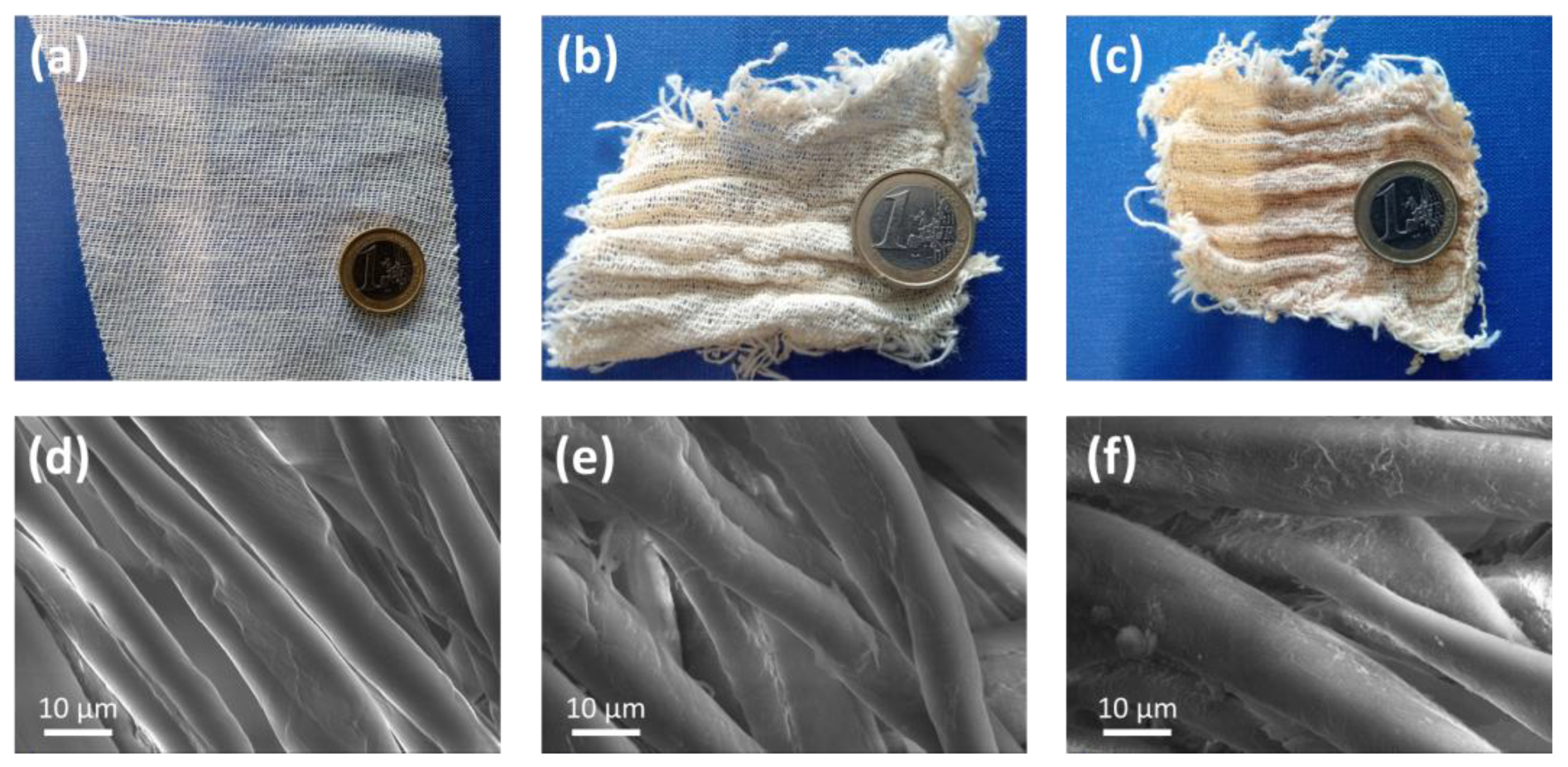
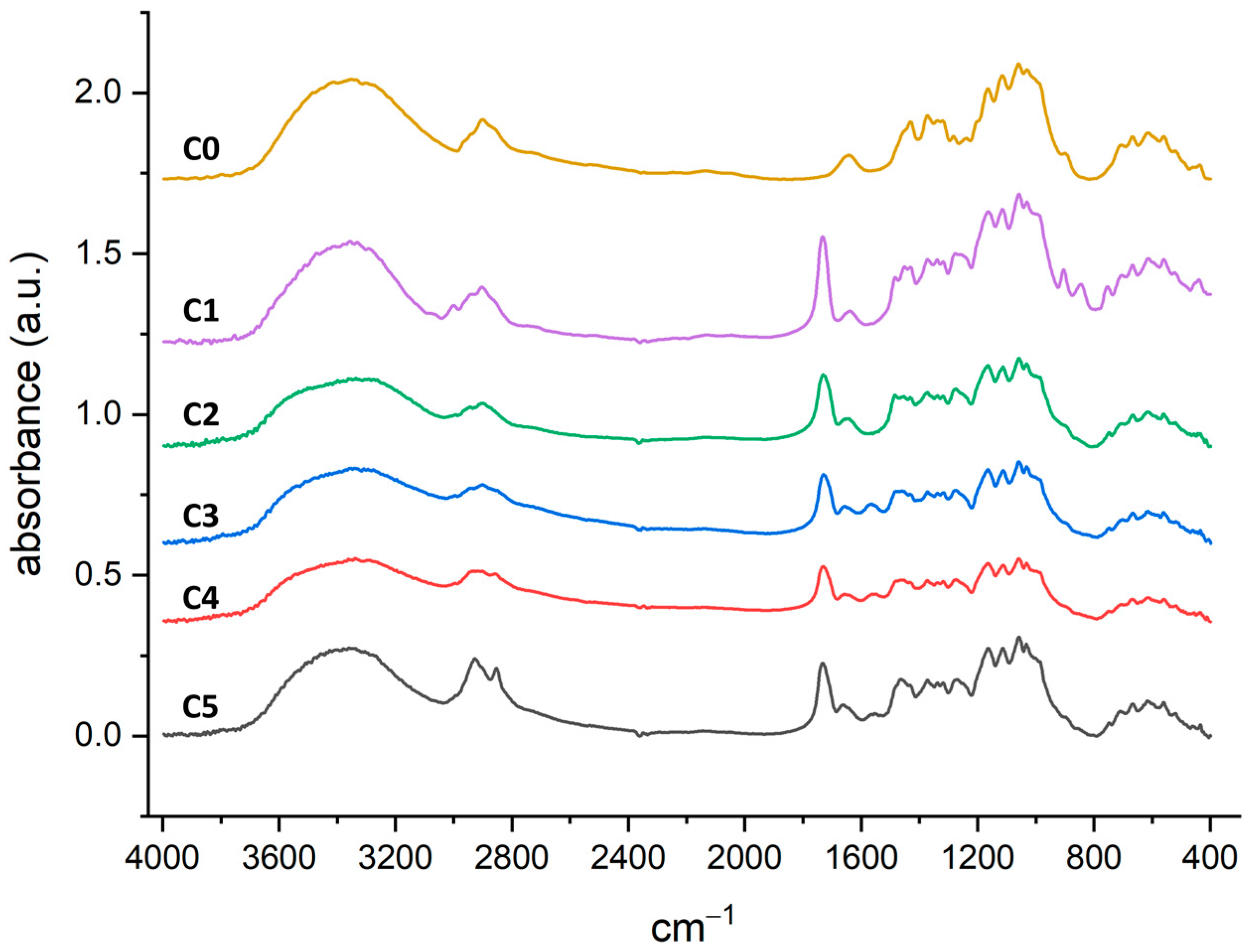
2.1. Preparation and Characterization of the Functionalised Cotton Gauzes
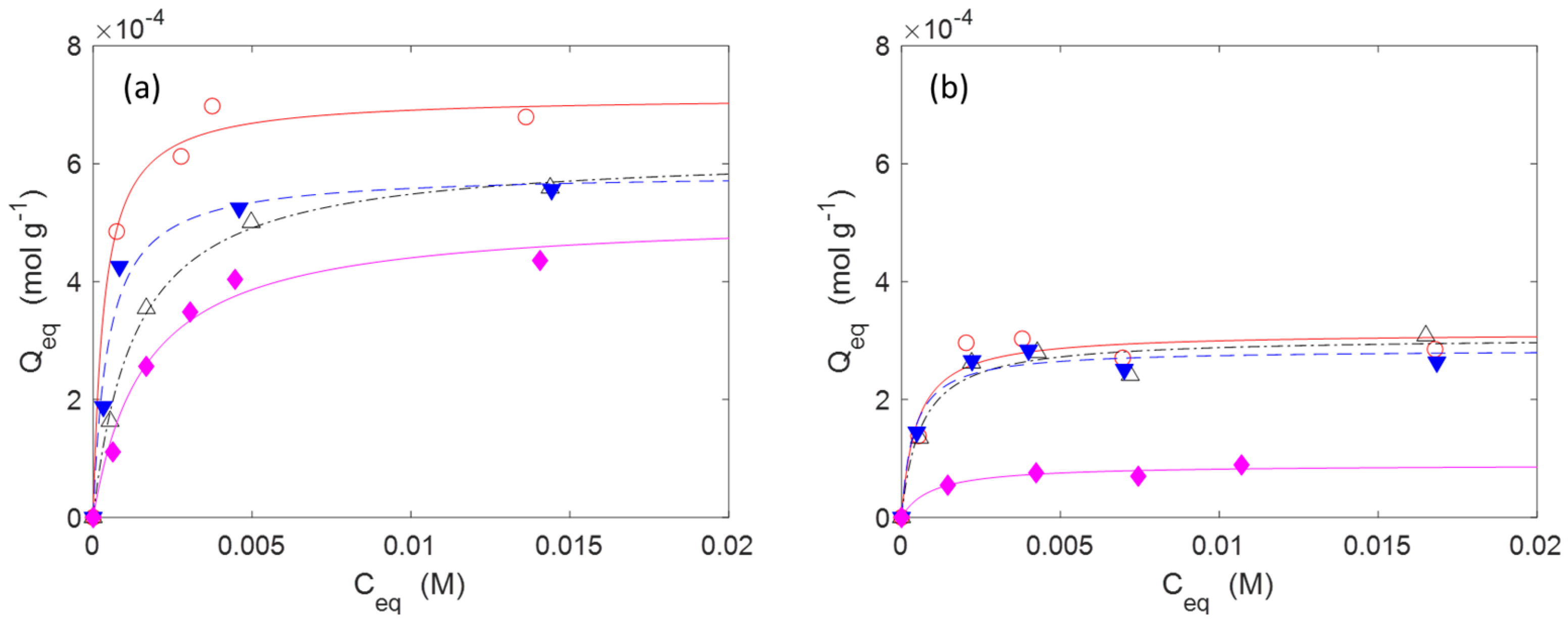
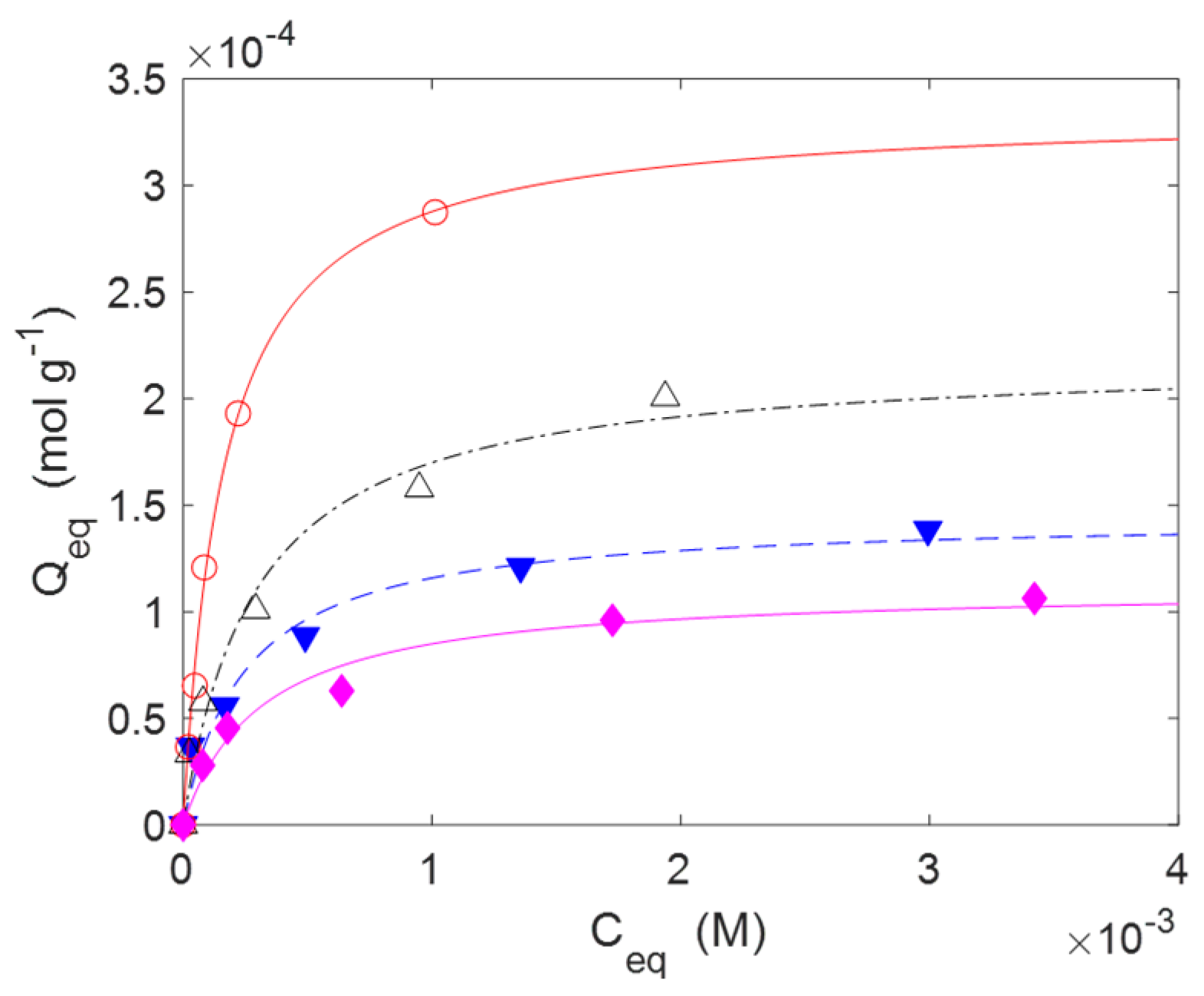
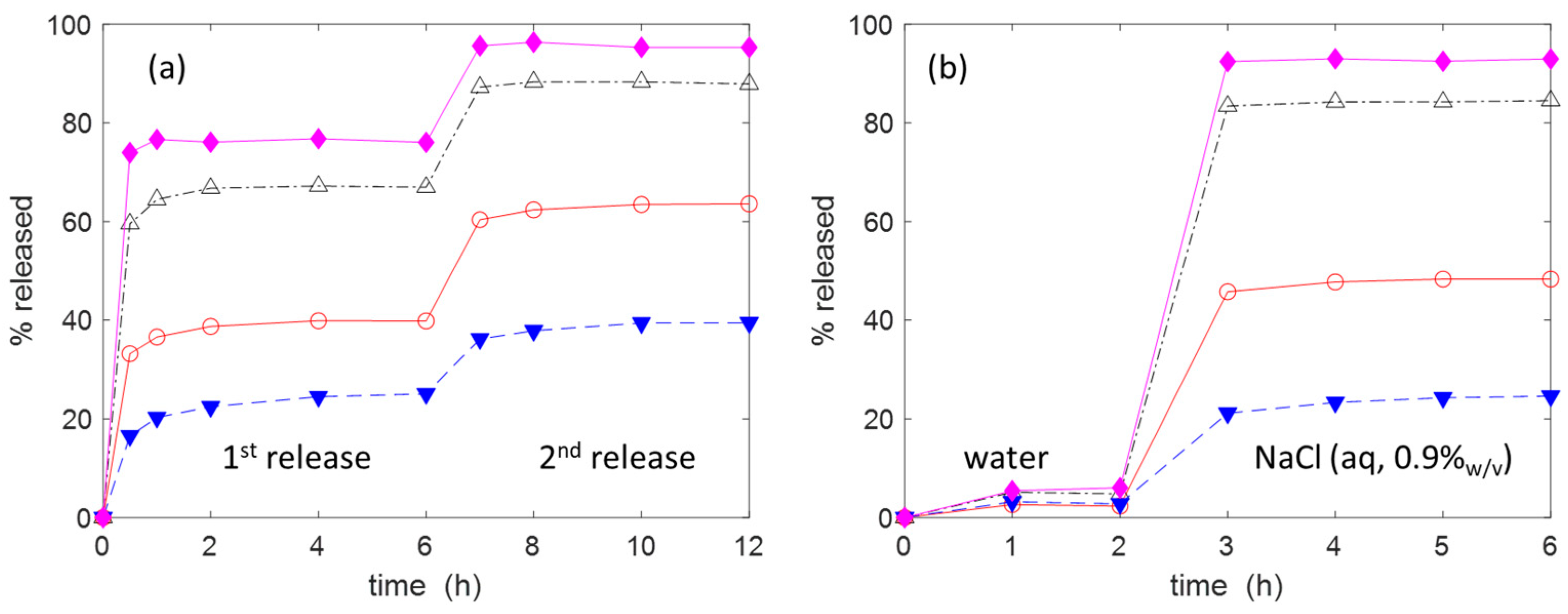
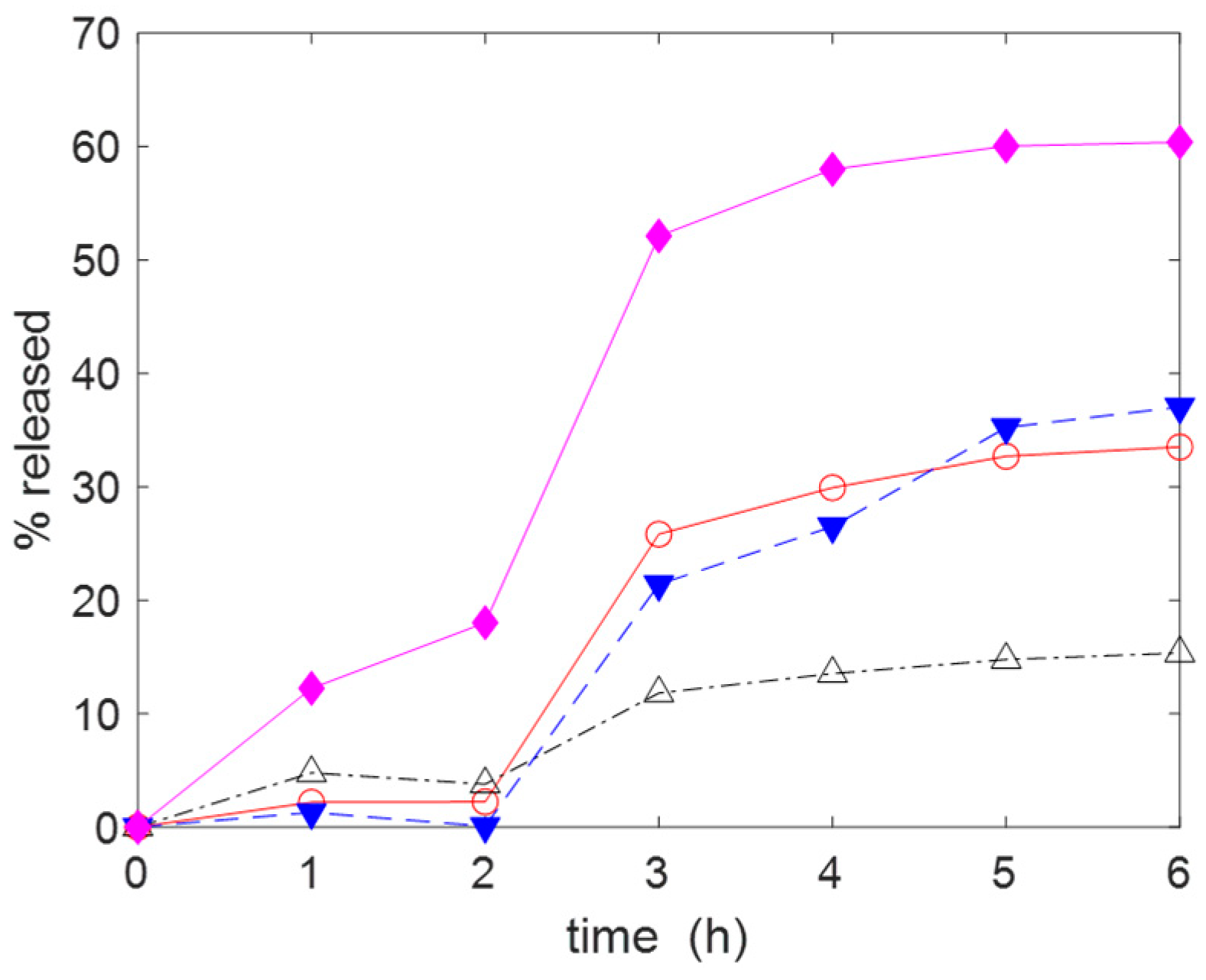
2.2. Adsorption and Release of Drugs
3. Materials and Methods
3.1. Materials Preparation
3.2. Characterization
3.3. Drug Adsorption and Release
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaishya, R.; Agarwal, A.K.; Tiwari, M.; Vaish, A.; Vijay, V.; Nigam, Y. Medical textiles in orthopedics: An overview. J. Clin. Orthop. Trauma 2018, 9, S26–S33. [Google Scholar] [CrossRef]
- Tassw, D.F.; Birlie, B.; Mamaye, T. Nanotechnologies past, present and future applications in enhancing functionality of medical textiles: A review. J. Text. Inst. 2024, 1–21. [Google Scholar] [CrossRef]
- Firmanda, A.; Mahardika, M.; Fahma, F.; Amelia, D.; Pratama, A.W.; Amalia, N.; Syafri, E.; El Achaby, M. Cellulose-enriched ascorbic acid for wound dressing application: Future medical textile. J. Appl. Polym. Sci. 2024, 141, e56013. [Google Scholar] [CrossRef]
- Shai, A.; Maibach, H.I. Wound Healing and Ulcers of the Skin. In Diagnosis and Therapy—The Practical Approach; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Zgonis, T.; Armstrong, D.G.; Driver, V.R.; Giurini, J.M.; Kravitz, S.R.; Landsman, A.S.; Lavery, L.A.; Moore, J.C.; Schuberth, J.M.; et al. Diabetic Foot Disorders: A Clinical Practice Guideline. J. Foot Ankle Surg. 2006, 45, S1–S66. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, D.; Staneva, D.; Grabchev, I. Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery. Materials 2021, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Ongarora, B.G. Recent technological advances in the management of chronic wounds: A literature review. Health Sci. Rep. 2022, 5, e641. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Adiban, H.; Rashidipour, M.; Eskandari, M.R. Bioactive Natural and Synthetic Polymers for Wound Repair. Macromol. Res. 2022, 30, 495–526. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef]
- Rehan, M.; Zaghloul, S.; Mahmoud, F.A.; Montaser, A.S.; Hebeish, A. Design of multi-functional cotton gauze with antimicrobial and drug delivery properties. Mater. Sci. Eng. C 2017, 80, 29–37. [Google Scholar] [CrossRef]
- Granados, A.; Pleixats, R.; Vallribera, A. Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures. Molecules 2021, 26, 3008. [Google Scholar] [CrossRef]
- Kopańska, A.; Brzeziński, M.; Gonciarz, W.; Draczyński, Z. Compatibilizing of cotton fabric with hydrophobic drug cover layer for anti-inflammatory performance with the implementation of ibuprofen. Sci. Rep. 2024, 14, 7310. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guinart, A.; Granados, A.; Gimbert-Suriñach, C.; Fernández, E.; Pleixats, R.; Vallribera, A. Coated Cotton Fabrics with Antibacterial and Anti-Inflammatory Silica Nanoparticles for Improving Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 14595–14604. [Google Scholar] [CrossRef]
- de Oliveira, C.S.F.; Tavaria, F.K. The impact of bioactive textiles on human skin microbiota. Eur. J. Pharm. Biopharm. 2023, 188, 66–77. [Google Scholar] [CrossRef]
- Martel, B.; Morcellet, M.; Ruffin, D.; Vinet, F.; Weltrowski, L. Capture and Controlled Release of Fragrances by CD Finished Textiles. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 439–442. [Google Scholar] [CrossRef]
- Hebeish, A.; Fouda, M.M.G.; Hamdy, I.A.; El-Sawy, S.M.; Abdel-Mohdy, F.A. Preparation of durable insect repellent cotton fabric: Limonene as insecticide. Carbohydr. Polym. 2008, 74, 268–273. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Sawy, S.M.; Ragaei, M.; Hamdy, I.A.; El-Bisi, M.K.; Abdel-Mohdy, F.A. New textiles of biocidal activity by introduce insecticide in cotton-poly (GMA) copolymer containing β-CD. Carbohydr. Polym. 2014, 99, 208–217. [Google Scholar] [CrossRef]
- Kvavadze, E.; Bar-Yosef, O.; Belfer-Cohen, A.; Boaretto, E.; Jakeli, N.; Matskevich, Z.; Meshveliani, T. 30,000-Year-Old Wild Flax Fibres. Science 2009, 325, 1359. [Google Scholar] [CrossRef] [PubMed]
- Shahriari-Khalaji, M.; Alassod, A.; Nozhat, Z. Cotton-based health care textile: A mini review. Polym. Bull. 2022, 79, 10409–10432. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, L.; Zang, C.; Chen, Y.; Lin, H. Antibacterial cotton fabric grafted with silver nanoparticles and its excellent laundering durability. Carbohydr. Polym. 2013, 92, 2088–2094. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef]
- Li, H.; Granados, A.; Fernández, E.; Pleixats, R.; Vallribera, A. Anti-inflammatory Cotton Fabrics and Silica Nanoparticles with Potential Topical Medical Applications. ACS Appl. Mater. Interfaces 2020, 12, 25658–25675. [Google Scholar] [CrossRef] [PubMed]
- Noorian, S.A.; Hemmatinejad, N.; Navarro, J.A.R. BioMOF@cellulose fabric composites for bioactive molecule delivery. J. Inorg. Biochem. 2019, 201, 110818. [Google Scholar] [CrossRef]
- Macha, I.J.; Muna, M.M.; Magere, J.L. In vitro study and characterization of cotton fabric PLA composite as a slow antibiotic delivery device for biomedical applications. J. Drug Deliv. Technol. 2018, 43, 172–177. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Ji, J. Antibacterial Properties of Cotton Fabrics Treated with Chitosan. Text. Res. J. 2003, 73, 1103–1106. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Application of a fiber-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Carbohydr. Polym. 2004, 56, 227–234. [Google Scholar] [CrossRef]
- Gupta, B.; Arora, A.; Saxena, S.; Sarwar, A.M. Preparation of chitosan–polyethylene glycol coated cotton membranes for wound dressings: Preparation and characterisation. Polym. Adv. Technol. 2009, 20, 58–65. [Google Scholar] [CrossRef]
- Ruan, H.; Aulova, A.; Ghai, V.; Pandit, S.; Lovmar, M.; Mijakovic, I.; Kádár, R. Polysaccharide-based antibacterial coating technologies. Acta Biomater. 2023, 168, 42–77. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles—Review. J. Control. Release 2016, 224, 146–157. [Google Scholar] [CrossRef]
- Wang, J.H.; Cai, Z. Incorporation of the antibacterial agent, miconazole nitrate into a cellulosic fabric grafted with b-cyclodextrin. Carbohydr. Polym. 2008, 72, 695–700. [Google Scholar] [CrossRef]
- Hedayati, N.; Montazer, M.; Mahmoudirad, M.; Toliyat, T. Ketoconazole and Ketoconazole/β-cyclodextrin performance on cotton wound dressing as fungal skin treatment. Carbohydr. Polym. 2020, 240, 116267. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Guan, Y.; Ziaee, Z.; He, B.; Zheng, A.; Xiao, H. Rendering cellulose fibres antimicrobial using cationic, β-cyclodextrin-based polymers included with antibiotics. Cellulose 2009, 16, 309–317. [Google Scholar] [CrossRef]
- Hiriart-Ramírez, E.; Contreras-García, A.; Garcia-Fernandez, M.J.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Radiation grafting of glycidyl methacrylate onto cotton gauzes for functionalization with cyclodextrins and elution of antimicrobial agents. Cellulose 2012, 19, 2165–2177. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S.; Alfaifi, A.Y.A. Cotton fabric finished with -cyclodextrin: Inclusion ability toward antimicrobial agent. Carbohydr. Polym. 2014, 102, 550–556. [Google Scholar] [CrossRef]
- Romi, R.; Lo Nostro, P.; Bocci, E.; Ridi, F.; Baglioni, P. Bioengineering of a Cellulosic Fabric for Insecticide Delivery via Grafted Cyclodextrin. Biotechnol. Prog. 2005, 21, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Fu, G.; He, B. Preparation and adsorption properties of PAA-grafted cellulose adsorbent for low-density lipoprotein from human plasma. Cellulose 2007, 14, 99–107. [Google Scholar] [CrossRef]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.T.; Chen, L.C.; Lee, C.C.; Pan, T.C.; You, B.X.; Yan, Q.F. Preparation of methacrylic acid-modified rice husk improved by an experimental design and application for paraquat adsorption. J. Hazard. Mater. 2009, 171, 465–470. [Google Scholar] [CrossRef]
- Lumbreras-Aguayo, A.; Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Alvarado-Canché, C.; Ledezma, A.; Romero-García, J.; Betancourt-Galindo, R. Poly(methacrylic acid)-modified medical cotton gauzes with antimicrobial and drug delivery properties for their use as wound dressings. Carbohydr. Polym. 2019, 205, 203–210. [Google Scholar] [CrossRef]
- Andreozzi, L.; Castelvetro, V.; Ciardelli, G.; Corsi, L.; Faetti, M.; Fatarella, E.; Zulli, F. Free radical generation upon plasma treatment of cotton fibers and their initiation efficiency in surface-graft polymerization. J. Colloid Interface Sci. 2005, 289, 455–465. [Google Scholar] [CrossRef]
- Alberti, A.; Bertini, S.; Gastaldi, G.; Iannaccone, N.; Macciantelli, D.; Torri, G.; Vismara, E. Electron beam irradiated textile cellulose fibres.: ESR studies and derivatisation with glycidyl methacrylate (GMA). Eur. Polym. J. 2005, 41, 1787–1797. [Google Scholar] [CrossRef]
- Elmaaty, T.A.; Okubayashi, S.; Elsisi, H.; Abouelenin, S. Electron beam irradiation treatment of textiles materials: A review. J. Polym. Res. 2022, 29, 117. [Google Scholar] [CrossRef]
- Vismara, E.; Melone, L.; Gastaldi, G.; Cosentino, C.; Torri, G. Surface functionalization of cotton cellulose with glycidyl methacrylate and its application for the adsorption of aromatic pollutants from wastewaters. J. Hazard. Mater. 2009, 170, 798–808. [Google Scholar] [CrossRef]
- Vismara, E.; Melone, L.; Torri, G. Surface Functionalizationed Cotton with Glycidyl Methacrylate: Physico-Chemical Aspects and Multitasking Applications. In Cotton: Cultivation, Varieties and Uses; Giuliano, B., Vinci, E.J., Eds.; Nova Science Publishers: New York, NY, USA, 2012; ISBN/ISSN: 978-1-61942-746-4. [Google Scholar]
- Dawlee, S.; Jayakrishnan, A.; Jayabalan, M. Studies on novel radiopaque methyl methacrylate: Glycidyl methacrylate based polymer for biomedical applications. J. Mater. Sci. Mater. Med. 2009, 20, S243–S250. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.F.R.; Torres-Labandeira, J.J.; Matthijs, N.; Coenye, T.; Concheiro, A.; Alvarez-Lorenzo, C. Functionalization of acrylic hydrogels with α-, β- or γ-cyclodextrin modulates protein adsorption and antifungal delivery. Acta Biomater. 2010, 6, 3919–3926. [Google Scholar] [CrossRef]
- Travan, A.; Marsich, E.; Donati, I.; Benincasa, M.; Giazzon, M.; Felisari, L.; Paoletti, S. Silver–polysaccharide nanocomposite antimicrobial coatings for methacrylic thermosets. Acta Biomater. 2011, 7, 337–346. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Bencherif, S.A.; Matyjaszewski, K. Graft Copolymers by a Combination of ATRP and Two Different Consecutive Click Reactions. Macromolecules 2007, 40, 4439–4445. [Google Scholar] [CrossRef]
- Zhang, Q.; Slavin, S.; Jones, M.W.; Haddleton, A.J.; Haddleton, D.M. Terminal functional glycopolymers via a combination of catalytic chain transfer polymerisation (CCTP) followed by three consecutive click reactions. Polym. Chem. 2012, 3, 1016. [Google Scholar] [CrossRef]
- Vismara, E.; Melone, L.; Torri, G.; Graziani, G.; Montanelli, A. Derivatised Polysaccharide Material for the Topic Antibacterial Activity. EP2182931 (B1) 6 July 2016. [Google Scholar]
- Vismara, E.; Melone, L.; Torri, G.; Graziani, G.; Montanelli, A. Method and Kit for Antibiogram. WO2010/010582 A1 28 January 2010. [Google Scholar]
- Bullen, J.C.; Saleesongsom, S.; Gallagher, K.; Weiss, D.J. A Revised Pseudo-Second-Order Kinetic Model for Adsorption, Sensitive to Changes in Adsorbate and Adsorbent Concentrations. Langmuir 2021, 37, 3189–3201. [Google Scholar] [CrossRef] [PubMed]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 1–2, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part II. Models with more than two parameters. J. Hazard. Mater. 2007, 1–2, 401–411. [Google Scholar] [CrossRef]
- Felix, I.M.B.; Moreira, L.C.; Chiavone-Filho, O.; Mattedi, S. Solubility measurements of amoxicillin in mixtures of water and ethanol from 283.15 to 298.15 K. Fluid Phase Equilib. 2016, 422, 78–86. [Google Scholar] [CrossRef]
- Fernandez, R.; Green, H.L.; Griffiths, R.; Atkinson, R.A.; Ellwood, L.J. Water for wound cleansing. Cochrane Database Syst. Rev. 2022, 9, CD003861. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef]
- Basha, S.; Barr, C.; Keane, D.; Nolan, K.; Morrissey, A.; Oelgemőllerd, M.; Tobin, J.M. On the adsorption/photodegradation of amoxicillin in aqueous solutions by an integrated photocatalytic adsorbent (IPCA): Experimental studies and kinetics analysis. Photochem. Photobiol. Sci. 2011, 10, 1014. [Google Scholar] [CrossRef] [PubMed]


| GMA/Cellulose Preparative Ratio (mL/g) | N (%) | C (%) | H (%) | DG † (mmol g−1) | |
|---|---|---|---|---|---|
| C1(L) | 2/6 | 2.44 ± 0.1 | 41.42 ± 0.5 | 8.98 ± 0.3 | 0.596 |
| C1(H) | 6/6 | 7.48 ± 0.2 | 43.41 ± 0.5 | 9.25 ± 0.3 | 1.929 |
| † DG = 1000 × (N(%)/100)/(42 − 43 × (N(%)/100) | (1) | ||||
| Diamine § | N (%) | C (%) | H (%) | DG(*) ‡ | Amine Content (mmol g−1) † | |
|---|---|---|---|---|---|---|
| C3(L) | DAE (60.10) | 1.08 ± 0.05 | 41.30 ± 0.5 | 9.19 ± 0.3 | 0.395 | 0.77 |
| C4(L) | DAH (116.20) | 1.08 ± 0.05 | 41.08 ± 0.5 | 9.65 ± 0.3 | 0.404 | 0.77 |
| C5(L) | DAD (200.36) | 0.87 ± 0.05 | 42.72 ± 0.5 | 9.32 ± 0.3 | 0.331 | 0.62 |
| C3(H) | DAE (60.10) | 3.91 ± 0.1 | 43.49 ± 0.5 | 10.30 ± 0.3 | 1.524 | 2.79 |
| C4(H) | DAH (116.20) | 3.14 ± 0.1 | 46.23 ± 0.5 | 10.19 ± 0.3 | 1.289 | 2.24 |
| C5(H) | DAD (200.36) | 2.56 ± 0.1 | 46.99 ± 0.5 | 11.77 ± 0.3 | 1.119 | 1.83 |
| ‡ DG(*) represents the DG that would be obtained if a single diamine molecule, having a molecular weight Mw (g mol−1), reacted with a single epoxide unit. | ||||||
| DG(*) = 1000 × (N(%)/100)/(28 − Mw × (N(%)/100)) | (2) | |||||
| § The molecular weight (g mol−1) is reported between commas. | ||||||
| † Amine content (mmol g−1) = 1000 × (%N/100)/14 | (3) | |||||
Ibuprofen | Amoxicillin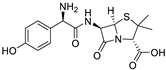 | |||
|---|---|---|---|---|
| k2 (g mol−1 h−1) | Qe (mol g−1) | k2 (g mol−1 h−1) | Qe (mol g−1) | |
| C2(H) | 6.77 × 10−5 | 2.253 × 10−4 | 1.03 × 10−4 | 7.842 × 10−5 |
| C3(H) | 1.34 × 10−5 | 2.941 × 10−4 | 3.86 × 10−4 | 1.130 × 10−4 |
| C4(H) | 5.15 × 10−5 | 3.664 × 10−4 | 1.32 × 10−4 | 1.165 × 10−4 |
| C5(H) | 1.35 × 10−5 | 3.754 × 10−4 | 2.90 × 10−4 | 1.013 × 10−4 |
| IB | AM | |||
|---|---|---|---|---|
| Qm (mol g−1) | b (M−1) | Qm (mol g−1) | b (M−1) | |
| C2(L) | 8.942 × 10−5 | 1.066 × 103 | n.a. | n.a. |
| C3(L) | 3.056 × 10−4 | 1.631 × 103 | n.a. | n.a. |
| C4(L) | 3.317 × 10−4 | 2.113 × 103 | n.a. | n.a. |
| C5(L) | 2.848 × 10−4 | 2.613 × 103 | n.a. | n.a. |
| C2(H) | 5.120 × 10−4 | 6.146 × 102 | 1.118 × 10−4 | 3.149 × 103 |
| C3(H) | 6.210 × 10−4 | 7.542 × 102 | 2.194 × 10−4 | 3.439 × 103 |
| C4(H) | 7.144 × 10−4 | 2.916 × 103 | 3.349 × 10−4 | 6.102 × 103 |
| C5(H) | 5.845 × 10−4 | 2.114 × 103 | 1.447 × 10−4 | 4.008 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melone, L. The Role of the Functionalization of Biomedical Fabrics on Their Ability to Adsorb and Release Drugs. Molecules 2025, 30, 552. https://doi.org/10.3390/molecules30030552
Melone L. The Role of the Functionalization of Biomedical Fabrics on Their Ability to Adsorb and Release Drugs. Molecules. 2025; 30(3):552. https://doi.org/10.3390/molecules30030552
Chicago/Turabian StyleMelone, Lucio. 2025. "The Role of the Functionalization of Biomedical Fabrics on Their Ability to Adsorb and Release Drugs" Molecules 30, no. 3: 552. https://doi.org/10.3390/molecules30030552
APA StyleMelone, L. (2025). The Role of the Functionalization of Biomedical Fabrics on Their Ability to Adsorb and Release Drugs. Molecules, 30(3), 552. https://doi.org/10.3390/molecules30030552




